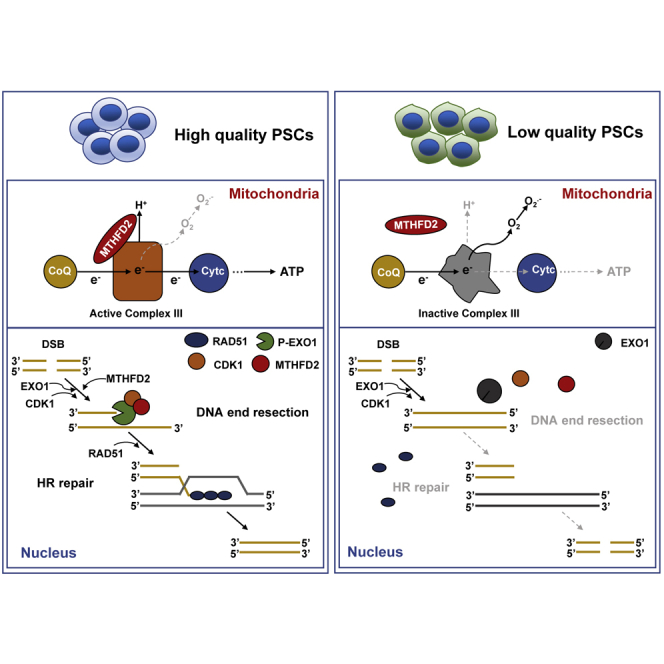
Summary
The pluripotency of stem cells determines their developmental potential. While the pluripotency states of pluripotent stem cells are variable and interconvertible, the mechanisms underlying the acquisition and maintenance of pluripotency remain largely elusive. Here, we identified that methylenetetrahydrofolate dehydrogenase (NAD+-dependent), methenyltetrahydrofolate cyclohydrolase (Mthfd2) plays an essential role in maintaining embryonic stem cell pluripotency and promoting complete reprogramming of induced pluripotent stem cells. Mechanistically, in mitochondria, Mthfd2 maintains the integrity of the mitochondrial respiratory chain and prevents mitochondrial dysfunction. In the nucleus, Mthfd2 stabilizes the phosphorylation of EXO1 to support DNA end resection and promote homologous recombination repair. Our results revealed that Mthfd2 is a dual-function factor in determining the pluripotency of pluripotent stem cells through both mitochondrial and nuclear pathways, ultimately ensuring safe application of pluripotent stem cells.
Keywords: DNA repair, mitochondrial dysfunction, pluripotency, pluripotent stem cells, Mthfd2
Graphical Abstract
Highlights
-
•
Mthfd2 improves complete reprogramming of mouse iPSCs
-
•
Mthfd2 regulates pluripotency of mESCs by enzyme-independent mechanisms
-
•
MTHFD2 directly interacts with CDK1 to modulates DNA repair in mESCs
-
•
Mthfd2 regulates both mitochondrial function and genomic stability in mESCs
In this article, Han and colleagues show that Mthfd2 is a powerful bifunctional regulator for pluripotency of pluripotent stem cells. Mechanistically, in mitochondria, Mthfd2 maintains the integrity of the mitochondrial respiratory chain and prevents mitochondrial dysfunction. In the nucleus, Mthfd2 stabilizes the phosphorylation of EXO1 to support DNA end resection and promote homologous recombination repair.
Introduction
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can self-renew or potentially differentiate into all cell types, a phenomenon known as pluripotency (Evans and Kaufman, 1981; Takahashi and Yamanaka, 2006). PSCs hold great promise for basic biomedical research, the production of genetically modified animals, and future clinical applications. Distinct pluripotent states, termed naive and primed pluripotency, have been described (Nichols and Smith, 2009). Naive PSCs resemble the preimplantation embryo inner cell mass (Boroviak et al., 2015) while primed PSCs resemble postimplantation embryonic epiblasts (Tesar et al., 2007). These states are reversible and interconvertible. The pluripotency of PSCs is known to determine the fate of embryonic development, and high-quality PSCs can produce chimeras with high efficiency when they are introduced into a blastocyst (Huang et al., 2012) and can develop into viable offspring by tetraploid complementation (Zhao et al., 2009; Zhong et al., 2019). Understanding the molecular mechanisms that influence pluripotency acquisition and maintenance is key to advancing therapeutic applications of PSCs.
