Abstract
Background
Nontuberculous mycobacterial lung disease (NTMLD) is a rare, progressive disease with an increasing incidence worldwide.
Aims
The aim of this retrospective study was to analyze the baseline characteristics and management of NTMLD in general and pneumologist practices in Germany.
Methods
This retrospective study included patients with a culture-confirmed diagnosis of NTMLD documented between October 1, 2014 and September 30, 2019 by 125 general practitioners (GP) and 31 office-based pulmonologists from the IMS Disease Analyzer Database (IQVIA).
Results
A total of 159 patients managed by German GPs (mean age 59 ± 19 years, 51% female) and 236 patients managed by pulmonologists (mean age 62 ± 14 years, 58% female) were analyzed. In total, 45% (72/159) and 40% (94/236) of patients managed by GPs and pulmonologists respectively received antibiotic therapy for NTMLD. This therapy lasted for ≥ 6 months in 42%, for ≥ 12 months in 24%, and ≥ 18 months in 8% of patients. The average therapy duration was longer in patients treated by pulmonologists (241 ± 196 days) than in patients treated by GPs (113 ± 152 days). A total of 27% of patients managed by GPs and 45% of those managed by pulmonologists respectively received guideline-based therapy (GBT), defined as combination therapy with macrolide (azi-/ clarithromycin) + ethambutol + rifabutin/rifampicin, at least once; however, almost all patients (100% in the GP group, 96% in the pulmonologist group) also received non-GBT regimens intermediately.
Conclusions
A considerable number of patients with NTMLD were not managed in accordance with the German guidelines and a substantial proportion also discontinue therapy prematurely. NTMLD management should be improved through appropriate referral pathways and collaboration between expert centers and primary or secondary care physicians.
Keywords: Nontuberculous mycobacterial lung disease, Outpatients, Antibiotic, Guideline-based therapy
1. Introduction
Nontuberculous mycobacterial lung disease (NTMLD) is a rare but progressive chronic disease that affects that affects mainly or almost exclusively vulnerable populations; many patients diagnosed with the disease have preexisting lung diseases or are immunodeficient [1]. NTMLD has a considerable impact on patients’ health-related quality of life [2]. It accelerates respiratory function decline in patients with underlying structural lung disease [3], and also increases the risk of hospitalization [4], [5] and the risk of death [6].
In recent decades, the incidence and prevalence of NTMLD have increased worldwide [1]. In line with this trend, the prevalence of NTMLD in Germany is reported to have increased from 2.3 to 3.3 per 100,000 persons in the period from 2009 to 2014 [7].
Non-specific symptoms and frequently coexisting pulmonary conditions as well as the rarity of the disease and lack of awareness concerning the disease among physicians often delay the diagnosis of NTMLD [8]. Furthermore, as treatment regimens consist of a combination of several drugs given over a prolonged period of time with frequently occurring adverse events, adherence to therapy is generally low [9]. In addition, previous studies [10], [11] have highlighted poor adherence by physicians to management guidelines for patients suffering from the disease. All of these factors contribute to poor treatment outcomes in real-world practice.
A previous study has shown that the majority of patients with NTMLD in Germany are managed in community care setting [7]. The goal of this study was to analyze the baseline patient characteristics and the real-world outpatient management for patients with NTMLD in in Germany.
2. Materials and methods
2.1. Database
This study was based on data from the Disease Analyzer database (IQVIA), which compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymized format from computer systems used in the practices of general practitioners and office-based specialists in Germany [12]. Diagnoses (International Classification of Diseases, 10th revision [ICD-10]), prescriptions (European Pharmaceutical Marketing Research Association [EphMRA] Anatomical Therapeutic Chemical [ATC] classification system), and the quality of the reported data are monitored by IQVIA based on a number of criteria (e.g., completeness of documentation and linkage between diagnoses and prescriptions) [12], [13]. The sampling method used to select physicians’ practices for the Disease Analyzer database is based on statistics from all doctors in Germany. These statistics are used to determine the panel composition according to the following strata: region, community size category, and physician age. This database has a coverage of 3%−4% of private practices in Germany and actually contains data from approximately 1200 general practitioners (GP) and 40 pneumologists. Other specialties like gynecologists, neurologists, psychiatrists, urologists, orthopedists are also included. The Disease Analyzer database has been shown to be representative of clinical practices in Germany [12], [13].
