Summary
The transcription factor (TF) GATA2 plays a key role in organ development and cell fate control in the central nervous, urogenital, respiratory, and reproductive systems, and in primitive and definitive hematopoiesis. Here, we generate a knockin protein reporter mouse line expressing a GATA2VENUS fusion from the endogenous Gata2 genomic locus, with correct expression and localization of GATA2VENUS in different organs. GATA2VENUS expression is heterogeneous in different hematopoietic stem and progenitor cell populations (HSPCs), identifies functionally distinct subsets, and suggests a novel monocyte and mast cell lineage bifurcation point. GATA2 levels further correlate with proliferation and lineage outcome of hematopoietic progenitors. The GATA2VENUS mouse line improves the identification of specific live cell types during embryonic and adult development and will be crucial for analyzing GATA2 protein dynamics in TF networks.
Key words: development, transcription factor networks, cell fate, GATA2, monocyte and mast cell lineage, hematopoietic stem and progenitor cell, transgenic mouse, fluorescent protein
Highlights
-
•
A novel GATA2VENUS fusion mouse line to report GATA2 protein expression
-
•
VENUS fusion does not alter GATA2 expression or disturb development or homeostasis
-
•
GATA2 expression identifies functionally distinct HSPC subpopulations
-
•
GATA2 expression unveils an earlier monocyte-mast cell lineage bifurcation point
In this study, Schroeder and colleagues generated a GATA2VENUS protein reporter mouse line with normal GATA2 expression and localization, e.g., in the urogenital, auditory, and nervous systems. The authors also profiled GATA2 protein expression across hematopoietic cell types, identified heterogeneity within populations currently assumed to be homogeneous, and demonstrate an earlier monocyte-mast cell lineage bifurcation point.
Introduction
Establishment and maintenance of cell states in multicellular organisms is regulated by a complex interplay of transcription factors (TFs). TFs exert their effects by fine-tuning gene expression programs. They control key cellular processes, including cellular homeostasis, metabolism, cell-cycle control, and cell fate determination, dictating the differentiation and development of complex tissues and organs. Misregulation of these transcriptional programs lead to a broad range of diseases, including developmental disorders and cancer (Lee and Young, 2013; Spitz and Furlong, 2012).
The TF GATA2 serves as a crucial regulator for development and function of several organs, e.g., the central nervous system (Nardelli et al., 1999), urogenital (Khandekar et al., 2004; Zhou, 1998) and reproductive organs (Siggers et al., 2002), respiratory and auditory systems (Suzuki et al., 2006), endothelial cells (Minami et al., 2004), and adipose tissues (Tong et al., 2000, 2005). Tissue-specific knockout of Gata2 leads to a reduction in number of thyrotropes suggesting its role in cell fate determination of pituitary glands as well (Charles et al., 2006; Dasen et al., 1999). GATA2 is also required for trophoblast differentiation and correct functioning of placenta (Ray et al., 2009).
GATA2 has a prominent role in hematopoiesis where it has been shown to be indispensable to the development of primitive and definitive hematopoiesis (Bresnick et al., 2010, 2005; Shimizu and Yamamoto, 2005). Gata2-null mouse embryos fail to survive beyond embryonic day 10–11 (E10–E11), due to severe anemia. Gata2-null embryonic stem cells (ESCs) display a deficit in definitive hematopoiesis owing to limited expansion of hematopoietic colonies (Tsai et al., 1994). Adult Gata2+/− hematopoietic stem cells (HSCs) exhibit a reduced reconstitution capacity in competitive transplantation assays (Ling et al., 2004; Rodrigues et al., 2005). In contrast, HSCs with increased GATA2 levels fail to contribute to multilineage hematopoietic reconstitution of transplanted mice (Persons et al., 1999). Together, this suggests a GATA2 dose-dependent regulation of hematopoiesis.
GATA2 positively reinforces mast cell and basophil differentiation (Cantor et al., 2008; Kauts et al., 2018; Li et al., 2015; Ohmori et al., 2012, 2015) and its downregulation enables the proper transition of hematopoietic progenitor cells into the megakaryocyte and erythrocyte lineage through the GATA2–GATA1 switch mechanism (Bresnick et al., 2010; Doré et al., 2012; Grass et al., 2003; Snow et al., 2011).
Hence, it is becoming increasingly evident that correct GATA2 levels are crucial in regulating and maintaining the pool and function of many cell types in different organs. However, quantitative measurements of GATA2 protein levels are often still missing. Also, little is known about its precise molecular regulation, and how its protein levels relate to future functional outcomes. Understanding the GATA2 protein concentrations in diverse living cell types will provide insights into the role and regulation of GATA2 in different tissues.
One main reason is the lack of a reporter mouse line that can accurately reflect the endogenous GATA2 protein levels in different tissues. Suzuki et al. (2006) generated a mutant mouse line with a knockin of a green fluorescent protein (GFP) gene followed by a poly(A) sequence into the first exon of Gata2 (Suzuki et al., 2006). This mouse line reports the transcriptional activity, not protein levels, of the Gata2 gene. Also, GFP fluorescence was restricted to only neural and hematopoietic cells and not present in other GATA2-expressing tissues. In addition, GFP has several drawbacks for imaging and multiplexing. Its overlapping emission spectrum prevents simultaneous use of cyan and yellow fluorescent proteins, and its excitation and emission spectra lead to high autofluorescence and low tissue penetrance (Okita et al., 2004). Recently, Kaimakis et al. (2016) generated a reporter for Gata2 mRNA by inserting an IRES-VENUS cassette in its 3′ UTR. VENUS has a higher relative fluorescence intensity, is less pH sensitive, and matures faster than eGFP and hence is better for live imaging of biological samples (Nagai et al., 2002; Okita et al., 2004). However, the IRES-VENUS reporter also does not report GATA2 protein, but only mRNA, levels (Kaimakis et al., 2016; Eich et al., 2018) and with differing stability of the endogenous GATA2 and VENUS reporter proteins.
Here, we generate the first reporter mouse line for the non-invasive quantification of GATA2 protein levels by an in-frame knockin of VENUS FP into the C terminus of the Gata2 genomic locus. These reporter mice are phenotypically normal, allow detection of heterogeneous GATA2 protein expression in different tissues during embryonic and adult development, and the identification, e.g., of novel hematopoietic stem and progenitor cell (HSPC) types, with distinct molecular and functional properties.
Results
Generation of a GATA2VENUS Protein Reporter Mouse Line
We generated a novel reporter mouse line with a linker and VENUS fluorescent protein reading frame knocked into the gene locus of Gata2 (Figures 1A and S1). VENUS was fused to the C terminus of GATA2 in exon 6, enabling the non-invasive quantification of GATA2 protein levels in all expressing cell types.
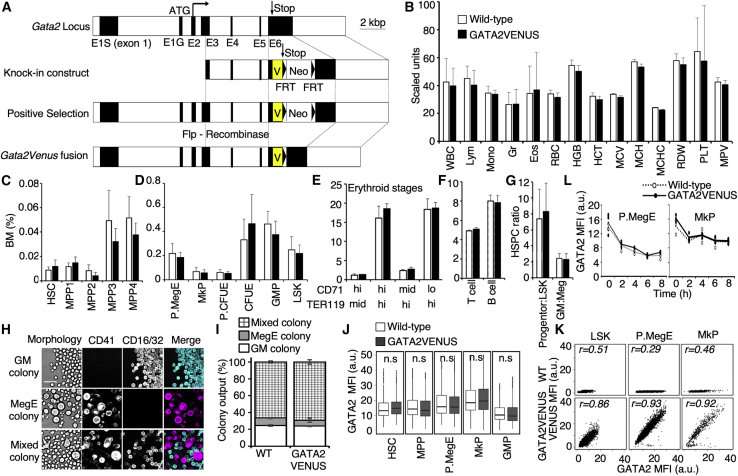
Figure 1.
Generation of a GATA2VENUS Knockin Protein Reporter Mouse Line with Normal Hematopoiesis
(A) Constructs used for GATA2VENUS knockin generation. The FRT PGK-Neo FRT was deleted by cross with a Flpe deleter mouse line. Black boxes indicate exons (also see Figure S1).
