Summary
The last 5 years have witnessed a significant increase in the number of clinical studies based on human pluripotent stem cells (hPSCs). In parallel, concern is increasing about the proliferation of unregulated stem cell treatments worldwide. Regulated clinical testing is a de facto standard to establish the safety and efficacy of new cell therapies, yet reliable information on clinical studies involving hPSCs is scattered. Our analysis of a multitude of resources found 54 clinical studies involving several types of hPSCs, which are performed in ten countries. While the majority of those studies is based on human embryonic stem cells (hESCs), clinical studies involving human induced pluripotent stem cells increased more strongly in the past 2 years than the number of hESC-based studies. A publicly accessible database was created using the human pluripotent stem cell registry (https://hpscreg.eu) platform, providing a steadily updated comprehensive overview on hPSC-based clinical studies performed worldwide.
Keywords: pluripotent stem cell, clinical study database, embryonic stem cell
Highlights
-
•
Establishment of a database for clinical studies based on pluripotent stem cells
-
•
54 clinical studies identified from public sources
-
•
Majority of studies based on embryonic stem cells
-
•
Strong increase in studies based on induced pluripotent stem cells in last 2 years
In this article, Kurtz and colleagues provide a comprehensive overview of clinical studies using cells derived from human pluripotent stem cells. They show that although the majority of studies still used embryonic stem cells, the number of studies based on induced pluripotent stem cells is rapidly increasing. An hPSC-focused clinical study database was established on the hPSCreg platform.
Introduction
Very soon after the first derivation of human pluripotent stem cell (hPSC) lines more than 20 years ago (Thomson et al., 1998), there were hopes in the scientific community that these cells could be the basis for future cell and tissue replacement therapies (Trounson and Pera, 2001). Over the past two decades, progenitor or somatic cells derived from hPSCs proved to be functional after transplantation in animals and could therefore serve as cell/tissue replacement in the treatment of a wide range of human degenerative diseases. Although the prospective clinical use of hPSC-derived cells/tissues still faces a range of safety, quality, functionality, and efficacy issues that need to be solved, a first safety study involving human embryonic stem cell (hESC)-derived oligodendrocyte progenitors for treatment of subacute spinal cord injury was already launched in 2007 and approved in 2009, after years of delay and a gargantuan 22,000-plus page Investigational New Drug application (Alper, 2009; Pollack, 2010). This study was followed by the first hESC-based clinical studies for treatment of macular degeneration (Liu et al., 2018; Schwartz et al., 2012, 2015; Song et al., 2015; Vitillo et al., 2020) with some early positive indications after 2–3 years of follow-up (https://theophthalmologist.com/subspecialties/a-promise-fulfilled), diabetes mellitus (Schulz, 2015), and ischemic heart disease (Menasche et al., 2018).
With the establishment of the first human induced pluripotent stem cells (hiPSCs) in 2007 (Takahashi et al., 2007; Yu et al., 2007), the prospect was held out that hiPSCs would facilitate personalized cell therapies for treating human diseases while solving the controversy over the destruction of embryos in the process of deriving hESCs. Indeed, due to strong promotion of hiPSC research especially in Japan, a first autologous hiPSC-based clinical study was initiated in 2014 for the treatment of age-related macular degeneration (Mandai et al., 2017), and shortly thereafter a whole battery of further clinical trials involving hiPSC-derived cells were either announced or initiated.
Since the translation of hPSC research to the clinic started in 2010, we and others have begun to systematically analyze and document the respective clinical studies to assess the progress in this field and provide an overview on planned, completed, and ongoing hPSC-based clinical trials (Desgres and Menasche, 2019; Guhr et al., 2018). Here we present the up-to-date status on the extent, subject, and localization of hPSC-based clinical studies currently under way and provide an easily accessible reference database on hPSC-based clinical studies. Data were collected from a multitude of publicly accessible sources such as clinical trial registries worldwide, press releases, and scientific papers, and a respective database was established, which is hosted on the hPSCreg platform (https://hpscreg.eu/browse/trials). This novel database combines data from relevant national and international clinical trial registries, provides for a constantly updated and comprehensive overview on the type of clinical studies, and also links clinical studies to the hPSC lines on which a cell or tissue therapy product is based. The criteria adopted to test the veracity of included data is discussed in terms of establishing minimum standards and necessary criteria for such international databases and ongoing refinement of the hPSCreg database.
Results
Current State of hPSC-Based Clinical Studies
Having studied research trends in the field of hPSCs over the past 15 years (Guhr et al., 2006, 2018; Kobold et al., 2015; Löser et al., 2010, 2012), we were now interested in tracing the latest developments of translating hPSC research to the clinic. Because of a lack of a comprehensive access platform for information on clinical studies, we searched clinical trial databases, press releases, scientific articles, and web pages of companies involved in hPSC research for novel clinical study data on a regular basis (for details see Experimental Procedures). Based on this continuous intensive long-term collection of publicly available information, we compiled a unique database of verified clinical studies based on hPSCs, including hESCs, hiPSCs, stem cells created by somatic cell nuclear transfer (SCNT-hPSCs), and parthenogenetically derived human pluripotent stem cells (hpPSCs).