2.2. Study population
This study included patients with an initial confirmed diagnosis of pulmonary mycobacterial infection (ICD-10: A31.0), other mycobacterial infection (ICD-10: A31.8), or unspecified mycobacterial infection (ICD-10: A31.9) documented between October 1, 2014 and September 30, 2019 by a GP or pulmonologist. Further inclusion criteria were an observation time of at least 12 months prior to and a follow-up time of at least 12 months after the index date (date of initial documented NTM diagnosis). A subgroup of patients who were observed for at least 18 months after the initiation of therapy was selected for the analysis of treatment patterns.
2.3. Study outcomes
The outcomes of the study included baseline characteristics of NTMLD patients (age, sex, health insurance coverage, and comorbidities), as well as treatment patterns (proportion of patients receiving guideline-based treatment (GBT) in accordance with the current German guidelines [14], time to therapy initiation, and therapy duration). GBT was defined as combination therapy with macrolide (azi-/clarithromycin) + ethambutol + rifabutin/rifampicin, which is the recommended therapy for the most common species causing NTMLD, i.e. Mycobacterium avium complex. Use of other antibiotics or use of macrolides, ethambutol and rifabutin/rifampicin as mono or dual therapy (and no triple combination) was considered Non-GBT.
Patients who initiated therapy between October 1, 2014 and March 30, 2018 (N = 130) and had a follow-up time of at least 18 months after the start of therapy were included in the analysis of the therapy duration. Two methods were used to analyze the overall therapy duration. The first of these involved deriving the overall therapy from the time between the first prescription until the last day’s supply of the last prescription. Using the second method, the overall therapy duration was defined as the time between the first prescription until the occurrence of a therapy gap of at least 60 days, if any, or the last day’s supply of the last prescription in cases where no gaps occurred. The number of drug switches that occurred before termination of therapy was estimated, and the periods when these switches occurred (0–3, 4–6, 7–9, 10–12, 13–18 months after therapy initiation) were shown.
2.4. Statistical analyses
Most of the analyses performed for the purposes of this study are of a descriptive nature and no hypotheses were tested. A multivariate logistic regression was used to study the association between defined variables (age, sex, COPD, tuberculosis and reflux disease) and the probability of GBT being applied in NTMLD patients. A p-value of <0.05 was considered statistically significant. All analyses were performed using SAS 9.4.
2.5. Ethics statement
The use of anonymous electronic medical records for research purposes is permitted by German law under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study, which contains no directly identifiable data. As patients were only studied as aggregates and no protected health information was available for queries, no institutional review board (IRB) approval was required for the use of this database or the completion of this study.
3. Results
A total of 395 individual patients (159 patients managed by 125 GPs and 236 patients managed by 31 pulmonologists) were available for analysis. The mean age of patients (±standard deviation) managed by GPs and pulmonologists was 59 (±19) and 62 (±14) years respectively; 51% (81/159) and 58% (138/236) of patients managed by GPs and pulmonologists respectively were female, and 95%-97% had statutory health insurance coverage. COPD was the most common comorbidity recorded (in 22% of patients treated by GPs and in 47% of patients treated by pulmonologists) (Table 1). No immunodeficiency diagnoses were identified in the sample used for this study.
Table 1.