(B) Peripheral blood counts are not altered in GATA2VENUS mouse line. WBC, white blood cells (200 cells per mm3); Lym, percent lymphocytes of WBC (%); Mono, percent monocytes of WBC (0.1%); Gr, percent granulocytes of WBC (%); Eos, percent eosinophils of WBC (0.2%); RBC, red blood cells (2 ×105 cells per mm3); HGB, hemoglobin (0.2 g/dL); HCT, hematocrit (%); MCV, mean corpuscular volume (μm3); MCH, mean corpuscular hemoglobin (0.2 pg); MCHC, mean corpuscular hemoglobin concentration (g/dL); RDW, red cell distribution width (0.2%); PLT, platelets (104 per mm3); MPV, mean platelet volume (0.1 μm3) (n = 9 mice per genotype).
(C–G) Fusion of VENUS to GATA2 does not alter bone marrow composition. Data indicate bone marrow percentage of (C) HSCs and multipotent progenitors (n = 7 mice per genotype), (D) lineage committed progenitors (n = 10 mice per genotype), (E) early and late erythrocyte progenitors (n = 3 mice per genotype), (F) T and B cells (n = 3 mice per genotype), and (G) ratio of multipotent progenitors to lineage committed progenitors and granulocyte-monocyte progenitors to megakaryocyte-erythrocyte (MegE) progenitors (n = 10 mice per genotype).
(H and I) Colony-forming potential and output of HSCs is not altered in GATA2VENUS mouse line. (H) Single HSCs sorted into 384-wells in IMDM, FCS, BIT, SCF, EPO, TPO, IL-3, and IL-6. Granulocyte-monocyte (GM) colonies identified by morphology and FCγR expression. MegE colonies identified by morphology and CD41 expression. Scale bar, 50 μm. (I) Types of colonies formed from HSCs (n = 3 independent mice per genotype).
(J) Protein levels of GATA2 are not altered in different cell types in GATA2VENUS mouse line. Data were acquired using quantitative immunostaining against endogenous GATA2 protein. Data represented by box and whisker plots with median of GATA2 intensity (n = 3 independent mice per genotype).
(K) Endogenous GATA2 protein levels correlate to VENUS fusion levels. Data were acquired using quantitative immunostaining against GATA2 and VENUS and represented by a 2D plot of GATA2 and VENUS intensities. Number (r) in the plots indicate Pearson correlation coefficient (n = 3 independent mice per genotype).
(L) Normal stability of GATA2 fusion proteins in pre-MegE progenitors (preMegEs) (left panel) and megakaryocyte progenitors (MkPs) (right panel). Data were acquired using quantitative immunostaining against GATA2 in indicated cell types after treatment with 50 μM cycloheximide (protein translation inhibitor) and sampling of cells at the indicated time points (n = 3 independent mice per genotype).
Error bars in (B)–(G) and (I) = SD. Data in (J)–(L) indicate mean fluorescence intensity (MFI) of GATA2 and VENUS. Difference between wild-type and GATA2VENUS samples is non-significant unless specified. Two-sample t test; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
To exclude the possible alteration of GATA2 function, stability, or expression due to the VENUS fusion, we first confirmed the absence of abnormal phenotypes in homozygous GATA2VENUS mice. As described previously, Gata2 deletion leads to embryonic lethality at the E10–E11 stage (Tsai et al., 1994), while altered expression levels result in a change, e.g., of the number and function of HSCs (Ling et al., 2004; Rodrigues et al., 2005; Persons et al., 1999). In contrast, homozygous GATA2VENUS mice showed no aberrant phenotype (Figures 1 and 2), were fertile and born at normal Mendelian ratios (Figure S2), and did not show increased mortality throughout adulthood (not shown). While altered GATA2 expression levels changes the composition of the HSPC pool (Kaimakis et al., 2016; Ling et al., 2004; Persons et al., 1999; Rodrigues et al., 2005), the frequencies of blood cells and HSPCs in bone marrow and peripheral blood from adult GATA2VENUS mice were unchanged (Figures 1B–1G and S3), and in vitro colony numbers (not shown) and types (Figures 1H and 1I) from GATA2VENUS HSCs were unaltered.
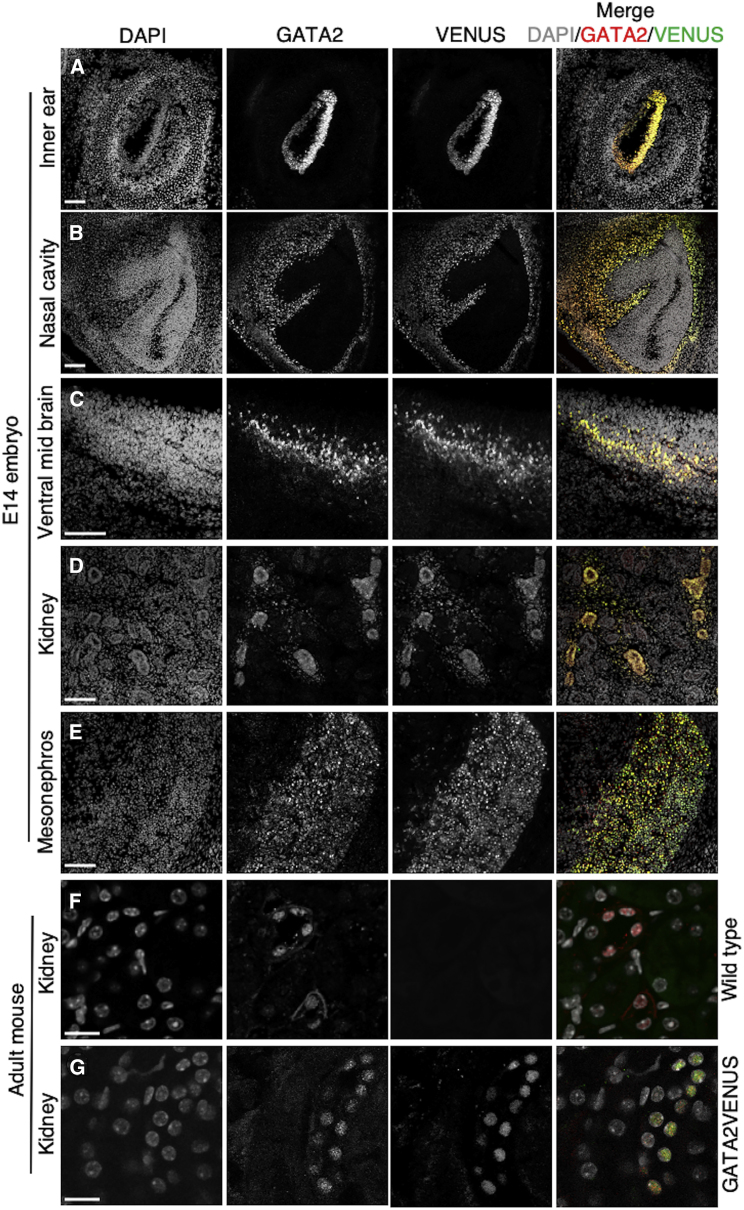
Figure 2.
Normal Expression and Localization of GATA2VENUS in Embryonic and Adult Tissues
Localization of GATA2VENUS is similar to GATA2 in nuclei of the inner ear (A), nasal cavity (B), ventral mesoderm (C), kidney (D), and mesonephros (E) from E14 embryo and kidney from adult wild-type (F) and GATA2VENUS (G) mice. Confocal images of E14 embryo (A–E) and 12-week-old adult mouse (F–G) sections stained with DAPI (nuclei), and anti-GATA2 and anti-GFP antibodies. Scale bars, 100 μm (A–E) and 10 μm (F–G).
Quantitative immunostaining against GATA2 did not show any changed expression levels (Figure 1J) or stability (Figure 1L) of the GATA2VENUS fusion in different hematopoietic cell types. Simultaneous quantitative immunostaining against GATA2 and VENUS demonstrated their high correlation of expression and localization in HSPC nuclei (Figures 1K and S4). Thus, the GATA2VENUS fusion does not alter GATA2 function, stability, or localization, and can be used as a reliable readout of GATA2 protein expression.
