Abstract
Cells in assemblages differentiate and perform distinct roles. Though many pathways of differentiation are understood at the molecular level in multicellular eukaryotes, the elucidation of similar processes in bacterial assemblages is recent and ongoing. Here, we discuss examples of bacterial differentiation, focusing on cases in which distinct metabolisms coexist and those that exhibit cross-feeding, with one subpopulation producing substrates that are metabolized by a second subpopulation. We describe several studies of single-species systems, then segue to studies of multi-species metabolic heterogeneity and cross-feeding in the clinical setting. Many of the studies described exemplify the application of new techniques and modeling approaches that provide insights into metabolic interactions relevant for bacterial growth outside the laboratory.
Keywords: microfluidics, biofilms, metabolite exchange, phenotypic diversity
Bacterial multicellular systems show metabolic differentiation and cooperation
When cells grow in groups, they can benefit from differentiating such that cellular subpopulations perform discrete tasks. Differentiation enables division of labor and may allow for cross-feeding (the exchange of metabolites between cells), thereby increasing metabolic efficiency. It can also enhance robustness because cells with different physiologies vary in their sensitivities to stress. These phenomena are familiar for cases of eukaryotic cells growing as organisms such as animals and plants [1–8]. Though bacterial and eukaryotic cells are physiologically quite distinct, bacterial multicellular systems also show patterns of differentiation and accrue similar benefits to those of the well-studied examples from multicellular eukaryotes [9,10,84]. An understanding of the mechanisms that support division of labor, metabolite cross-feeding, and group-level responses to stress in bacterial communities is critical to modeling and exploiting bacterial activities as they relate to human welfare.
In this review, we discuss examples from the recent literature that showcase the metabolic heterogeneity and cross-feeding that can arise in bacterial systems. We strove to select a complement of studies that demonstrate the diversity of growth parameters and bacterial lifestyles that can exhibit these properties. However, we place an emphasis on the pathogen Pseudomonas aeruginosa, our major organism of interest, and growth as biofilms, assemblages of bacteria in a self-produced matrix that are notorious for their roles in the establishment and persistence of infections. We also describe studies in which metabolic interactions have implications for our understanding of the physiology of pathogens during host colonization and their responses to treatment.
Metabolic heterogeneity in bacterial multicellular systems
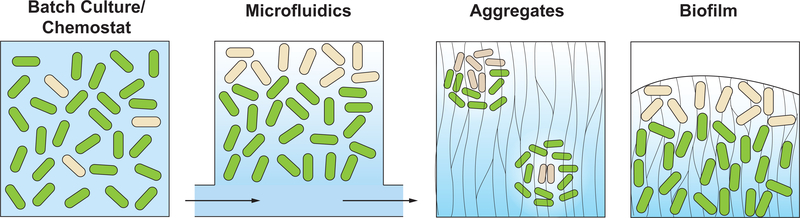
Laboratory culturing systems show a wide variation in the physical and chemical interactions that occur between individual cells and between cells and their environments (Figure 1). For metabolic differentiation, an important feature of the growth environment is whether it promotes chemical gradient formation. Bacteria suspended in well-mixed liquid media, such as those growing in batch cultures or chemostats, are usually dispersed and experience homogeneous environments. In contrast, bacteria growing in structured environments such as microfluidic devices typically grow at high density and experience chemical gradients because resources are provided from specific sources. The close associations between cells in these models may mirror those they experience in environmental contexts, where bacteria grow on decaying organic matter such as plant detritus or marine snow, in confined soil or poor water spaces, or on mammalian tissues [11]. Bacteria can also excrete polymeric substances, which constitute a matrix that promotes adhesion and establishes the structures of biofilms [12]. By producing matrix and forming biofilms, bacteria exert control over their physical interactions with other cells and objects and participate in a process that promotes chemical gradient formation. Studies have shown that bacterial metabolic differentiation and metabolite cross-feeding can arise in all growth regimes but that the architecture of the system and its bacterial interactions influence the cues and mechanisms that promote these processes.
Figure 1. Conditions of various bacterial growth models.
Metabolic heterogeneity arises in bacterial systems due to innate and stochastic effects. However, differentiation can be further promoted by chemical gradients and spatial constraints that result from endogenous structure formation or imposed architectures. In well-mixed liquid cultures (e.g., batch cultures or chemostats) cells with different metabolisms (represented by green and beige coloration) are evenly distributed throughout the suspension. In microfluidic devices such as the one depicted, medium flows across one side of the population, leading to chemical gradient formation and metabolic differentiation along the gradient. When bacterial aggregates are suspended in a provided matrix (such as an agar block or sputum in the lung of a patient with cystic fibrosis), gradients can be present in the external matrix, leading to the differentiation seen in the upper aggregate, but can also form inside aggregates due to the activities of the cells, as seen in the lower aggregate. In biofilms, cells reside in a self-produced matrix and promote gradient formation and thereby metabolic differentiation.
During growth in homogeneous environments, metabolic heterogeneity can arise from innate stochasticity [13–21]. In an example of this, Nikolic et al. used isotope labeling and nanometer-scale secondary ion mass spectrometry (NanoSIMs) to measure anabolism of two different carbon sources, glucose and arabinose, in Escherichia coli grown in carbon-limited chemostats [22]. Most cells in these cultures specialized in assimilation of arabinose over glucose, but individuals varied in the degree of specialization. By monitoring expression of genes for arabinose and glucose uptake systems, the authors also showed a correlation between the degree to which an individual cell specialized in expression of arabinose uptake and its specialization for arabinose assimilation. Nikolic et al. therefore concluded that heterogeneity in transcription could at least partially account for the metabolic heterogeneity they observed. These findings demonstrate metabolic heterogeneity in a well-mixed liquid culture, suggesting that even in the absence of gradients that create spatially separated microniches, a clonal system benefits from forming metabolic subpopulations. Indeed, metabolic heterogeneity is thought to contribute to increased resilience in the face of environmental changes and allow specialized cells to increase the efficiencies with which they perform specific reactions [23].