Cells produce ATP by exploiting glycolysis and oxidative phosphorylation (OXPHOS) in different proportions to obtain energy for survival. Somatic cell reprogramming resets cellular metabolism to a state of relatively high glycolysis and low OXPHOS (Folmes et al., 2011). PSCs in a naive state are highly oxidative compared with primed cells. The progression from naive to primed pluripotency is characterized by a metabolic shift from OXPHOS to glycolysis (Zhou et al., 2012), and resetting primed pluripotent cells to a naive state necessitates a reversal of the metabolic switch (Carbognin et al., 2016; Takashima et al., 2014). Mitochondria are essential organelles in all nucleated cells that function mainly to generate cellular ATP by OXPHOS via the electron transport chain (ETC, complexes I–IV) and ATP synthase (complex V). Primed PSCs maintain lower mitochondrial function than naive cells (Zhang et al., 2016). In addition, the mitochondrial membrane potential (Sukumar et al., 2016), ATP (Zhang et al., 2018), reactive oxygen species (ROS) (Ryu et al., 2015; Zhou et al., 2016), and mitochondrial dynamics (Khacho et al., 2016; Zhong et al., 2019) were reported as the key factors responsible for cell-fate changes. However, the factors that determine mitochondrial function in PSCs and their regulatory mechanism remain unclear.
Genetic lesions of either endogenous or exogenous origin are major threats to the function of PSCs (Weissbein et al., 2014; Yoshihara et al., 2017). PSCs rely on a very robust DNA damage response (DDR) to detect and control specific types of DNA damage. Once DNA damage is unresolvable, PSCs avoid the propagation of genetic lesions by undergoing regulated cell death (Desmarais et al., 2016; Liu et al., 2013) or losing pluripotency (Li et al., 2012; Lin et al., 2005). Among all types of DNA lesions, DNA double-strand breaks (DSBs) are arguably the most dangerous (Jackson, 2002), and PSCs predominantly use homologous recombination (HR) to repair DSBs (Tichy et al., 2010). Failure of HR repair systems typically leads to severe consequences, such as genomic instability in cellular reprogramming (Gonzalez et al., 2013; Lee et al., 2016). Thus, efficacious DSB repair is a key element in the maintenance of high genomic integrity. However, the mechanisms that protect genomic integrity and the key regulators involved are not completely clear.
In this study, we identified methylenetetrahydrofolate dehydrogenase (NAD+-dependent), methenyltetrahydrofolate cyclohydrolase (Mthfd2) as an important regulator of pluripotency in mouse PSCs. Mthfd2 is a bifunctional enzyme with methylene dehydrogenase and cyclohydrolase activity involved in mitochondrial folate one-carbon metabolism (Tibbetts and Appling, 2010). Mthfd2 plays an essential role in mouse embryonic development, because inactivation of this gene in mice was demonstrated to be lethal (Di Pietro et al., 2002). MTHFD2 is markedly elevated in many cancers and positively correlated with poor prognosis in patients with cancer (Lin et al., 2018; Liu et al., 2014a; Pikman et al., 2016). In addition, MTHFD2 is localized to the nucleus and affects proliferation independent of its enzymatic activity in cancer cells (Gustafsson Sheppard et al., 2015). However, the function of Mthfd2 in PSCs has not been reported. Here, we demonstrated that Mthfd2 mediates both mitochondrial function and DNA repair to determine the pluripotency state of PSCs, ultimately improving their potential use in various applications and their safety.
Results
A Microarray Assay Identifies Putative New Pluripotency-Regulating Genes in iPSCs
We reanalyzed the microarray data from iPSCs of different quality initially generated in our laboratory and demonstrated the quality of the iPSCs based on their in vitro developmental potential (Han et al., 2010; Heng et al., 2010). High-quality iPSCs underwent germline transmission or produced mice derived completely from iPSCs by tetraploid complementation, while low-quality iPSCs produced only chimeras with a low coat color contribution. Candidate genes were selected based on their higher expression in high-quality iPSCs than in low-quality iPSCs (Table S1). Among these candidates, Esrrb, Sall4, Gadd45a, and Rab32 were previously reported to be important for enhancing iPSC generation and modulating ESC pluripotency (Chen et al., 2016; Feng et al., 2009; Pei et al., 2015; Zhang et al., 2006). Besides, some new potential regulators such as Mthfd2 were identified (Figure S1A).