GATA2VENUS Expression in Embryonic and Adult Organs
In addition to the hematopoietic system, GATA2 is expressed in numerous solid organs during embryonic development and in the adult (Charles et al., 2006; Dasen et al., 1999; Khandekar et al., 2004; Minami et al., 2004; Nardelli et al., 1999; Siggers et al., 2002; Suzuki et al., 2006; Tong et al., 2005, 2000; Zhou, 1998). Here, to analyze GATA2 expression in non-hematopoietic tissues, we established in situ immunostaining of GATA2 in various embryonic and adult organs. As expected (Charles et al., 2006; Dasen et al., 1999; Khandekar et al., 2004; Minami et al., 2004; Nardelli et al., 1999; Siggers et al., 2002; Suzuki et al., 2006; Tong et al., 2005, 2000; Zhou, 1998), GATA2 and GATA2VENUS expression was predominant in the embryonic nasal cavity, inner ear, ventral mid brain, urogenital system (Figures 2A–2E), and in the adult mouse kidney (Figures 2F and 2G). Co-immunostaining against GATA2 and VENUS in different organs confirmed colocalization of GATA2 and VENUS signal in nuclei, demonstrating normal GATA2VENUS localization (Figure 2). Thus, the GATA2VENUS mouse line is a faithful reporter of GATA2 protein expression in different tissues.
Heterogeneous GATA2 Expression in Adult HSPCs
To date, GATA2 expression is largely analyzed at the RNA level. Its protein expression was only quantified in developmental hematopoiesis or by using population average biochemical assays, masking GATA2 protein levels and heterogeneity at the single-cell level and thus hampering our understanding of GATA2 regulation and function during HSC differentiation (Etzrodt et al., 2014; Hoppe et al., 2014). Here, we profiled the single-cell expression of GATA2 protein in 20 adult HSPC populations encompassing the whole myeloid lineage, including the erythrocyte, megakaryocyte, mast cell, basophil, eosinophil, neutrophil, and monocyte lineages (Figures 3, S3, and S5).
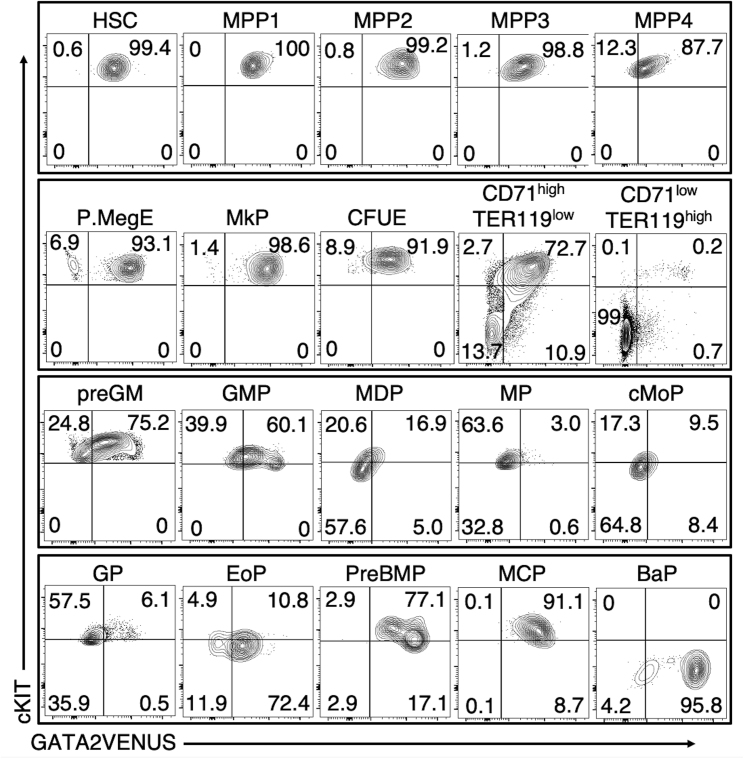
Figure 3.
Cell-Type-Specific and Heterogeneous GATA2 Protein Expression in Adult HSPCs
Flow-cytometric quantification of GATA2VENUS expression in adult HSPCs. GATA2VENUS gate was set using wild-type cells (see Figure S5). Numbers in quadrants represent percentage of cells. See Figure S3 for gating schemes for these cell types: HSC, hematopoietic stem cell; MPP 1–4, multipotent progenitors 1–4; P.MegE, pre-megakaryocyte-erythrocyte; MkP, megakaryocyte progenitor; CFUE, colony-forming unit erythrocyte; CD71highTER119low and CD71lowTER119high, erythrocyte differentiation stages; preGM, pre-granulocyte-monocyte; GMP, granulocyte-monocyte progenitor; MDP, monocyte dendritic cell progenitor; MP, monocyte progenitor; cMoP, common monocyte progenitor; GP, granulocyte progenitor; EoP, eosinophil progenitor; preBMP, pre-basophil mast cell progenitor; MCP, mast cell progenitor; BaP, basophil progenitor.
GATA2 is homogeneously expressed at low level in long-term HSCs , and heterogeneously in different MPP populations (Cabezas-Wallscheid et al., 2014; Wilson et al., 2008). Of all the populations analyzed, pre-megakaryocyte-erythrocyte progenitors (Pronk et al., 2007), megakaryocyte progenitors (Pronk et al., 2007), mast cell progenitors (Chen et al., 2005), and basophil progenitors (Arinobu et al., 2005) exhibit the highest expression of GATA2. In contrast, monocyte progenitors (MPs) (Yanez et al., 2017) and monocyte dendritic cell progenitors (Hettinger et al., 2013) show lowest expression of GATA2. GATA2 is downregulated from the CD71high TER119low to the CD71low TER119high stage (Pop et al., 2010), in line with the proposed GATA2 downregulation during erythrocyte differentiation (Bresnick et al., 2010; Doré et al., 2012; Grass et al., 2003; Snow et al., 2011) (Figures 3, S3, and S5).
Interestingly, we also observed large heterogeneity of GATA2 expression in the pre-granulocyte-monocyte progenitor (preGM) and granulocyte-monocyte progenitor (GMP) (Pronk et al., 2007) populations (Figure 3), indicating novel GATA2-based subpopulations. This is reminiscent of previous studies highlighting heterogeneous GATA1 expression in preGMs and GMPs (Drissen et al., 2016; Hoppe et al., 2016).
GATA2 Protein Expression Identifies HSPC Subsets with Distinct TF Networks
The heterogeneous GATA2 expression in preGMs and GMPs suggested the existence of subtypes with specific molecular and functional properties. To analyze this further in a quantitative way, we used a simple, robust, and efficient multiplexed immunostaining protocol. It has high enough throughput and sensitivity to work with rare cell types and permits automated imaging and multiplexed single-cell TF protein quantification in primary HSPCs. In addition, it is quantitative enough to detect minor variations in TF levels (Figures 4B and 4C).
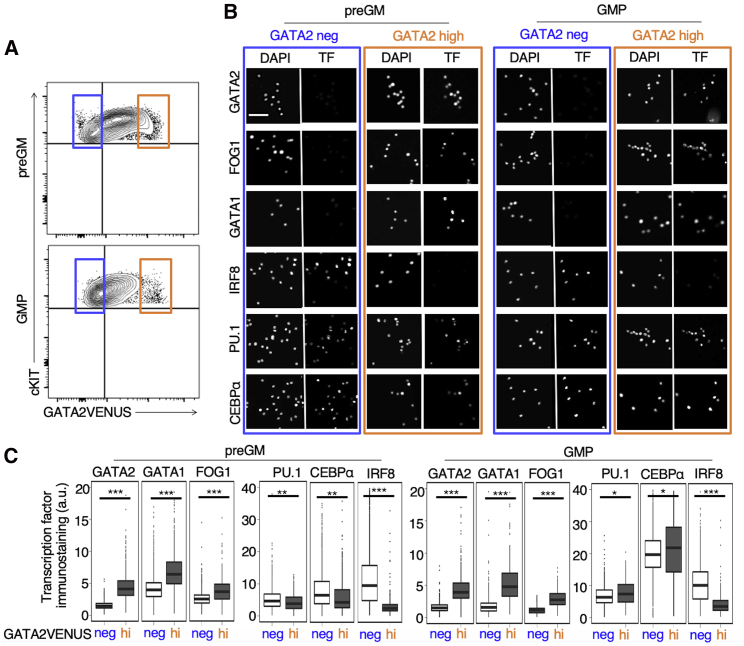
Figure 4.
GATA2 Protein Expression Identifies preGM and GMP Subpopulations with Differential Hematopoietic TF Expression
(A) GATA2-negative and -high preGMs and GMPs were sorted before immunostaining, imaging, and quantification of core hematopoietic TFs.