Growth of bacteria in engineered devices or as suspended aggregates can allow researchers to examine the effects of imposed structural constraints on chemical topography and cellular behavior in assemblages that are less complex than biofilms. Microscale devices and models of suspended aggregates do not always favor matrix production and typically produce assemblages of fewer cells [24–26] than biofilms. Furthermore, it has been argued that suspended aggregates better approximate the conditions inside some types of infections than other popular models of bacterial multicellularity [26–29].
Wessel et al. used an elegant model system to examine the physiology of P. aeruginosa strain PAO1 grown in gelatin microtraps on glass surfaces [30]. The microtraps allowed nutrients and O2 to diffuse into the bacterial aggregates but limited their growth to a radius of 27 μm (~104–105 cells). To test whether these aggregates would develop O2 gradients, the authors generated a strain that expressed gfp under the control of a promoter that is activated by the switch from high-to low-O2 conditions. When grown in microtraps, this strain produced GFP specifically at the aggregate base, and elevation of the aggregate off the glass surface abolished GFP production. Though O2 gradient formation had been demonstrated in larger assemblages containing >108 cells, this study stood out at the time of publication due to its focus on smaller aggregates, which could represent the primary modes of growth for many bacterial species and for pathogens during transmission or infection [26,31–33]. Recently, another group introduced and employed an “agar block biofilm assay” to examine physiological heterogeneity in aggregates suspended in agar-solidified media [29,34]. They found variation in the responses of cells to tobramycin and chlorate and confirmed heterogeneity in gene expression for aggregates ~50 μm in size.
Biofilms represent the most complex multicellular bacterial systems due to their high cell numbers and densities and production of matrix that contains polysaccharides, proteins, and DNA [12]. A long-running theme in the study of physiological heterogeneity in biofilms is the role of O2 gradients [35] in promoting metabolic differentiation. Xu et al. detected metabolic activity in biofilms of P. aeruginosa strain ERC1, grown in drip-flow reactors under an atmosphere of air, using a substrate that forms a fluorescent intracellular precipitate after cleavage by alkaline phosphatase [36]. They found that only the top ~30 μm of ~130 μm-thick biofilms exhibited alkaline phosphatase activity, but that exposing the biofilms to pure O2 increased this region by ~1.5-fold. Using P. aeruginosa strain PA14, our group has focused on the expression of genes encoding electron transport chain components in colony biofilms. P. aeruginosa shows versatility in aerobic respiration due to its ability to produce five major enzymes that can catalyze the reduction of O2. We have used strains that express gfp under the control of promoters for each of these distinct terminal oxidases and found that some are differentially expressed over biofilm depth and that they are also affected by the identity of the electron donor provided in the growth medium [37,38]. These and additional studies described in subsequent sections underscore the point that systems with structural stability and gradient formation are predestined to give rise to metabolic heterogeneity. The structure itself, which can include self-produced exopolysaccharide matrix, host-derived matrix, or in vitro physical barriers, can be provided in diverse ways.
Metabolite cross-feeding in bacterial multicellular systems
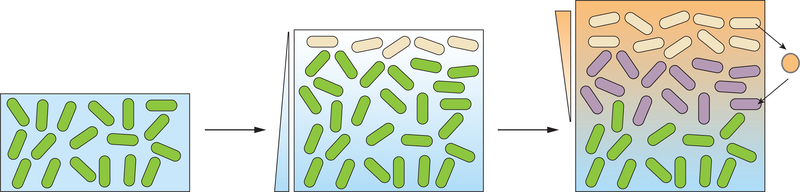
When subpopulations carry out distinct metabolisms, the opportunity arises for them to exchange metabolites and transform them further in pathways that were not active in their producers. This phenomenon is termed “cross-feeding” and is well-studied in evolved single-species and multispecies bacterial systems (for a detailed discussion of cross-feeding in systems with genotypic diversity, we refer the reader to Smith et al. [39]). Our discussion in this section will be limited to examples of cross-feeding in single-species, clonal models. Figure 2 depicts a generic example of how heterogeneity in chemical gradients can lead to cross-feeding and a potential role for cross-feeding in promoting the formation of a distinct metabolic subpopulation. For both clonal and genotypically diverse systems, cross-feeding can be beneficial when it allows for: (1) increased efficiency through the separation of metabolic pathways into discrete cells, (2) the use of waste products as metabolic inputs to other cells, or (3) the ability to access metabolisms that would be unachievable within a single bacterial cell due to biochemical conflicts [40,41]. Perhaps the most striking evidence that the benefits of cross-feeding cannot be realized in one cell is the observation that the number of regulatory genes increases according to the possible number of pairwise interactions amongst genes [42], suggesting that biochemical conflict is common and strongly deleterious. In support of (1), experimental work has shown that separating amino acid synthesis into engineered cross-feeding cells leads to fitness benefits against the wild type [43]. This occurs because the benefit of receiving an amino acid from a partner is greater than the cost to the partner of overproducing that amino acid, such that reciprocating pairs have an advantage.
Figure 2. Niche formation can enable cross-feeding, and cross-feeding can promote niche formation, in bacterial systems.