Baseline characteristics of study patients with NTMLD diagnosis.
| Variable | GPs | PNs |
|---|---|---|
| N | 159 | 236 |
| Age | ||
| Mean (±SD) | 59,3 (±19.1) | 61,7 (±14.3) |
| <50 | 38 (24%) | 33 (14%) |
| 50–59 | 32 (20%) | 57 (24%) |
| 60–69 | 32 (20%) | 69 (29%) |
| 70–79 | 40 (25%) | 64 (27%) |
| ≥80 | 17 (11%) | 13 (6%) |
| Female | 81 (51%) | 138 (58%) |
| Male | 78 (49%) | 98 (42%) |
| Private health insurance coverage | 8 (5%) | 16 (7%) |
| Statutory health insurance coverage | 151 (95%) | 220 (93%) |
| Diagnosis documented within 12 months prior to the index date | ||
| COPD | 46 (29%) | 99 (42%) |
| Tuberculosis | 30 (19%) | 52 (22%) |
| Reflux Disease | 27 (17%) | 14 (6%) |
| Asthma | 14 (9%) | 33 (14%) |
| Coronary Heart Disease | 14 (9%) | 0 (0%) |
| Chronic bronchitis, emphysema | 11 (7%) | 35 (15%) |
| Bronchiectasis | 8 (5%) | 33 (14%) |
| Rheumatoid Arthritis | 8 (5%) | 0 (0%) |
| Lung Cancer | 3 (2%) | 9 (4%) |
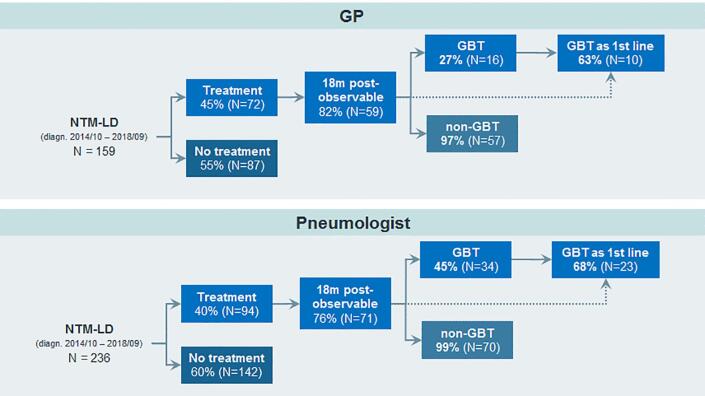
A total of 45% (72/159) of study patients diagnosed by GPs and 40% (94/236) of patients diagnosed by pulmonologists received antibiotic therapy for NTMLD (Fig. 1). While 27% (16/59) and 48% (34/71) of NTMLD patients managed by GPs and pulmonologists respectively, received GBT at least once, almost all patients (100% in GP, 96% in pulmonology practice) were also treated with a non-GBT regimen (Fig. 1).
Fig. 1.
Proportions of NTMLD patients receiving antibiotic treatment and GBT.
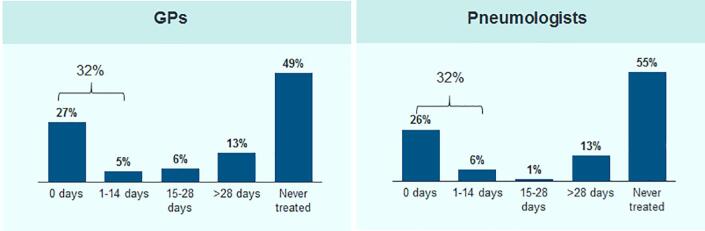
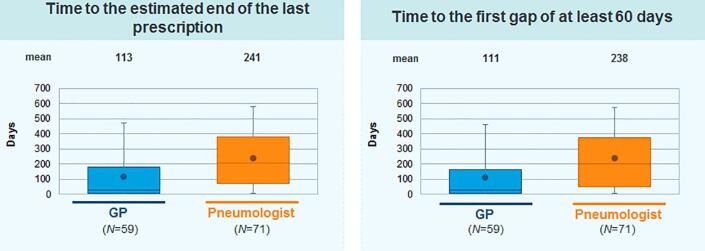
For the majority of patients treated in both specialties, treatment was started within 2 weeks after initial NTMLD diagnosis (Fig. 2). In 42% of the patients treated, therapy lasted more than 6 months, while only 24% of the treated patients received medication for at least 12 months and only 8% were still receiving therapy after 18 months (Fig. 3). The average therapy duration was longer in patients treated by pulmonologists [241 (±196) days] than in patients treated by GPs [113 (±152) days] (Fig. 4).