(B) Representative fluorescence images of preGMs (left panel) and GMPs (right panel) stained with DAPI and anti-TF antibodies. Scale bar: 50 µm.
(C) Quantification of TF levels in GATA2-negative and -high preGMs and GMPs. Data represented by box and whisker plots with median (n = 3 independent mice). Two-tailed t test; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
We quantified the protein expression of core hematopoietic TFs in freshly sorted GATA2-negative and -high preGMs and GMPs (Figure 4A). GATA2-negative preGMs and GMPs showed no GATA1 and FOG-1 expression, whereas GATA2 -high preGMs and GMPs showed high expression. IRF8, a monocyte and dendritic cell marker, displayed an inverse pattern of expression, with high IRF8 expression in GATA2-negative cells. No or only minor correlations of PU.1 and CEBPα with GATA2 expression could be observed, despite a >2-fold increase in CEBPα expression during preGM to GMP transition (Figures 4B and 4C).
Thus, the GATA2VENUS mouse line enables identification of HSPC subsets with distinct TF networks within populations previously assumed to be homogeneous and allows better understanding of molecular pathways regulating hematopoietic cell fate.
GATA2 Expression Identifies Early Segregation of Monocyte and Mast Cell Lineages
TF networks determine cell fate of HSPCs (Krumsiek et al., 2011). The differential expression of core hematopoietic TFs, such as GATA1, FOG1, and IRF8 in heterogeneous preGMs and GMPs suggests distinct differentiation potentials.
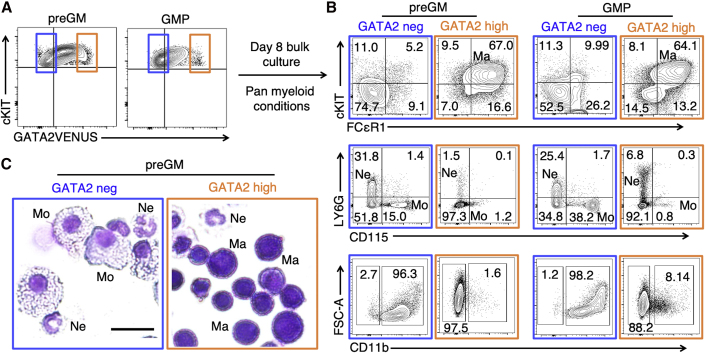
To determine whether GATA2 expression can identify HSPC subsets with different lineage potential, we cultured freshly sorted GATA2-negative and -high preGMs and GMPs under pan-myeloid conditions (Dahlin et al., 2018; Drissen et al., 2016) (with cytokines stem cell factor, interleukin-3 [IL-3], IL-9, and granulocyte-monocyte colony-stimulating factor [GM-CSF]) for 8 days and quantified the resulting cultures by morphology and molecular markers (Figures 5A and S6). Morphological analysis indicated that GATA2-negative versus -high progenitors predominantly generated monocytes and macrophages, versus mast cells, respectively. In contrast, neutrophils were generated from both progenitors, albeit with lower frequency from GATA2-high progenitors (Figure 5C). Consistent with the morphological observations, flow cytometry of GATA2-high cultures revealed a high frequency of cKIT+ FCεR1+ mast cells with no CD115 expression, and some CD11b+ LY6G+ neutrophils. In contrast, GATA2-negative progenitors mostly produced CD11b+ CD115+ monocytes and CD11b+ LY6G+ neutrophils and no mast cells (Figure 5B).
Figure 5.
GATA2 Protein Levels Identify Early Segregation of Monocyte and Mast Cell Lineages but Not Neutrophils
(A) GATA2-negative and -high preGMs and GMPs were sorted and cultured in pan-myeloid medium (IMDM + FCS + BIT + SCF + GM-CSF + IL-3 + IL-9).
(B) Quantification of mature cell types at day 8 by flow cytometry. GATA2-negative preGMs and GMPs mainly generate CD11b+ CD115+ FCεR1− monocytes and CD11b+ LY6G+ FCεR1− neutrophils while GATA2-high preGMs and GMPs generate cKIT+ FCεR1+ CD11b− CD115− LY6G− mast cells and few neutrophils. Numbers in quadrants represent percentage of cells.
(C) Representative images of May-Grünwald Giemsa morphology of cells from day 8 culture preGMs. GATA2-negative versus -high preGMs generate monocytes and neutrophils versus mast cells and neutrophils, respectively. Scale bar, 20 μm.
Thus, GATA2 expression in preGM and GMP populations identifies cells with mast cell commitment (Ohmori et al., 2015) and demonstrates early segregation of monocyte and mast cell lineages, but not neutrophils, before the preGM and GMP stage.
GATA2 Protein Levels Predict preGM and GMP Proliferation and Lineage Potential
Next, we analyzed if the expression levels of GATA2 protein in preGMs and GMPs can predict their proliferation and lineage potential. We single-cell-sorted preGM and GMP populations into negative, low, mid, and high GATA2 expressers, and cultured them under pan-myeloid conditions (Dahlin et al., 2018; Drissen et al., 2016) (Figure 6).
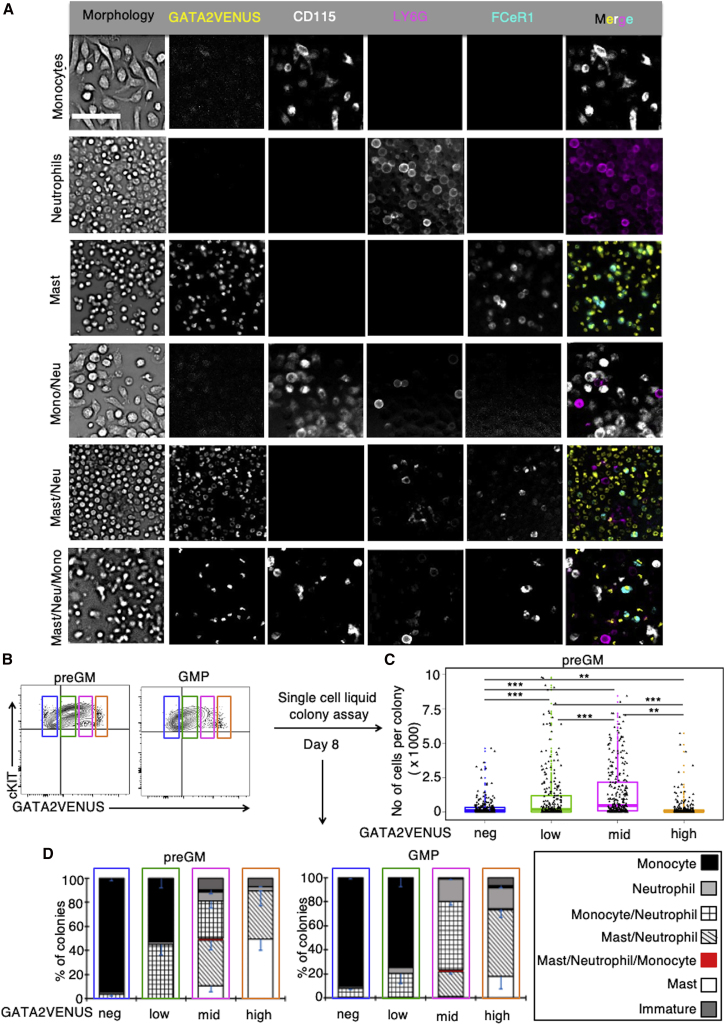
Figure 6.
Variations in GATA2 Protein Levels Correlate with HSPC Proliferation and Lineage Potential
(A) Quantification of liquid colonies from single preGMs or GMPs by quantitative imaging of cell morphology, nuclear shape, and expression of GATA2VENUS, CD115 (monocytes), LY6G (neutrophils), and FCεR1 (mast cells). Examples shown for monocyte, neutrophil, mast cell, bipotent monocyte-neutrophil, bipotent mast cell-neutrophil, and tripotent monocyte-neutrophil-mast cell colonies. Immature colonies do not express any surface markers. Scale bar, 50 μm.
(B) Four different preGM and GMP fractions based on GATA2 levels were single-cell sorted into 384-well plates and cultured for 8 days in pan-myeloid media (IMDM + FCS + BIT + SCF + GM-CSF + IL-3 + IL-9).