An example showing the development of resource gradients and cross-feeding is depicted. As the bacterial population grows, a resource gradient (blue) forms that promotes metabolic differentiation (represented by green and beige coloration of the cells). Bacteria carrying out the “beige” metabolism release a product (closed orange circle) that can be used by cells in a specific subzone, leading to further metabolic differentiation (purple cells).
Numerous studies of cross-feeding in single-species bacterial systems have examined the exchange of acetate in E. coli or Bacillus subtilis cultures and assemblages grown on glucose. Under some conditions, these species do not fully oxidize glucose to CO2, but instead release some of the carbon as acetate (Figure 3A). In E. coli homogeneous liquid batch cultures, the acetate can be oxidized to CO2 later in the incubation period, after most of the glucose has been consumed [44–46]. In metabolically differentiated systems of E. coli, the acetate can be consumed by a distinct subpopulation that oxidizes it to CO2 [16]. In B. subtilis, this phenomenon has been described specifically for cultures that are coutilizing glucose and malate. Metabolic differentiation can be observed in the development of a B. subtilis subpopulation that converts the acetic acid to acetoin. By carrying out this activity, B. subtilis transforms an acid, which threatens to alter the environmental pH, into a pH-neutral compound that could potentially be used for growth after the glucose and malate have been consumed [47].
Figure 3. Examples of cross-feeding in biofilms.
Four distinct cases of metabolite cross-feeding are depicted as biofilms that each contain two metabolic subpopulations (beige and green). Metabolic pathways for each subpopulation are indicated by selected intermediates (arrows may represent multiple pathway steps). All biofilms shown are growing under an oxic atmosphere and form an O2 gradient such that cells are O2-limited at the biofilm base. In the biofilms shown in (A), cells at the base convert glucose into an intermediate that is released. The intermediate is taken up by cells in the oxic zone and provides a source of carbon for the tricarboxylic acid (TCA) cycle. In the biofilms shown in (B), cells are releasing compounds that can be used as electron acceptors. Left: In our group’s model of P. aeruginosa biofilms growing under an oxic atmosphere, on medium with nitrate (NO 3-), the first step of denitrification is carried out in the oxic zone, and subsequent steps are carried out in the O2-limited zone. Right: Some P. aeruginosa phenazines are specifically produced in the oxic zone of the biofilm, then oxidized by O2 (blue arrow). Phenazines are reduced by cells at the biofilm base and can serve to balance the intracellular redox state.
Cole et al. assessed the potential for cross-feeding in simulated and experimental E. coli colonies (composed of ~109 cells) grown on a thin (1 mm-thick) layer of a defined medium containing glucose [48]. This group used an in silico technique called 3-Dimensional dynamic Flux Balance Analysis (3DdFBA) to predict the development of metabolic subpopulations and growth rates in colonies. Their simulations indicated that the formation of glucose and O2 gradients would result in a large region of relatively inactive cells at the colony center and three metabolically distinct populations in other regions of the colony: (1) cells at the colony edge that are consuming glucose and respiring; (2) cells at the colony base that are fermenting glucose and producing acetate; and (3) cells near the top of the colony that are consuming acetate and respiring. The potential for acetate cross-feeding in colonies was confirmed using a reporter strain that expresses gfp under the control of an acetate-sensitive promoter. Based on modeling, this cross-feeding should allow for faster overall colony growth than would be predicted in the absence of cross-feeding. A follow-up study by Wolfsberg et al. used 13C metabolic flux analysis (13C-MFA) to independently test predictions regarding the relative sizes of the acetate-producing and acetate-utilizing subpopulations in E. coli colonies grown on 13C-labeled glucose [49]. They measured incorporation of 13C into proteinogenic amino acids in wild-type E. coli and a ∆ackA mutant, which is defective in acetate production. They found that an E. coli monoculture network model was sufficient to describe the metabolism of the ∆ackA mutant, consistent with its inability to establish cross-feeding subpopulations. This network model was not sufficient to describe the metabolism of the wild type. Modification of the model to include “coculturing” of distinct subpopulations and acetate cross-feeding provided a statistically acceptable fit for the wild type, supporting the models generated by simulations in the Cole et al. study. In E. coli colonies growing on glucose, therefore, production of acetate and its diffusion from the anoxic to the oxic colony subzone allows the whole colony to consume more glucose than would have been possible without cross-feeding (Figure 3A).
Recent studies from our group have described the potential for cross-feeding in biofilms formed by P. aeruginosa strain PA14 (Figure 3). P. aeruginosa releases lactate as a product of pyruvate fermentation, which can support survival under anoxic conditions, via activity of the enzyme LdhA [50]. Under oxic conditions lactate activates expression of lldE, which codes for a lactate dehydrogenase, allowing it to be used as a carbon source for growth. By using strains that express fluorescent reporters for genes involved in anaerobic lactate production and aerobic lactate consumption, our group identified metabolically distinct subzones in P. aeruginosa colony biofilms. Cells in the anoxic region expressed ldhA and produced lactate, which activated expression of lldE [51,68]. Similar to acetate cross-feeding in E. coli biofilms, these observations suggest that P. aeruginosa lactate cross-feeding allows for the metabolism of a carbon source that otherwise might not be utilized due to spatial separation of the oxic zone from the growth medium. In a separate study, our group examined the utilization of electron acceptors by subpopulations in P. aeruginosa PA14 colony biofilms. We had previously found that PA14 biofilms grown on NO3- under an oxic atmosphere carry out a denitrification pathway that contributes to redox balancing [52]. The first step in this pathway is carried out by Nap, a periplasmic NO3- reductase that produces NO2-. Then NO2- is reduced to NO- by Nir, and reduced via two subsequent steps to N2. Interestingly, fluorescent reporters of nap and nir expression in P. aeruginosa colony biofilms show that nap is expressed in the top, oxic region of the biofilm, whereas nir is expressed in the bottom anoxic region [53]. This suggests that when NO3- is available, biofilm cells employ a division-of-labor cross-feeding strategy to complete the denitrification pathway (Figure 3B).