Fig. 2.
Time from first NTMLD diagnosis to onset of antibiotic treatment (for patients with at least 18 months follow-up time after diagnosis).
Fig. 3.
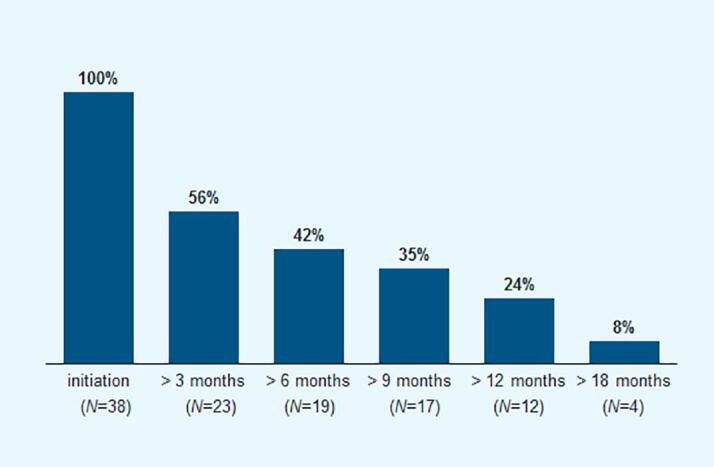
Treatment persistence over time (for patients with at least 18 months follow-up time after diagnosis).
Fig. 4.
Average antibiotic therapy duration in patients with NTMLD.
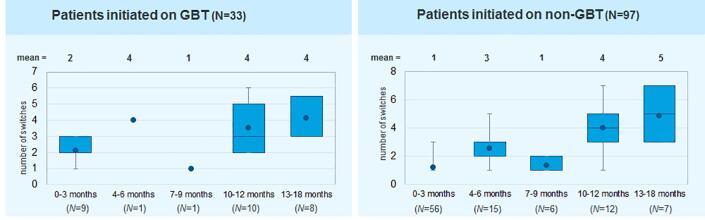
The number of therapy switches before the termination of treatment for patients initiated on GBT or a non-GBT regimen is presented in Fig. 5. In the early phase of treatment, patients treated with GBT switched therapy regimen slightly more often than those on a non-GBT regimen.
Fig. 5.
Number of therapy switches before termination of therapy.
In the multivariate logistic regression, age, sex, physician specialty, and COPD as most frequent co-diagnosis were not significantly associated with the probability of prescribing a GBT regimen (Table 2).
Table 2.
Association between predefined variables and the probability of GBT (multivariate regression model).
| Variable | Odds Ratio (95% confidence intervals) | P-value |
|---|---|---|
| Age (per year) | 1.00 (0.98–1.02) | 0.877 |
| Male versus female | 1.21 (0.67–2.20) | 0.535 |
| PN versus GP | 1.52 (0.78–2.93) | 0.216 |
| COPD diagnosis | 1.01 (0.54–1.91) | 0.967 |
4. Discussion
Real-world treatment patterns for NTMLD patients were analyzed in this study. A considerable number of the patients identified were continuously treated by GPs. In total, less than half of patients (166/395) received any antibiotic treatment for NTMLD. Adherence to guideline-based therapy was low, particularly in the group of patients treated by GPs. Moreover, the majority of patients treated stopped therapy prematurely, and the mean treatment duration was more than 50% shorter for patients treated by GPs than those treated by pulmonologists (113 days compared to 241 days respectively).