(C) GATA2 -low and -mid preGMs exhibit higher proliferation and colony-forming potential than GATA2 -negative and -high cells. Data represented by box and whisker plots with median. Dots indicate individual measurement per cell (n = 3 independent mice).
(D) GATA2 protein expression correlates with different preGM and GMP lineage potential. Mean percentage of different colony types identified in (A) (n = 3 independent mice). Error bars = SD. Number of colonies, preGMs: GATA2-neg, 155; GATA2-low, 225; GATA2-mid, 157; GATA2-high, 152. GMPs: GATA2-neg, 173; GATA2-low, 153; GATA2-mid, 161; GATA2-high, 91. Two-sided Wilcoxon rank-sum test; ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
Fluorescent antibodies against surface markers CD115, LY6G, and FCεR1 were added to live colonies to identify monocytes, neutrophils, and mast cells, respectively (Eilken et al., 2011, 2009). After 8 days, we observed different colonies, including unipotent (monocyte, neutrophil, and mast cell), bipotent (monocyte-neutrophil and mast cell-neutrophil), and extremely rare tripotent (monocyte-neutrophil-mast cell) colonies (Figure 6A). Unipotent monocyte colonies were exclusively generated from GATA2-negative preGMs and GMPs, while unipotent mast cell colonies were exclusively generated from GATA2 higher progenitors. GATA2-low and -mid cells predominantly generated bipotent colonies, including monocyte-neutrophil and mast cell-neutrophil colonies. These results hence rule out the existence of a bipotent monocyte-mast cell progenitor (Figure 6D). Interestingly, GATA2-low and -mid preGMs generated colonies with higher numbers of cells than GATA2-negative or -high preGMs (Figure 6C). These data establish low GATA2 protein levels as a marker of unipotent monocyte and high GATA2 protein levels as a marker for mast cell progenitors. Thus, specific GATA2 protein expression levels identify and possibly regulate distinct proliferation and lineage potentials for early myeloid progenitors.
Discussion
GATA2 TF has long been known as a crucial regulator of the development, differentiation, and function of numerous tissues, including the central nervous system, pituitary glands, adipose tissues, endothelial cells, and urogenital and reproductive systems (Charles et al., 2006; Dasen et al., 1999; Khandekar et al., 2004; Minami et al., 2004; Nardelli et al., 1999; Siggers et al., 2002; Tong et al., 2005, 2000; Zhou, 1998). A prominent role of GATA2 in regulating the emergence, maintenance, function, and differentiation of HSCs, both during embryonic and adult phases of definitive hematopoiesis, has been well documented (de Pater et al., 2013; Gao et al., 2013; Kaimakis et al., 2016). Correct GATA2 expression levels are required for the normal development of numerous cell types (Ling et al., 2004; Persons et al., 1999; Rodrigues et al., 2005).
However, little is known about the precise protein levels and molecular regulation of GATA2 protein and how this relates to the functional outcome during cell fate determination and development of different organs. To better quantify the heterogeneity and dynamics of TFs, such as GATA2, which play a crucial role in regulating, e.g., hematopoietic lineage choice (Etzrodt et al., 2014; Hoppe et al., 2014; Loeffler and Schroeder, 2019; McIvor et al., 2003; Schroeder, 2005), requires reporter mice enabling the non-invasive quantification of their protein levels at the single-cell level (Etzrodt and Schroeder, 2017), e.g., by TF-fluorescent protein fusion reporters. Since these fusions could potentially change the TF's function or stability, the absence of these changes has to be confirmed before further using them (Filipczyk et al., 2015; Hoppe et al., 2016).
Here, we therefore generated a GATA2VENUS knockin protein reporter mouse line. Although Gata2-null embryos are non-viable (Tsai et al., 1994), have severe deformations of the urogenital system (Khandekar et al., 2004; Zhou, 1998), and GATA2-haploinsufficiency results in altered hematopoiesis (Ling et al., 2004; Rodrigues et al., 2005), homozygous GATA2VENUS mice are normal, without urogenital deformations, fertile, and born at expected Mendelian frequencies. GATA2VENUS has normal protein stability, and the same expression as GATA2 across different cell types. While even minor changes in GATA2 expression levels lead to changed HSPC pool sizes (Ling et al., 2004; Rodrigues et al., 2005), we could not detect changes in hematopoiesis or the morphology of other organs expressing GATA2 in homozygous GATA2VENUS mice. Thus, the GATA2VENUS fusion does not change GATA2 function, stability, or expression.
Although previous studies highlighted the expression of GATA2 in different organs, the correlation of GATA2 protein levels and function in regulating the development and homeostasis of these organs is not well understood. Therefore, the produced reporter line can be used to better analyze the role of GATA2 in the many live tissues during embryonic and adult development stages where it is expressed and functionally important. These include, for example, motor neuron precursors and the olfactory bulb in the embryonic central nervous system (Nardelli et al., 1999), epithelium of developing ureteric buds destined to become collecting tubules of kidney (Khandekar et al., 2004; Zhou, 1998), and trophoblast cells covering the inner cell mass and blastocoel of developing embryo (Ray et al., 2009). The GATA2VENUS mouse can also aid in identifying and further characterizing the endothelial cells undergoing EHT during HSC generation at E10.5, all long-term repopulating HSCs and a large fraction of hematopoietic progenitors and erythromyeloid progenitors in mid-gestation embryo (Kaimakis et al., 2016; McGrath et al., 2015) and distinct erythroid differentiation stages in the fetal liver (Pop et al., 2010).
GATA2 protein expression in 20 different HSPC types showed unique patterns and heterogeneity in different populations suggesting distinct molecular pathways affecting, and regulated by, GATA2 expression. The observed GATA2 heterogeneity in preGMs and GMPs allowed identification of novel subtypes within these phenotypically identical (Akashi et al., 2000; Pronk et al., 2007) populations. Importantly, since GATA2 is expressed in all HSCs while many other TFs, such as GATA1, are expressed later in differentiation (Drissen et al., 2016; Hoppe et al., 2016), GATA2 is likely a central TF in the hematopoietic lineage decision TF network. The GATA2-GATA1 switch during erythrocyte differentiation (Bresnick et al., 2010; Doré et al., 2012; Grass et al., 2003; Snow et al., 2011), and the cooperation of GATA2 with PU.1 and CEBPα during the specification of mast cell and basophil lineages (Iwasaki et al., 2006; Ohmori et al., 2015; Walsh et al., 2002) are two examples of GATA2-controlled lineage choice. We have established a multiplexed immunostaining protocol and used it to screen core hematopoietic TFs in GATA2-negative and -high preGMs and GMPs. The observed high levels of GATA1 and FOG-1 expression in GATA2-high progenitors suggest distinct molecular programs in GATA2-negative and -high preGMs and GMPs with distinct potential. Indeed, GATA2-negative and -high preGM and GMP populations generate monocytes and mast cells, respectively. This is in line with a recent report on early segregation of monocyte and mast cell lineages using a GATA1 reporter mouse line (Drissen et al., 2016). The new reporter line allows to sort for monocyte and mast cell progenitors based on GATA2VENUS levels and therefore will aid their molecular characterization.