A summary of our data is shown in Table 1. By the end of 2019, there were reports on at least 54 clinical studies based on hPSCs aimed at treating 22 different diseases. It should be noted that all of these 54 studies include the transfer of a cell product to patients. Other studies also listed in registries such as clinicaltrials.gov and which, for example, only plan the production of patient-specific clinical-grade hPSC lines, were excluded from our analyses.
Table 1.
Clinical Trials on the Basis of hPSCs by the End of 2019
| ICD-10 Main Chapter | Disease ICD-10 | Cellular Origin of Cell Product |
||||
|---|---|---|---|---|---|---|
| hESC | hiPSC | hpPSC | SCNT-hESC | Total | ||
| Neoplasms | malignant neoplasms (advanced solid tumors) | 0 | 2 | 0 | 0 | 5 |
| malignant neoplasm of bronchus and lung | 1 | 0 | 0 | 0 | ||
| myeloid leukemia | 0 | 1 | 0 | 0 | ||
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | beta-thalassemia | 0 | 2 | 0 | 0 | 2 |
| Endocrine, nutritional, and metabolic diseases | type 1 diabetes mellitus | 4 | 1 | 0 | 0 | 6 |
| primary ovarian failure | 1 | 0 | 0 | 0 | ||
| Diseases of the nervous system | Parkinson's disease | 0 | 3 | 2 | 0 | 6 |
| motor neuron disease | 1 | 0 | 0 | 0 | ||
| Diseases of the eye and adnexa | other retinal disorders | 4 | 0 | 0 | 0 | 25 |
| degeneration of macula and posterior pole: senile macular degeneration, dry age-related macular degeneration | 10 | 1 | 0 | 1 | ||
| hereditary retinal dystrophy: Retinitis pigmentosa | 2 | 0 | 0 | 0 | ||
| hereditary retinal dystrophy: Stargardt disease | 5 | 0 | 0 | 0 | ||
| other specified disorders of cornea (limbal stem cell deficiency) | 0 | 1 | 0 | 0 | ||
| degeneration of macula and posterior pole: wet age-related macular degeneration | 0 | 1 | 0 | 0 | ||
| Diseases of the circulatory system | ischemic heart diseases | 1 | 4 | 0 | 0 | 7 |
| cerebral infarction, unspecified | 0 | 2 | 0 | 0 | ||
| Diseases of the musculoskeletal system and connective tissue | derangement of meniscus due to old tear or injury | 1 | 0 | 0 | 0 | 1 |
| Injury, poisoning, and certain other consequences of external causes | injury of spinal cord, level unspecified | 2 | 0 | 0 | 0 | 3 |
| bone marrow transplant rejection | 0 | 1 | 0 | 0 | ||
| 32 | 19 | 2 | 1 | 54 | ||
Given are diseases targeted by the respective studies and the number of studies involving hESC-, hiPSC-, hpPSC-, and SCNT-hESC-derived cell products. Two Food and Drug Administration-approved hESC studies withdrawn before enrollment are not included.
Nearly half of these studies (n = 24) aim to develop new therapies for eye diseases, mainly for different kinds of macular degeneration and retinal dystrophies. It is not surprising that the eye is the major target of first hPSC-based trials in humans, since it is immune-privileged, is easily accessible for transplantation, and capable of direct observation for assessment of engraftment. Moreover, differentiation of retinal pigment epithelia cells is highly efficient, and therapeutic effects in the eye may require only a rather small number of cells, reducing the risks of tumorigenicity (Zarbin et al., 2019). In addition, transplants in the eye can be monitored non-invasively, allowing for detection of potential aberrations at an early stage. Beyond that, macular degeneration in particular is a very frequent disease in the developed world, and adequate therapies are not available. The next most popular indications for hPSC-derived cell therapy were aimed at diabetes mellitus (four clinical studies sponsored by Viacyte) and ischemic heart diseases (also four studies): both diseases also represent major health problems in Western countries. Other clinical studies performed so far aim to treat neoplasms, neuronal degenerative diseases such as Parkinson's disease, or spinal cord injuries. Details of all 54 clinical trials are given in Table S1.