In this section, we have discussed examples of cross-feeding in both well-mixed and spatially structured systems. Since living in a biofilm is a common and sometimes dominant lifestyle for many bacterial species, we would expect there to be strong evolutionary pressures for the appropriate metabolic and physiological responses within various regions of the biofilm. Thus, one should not be surprised to see complex cross-feeding strategies readily developing within a biofilm, because at a minimum, evolution should enforce the maintenance of genes that allow for survival in various regions of this large, heterogeneous structure. However, a wide variety of theoretical and experimental studies have shown that complex mutually beneficial responses, including cross-feeding, are almost never stable in well-mixed scenarios and often require persistent interaction. The spatial structure of biofilms would provide this and thereby enforce reciprocation and cooperation [54–57].
In addition, it has recently been shown that diffusive processes and gradients are essential for establishing the coexistence of cells with distinct physiologies [57]. In cases without diffusive gradients, cross-feeding and coexistence are possible but only under a narrower set of physiological trait combinations. Since the structural arrangements of biofilms are conducive to the establishment of gradients and diffusion-based processes, cross-feeding is likely to be a common trait in diverse biofilm-forming species [52,58]. Finally, although we have focused in this section on single-species cross-feeding and cases in which metabolite exchange is “unidirectional”, more complex scenarios involving syntrophies, in which substrate release and take-up are “bidirectional”, and multi-species cross-feeding are prevalent in natural settings [59,60]. In the next section, we include examples of multi-species communities that exhibit metabolic diversity and cross-feeding in the context of clinically relevant phenomena such as pathogenesis and antibiotic resistance.
Implications of metabolic heterogeneity and cross-feeding for infection and antibiotic tolerance
Initial studies investigating the physiology of cells in biofilms used global techniques, such as transcriptomics and proteomics, to profile whole-biofilm or homogenized samples [61–66]. Though these studies allowed researchers to compare biofilm-grown cells with those grown under other conditions, such as shaken liquid cultures, and identify biofilm-specific features, they could not provide information about physiological differences between cells in distinct biofilm subzones. One technique that has allowed for selective sampling of regions within biofilms is laser capture microdissection. Williamson et al. used this method to sample from the top or bottom 30 μm of ~350 μm-tall P. aeruginosa PAO1 colony biofilms and examined localized mRNA abundances [67]. They found that overall mRNA abundances were higher for cells at the top of the biofilm than for cells at the bottom, consistent with models regarding the overall levels of metabolic activity in these two biofilm subzones. In a separate experiment, the authors used an arabinose-inducible promoter to selectively GFP-label biofilm subpopulations and assess the correlation between metabolic activity and antibiotic susceptibility in PAO1 biofilms (Figure 4B). To label the metabolically active subpopulation, they grew the biofilms without arabinose and then transferred them to medium containing both arabinose and antibiotic. To label the metabolically inactive subpopulation, they grew the biofilms on arabinose, then transferred them to medium without arabinose before exposing them to antibiotic. Biofilms were then homogenized and subjected to FACS sorting and plating for CFUs. The results showed that cells in the metabolically active subpopulation were more susceptible to antibiotic treatment than those that were metabolically inactive.
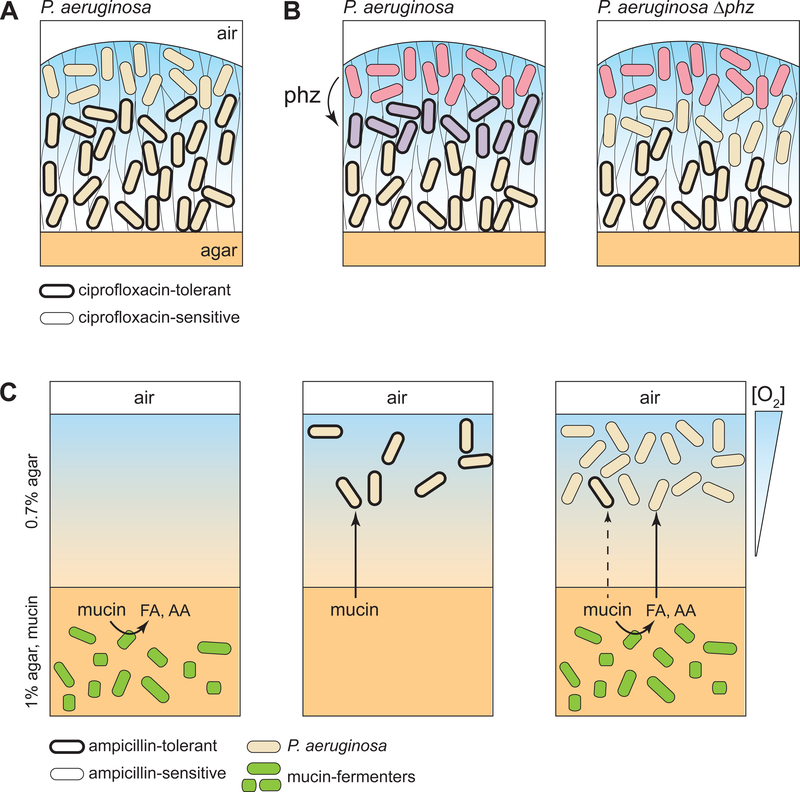
Figure 4. Implications of metabolic heterogeneity for antibiotic tolerance of P. aeruginosa populations.