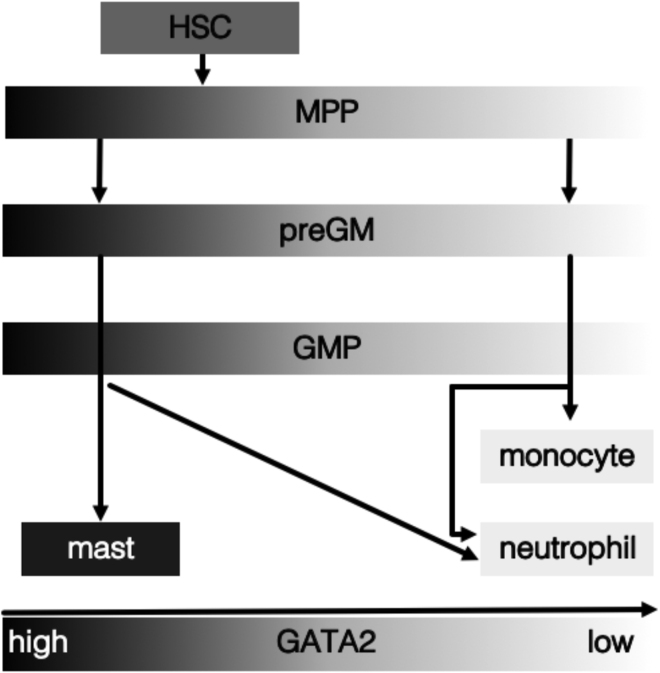
Despite homogeneous low GATA2 expression in HSCs, we find heterogeneity in GATA2 expression already in MPPs. Together with the segregation of monocyte and mast cell lineages with GATA2 expression at the preGM stage, this suggests monocyte-mast cell lineage bifurcation already at the preGM and likely MPP stage. This is in agreement with recent single-cell RNA sequencing analysis confirming heterogeneous MPP Gata2 expression at the mRNA level (Weinreb et al., 2020). High Gata2 mRNA-expressing MPPs (likely corresponding to GATA2 protein high MPP2 in our study) predominantly differentiated to the mast cells and the megakaryocyte-erythrocyte lineage, whereas Gata2-low MPPs (corresponding to GATA2-low MPP3 and 4 in our study) differentiated to monocytes and neutrophils, respectively. Altogether, this suggests lineage commitment at the MPP stage and thus earlier than previously thought. It suggests GATA2 as a potential regulator of this lineage choice (Figure 7) (Akashi et al., 2000; Cabezas-Wallscheid et al., 2014; Pietras et al., 2015; Pronk et al., 2007; Weinreb et al., 2020).
Figure 7.
Early Segregation of Monocyte and Mast Cell Lineages
Based on GATA2 expression, monocyte, and mast cell lineages bifurcate already within the preGM and likely the MPP compartment. Neutrophil fate is shared between GATA2-low and -high pathways. Only mast, neutrophil, and monocyte lineages are shown. Infrequent transition between GATA2-low and -high states in MPPs, preGMs, and GMPs may be possible.
Concentrations of TFs regulate differentiation and developmental pathways. This includes, for example, the differentiation of HSCs toward GM lineage in response to above-threshold levels of PU.1 TF and the transition of fetal liver progenitors into B cells versus macrophages governed by relative concentration of PU.1 (Graf and Enver, 2009; Hoppe et al., 2016; Kueh et al., 2013). Indeed, sub-fractionation of preGMs and GMPs into four subsets based on GATA2 protein levels demonstrated high fractions of bipotent colonies with increased proliferation potential in GATA2-low and -mid progenitors compared with GATA2-negative and -high progenitors, which suggests that variations in GATA2 levels correlate to distinct proliferation and lineage potential. However, whether GATA2 levels are a cause or consequence of different functional outcomes is a matter of future interest.
It will be interesting to know how GATA2 levels and dynamics are involved in the lineage choice of early myeloid progenitors, its role in controlling the decision between mast cell and monocyte lineages, how the levels and dynamics of GATA2 modulate the onset of the GATA2-GATA1 switch, and if and how GATA2 regulates other master regulators of hematopoiesis, including PU.1 and CEBPα. In addition, the novel reporter line will be invaluable for better analyzing GATA2 regulation and function in many solid tissues it regulates.
Experimental Procedures
Animals
Experiments were performed with 12- to 16-week-old wild-type, male C57BL/6J mice from Janvier Labs and in-house generated GATA2VENUS reporter mice. Animal experiments were approved according to Institutional guidelines of ETH Zurich and Swiss Federal Law by veterinary office of Canton Basel-Stadt, Switzerland (approval no. 2655).
Generation of GATA2VENUS Knockin Reporter Mouse Line
The GATA2VENUS knockin construct consists of a 5.0-kbps 5′ end homology arm lasting until the last codon of Gata2 (skipping the endogenous stop-codon) followed by a short linker sequence, the coding sequence of Venus, FRT (Flp recognition target)-flanked neomycin resistance gene cassette, and a 5-kbps 3′ end homology arm. JM8.A3 ESCs (C57BL/6J background) were electroporated with the targeting vector and selected using 0.2 mg/mL G418 and 2 μM ganciclovir. Colonies were screened by Southern blot for correct integration events using 5′ and 3′ end external probes. Germline chimeras were generated from correct ESC clone by ESC aggregation. FRT-flanked neo-selection cassette was excised in vivo by crossing with an Flpe deleter strain (Dymecki, 1996). The resulting GATA2VENUS offspring were backcrossed for more than five generations with C57BL/6J animals.
Peripheral Blood Analysis
Male mice for peripheral blood analysis were euthanized, 0.5 mL blood was collected through cardiac puncture, and blood counts were analyzed using a VetABC Plus+ analyzer machine.
HSPC Analysis and Isolation
Analysis and isolation of primary HSPCs was performed according to previously described protocols (Arinobu et al., 2005, Cabezas-Wallscheid et al., 2014, Chen et al., 2005, Hettinger et al., 2013, Iwasaki et al., 2005, Kiel et al., 2005, Pop et al., 2010, Pronk et al., 2007, Qi et al., 2013, Wilson et al., 2008, Yanez et al., 2017) using BD FACSAria III (BD Biosciences).
Bulk- and Single-Cell HSPC Culture and Analysis
HSPC culture was performed in pan-myeloid conditions as described previously (Drissen et al., 2016). For bulk colony assays, 100–200 HSPCs were seeded in 24-well plates (Thermo Scientific) and resulting cultures after 8 days were analyzed either by morphology (May-Grünwald Giemsa staining) or flow cytometry of lineage-specific surface markers. For single-cell colony assays, HSPCs were single-cell sorted into plastic-bottom 384-well plates (Greiner Bio-One) using BD FACSAria III (BD Biosciences). Analyses of single-cell colony assays after 8 days were performed using live-in culture antibody staining (Eilken et al., 2011; Endele et al., 2017; Hoppe et al., 2016; Loeffler et al., 2018) and fluorescence imaging using a Nikon Eclipse Ti-E microscope. Quantification of the number of cells per colony was performed using fastER (Hilsenbeck et al., 2017).
Immunostaining of TFs
Freshly sorted HSPCs were seeded in poly-L-lysine- (Sigma Aldrich)-coated 384-well plates (Greiner Bio-One) and immunostaining of TFs was performed according to protocols as described previously (Etzrodt et al., 2018; Hoppe et al., 2016) using the indicated antibodies (Table S5). Images were acquired using a Nikon Eclipse Ti-E microscope and quantification of TF intensities was performed using a BaSiC background analyzer (Peng et al., 2017) and fastER segmentation tool (Hilsenbeck et al., 2017). For in situ imaging of embryos, E14 embryos were frozen, embedded on PolyFreeze, sliced in a cryostat on glass slides, fixed with 4% paraformaldehyde for 20 min at room temperature, and immunostained using indicated antibodies. Sections were imaged on a Leica TCS SP5 confocal microscope. In situ immunostaining of GATA2 TF in kidney was performed according to protocols described previously (Coutu et al., 2017, 2018) and imaged on a Leica TCS SP8 confocal microscope.
Protein Stability Assay of TFs
HSPCs were cultured as described above and treated with 50 μM cycloheximide (Sigma Aldrich). Cells were fixed at the indicated time points using 4% paraformaldehyde (Sigma Aldrich) and subjected to the standard immunostaining protocol (as described above) (Hoppe et al., 2016).
Statistical Analyses
The error bars in this report indicate SDs. Sample means with SDs were derived from the indicated numbers of mice. Each mouse represents an independent replicate. The difference between two samples was analyzed by using either two-sample t test or two-sided Wilcoxon rank-sum test with continuity correction after normality analysis with custom written codes in R.
Author Contributions
N.A. planned and performed the experiments and analyzed data. P.S.H. cloned knockin constructs and provided technical support for TF immunostaining protocols with M.E. L.K. and G.C.O. performed in situ immunostaining of adult and embryonic tissues, respectively. D.L. supported the imaging. O.H. programmed the software. K.A. generated GATA2VENUS ESCs. T.S. designed and supervised the study, analyzed data, and wrote the manuscript with N.A. All authors read and commented on final manuscript.
Acknowledgments
We are grateful to G. Camenisch and M. Hussherr of the D-BSSE mouse facility for animal handling, the EPIC facility of ETH Zurich for GATA2 mouse line generation from ESCs and the D-BSSE SCU for flow cytometry and imaging support. We thank P. Dettinger for reviewing the manuscript. This work was supported by Swiss National Science Foundation grant to T.S. and EMBO long-term fellowship to M.E. T.S. and O.H. acknowledge financial support from SystemsX.ch.