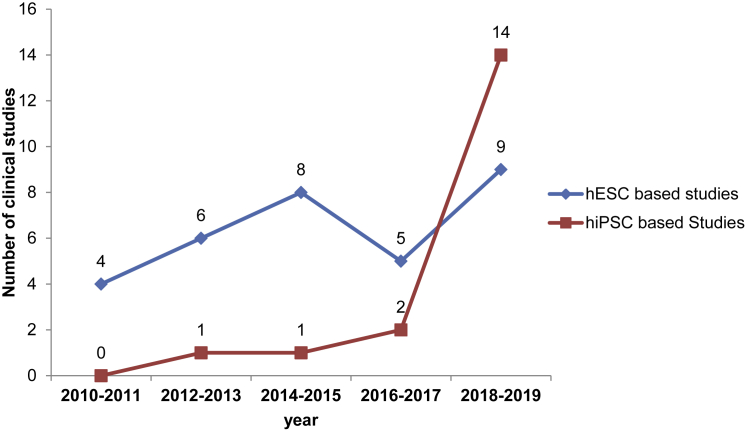
While 32 of the clinical studies identified are performed with hESC-derived cell products, 19 studies are based on hiPSCs. Figure 1 shows the temporal distribution of the initiation of hESC- and hiPSC-based clinical studies within the past 10 years. Not surprisingly, since hESCs entered the field nearly 10 years before hiPSCs, hESC-based cell products found their way into clinical trials earlier than hiPSCs. However, while the annual number of newly started hESC-based clinical studies did not markedly increase after 2014–2015, we observed a significant increase in hiPSC-based studies initiated in the last 2-year period analyzed here (from only two new studies in 2016–2017 to 14 new studies in 2018–2019). In addition, there are two clinical studies in which a cell product based on hpPSCs is tested in patients with Parkinson's disease and one study in which retinal pigment epithelial cells derived from SCNT-hPSCs are used for treatment of dry age-related macular degeneration.
Figure 1.
Temporal Distribution of the Initiation of Clinical Studies Based on either hESCs or hiPSCs within the Past 10 Years
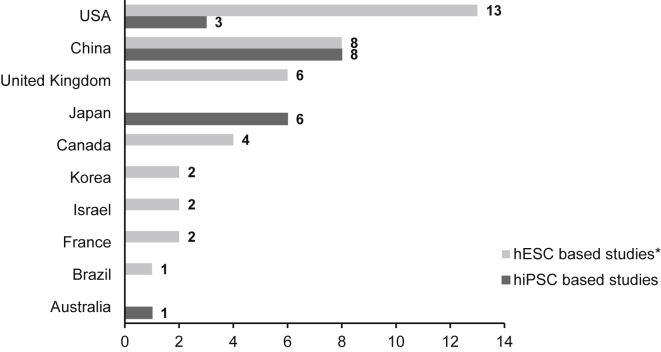
Clinical studies based on hPSC are being performed in at least ten countries (Figure 2). Most studies were initiated in the United States and China, with at least 16 clinical trials started until the end of 2019 in each of these two countries. Clinics in the United Kingdom as well as in Canada are involved in six and four hESC-based clinical trials, respectively, while seven clinical studies based on hiPSCs were or are being performed in Japan. It should be noted that the number of clinical studies initiated in the respective countries correlates with the national research activities in the hPSC field (reported in Guhr et al., 2018), indicating that intensively funded basic research is the basis for the successful development of cell products for clinical use.
Figure 2.
hPSC-Based Clinical Studies by Countries Worldwide
Shown is the number of clinical trials based on either hESCs or hiPSCs which were performed/initiated within the past decade. ∗Several studies were/are performed in more than one country so that in some cases the same study was assigned to more than one country.
It is also of interest to know from which specific hPSC line the cell product used in a given clinical trial was developed: the use of cell products derived from a particular cell line in numerous trials would increase the body of data to establish a safe history of use and promote the exchange and harmonization of applicable quality requirements between different lines. While there is only scarce data publicly available as to which specific cell lines were used in hiPSC-based studies, this information can be provided for most (27 out of 32) hESC-based trials (Table 2). According to data publicly available, cell products derived from hESC line MA09 were used in 11 studies, which all aim at therapy development for degenerative eye diseases, while 16 studies are based on one of the nine other hESC lines listed in Table 2. Of the ten known hESC cell lines used as a starting material for cell products to be transferred into patients, four cell lines (HDAC100, HDAC112, Q-CTS-hESC-2, and RC-09) were originally derived as clinical-grade cell lines or as GMP-compliant cell lines, respectively (Kaupisch et al., 2012; Liu et al., 2018; Tannenbaum et al., 2012). The remaining six hESC lines were established as laboratory-grade lines and were only retrospectively converted into clinical-grade material (Amit and Itskovitz-Eldor, 2002; D'Amour et al., 2006; Draper et al., 2004; Lund et al., 2006; Thomson et al., 1998). In this context it should be noted that the use of standard pluripotent stem cell line names, as promoted by hPSCreg (Seltmann et al., 2016), would enable unambiguous and transparent tracking of these clinically important lines from research to clinical application.