Three studies addressing the effects of metabolic heterogeneity on antibiotic tolerance are represented. (A, B) Tolerance to ciprofloxacin in P. aeruginosa colony biofilms. (A) Williamson et al. used selective GFP labeling of metabolically active or inactive cells, followed by antibiotic exposure, FACS, and CFU counting, to show that the air-exposed metabolically active subpopulation is more susceptible to antibiotics [67]. (B) Schiessl et al. used SRS microscopy to show that phenazine-producing biofilms contain two bands of metabolically active cells (red and purple), while phenazine-null biofilms contain one pronounced band of metabolically active cells (red) [68]. Based on CFU counts, phenazine-producing biofilms show less antibiotic susceptibility, suggesting that the lower band of metabolic activity in these biofilms contains cells in a differentiated state with higher antibiotic tolerance. (C) An anaerobic community of mucin fermenters embedded in agar produces short-chain fatty acids (FA) and amino acids (AA). These products can serve as carbon sources and support replication of a P. aeruginosa population present in the air-exposed portion of the agar block. Interestingly, while the fermenters are sensitive to ampicillin in mono-and co-culture (left and right panels, respectively), P. aeruginosa is only sensitive in co-culture [78, 79].
Our group has developed an independent technique that enables in situ visualization of metabolic activity over biofilm depth [68] (Figure 4B). This approach employs stable isotope labeling and stimulated Raman scattering (SRS) microscopy [69]. We have optimized a method for applying SRS microscopy to P. aeruginosa PA14 colony biofilms and have used it to examine the effects of phenazines, redox-active secondary metabolites, on the physiology of resident cells. PA14 produces several different types of phenazines, but the ones that most dramatically affect gene expression and biofilm development are those with relatively high redox potentials that are produced specifically under oxic conditions [70,71]. A long-standing model has been that these high-potential phenazines participate in a form of “cross-feeding” in which they are produced in oxic biofilm subzones and migrate to oxygen-limited subzones, where they act as alternate electron acceptors (Figure 3B) [72,73]. These reduced phenazines can then act as mediators and shuttle electrons to other, oxidized phenazines or to O2 available in regions closer to the biofilm periphery. While various lines of evidence had indicated that phenazines facilitate metabolism in hypoxic biofilm regions [37,52,74], this had not been directly observed. We therefore examined SRS images of sections from paraffin-embedded biofilms incubated on deuterium-labeled water and compared the wild type to a mutant unable to produce phenazines (Δphz). Wild-type sections showed incorporation of the deuterium label, indicating metabolic activity, in two discrete bands across biofilm depth: one at ~30–40 μm from the air-interface, and one below 50 μm from the air-interface (where O2 is undetectable). Δphz sections, in contrast, showed one pronounced band of label at a depth of ~30–40 μm and a significantly attenuated band deeper down in the biofilm. These results suggest that phenazines indeed support metabolic activity in microoxic biofilm subzones.
Because the metabolic state is a determinant of antibiotic sensitivity [75], we tested whether phenazines affect the antibiotic tolerance of cells in PA14 biofilms and found that cells in Δphz biofilms are significantly more sensitive to several different types of antibiotics, including ciprofloxacin, than cells in wild-type biofilms. We also applied our SRS microscopy method to ciprofloxacin-treated biofilms and found that phenazine production altered the effect of ciprofloxacin on biofilm metabolic patterning [68]. These observations revealed that endogenous metabolites can participate in “drug-drug” interactions that could influence our ability to treat infections. They also introduced a new method for the study of metabolic heterogeneity in intact microbial multicellular systems.
Another recent study that examined metabolic heterogeneity and that uncovered effects relevant for our ability to treat infections was performed by Dal Co et al. [83]. This group grew E. coli in two-dimensional microfluidic chambers in which the exposure to fresh medium was limited to cells along one edge. They found that the cells differentiated into two subpopulations such that those exposed to fresh medium consumed glucose and produced acetate, while the cells deeper in the chamber and further from the medium source consumed the acetate. When an antibiotic was added to the medium, it preferentially killed the glucose-consuming cells. Interpretation of these results is hindered by the caveat that the antibiotic may not have diffused evenly into the chamber, but they nevertheless raise the possibility that metabolic differentiation contributed to antibiotic tolerance in this multicellular system. During infections, the causative pathogen is usually found coexisting with other microbial species. In recognition of this, researchers are moving on from studies of pathogens in monocultures. By coculturing pathogens with other species, they are studying how metabolic heterogeneity, cross-feeding, and/or interspecies effects on metabolism can enhance pathogenicity, survival in hosts, and antibiotic tolerance.