Published: July 09, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.06.008.
Data and Code Availability
Data is available upon request.
Supplemental Information
References
- Akashi K., Traver D., Miyamoto T., Weissman I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Arinobu Y., Iwasaki H., Gurish M.F., Mizuno S., Shigematsu H., Ozawa H., Tenen D.G., Austen K.F., Akashi K. Developmental checkpoints of basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl. Acad. Sci. U S A. 2005;102:1–6. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E.H., Martowicz M., Pal S., Johnson K.D. Developmental control via GATA factor interplay at chromatin domains. J. Cell. Physiol. 2005;205:1–9. doi: 10.1002/jcp.20393. [DOI] [PubMed] [Google Scholar]
- Bresnick E.H., Lee H.Y., Fujiwara T., Johnson K.D., Keles S. GATA switches as developmental drivers. J. Biol. Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas-Wallscheid N., Klimmeck D., Hansson J., Lipka D.B., Reyes A., Wang Q., Weichenhan D., Lier A., Von Paleske L., Renders S. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Cantor A.B., Iwasaki H., Arinobu Y., Moran T.B., Shigematsu H., Sullivan M.R., Akashi K., Orkin S.H. Antagonism of FOG-1 and GATA factors in fate choice for the mast cell lineage. J. Exp. Med. 2008;205:611–624. doi: 10.1084/jem.20070544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles M.A., Saunde T.L., Wood W.M., Owens K., Parlow A.F., Camper S.A., Ridgway E.C., Gordon D.F. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol. Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- Chen C.-C., Grimbaldeston M.A., Tsai M., Weissman I.L., Galli S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. U S A. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu D.L., Kokkaliaris K.D., Kunz L., Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat. Biotechnol. 2017;35:1202–1210. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- Coutu D.L., Kokkaliaris K.D., Kunz L., Schroeder T. Multicolor quantitative confocal imaging cytometry. Nat. Methods. 2018;15:39–46. doi: 10.1038/nmeth.4503. [DOI] [PubMed] [Google Scholar]
- Dahlin J.S., Hamey F.K., Pijuan-Sala B., Shepherd M., Lau W.W.Y., Nestorowa S., Weinreb C., Wolock S., Hannah R., Diamanti E. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 2018;131:e1–e11. doi: 10.1182/blood-2017-12-821413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen J.S., O’Connell S.M., Flynn S.E., Treier M., Gleiberman A.S., Szeto D.P., Hooshmand F., Aggarwal A.K., Rosenfeld M.G. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient- induced determination of pituitary cell types. Cell. 1999;97:587–598. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- Doré L.C., Chlon T.M., Brown C.D., White K.P., Crispino J.D. Chromatin occupancy analysis reveals genome-wide GATA factor switching during hematopoiesis. Blood. 2012;119:3724–3733. doi: 10.1182/blood-2011-09-380634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drissen R., Buza-Vidas N., Woll P., Thongjuea S., Gambardella A., Giustacchini A., Mancini E., Zriwil A., Lutteropp M., Grover A. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat. Immunol. 2016;17:666–676. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymecki S.M. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc. Natl. Acad. Sci. U S A. 1996;93:6191–6196. doi: 10.1073/pnas.93.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich C., Arlt J., Vink C.S., Solaimani Kartalaei P., Kaimakis P., Mariani S.A., van der Linden R., van Cappellen W.A., Dzierzak E. In vivo single cell analysis reveals Gata2 dynamics in cells transitioning to hematopoietic fate. J. Exp. Med. 2018;215:233–248. doi: 10.1084/jem.20170807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken H., Rieger M., Hoppe P., Hermann A., Smejkal B., Drew E., Thum M., Ninkovic J., Beckervordersandforth R. Continuous long-term detection of live cell surface markers by ‘in culture’ antibody staining. Protocol Exchange. 2011 doi: 10.1038/protex.2011.205. [DOI] [Google Scholar]
- Eilken H.M., Nishikawa S.I., Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Endele M., Loeffler D., Kokkaliaris K.D., Hilsenbeck O., Skylaki S., Hoppe P.S., Schambach A., Stanley E.R., Schroeder T. CSF-1-induced Src signaling can instruct monocytic lineage choice. Blood. 2017;129:1691–1701. doi: 10.1182/blood-2016-05-714329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzrodt M., Schroeder T. Illuminating stem cell transcription factor dynamics: long-term single-cell imaging of fluorescent protein fusions. Curr. Opin. Cell Biol. 2017;49:77–83. doi: 10.1016/j.ceb.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Etzrodt M., Endele M., Schroeder T. Quantitative single-cell approaches to stem cell research. Cell Stem Cell. 2014;15:546–558. doi: 10.1016/j.stem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Etzrodt M., Ahmed N., Hoppe P.S., Loeffler D., Skylaki S., Hilsenbeck O., Kokkaliaris K.D., Kaltenbach H.-M., Stelling J., Nerlov C. Inflammatory signals directly instruct PU.1 in HSCs via TNF. Blood. 2018;133:816–819. doi: 10.1182/blood-2018-02-832998. [DOI] [PubMed] [Google Scholar]
- Filipczyk A., Marr C., Hastreiter S., Feigelman J., Schwarzfischer M., Hoppe P.S., Loeffler D., Kokkaliaris K.D., Endele M., Schauberger B. Network plasticity of pluripotency transcription factors in embryonic stem cells. Nat. Cell Biol. 2015;17:1235–1246. doi: 10.1038/ncb3237. [DOI] [PubMed] [Google Scholar]
- Gao X., Johnson K.D., Chang Y.-I., Boyer M.E., Dewey C.N., Zhang J., Bresnick E.H. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 2013;210:2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- Grass J.A., Boyer M.E., Pal S., Wu J., Weiss M.J., Bresnick E.H. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U S A. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger J., Richards D.M., Hansson J., Barra M.M., Joschko A.C., Krijgsveld J., Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- Hilsenbeck O., Schwarzfischer M., Loeffler Di., DImopoulos S., Hastreiter S., Marr C., Theis F.J., Schroeder T. FastER: a user-friendly tool for ultrafast and robust cell segmentation in large-scale microscopy. Bioinformatics. 2017;33:2020–2028. doi: 10.1093/bioinformatics/btx107. [DOI] [PubMed] [Google Scholar]
- Hoppe P.S., Coutu D.L., Schroeder T. Single-cell technologies sharpen up mammalian stem cell research. Nat. Cell Biol. 2014;16:919–927. doi: 10.1038/ncb3042. [DOI] [PubMed] [Google Scholar]
- Hoppe P.S., Schwarzfischer M., Loeffler D., Kokkaliaris K.D., Hilsenbeck O., Moritz N., Endele M., Filipczyk A., Gambardella A., Ahmed N. Early myeloid lineage choice is not initiated by random PU.1 to GATA1 protein ratios. Nature. 2016;535:299–302. doi: 10.1038/nature18320. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Mizuno S., Mayfield R., Shigematsu H., Arinobu Y., Seed B., Gurish M.F., Takatsu K., Akashi K. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 2005;201:1891–1897. doi: 10.1084/jem.20050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Mizuno S.I., Arinobu Y., Ozawa H., Mori Y., Shigematsu H., Takatsu K., Tenen D.G., Akashi K. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20:3010–3021. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimakis P., De Pater E., Eich C., Kartalaei P.S., Kauts M., Vink C.S., Van Der Linden R., Jaegle M., Yokomizo T., Meijer D. Functional and molecular characterization of mouse Gata2-independent hematopoietic progenitors (blood-2015-10-673749.full) Blood. 2016;127:1426–1438. doi: 10.1182/blood-2015-10-673749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauts M.L., De Leo B., Rodríguez-Seoane C., Ronn R., Glykofrydis F., Maglitto A., Kaimakis P., Basi M., Taylor H., Forrester L. Rapid mast cell generation from Gata2 reporter pluripotent stem cells. Stem Cell Reports. 2018;11:1009–1020. doi: 10.1016/j.stemcr.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar M., Suzuki N., Lewton J., Yamamoto M., Douglas Engel J. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developing urogenital system. Mol. Cell. Biol. 2004;24:10263–10276. doi: 10.1128/MCB.24.23.10263-10276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz O.