The demographic characteristics of patients with NTMLD in the current study were in line with those in a previous study by Ringshausen et al. [7]. The proportions of patients with COPD or chronic bronchitis or emphysema (42%) were lower in the present study, compared to Ringshausen et al. [7] (69%), however.
In the present study, 45% (GP) and 40% (pulmonologist) of patients received antibiotic therapy for NTMLD, and 27% (GP) and 26% (pulmonologist) began treatment immediately at the time of diagnosis. In the study by Diel et al. (2017), which covered both inpatient and outpatient settings, 54% of patients began antibiotic treatment at the time of diagnosis, whereby the overall median time between diagnosis and antibiotic therapy initiation was 32 days [15]. The main differences between the results of the two studies are likely driven by differences in the databases used.
In the current study, GPT was prescribed at least once in 27% of patients treated in GP practices and in 48% of those treated in pulmonology practices. Rare diseases in Germany are usually treated by office-based specialists and in specialized outpatient units and hospitals. Pulmonologists have specific knowledge and more experience in treating patients with NTMLD than GPs. This is likely the reason for the finding that pulmonologists prescribed GBT much more often than GPs. Our results are in line with published work from other countries showing low rates of adherence to GBT [10], [11], [16]. Based on the low patient numbers in this study, however, the association between physician specialty and the likelihood of prescribing GBT cannot be considered significant.
Treatment persistence in patients treated by both GPs and pulmonologists was generally poor. This low persistence may have been due to several reasons including poor tolerability of antibiotic combination therapy, comorbidities of patients associated with polypharmacy and drug interactions, as well as lack of experience in physicians with regard to managing these patients appropriately. This finding suggests that there is a high risk that treatment success will not be achieved, resulting in poor patient outcomes. Finally, those patients who had continuous therapy of over 12 months had to change therapy regimen frequently, with a mean of 5 therapy switches per treatment course. This highlights the fact that treatment of NTMLD is complicated and should be done with the involvement of an expert.
The strengths of this study include the use of real-world data, which allowed for an unbiased exposure assessment using a representative outpatient database. However, the study findings must be interpreted taking into account several limitations. First, the types of species isolated (mycobacteria) were not known. Second, diagnoses relied on ICD 10 codes only, and no information was available on disease severity at the time of diagnosis. Third, several variables (e.g., smoking behavior, physical activity, spirometry results) were lacking in the baseline characteristics. Fourth, this study included patients managed in general practices and office-based pulmonologists only, whereas patients managed in hospital departments s that perform outpatient care were not captured. Fifth, the definition of GBT used in this study is specific for Mycobacterium avium complex, which represents approximately half of all NTMLD cases in Germany. Nevertheless, this triple regimen is also one of the recommended treatment regimens for other species, such as Mycobacterium kansasii and Mycobacterium xenopi which represent large part of the remaining species causing NTMLD in Germany. [14], [16] Finally, non-respiratory comorbidities may be not captured consistently in pulmonology practices.
5. Conclusion
This is the first study to provide insights into the real-world management of NTMLD in general and pneumologist practices in Germany. Many patients were not managed in accordance with the relevant guidelines and many also discontinue therapy prematurely. NTMLD management should be improved through ensuring appropriate referral pathways and collaboration between expert centers and primary or secondary care physicians. There is a need for inter-sectorial management and stronger involvement of expert centers.
Acknowledgements
This study received financial support from Insmed Germany GmbH. R.D. received fees for lecturing at symposia sponsored by INSMED Germany GmbHand for consulting. M.O. and S.T. are employees of INSMED Germany GmbH. E.J. and K.K. are employees of IQVIA that received financial support for conducting this study.