Mthfd2 Plays a Key Role in Mouse ESCs to Maintain Self-Renewal
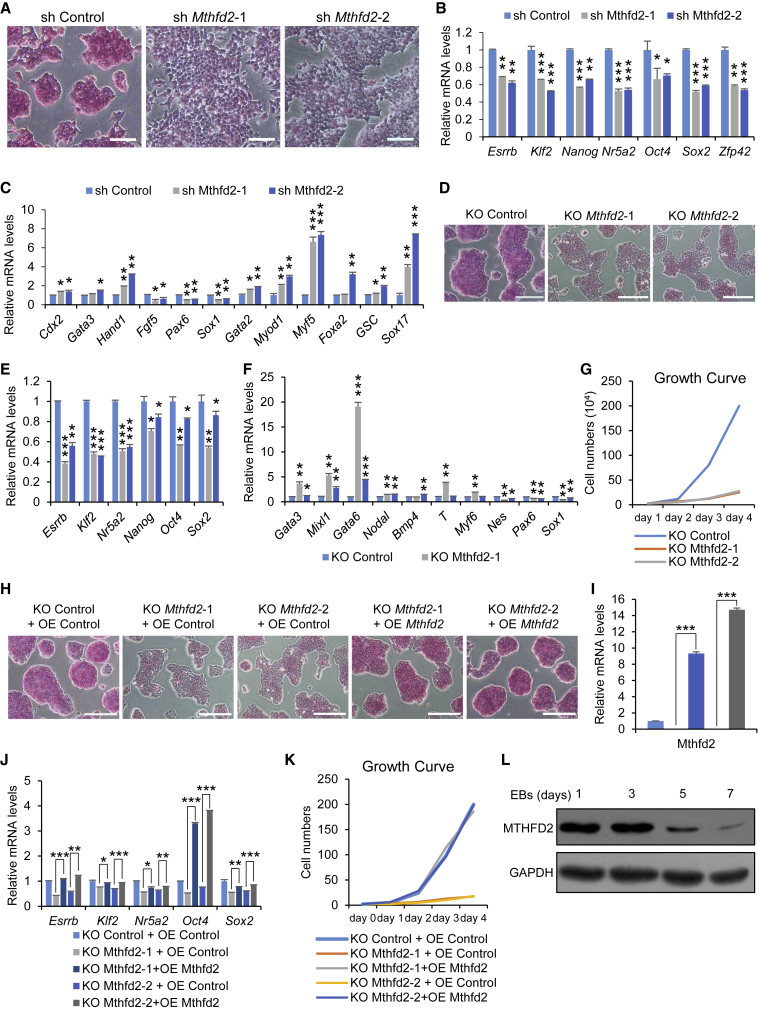
To validate the role of the candidate genes in regulating mouse ESC (mESC) self-renewal, we separately used short hairpin RNAs (shRNAs) to suppress the expression of candidate genes in E14 mESCs. Mthfd2 knockdown (KD) resulted in loss of typical stem cell morphology (Figures S1B–S1D), with reduced alkaline phosphatase (AP) staining (Figure 1A). The expression of pluripotency marker genes was downregulated and that of lineage marker genes upregulated (Figures 1B, 1C, and S1E), showing that Mthfd2 depletion results in differentiation of mESCs. We then knocked down Mthfd2 in another G4 mESC line and found that the results were consistent with those in Mthfd2 KD E14 mESCs (Figures S1F and S1G). Additionally, homozygous Mthfd2 knockout (KO) mESCs were characterized by the loss of typical mESC morphology, abnormal expression of marker genes, and compromised cell proliferation (Figures 1D–1G and S1H). Forced expression of Mthfd2 rescued the Mthfd2 KO-induced differentiation and compromised cell proliferation (Figures 1H–1K). In addition, MTHFD2 protein expression was gradually silenced during the differentiation of mESCs into embryoid bodies (EBs) (Figure 1L). These results demonstrate a key role of Mthfd2 in the maintenance of mESC self-renewal.
Figure 1.
Mthfd2 Is Important for mESCs to Maintain Self-Renewal
(A) Representative results of Mthfd2 KD mESCs with AP staining. Scale bars, 100 μm.
(B and C) qRT-PCR analysis of mRNA levels of pluripotency marker genes (B) and lineage marker genes (C) in Mthfd2 KD mESCs.
(D) Representative results of Mthfd2 KO mESCs with AP staining. Scale bars, 200 μm.
(E and F) qRT-PCR analysis of mRNA levels of pluripotency marker genes (E) and lineage marker genes (F) in Mthfd2 KO mESCs.
(G) Representative growth curve of Mthfd2 KO mESCs.