Table 2.
hESC Lines Used for Production of Cell Products Used in Clinical Trials So Far
| hESC Line (Provider) | hESC Line (hPSCreg) | First Publication of Cell Line | No. of Clinical Trials |
|---|---|---|---|
| MA09 | ACTe002-A | 2006 | 11 |
| Cyt49 | VCYTe001-A | 2006 | 4 |
| H1 | WAe001-A | 1998 | 3 |
| H9 | WAe009-A | 1998 | 2 |
| Shef-1 | AXORe001-A | 2004 | 2 |
| HADC102 | HADe008-A | 2012 | 1 |
| HADC100 | HADe007-A | 2012 | 1 |
| I6 | TECHe003-A | 2002 | 1 |
| Q-CTS-hESC-2 | not yet registered | 2016 | 1 |
| RC-09 | RCe013-A | 2012 | 1 |
| Data NA | 5 |
Given are the provider's and hPSCreg's designation of hESC lines, the year of its first publication in the scientific literature, and the number of clinical trials based on the respective cell line. Please note that for five studies performed in China, cell line information cannot be provided.
Although there are no detailed data publicly available on the specific hiPSC lines used as a starting material for the production of hiPSC-derived cells/tissues used in clinical trials, information on the autologous or allogenic use of the hiPSC-derived material was assessable. According to this information, in 8 out of 19 studies (mainly based in China), autologous hiPSCs were used to produce the cell product for transplantation, whereas in 11 cases allogenic cells were used as a starting material. The allogenic use of hiPSC-derived cell products may reflect the growing realization that patient-specific therapies using personalized cell products is currently extremely expensive and time-consuming and therefore may not be broadly applicable in the near future.
The Clinical Studies Database on hPSCreg
Currently there is no publicly available comprehensive overview on hPSC-based clinical trials, and collecting the relevant scattered information is cumbersome. The lack of information access and transparency may hamper clinical study communication, exchange of knowledge, and development (https://uottawa.scholarsportal.info/ottawa/index.php/uojm-jmuo/article/view/2021 and https://pharmafield.co.uk/pharma_news/fewer-than-half-of-us-clinical-trials-comply-with-law-on-reporting-results/). Therefore, we initiated a clinical study database to meet the need for easily accessible comprehensive and up-to-date information on clinical studies. This database is part of the Human Pluripotent Stem Cell Registry (hPSCreg) platform, a global and freely accessible registry for hPSC lines that has been progressively developing its procedures for management of ethical and scientific data since 2006 (Isasi et al., 2019; Seltmann et al., 2016).
The aim of the clinical study database is to provide a regularly updated overview on planned, ongoing, and completed clinical studies in which cell products derived from hPSCs, including hESCs, hiPSCs, SCNT-hPSCs, or hpPSCs, are tested in patients. Therefore, the database comprises only those trials where a stem cell-based product is used to treat patients as well as the respective (observational) follow-up studies. Some other clinical study databases/registries list, for example, the donation of tissues aimed to generate clinical-grade hiPSC lines (and therefore involve patients as donors and not as recipients of cells/tissues). These data are excluded from the clinical study database.
Although it is envisaged to provide a broad range of information on hPSC-based clinical studies, available data are sometimes restricted. As a consequence and to ensure a specified data consistency, a minimum information package was defined and is stringently required, including a meaningful study title, current study phase, starting date, estimated number of participants, and recruitment status. Furthermore, a contact including name and email address is required together with listing information about the person, company, or organization that takes responsibility for the initiation, management, and/or financing of the clinical trial (“sponsor”). The database also provides links to the full original source public records (e.g., to the respective record in the primary registries as listed in the “material and methods” sections) to facilitate the identification of trials by joining multiple records on the same trial. Currently, the database provides detailed information on 42 studies involving hPSC-derived material. To ensure consistent and valid data, the remaining 12 studies identified so far will only be included after all information required for listing in the database is obtained.
In addition to the obligatory information, a range of further data is included, for example on the specific aims with regard to the disease targeted by the respective cell product. Moreover, the original hPSC line on which a study is based, the name of cell or tissue product used in the study, and published scientific papers connected to a given clinical trial are listed. This latter information will provide links to the extensive data on hPSC lines registered in hPSCreg as well as to the comprehensive collection of scientific papers on derivation and use of hPSC lines present in hPSCreg.
Currently, information on clinical studies from publicly available national and international registries is manually curated by hPSCreg staff to ensure that data are recorded in a consistent manner. In addition, hPSCreg seeks the participation of principal investigators/sponsors of the respective studies to confirm the clinical study records whenever possible. Furthermore, voluntary text/uploads are possible, for example to better describe the clinical study and its outcomes or to provide more details on the potency assays and safety testing for the used hPSC lines as well as their derivatives. Records reconfirmed by the qualified investigator in charge of a given study are marked, indicating the extra level of validation. This process of data validation assures that registered data in hPSCreg are complete, accurate, and valid.