P. aeruginosa is notorious for causing infections in the lungs of people with cystic fibrosis (CF). In CF, a mutation in a chloride transporter impairs the ability to clear particles from the airway and leads to the accumulation of thick mucus, which is chronically colonized by bacteria [76]. P. aeruginosa is one of the major species represented in the CF lung, which can contain bacterial loads on the order of 108 cells/gram of sputum [77]. However, until recently, little was known about the carbon sources that support P. aeruginosa growth in this environment. Flynn et al. investigated whether mucin, a major source of organic carbon in the CF lung, could support growth of P. aeruginosa strain PA14 and several clinical isolates [78]. They obtained low growth yields when these strains were grown in monoculture on mucin. To investigate whether coculturing with other species could enhance growth, they designed an agar-suspension system in which a consortium of mucin-degrading anaerobes that are also found in CF lungs could be grown at the base of an O2 gradient, while P. aeruginosa could be incubated on top, at the interface with air. Conversion of mucin into propionate and acetate supported growth of the P. aeruginosa population, leading to higher growth yields than mucin alone. These short-chain fatty acids were also detected in CF sputum, and the authors demonstrated that P. aeruginosa genes for propionate utilization are expressed in this host environment. These observations indicate that cross-feeding enables P. aeruginosa to utilize mucin as a carbon source during chronic CF lung infection. A follow-up study by Adamowicz et al. investigated whether this cross-feeding arrangement has implications for the effects of antibiotics on the viability of P. aeruginosa cultures grown on mucin (Figure 4C) [79]. They found that, though P. aeruginosa grows only modestly on mucin, this growth was unaffected by ampicillin at all concentrations tested due to P. aeruginosa’s inherent ampicillin resistance. In contrast, the growth of P. aeruginosa that is achieved when it is in coculture is sensitive to ampicillin, due to the sensitivity of the anaerobic mucin-degraders providing other carbon sources. This result suggests that the success of a pathogen that is antibiotic-resistant can be dampened by the effects of antibiotics on other species that provide critical substrates and that these effects should be considered in the design of therapeutic strategies.
Another model system used for investigating the effects of interspecies interactions on pathogen physiology is one in which the pathogen Aggregatibacter actinomycetemcomitans is cocultured with the commensal Streptococcus gordonii. These organisms are both found in the human oral cavity, where A. actinomycetemcomitans is associated with localized aggressive periodontitis [80]. Growth in a murine thigh abscess model showed that S. gordonii produces L-lactate, which serves as a carbon source for A. actinomycetemcomitans and enhances its pathogenicity [81]. An additional study by Stacy et al. used transposon mutant pools (Tn-seq) to identify loci required for growth of A. actinomycetemcomitans during monoinfection in the abscess model and compared these to loci required for growth during coinfection with S. gordonii [82]. Their results showed dramatic differences in the physiology of A. actinomycetemcomitans depending on whether it colonized as a monoculture or in coculture with S. gordonii. By cross-referencing their in vivo results with Tn-seq data obtained from in vitro growth of A. actinomycetemcomitans under oxic and anoxic conditions, the authors were also able to infer that the presence of S. gordonii increased the availability of O2 in abscesses [82]. Together, observations from these studies demonstrate the importance of incorporating cross-species interactions into our models of pathogen behavior in hosts.
Concluding remarks
In summary, the studies discussed in this review show that metabolic heterogeneity and cross-feeding can arise in both simple and complex bacterial systems. However, the spatial structuring and genotypic diversity found in complex systems predispose them to the development of these features. The examples we describe from clinically relevant work, which are focused on antibiotic resistance and pathogenicity, show that biofilm formation and multi-species interactions can critically affect our ability to treat infections. Furthermore, in other environmental settings, the prevalence of the biofilm lifestyle and the diversity of microbial ecosystems suggest that metabolic heterogeneity and cross-feeding are broad determinants of biological potential. New technical approaches, such as 3DdFBA, 13C-MFA, growth in microfluidic devices, and SRS imaging, allow us to incorporate spatial structuring and metabolic heterogeneity into models of bacterial growth, yielding some of the insights described here. These hold great promise in our efforts to characterize complex systems that are increasingly analogous to those found outside the laboratory.
Outstanding questions.
How can we apply knowledge regarding the metabolic heterogeneity of pathogenic bacteria toward effective treatment strategies?
To what extent is our categorization of excreted compounds--i.e., as exchanged metabolites versus intercellular signals--an artificial division? What effects do exchanged metabolites have on gene expression? Are intercellular signals metabolized by their producers?
Given that they are capable of cross-feeding and intercellular signaling, why haven’t prokaryotic cells evolved to generate macroscopic systems with reproducible morphologies on the scale of animals and plants?
Highlights.
Metabolic diversification in bacterial systems arises from innate stochasticity and can be further promoted by growth in chemical gradients and/or constrained spaces.
Metabolic heterogeneity can enable metabolite cross-feeding, and cross-feeding itself can promote the development of metabolically differentiated subpopulations.
New techniques for characterizing metabolically diversified subpopulations in situ include those that incorporate 3-dimensional dynamic flux balance analysis, 13C metabolic flux analysis, growth in microfluidic devices, and stimulated Raman scattering microscopy.
Metabolic heterogeneity is an important factor to incorporate into models of infection because it influences virulence and antibiotic susceptibility.