Contributor Information
R. Diel, Email: roland.diel@epi.uni-kiel.de.
M. Obradovic, Email: marko.obradovic@insmed.com.
References
- 1.Haworth C.S., Banks J., Capstick T., Fisher A.J., Gorsuch T., Laurenson I.F., Leitch A., Loebinger M.R., Milburn H.J., Nightingale M., Ormerod P., Shingadia D., Smith D., Whitehead N., Wilson R., Floto R.A. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72(Suppl 2) doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 2.Mehta M., Marras T.K. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. 2011;105(11):1718–1725. doi: 10.1016/j.rmed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Huang C.T., Tsai Y.J., Wu H.D., Wang J.Y., Yu C.J., Lee L.N. Impact of non-tuberculous mycobacteria on pulmonary function decline in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2012 Apr;16(4):539–545. doi: 10.5588/ijtld.11.0412. [DOI] [PubMed] [Google Scholar]
- 4.Diel R., Jacob J., Lampenius N., Loebinger M., Nienhaus A., Rabe K.F., Ringshausen F.C. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J. 2017;49(4):1602109. doi: 10.1183/13993003.02109-2016. [DOI] [PubMed] [Google Scholar]
- 5.Marras T.K., Mirsaeidi M., Chou E., Eagle G., Zhang R., Leuchars M. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. J Manag Care Spec Pharm. 2018 Oct;24(10):964–974. doi: 10.18553/jmcp.2018.18122. Epub 2018 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diel R., Lipman M., Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1) doi: 10.1186/s12879-018-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringshausen F.C., Wagner D., de Roux A., Diel R., Hohmann D., Hickstein L., Welte T., Rademacher J. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009–2014. Emerg Infect Dis. 2016;22(6):1102–1105. doi: 10.3201/eid2206.151642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevots D.R., Marras T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2015;36(1):13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson L.O., Polverino E., Hoefsloot W., Codecasa L.R., Diel R., Jenkins S.G. Pulmonary disease by non-tuberculous mycobacteria – Clinical management, unmet needs and future perspectives. Expert Rev Respir Med. 2017 Dec;11(12):977–989. doi: 10.1080/17476348.2017.1386563. [DOI] [PubMed] [Google Scholar]
- 10.van Ingen J, Wagner D, Gallagher J, Morimoto K, Lange C, Haworth CS, Floto RA, Adjemian J, Prevots DR, Griffith DE; NTM-NET. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J. 2017 Feb 15;49(2). pii: 1601855. [DOI] [PubMed]
- 11.Adjemian J., Prevots D.R., Gallagher J. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann ATS. 2014;11:9–16. doi: 10.1513/AnnalsATS.201304-085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathmann W., Bongaerts B., Carius H.-J., Kruppert S., Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56:459–466. [PubMed] [Google Scholar]
- 13.Becher H., Kostev K., Schröder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):61. doi: 10.5414/cpp47617. [DOI] [PubMed] [Google Scholar]
- 14.Schönfeld N., Haas W., Richter E. Recommendations for diagnosis and treatment of nontuberculous mycobacterioses of the German Central Committee against tuberculosis and the German Respiratory Society. Pneumologie. 2013;67(11):605–633. doi: 10.1055/s-0033-1344790. [DOI] [PubMed] [Google Scholar]
- 15.Marras T.K., Mirsaeidi M., Vinnard C., Chan E.D., Eagle G., Zhang R. Guidelines-based treatment associated with improved economic outcomes in nontuberculous mycobacterial lung disease. J Med Econ. 2019 Nov;22(11):1126–1133. doi: 10.1080/13696998.2019.1620243. Epub 2019 Jun 10. [DOI] [PubMed] [Google Scholar]
- 16.Matthiessen W., Schmidt C., Rüsch-Gerdes S., Schönfeld N. Significance of local and general risk factors for the pathogenesis of pulmonary non-tuberculous mycobacteriosis in non-AIDS patients. Pneumologie. 2010 May;64(5):281–290. doi: 10.1055/s-0029-1244069. [DOI] [PubMed] [Google Scholar]