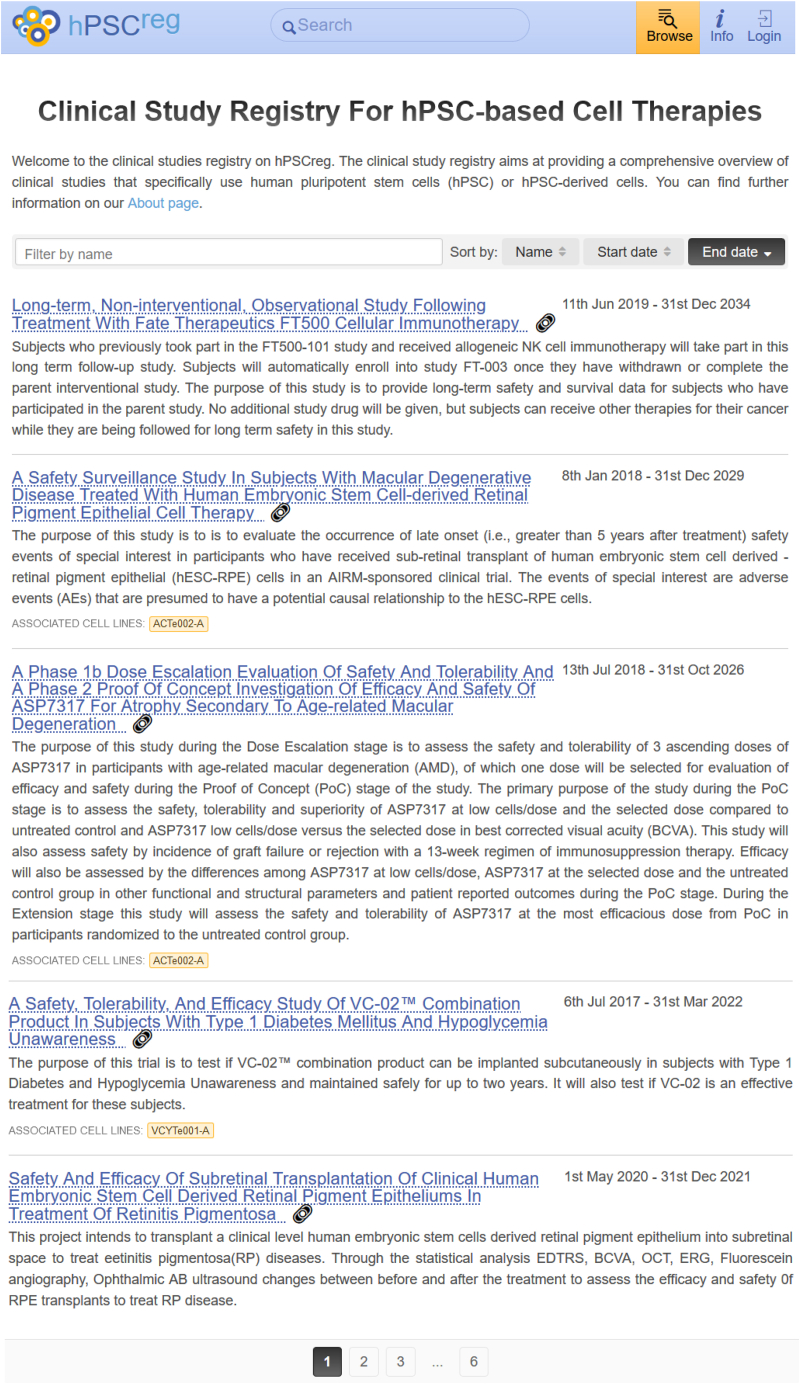
Figure 3 shows a screenshot of the searchable interface that currently displays all studies indexed in the database by descending study name as default. Currently, a sorting function allows displaying of the indexed studies by study name as well as starting and ending dates. For the future, additional filter options are planned so that it will be possible to find studies by study name, disease indication, sponsors, keywords, the hPSC line on which a given study is based, and scientific papers linked to a specific clinical trial.
Figure 3.
Screenshot of the Clinical Study Database for hPSC-Based Cell Therapies Interface
Discussion
Reliable information about ongoing clinical studies using hPSCs is required to assess the development and progress made in the field, which has made promises of cures for untreatable diseases. This is relevant to realistically inform not only the public but also translational researchers, regulators, and stakeholders. The provision of dependable, validated knowledge makes it also more difficult for dubious stem cell clinics to be successful. Moreover, during this early stage of clinical study development it is of high relevance to have information available on the cells being used and their characterization, quality assessment, application areas, details on clinical study design, recruitment, treatment, and the applicable regulatory and ethical backgrounds. This information should be presented in a way that would allow for at least a basic level of comparativeness and insights into developing standards. However, such information is currently not findable and accessible in a comprehensive, focused way but rather scattered in multiple databases and registries, and punctually described in some publications on planned and ongoing clinical use of hPSC-derived cell products for specific clinical applications (Parmar et al., 2020; Senior and Pettus, 2019; Silvestro et al., 2020; Wattanapanitch, 2019). Therefore, we have collected information on clinical studies from a wide array of sources. As reported previously for searching of literature (Guhr et al., 2018; Kobold et al., 2015), scouting clinical study databases or the web by the mere use of keywords without manual review of results for validation will result in a significant amount of false-positive results. For example, screening of clinicaltrials.gov with terms such as “embryonic stem cells” or “induced pluripotent stem cells” resulted in nearly 160 alleged clinical studies involving these cell types and would cause a considerable overestimation of the actual number of clinical studies registered. Instead, we identified 53 unique and actual clinical studies from the scouted resources, conducted in ten countries. However, this number might be an underestimation, as our searches rely mostly on English-language resources. Interestingly, 29 of the 53 clinical studies were sponsored by nine commercial entities. These companies sponsored clinical studies mainly for eye diseases and diabetes mellitus; study indications included spinal cord injury, amyotrophic lateral sclerosis, Parkinson's disease, and ischemic stroke, among others.