ACKNOWLEDGMENTS
The authors are grateful to members of the Dietrich lab who provided feedback on an earlier draft of this review. Research in the Dietrich lab is supported by NIH/NIAID R01AI103369 and an NSF CAREER award to L.E.P.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Batsivari A. et al. (2020) Dynamic responses of the haematopoietic stem cell niche to diverse stresses. Nat. Cell Biol 22, 7–17 [DOI] [PubMed] [Google Scholar]
- 2.Woods HA (2014) Mosaic physiology from developmental noise: within-organism physiological diversity as an alternative to phenotypic plasticity and phenotypic flexibility. J. Exp. Biol 217, 35–45 [DOI] [PubMed] [Google Scholar]
- 3.Dueck A. et al. (2016) Gene silencing pathways found in the green alga Volvox carteri reveal insights into evolution and origins of small RNA systems in plants. BMC Genomics 17, 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet T. and King N. (2017) The Origin of Animal Multicellularity and Cell Differentiation. Dev. Cell 43, 124–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang C. et al. (2019) Metabolite Exchange between Mammalian Organs Quantified in Pigs. Cell Metab. 30, 594–606.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueira JA et al. (2018) Unraveling Interfaces between Energy Metabolism and Cell Cycle in Plants. Trends Plant Sci. 23, 731–747 [DOI] [PubMed] [Google Scholar]
- 7.Swietach P. and Monterisi S. (2019) A Barter Economy in Tumors: Exchanging Metabolites through Gap Junctions. Cancers 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui S. et al. (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza G. et al. (2018) Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep 35, 455–488 [DOI] [PubMed] [Google Scholar]
- 10.Schreiber F. and Ackermann M. (2019) Environmental drivers of metabolic heterogeneity in clonal microbial populations. Curr. Opin. Biotechnol 62, 202–211 [DOI] [PubMed] [Google Scholar]
- 11.Flemming H-C and Wuertz S. (2019) Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol 17, 247–260 [DOI] [PubMed] [Google Scholar]
- 12.Flemming H-C and Wingender J. (2010) The biofilm matrix. Nat. Rev. Microbiol 8, 623–633 [DOI] [PubMed] [Google Scholar]
- 13.Elowitz MB et al. (2002) Stochastic gene expression in a single cell. Science 297, 1183–1186 [DOI] [PubMed] [Google Scholar]
- 14.Silander OK et al. (2012) A genome-wide analysis of promoter-mediated phenotypic noise in Escherichia coli. PLoS Genet. 8, e1002443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mars RAT et al. (2015) Small regulatory RNA-induced growth rate heterogeneity of Bacillus subtilis. PLoS Genet. 11, e1005046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolic N. et al. (2013) Analysis of fluorescent reporters indicates heterogeneity in glucose uptake and utilization in clonal bacterial populations. BMC Microbiol. 13, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takhaveev V. and Heinemann M. (2018) Metabolic heterogeneity in clonal microbial populations. Curr. Opin. Microbiol 45, 30–38 [DOI] [PubMed] [Google Scholar]
- 18.Kopf SH et al. (2016) Trace incorporation of heavy water reveals slow and heterogeneous pathogen growth rates in cystic fibrosis sputum. Proc. Natl. Acad. Sci. U. S. A 113, E110–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannan AA et al. (2015) Integrating Kinetic Model of E. coli with Genome Scale Metabolic Fluxes Overcomes Its Open System Problem and Reveals Bistability in Central Metabolism. PLoS One 10, e0139507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiviet DJ et al. (2014) Stochasticity of metabolism and growth at the single-cell level. Nature 514, 376–379 [DOI] [PubMed] [Google Scholar]
- 21.Tonn MK et al. (2019) Stochastic modelling reveals mechanisms of metabolic heterogeneity. Commun Biol 2, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolic N. et al. (2017) Cell-to-cell variation and specialization in sugar metabolism in clonal bacterial populations. PLoS Genet. 13, e1007122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M. (2015) A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol 13, 497–508 [DOI] [PubMed] [Google Scholar]
- 24.Nadell CD et al. (2017) Flow environment and matrix structure interact to determine spatial competition in Pseudomonas aeruginosa biofilms. Elife 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yawata Y. et al. (2016) Microfluidic Studies of Biofilm Formation in Dynamic Environments. J. Bacteriol 198, 2589–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staudinger BJ et al. (2014) Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 189, 812–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolenbrander PE et al. (1995) Intergeneric coaggregation of oral Treponema spp. with Fusobacterium spp. and intrageneric coaggregation among Fusobacterium spp. Infect. Immun. 63, 4584–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sønderholm M. et al. (2017) Pseudomonas aeruginosa Aggregate Formation in an Alginate Bead Model System Exhibits In Vivo-Like Characteristics. Appl. Environ. Microbiol 83, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spero MA and Newman DK (2018) Chlorate Specifically Targets Oxidant-Starved, Antibiotic-Tolerant Populations of Pseudomonas aeruginosa Biofilms. MBio 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessel AK et al. (2014) Oxygen limitation within a bacterial aggregate. MBio 5, e00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faruque SM et al. (2006) Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. U. S. A. 103, 6350–6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamruzzaman M. et al. (2010) Quorum-regulated biofilms enhance the development of conditionally viable, environmental Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 107, 1588–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kragh KN et al. (2014) Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect. Immun. 82, 4477–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorth P. et al. (2019) Quantitative Visualization of Gene Expression in Mucoid and Nonmucoid Pseudomonas aeruginosa Aggregates Reveals Localized Peak Expression of Alginate in the Hypoxic Zone. MBio 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Beer D. et al. (1994) Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 43, 1131–1138 [DOI] [PubMed] [Google Scholar]
- 36.Xu KD et al. (1998) Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol 64, 4035–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo J. et al. (2017) An orphan cbb3-type cytochrome oxidase subunit supports Pseudomonas aeruginosa biofilm growth and virulence. Elife 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo J. et al. (2020) Interdependency of Respiratory Metabolism and Phenazine-Associated Physiology in Pseudomonas aeruginosa PA14. J. Bacteriol. 202, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith NW et al. (2019) The Classification and Evolution of Bacterial Cross-Feeding. Frontiers in Ecology and Evolution 7, 153 [Google Scholar]