(H) Representative results of overexpressed (OE) Mthfd2-Mthfd2 KO mESCs with AP staining. Scale bar, 200 μm.
(I and J) qRT-PCR analysis of mRNA levels of Mthfd2 (I) and pluripotency marker genes (J) in OE Mthfd2-Mthfd2 KO mESCs.
(K) Representative growth curve of OE Mthfd2-Mthfd2 KO mESCs.
(L) Western blot analysis of the levels of the MTHFD2 protein during differentiation of mESCs. GAPDH was used as a loading control.
Data in (B), (C), (E), (F), (I) and (J) are pooled from three independent experiments (mean ± SD) relative to EF1-α and the control mESCs. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control.
See also Figure S1.
Mthfd2 Facilitates Mouse iPSC Induction
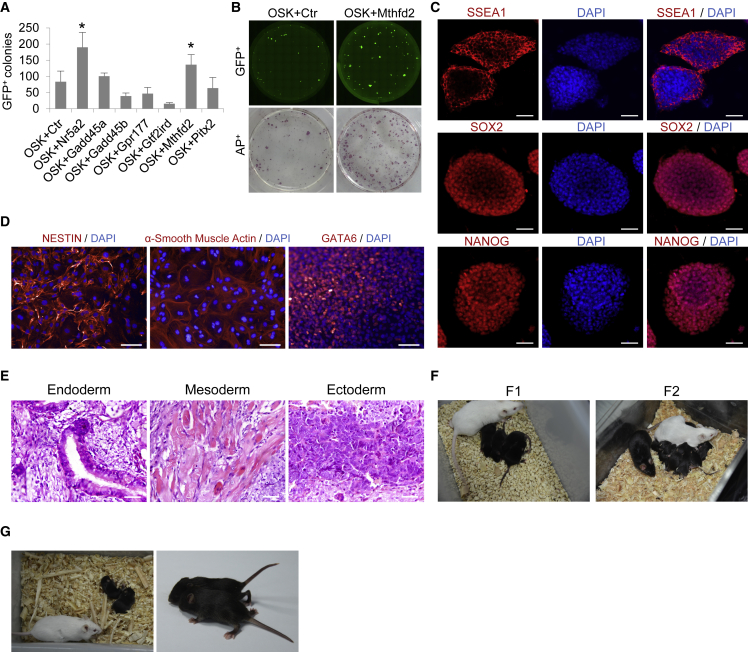
We used Oct4, Sox2, and Klf4 (OSK) combined with various candidate factors, including Mthfd2, in a subsequent reprogramming assay (Takahashi and Yamanaka, 2006). Mouse embryonic fibroblasts (MEFs) expressing a green fluorescent protein (GFP) reporter driven by an Oct4 promoter and enhancer (termed OG2 MEFs) were used for iPSC induction. To ensure the reliability of our results, we tested two culture conditions, serum + LIF (leukemia inhibitory factor) mESC medium and KOSR medium, the latter of which has been reported to enhance iPSC induction (Liu et al., 2014b). The number of Oct4-GFP+ colonies generated by coinfection of Mthfd2 and OSK was increased approximately 2-fold relative to that in control cells which coinfection of empty vector and OSK (p < 0.05) in both mESC medium (Figures S2A and S2B) and KOSR medium (Figures 2A and 2B). We used doxycycline-inducible MEFs expressing Oct4, Sox2, Klf4, and c-Myc (OSKC) reprogramming factor TF4 (Gao et al., 2013) for reprogramming to further confirm the Mthfd2-mediated improvements. The number of AP+ colonies was increased in the Mthfd2-overexpressing group compared with that in the control group (Figures S2C and S2D) (p < 0.05). These results indicated that Mthfd2 facilitates the induction of iPSCs.
Figure 2.
Mthfd2 Promotes Complete Reprogramming of iPSCs
(A) The reprogramming efficiencies in KOSR medium. OSK + control (Ctr) cells were used as a control. Data are pooled from three independent experiments (mean ± SD). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control.
(B) Full-well mosaic images of Oct4-GFP+ cells and AP+ colonies are shown for OSK+Ctr and OSKM2 iPSCs in KOSR medium.
(C) Immunofluorescence (IF) staining for pluripotency marker proteins in OSKM2 iPSCs. DAPI was used to indicate the nuclei. Scale bars, 100 μm.
(D) IF staining for GATA6 (endoderm), NESTIN (ectoderm), and α-smooth muscle actin (mesoderm) in EBs derived from OSKM2 iPSCs. DAPI was used to indicate the nuclei. Scale bars, 100 μm.