Not surprisingly, the majority of the studies performed are using hESCs as source material; only in the period 2018–2019 did the number of new studies using hiPSCs exceed those using hESCs as starting material. At least ten specific hESC lines were identified to be used in 27 clinical studies, while no information on the specific hESC line used as starting material could be acquired for five studies. In contrast to hESCs, for allogenic application of hiPSC-based cells (n = 11), none of the lines were unambiguously identifiable for the recorded studies. Thus, there is some lack of information with respect to the source material used especially for the clinically used hiPSC lines. However, information on cell lines used may be very valuable, as would be information about applied quality control and testing regimens for these cells and the differentiated therapeutic cell products. In addition, findability of clinically applied hPSC lines would be markedly increased by consequently using standard identifiers for these cell lines (Kurtz et al., 2018).
To make the clinical studies more findable and data on these accessible and interoperable, we establish a dedicated database for hPSC-based clinical studies. This database uses the hPSCreg platform and thus provides links to the data resources on hPSC lines registered in this database. As much as possible, standardized nomenclatures and ontologies are used for description of information to improve interoperability. Clinical studies in the database will be interlinked to other public resources hosting information on the same trial, thus creating a rich network of data. To be robust and thus reliable, the information provided on clinical studies in the hPSCreg database must satisfy a set of minimal criteria to be registered in the database and be validated for public access. This is required to allow for a meaningful evaluation and comparison of data. Preferentially, clinical study data obtained through the described search algorithms are manually entered by the hPSCreg team, which seeks verification by the team performing the respective clinical studies. In addition, data and information can be entered to enrich the information on a specific study, although all data will be independently validated by the hPSCreg to maintain a high level of reliability and completeness. The database does also allow for registration of new clinical studies by hPSCreg registered users. However, to ensure a high quality of the clinical study database, all newly entered studies will be manually verified by the hPSCreg and by the investigators performing the respective clinical studies before their public release. To assure transparency of database content development and reduce the risk of faulty transcription, the used standard protocols for data entry will be made available on the hPSCreg website.
The resource described here may be used for periodic updates to track trends over time regarding hiPSC, hESC, or other hPSC type of clinical use, type of product, potency and safety assays, commonly used cell lines, and quality assessment, and thus provide a platform for exchange of information. The clinical studies database at hPSCreg aims not to replace but rather to supplement established registries such as the World Health Organization (WHO) registry, which has an international regulatory utility and governance function. The main benefits of having a transparent overview on past and ongoing clinical studies with hPSCs is the mutual exchange of information, a platform for comparing and finding of applied standards and assays and to analyze trends in the field. Eventually, it may be used for comparison with other non-hPSC-based cell therapies. Finally, the high level of reliability and validation of information makes it a resource to distinguish studies having proper regulatory oversight from those aimed at providing commercial non-regulated clinical applications or treatments without proven therapeutic and safety profiles.
Experimental Procedures
Retrieving the Number of Clinical Studies Involving hPSCs
Identification of clinical studies was performed as described previously (Guhr et al., 2018). Please note that both terms “clinical study” and “clinical trial” were used to identify clinical studies/trials from public sources and include also designations such as clinical research studies within a regulated environment; therefore, the terms clinical study and clinical trial are used synonymously to describe a clinical application in a regulatory framework (see also Li et al., 2014).
In brief, the following resources were sourced: ClinicalTrials.gov, by the US National Library of Medicine (https://clinicaltrials.gov/), International Clinical Trials Registry Platform of the WHO (http://apps.who.int/trialsearch/), University Hospital Medical Information Network (UMIN), Japan (https://www.umin.ac.jp/ctr/), Chinese Clinical Trial Registry (ChiCTR), China (http://www.chictr.org.cn/searchprojen.aspx), the EU Clinical Trials Register by the European Medicines Agency, the Netherlands (https://www.clinicaltrialsregister.eu/ctr-search/search), German Clinical Trials Register (Deutsches Register Klinischer Studien [DRKS]) by the German Institute for Medical Documentation and Information, Germany (https://www.drks.de/drks_web/).
In addition, the sponsor's web pages were evaluated for the additional information on the respective clinical trials, and public information in the press was obtained by regular examination of Google News Alerts containing the terms stem cell or stem cells and clinical trials. Moreover, stem cell newsletters, such as Stem Cell Research News and Stem Cell Business News (published every 2 weeks by DataTrends Publications) were monitored on a regular basis. Extensive searches of the scientific literature were frequently performed in the PubMed database accessible through the NIH National Library of Medicine using the search strings described in Kobold et al. (2015), supplemented by the terms “clinical stud∗” or “clinical trial∗.”