- 40.Johnson DR et al. (2012) Metabolic specialization and the assembly of microbial communities. ISME J. 6, 1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson JR et al. (2017) Parametric studies of metabolic cooperativity in Escherichia coli colonies: Strain and geometric confinement effects. PLoS One 12, e0182570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Nimwegen E. (2003) Scaling laws in the functional content of genomes. Trends Genet. 19, 479–484 [DOI] [PubMed] [Google Scholar]
- 43.Pande S. et al. (2014) Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J. 8, 953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monod J. (1949) THE GROWTH OF BACTERIAL CULTURES. Annu. Rev. Microbiol 3, 371–394 [Google Scholar]
- 45.Helling RB et al. (1987) Evolution of Escherichia coli during growth in a constant environment. Genetics 116, 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotte O. et al. Phenotypic bistability in Escherichia coli ‘s central carbon metabolism., Molecular Systems Biology, 10 (2014), 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal AZ et al. (2018) Metabolic interactions between dynamic bacterial subpopulations. Elife 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole JA et al. (2015) Spatially-resolved metabolic cooperativity within dense bacterial colonies. BMC Syst. Biol 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfsberg E. et al. (2018) Metabolism in dense microbial colonies: 13C metabolic flux analysis of E. coli grown on agar identifies two distinct cell populations with acetate cross-feeding. Metab. Eng. 49, 242–247 [DOI] [PubMed] [Google Scholar]
- 50.Eschbach M. et al. (2004) Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J. Bacteriol. 186, 4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y-C et al. (2018) The Pseudomonas aeruginosa Complement of Lactate Dehydrogenases Enables Use of d-and l-Lactate and Metabolic Cross-Feeding. MBio 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietrich LEP et al. (2013) Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 195, 1371–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y-C et al. (2018) Phenazines regulate Nap-dependent denitrification in Pseudomonas aeruginosa biofilms. J. Bacteriol. DOI: 10.1128/JB.00031-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nadell CD et al. (2016) Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol 14, 589–600 [DOI] [PubMed] [Google Scholar]
- 55.Xavier JB and Foster KR (2007) Cooperation and conflict in microbial biofilms. Proc. Natl. Acad. Sci. U. S. A. 104, 876–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pande S. et al. (2016) Privatization of cooperative benefits stabilizes mutualistic cross-feeding interactions in spatially structured environments. ISME J. 10, 1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kallus Y. et al. (2017) Paradoxes in leaky microbial trade. Nat. Commun. 8, 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kempes CP et al. (2014) Morphological optimization for access to dual oxidants in biofilms. Proc. Natl. Acad. Sci. U. S. A. 111, 208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.San Roman M. and Wagner A. (2018) An enormous potential for niche construction through bacterial cross-feeding in a homogeneous environment. PLoS Comput. Biol 14, e1006340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Embree M. et al. (2015) Networks of energetic and metabolic interactions define dynamics in microbial communities. Proc. Natl. Acad. Sci. U. S. A. 112, 15450–15455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauer K. et al. (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184, 1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ram RJ et al. (2005) Community proteomics of a natural microbial biofilm. Science 308, 1915–1920 [PubMed] [Google Scholar]
- 63.Resch A. et al. (2006) Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6, 1867–1877 [DOI] [PubMed] [Google Scholar]
- 64.Mikkelsen H. et al. (2007) Interrelationships between colonies, biofilms, and planktonic cells of Pseudomonas aeruginosa. J. Bacteriol. 189, 2411–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folsom JP et al. (2010) Physiology of Pseudomonas aeruginosa in biofilms as revealed by transcriptome analysis. BMC Microbiol. 10, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patell S. et al. (2010) Comparative microarray analysis reveals that the core biofilm-associated transcriptome of Pseudomonas aeruginosa comprises relatively few genes. Environ. Microbiol. Rep. 2, 440–448 [DOI] [PubMed] [Google Scholar]
- 67.Williamson KS et al. (2012) Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol. 194, 2062–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiessl KT et al. (2019) Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 10, 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freudiger CW et al. (2008) Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science 322, 1857–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakhtah H. et al. (2016) The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc. Natl. Acad. Sci. U. S. A. 113, E3538–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellin DL et al. (2016) Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms. Nat. Commun. 7, 10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez ME and Newman DK (2001) Extracellular electron transfer. Cell. Mol. Life Sci. 58, 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okegbe C. et al. (2012) Redox eustress: roles for redox-active metabolites in bacterial signaling and behavior. Antioxid. Redox Signal. 16, 658–667 [DOI] [PubMed] [Google Scholar]
- 74.Wang Y. et al. (2010) Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 192, 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stokes JM et al. (2019) Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 30, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibson RL et al. (2003) Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168, 918–951 [DOI] [PubMed] [Google Scholar]
- 77.Bauernfeind A. et al. (1987) Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection 15, 270–277 [DOI] [PubMed] [Google Scholar]
- 78.Flynn JM et al. (2016) Evidence and Role for Bacterial Mucin Degradation in Cystic Fibrosis Airway Disease. PLoS Pathog. 12, e1005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adamowicz EM et al. (2018) Cross-feeding modulates antibiotic tolerance in bacterial communities. ISME J. 12, 2723–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fine DH et al. (2007) Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol 45, 3859–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramsey MM et al. (2011) Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 7, e1002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stacy A. et al. (2016) A Commensal Bacterium Promotes Virulence of an Opportunistic Pathogen via Cross-Respiration. MBio 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dal Co A. et al. (2019) Emergent microscale gradients give rise to metabolic cross-feeding and antibiotic tolerance in clonal bacterial populations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dragoš A. et al. (2018) Division of labor during biofilm matrix production. Curr. Biol 28 1903–1913.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]