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Krumsiek J., Marr C., Schroeder T., Theis F.J. Hierarchical differentiation of myeloid progenitors is encoded in the transcription factor network. PLoS One. 2011;6:e22649. doi: 10.1371/journal.pone.0022649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh H.Y., Champhekhar A., Nutt S.L., Elowitz M.B., Rothenberg E.V. Positive feedback between PU.1 and the cell cycle controls myeloid differentiation. Science. 2013;341:670–673. doi: 10.1126/science.1240831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.I., Young R.A. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–1251. doi: 10.1016/j.cell.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Qi X., Liu B., Huang H. The STAT5–GATA2 pathway is critical in basophil and mast cell differentiation and maintenance. J. Immunol. 2015;194:4328–4338. doi: 10.4049/jimmunol.1500018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K.-W., Ottersbach K., van Hamburg J.P., Oziemlak A., Tsai F.-Y., Orkin S.H., Ploemacher R., Hendriks R.W., Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler D., Schroeder T. Understanding cell fate control by continuous single cell quantification. Blood. 2019;133:1406–1414. doi: 10.1182/blood-2018-09-835397. [DOI] [PubMed] [Google Scholar]
- Loeffler D., Wang W., Hopf A., Hilsenbeck O., Bourgine P.E., Rudolf F., Martin I., Schroeder T. Mouse and human HSPC immobilization in liquid culture by CD43- or CD44-antibody coating. Blood. 2018;131:1425–1429. doi: 10.1182/blood-2017-07-794131. [DOI] [PubMed] [Google Scholar]
- McGrath K.E., Frame J.M., Fegan K.H., Bowen J.R., Conway S.J., Catherman S.C., Kingsley P.D., Koniski A.D., Palis J. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor Z., Hein S., Fiegler H., Schroeder T., Stocking C., Just U., Cross M. Transient expression of PU.1 commits multipotent progenitors to a myeloid fate whereas continued expression favors macrophage over granulocyte differentiation. Exp. Hematol. 2003;31:39–47. doi: 10.1016/s0301-472x(02)01017-2. [DOI] [PubMed] [Google Scholar]
- Minami T., Murakami T., Horiuchi K., Miura M., Noguchi T., Miyazaki J.I., Hamakubo T., Aird W.C., Kodama T. Interaction between Hex and GATA transcription factors in vascular endothelial cells inhibits flk-1/KDR-mediated vascular endothelial growth factor signaling. J. Biol. Chem. 2004;279:20626–20635. doi: 10.1074/jbc.M308730200. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Nardelli J., Thiesson D., Fujiwara Y., Tsai F.Y., Orkin S.H. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev. Biol. 1999;210:305–321. doi: 10.1006/dbio.1999.9278. [DOI] [PubMed] [Google Scholar]
- Ohmori S., Takai J., Ishijima Y., Suzuki M., Moriguchi T., Philipsen S., Yamamoto M., Ohneda K. Regulation of GATA factor expression is distinct between erythroid and mast cell lineages. Mol. Cell. Biol. 2012;32:4742–4755. doi: 10.1128/MCB.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori S.N.Y., Moriguchi T., Noguchi Y., Ikeda M., Kobayashi K., Tomaru N., Ishijima Y., Ohneda O., Yamamoto M., Ohneda K. GATA2 is critical for the maintenance of cellular identity in differentiated mast cells derived from mouse bone marrow. Blood. 2015;125:3306–3315. doi: 10.1182/blood-2014-11-612465. [DOI] [PubMed] [Google Scholar]
- Okita C., Sato M., Schroeder T. Generation of optimized yellow and red fluorescent proteins with distinct subcellular localization. Biotechniques. 2004;36:418–424. doi: 10.2144/04363ST01. [DOI] [PubMed] [Google Scholar]
- de Pater E., Kaimakis P., Vink C.S., Yokomizo T., Yamada-Inagawa T., van der Linden R., Kartalaei P.S., Camper S.A., Speck N., Dzierzak E. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013;210:2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T., Thorn K., Schroeder T., Wang L., Theis F.J., Marr C., Navab N. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 2017;8:1–7. doi: 10.1038/ncomms14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons B.D.A., Allay J.A., Allay E.R., Ashmun R.A., Orlic D., Jane S.M., Cunningham J.M., Nienhuis A.W. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. In Vitro. 1999;93:488–499. [PubMed] [Google Scholar]
- Pietras E.M., Reynaud D., Kang Y.-A., Carlin D., Calero-Nieto F.J., Leavitt A.D., Stuart J.M., Gottgens B., Passegué E. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop R., Shearstone J.R., Shen Q., Liu Y., Hallstrom K., Koulnis M., Gribnau J., Socolovsky M. A key commitment step in erythropoiesis is synchronized with the cell cycle clock through mutual inhibition between PU.1 and S-phase progression. PLoS Biol. 2010;8:e1000484. doi: 10.1371/journal.pbio.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk C.J.H., Rossi D.J., Månsson R., Attema J.L., Norddahl G.L., Chan C.K.F., Sigvardsson M., Weissman I.L., Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Qi X., Hong J., Chaves L., Zhuang Y., Chen Y., Wang D., Chabon J., Graham B., Ohmori K., Li Y. Antagonistic regulation by the transcription factors C/EBPα and MITF specifies basophil and mast cell fates. Immunity. 2013;39:97–110. doi: 10.1016/j.immuni.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Dutta D., Karim Rumi M.A., Kent L.N., Soares M.J., Paul S. Context-dependent function of regulatory elements and a switch in chromatin occupancy between GATA3 and GATA2 regulate Gata2 transcription during trophoblast differentiation. J. Biol. Chem. 2009;284:4978–4988. doi: 10.1074/jbc.M807329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N.P., Janzen V., Forkert R., Dombkowski D.M., Boyd A.S., Orkin S.H., Enver T., Vyas P., Scadden D.T. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- Schroeder T. Tracking hematopoiesis at the single cell level. Ann. N. Y. Acad. Sci. 2005;1044:201–209. doi: 10.1196/annals.1349.025. [DOI] [PubMed] [Google Scholar]
- Shimizu R., Yamamoto M. Gene expression regulation and domain function of hematopoietic GATA factors. Semin. Cell Dev. Biol. 2005;16:129–136. doi: 10.1016/j.semcdb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Siggers P., Smith L., Greenfield A. Sexually dimorphic expression of Gata-2 during mouse gonad development. Mech. Dev. 2002;111:159–162. doi: 10.1016/s0925-4773(01)00602-5. [DOI] [PubMed] [Google Scholar]
- Snow J.W., Trowbridge J.J., Johnson K.D., Fujiwara T., Emambokus N.E., Grass J.A., Orkin S.H., Bresnick E.H., Dc W. Context-dependent function of “GATA switch” sites in vivo. Blood. 2011;117:4769–4772. doi: 10.1182/blood-2010-10-313031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F., Furlong E.E.M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Ohneda O., Minegishi N., Nishikawa M., Ohta T., Takahashi S., Engel J.D., Yamamoto M. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc. Natl. Acad. Sci. U S A. 2006;103:2202–2207. doi: 10.1073/pnas.0508928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q., Dalgin G., Xu H., Ting C.N., Leiden J.M., Hotamisligil G.S. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- Tong Q., Tsai J., Tan G., Dalgin G., Hotamisligil G.S. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol. Cell. Biol. 2005;2:706–715. doi: 10.1128/MCB.25.2.706-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F.-Y., Keller G., Kuo F.C., Weiss M., Chen J., Rosenblatt M., Alt F.W., Orkin S.H. An early hematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Walsh J.C., DeKoter R.P., Lee H.J., Smith E.D., Lancki D.W., Gurish M.F., Friend D.S., Stevens R.L., Anastasi J., Singh H. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- Weinreb C., Rodriguez-Fraticelli A., Camargo F.D., Klein A.M. Lineage tracing on transcriptional landscapes links state to fate during differentiation Caleb. Science. 2020;367:eaaw3381. doi: 10.1126/science.aaw3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Oser G., van der Wath R.C., Blanco-Bose W., Jaworski M., Offner S., Dunant C.F., Eshkind L., Bockamp E. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Yanez A., Ng M.Y., Hassanzadeh-kiabi N., Goodridge H.S. IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Comparative Study. 2017;125:1452–1460. doi: 10.1182/blood-2014-09-600833. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 1998;17:6689–6700. doi: 10.1093/emboj/17.22.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request.