Establishing the Clinical Study Database
The clinical study database is embedded in the hPSCreg platform. It is a web application composed of a user interface provided by an intermediary server application, which accesses the data storage through Java/Tomcat web services. The clinical study database described here uses the same technologies that hPSCreg employs and crosslinks to relevant information available in hPSCreg and worldwide clinical trial registries, including ClinicalTrials.gov (United States), European Union Drug Regulating Authorities Clinical Trials Database (EudraCT), the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; Japan), the ChiCTR (China), and the International Clinical Trials Registry Platform (WHO). To define clinical features and cell types, ontologies are used, including Orphanet Rare Disease Ontology, Disease Ontology, and Experimental Factor Ontology for clinical features, as well as Cell Ontology and Foundational Model of Anatomy for cells or anatomic locations.
The interface for recording the study information was designed to include the majority of the Trial Registration DataSet (TRDS) fields as defined by the International Standards for Clinical Trial Registries (https://apps.who.int/iris/handle/10665/274994). To increase data interoperability, hPSCreg stores existing clinical trial identifiers from other databases, such as NCT identifiers, EudraCT number, or sponsor-supplied identifiers.
Management, Maintenance, and Updating of Database
Initial datasets are sourced from international and national clinical trial registries that participate in the WHO Registry Network, whose members must adhere to the WHO Registry criteria, some of which include standards and guidelines for clinical trials (and their data) set out by the International Committee of Medical Journal Editors and the Declaration of Helsinki, among others. (International Standards for Clinical Trial Registries, Version 3.0. Geneva: WHO, 2018. License: CC BY-NC-SA 3.0 IGO.) To record data in a consistent manner, datasets and metadata annotations are entered into the database by hPSCreg staff. Registered users of hPSCreg are also allowed to enter new clinical studies; however, hPSCreg staff will check the information before it will be publicly displayed on the hPSCreg clinical study website. In the case of clinical studies involving hPSCs, information pertinent to the respective cell products, such as the cell line provenance from the source hPSC line to its hPSC-derived therapeutic product, are not mandatory information in the TRDS. This value-added information is manually collected by hPSCreg staff through public sources. Being currently not mandatory because this information is not available for some hiPSC-based cell products, hPSCreg aims at making this source cell information mandatory for new trials, and retrospectively for already registered trials. Finally, hPSCreg reaches out to qualified investigators of the clinical studies for verification of the collected data and additional updates. Data records, which have been further validated by contact with qualified investigators of the studies, are marked as such in the database to demonstrate the extra level of confirmation. A complete overview of the clinical study data sourcing process is shown in Figure S1.
Persons in charge of a study who would like to add a new clinical study to the database are encouraged to visit the hPSCreg web page and to contact hPSCreg through the respective form (https://hpscreg.eu/contact).
Author Contributions
A.G.: Collection and assembly of data, data analysis. S.K. Collection and assembly of data, data analysis. N.M.: Conception and design of database, data analysis and interpretation, manuscript writing. N.B.: Conception and design of database, data analysis and interpretation. S.S.: Conception and design of database. G.S.: Conception and design, data analysis and interpretation, manuscript writing. H.J.: Data verification. W.L.: Data verification. A.E.M.S.W.: Conception and design, data analysis and interpretation, manuscript writing. A.K.: Conception and design, data analysis and interpretation, manuscript writing. P.L.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Acknowledgments
This work was supported by the European Union Horizon 2020 Societal Challenges program under grant agreement ID 726320 (hPSCreg).
Published: July 16, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.06.014.
Contributor Information
Peter Löser, Email: loeserp@rki.de.
Andreas Kurtz, Email: andreas.kurtz@charite.de.
Supplemental Information
References
- Alper J. Geron gets green light for human trial of ES cell-derived product. Nat. Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- Amit M., Itskovitz-Eldor J. Derivation and spontaneous differentiation of human embryonic stem cells. J. Anat. 2002;200:225–232. doi: 10.1046/j.1469-7580.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G., Moorman M.A., Kroon E., Carpenter M.K., Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Desgres M., Menasche P. Clinical translation of pluripotent stem cell therapies: challenges and considerations. Cell Stem Cell. 2019;25:594–606. doi: 10.1016/j.stem.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Draper J.S., Moore H.D., Ruban L.N., Gokhale P.J., Andrews P.W. Culture and characterization of human embryonic stem cells. Stem Cell Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- Guhr A., Kobold S., Seltmann S., Seiler Wulczyn A.E.M., Kurtz A., Löser P. Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Reports. 2018;11:485–496. doi: 10.1016/j.stemcr.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhr A., Kurtz A., Friedgen K., Löser P. Current state of human embryonic stem cell research: an overview of cell lines and their use in experimental work. Stem Cells. 2006;24:2187–2191. doi: 10.1634/stemcells.2006-0053. [DOI] [PubMed] [Google Scholar]
- Isasi R., Namorado J., Mah N., Bultjer N., Kurtz A. A pathway for attesting ethical provenance of cell lines: lessons from the European Human Pluripotent Stem Cell Registry (hPSC(reg)) Stem Cell Res. 2019;40:101539. doi: 10.1016/j.scr.2019.101539. [DOI] [PubMed] [Google Scholar]
- Kaupisch A., Kennedy L., Stelmanis V., Tye B., Kane N.M., Mountford J.C., Courtney A., Baker A.H. Derivation of vascular endothelial cells from human embryonic stem cells under GMP-compliant conditions: towards clinical studies in ischaemic disease. J. Cardiovasc. Transl. Res. 2012;5:605–617. doi: 10.1007/s12265-012-9379-2. [DOI] [PubMed] [Google Scholar]
- Kobold S., Guhr A., Kurtz A., Löser P. Human embryonic and induced pluripotent stem cell research trends: complementation and diversification of the field. Stem Cell Reports. 2015;4:914–925. doi: 10.1016/j.stemcr.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Seltmann S., Bairoch A., Bittner M.S., Bruce K., Capes-Davis A., Clarke L., Crook J.M., Daheron L., Dewender J. A standard nomenclature for referencing and authentication of pluripotent stem cells. Stem Cell Reports. 2018;10:1–6. doi: 10.1016/j.stemcr.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.D., Atkins H., Bubela T. The global landscape of stem cell clinical trials. Regen. Med. 2014;9:27–39. doi: 10.2217/rme.13.80. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu H.W., Wang L., Li S.Y., Zhao C.J., Hao J., Li Q.Y., Zhao T.T., Wu W., Wang Y. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018;4:50. doi: 10.1038/s41421-018-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser P., Kobold S., Guhr A., Muller F.J., Kurtz A. Scope and impact of international research in human pluripotent stem cells. Stem Cell Rev. Rep. 2012;8:1048–1055. doi: 10.1007/s12015-012-9409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser P., Schirm J., Guhr A., Wobus A.M., Kurtz A. Human embryonic stem cell lines and their use in international research. Stem Cells. 2010;28:240–246. doi: 10.1002/stem.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund R.D., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauve Y., Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Menasche P., Vanneaux V., Hagege A., Bel A., Cholley B., Parouchev A., Cacciapuoti I., Al-Daccak R., Benhamouda N., Blons H. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 2020;21:103–115. doi: 10.1038/s41583-019-0257-7. [DOI] [PubMed] [Google Scholar]
- Pollack A. New York Times; 2010. Stem Cell Trial Wins Approval of F.D.A. [Google Scholar]
- Schulz T.C. Concise review: manufacturing of pancreatic endoderm cells for clinical trials in type 1 diabetes. Stem Cell Transl. Med. 2015;4:927–931. doi: 10.5966/sctm.2015-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z., Hubschman J.P., Davis J.L., Heilwell G., Spirn M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Seltmann S., Lekschas F., Muller R., Stachelscheid H., Bittner M.S., Zhang W., Kidane L., Seriola A., Veiga A., Stacey G. hPSCreg—the human pluripotent stem cell registry. Nucleic Acids Res. 2016;44:D757–D763. doi: 10.1093/nar/gkv963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P.A., Pettus J.H. Stem cell therapies for Type 1 diabetes: current status and proposed road map to guide successful clinical trials. Diabetic Med. 2019;36:297–307. doi: 10.1111/dme.13846. [DOI] [PubMed] [Google Scholar]
- Silvestro S., Bramanti P., Trubiani O., Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.K., Park K.M., Kim H.J., Lee J.H., Choi J., Chong S.Y., Shim S.H., Del Priore L.V., Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S.E., Turetsky T.T., Singer O., Aizenman E., Kirshberg S., Ilouz N., Gil Y., Berman-Zaken Y., Perlman T.S., Geva N. Derivation of xeno-free and GMP-grade human embryonic stem cells--platforms for future clinical applications. PLoS One. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Trounson A., Pera M. Human embryonic stem cells. Fertil. Steril. 2001;76:660–661. doi: 10.1016/s0015-0282(01)02880-1. [DOI] [PubMed] [Google Scholar]
- Vitillo L., Tovell V.E., Coffey P. Treatment of age-related macular degeneration with pluripotent stem cell-derived retinal pigment epithelium. Curr. Eye Res. 2020;45:361–371. doi: 10.1080/02713683.2019.1691237. [DOI] [PubMed] [Google Scholar]
- Wattanapanitch M. Recent updates on induced pluripotent stem cells in hematological disorders. Stem Cell Int. 2019;2019:5171032. doi: 10.1155/2019/5171032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zarbin M., Sugino I., Townes-Anderson E. Concise review: update on retinal pigment epithelium transplantation for age-related macular degeneration. Stem Cell Transl. Med. 2019;8:466–477. doi: 10.1002/sctm.18-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.