(E) H&E staining of teratomas derived from OSKM2 iPSCs. Scale bars, 100 μm.
(F) Representative images of chimeric mice (F1) and their offspring (F2).
(G) Live-born pups obtained from OSKM2 iPSCs were tested by tetraploid complementation.
See also Figure S2.
Mthfd2 Improves the Quality of iPSCs
All iPSCs induced with Mthfd2 (OSKM2 iPSCs) showed typical mESC-like morphology and expressed Oct4-driven GFP (Figure S2E). Exogenous retroviral expression of OSK and Mthfd2 was silenced (Figure S2F), and the expression of pluripotency marker genes was similar in OSKM2 iPSCs and mESCs (Figure S2G). In addition, two master transcription factors (Nanog and Sox2) and the mESC-specific surface marker SSEA1 were expressed in OSKM2 iPSCs (Figure 2C).
We conducted EB and teratoma formation assays to assess the ability of OSKM2 iPSCs to differentiate in vitro and in vivo. The EBs and teratomas derived from OSKM2 iPSCs differentiated into cells of all three germ layers (Figures 2D and 2E). Furthermore, the developmental potential of OSKM2 iPSCs in vivo was tested via 8-cell embryo injection (Xiang et al., 2018), and chimeric mice derived almost completely from iPSCs were produced and had the ability of germline transmission (Figure 2F). Next, we tested the developmental potential of OSKM2 iPSCs in vivo by tetraploid complementation (Zhao et al., 2009). Remarkably, OSKM2 iPSCs gave rise to viable all-iPSC-derived pups (Figure 2G) with higher efficiency than control OSKC iPSCs (Figure S2H). Collectively, these results indicated that Mthfd2 improves the quality of iPSCs.
Mthfd2 Depletion Shifts Glucose Metabolism from OXPHOS to Glycolysis in mESCs
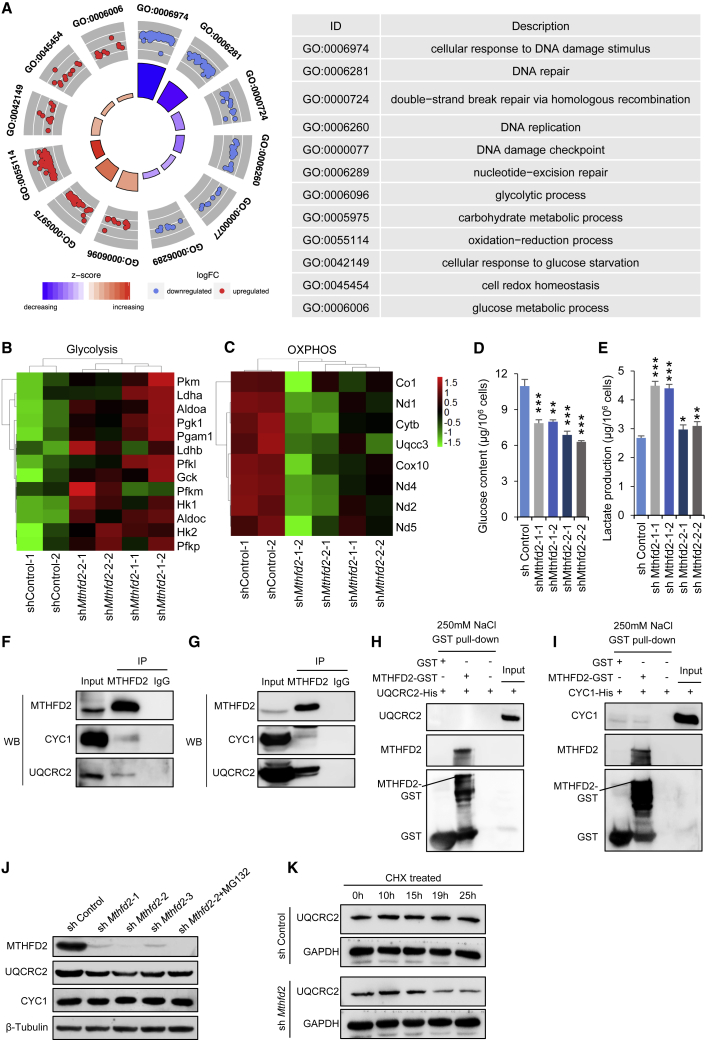
MTHFD2 was reported to be an important enzyme in mitochondrial one-carbon metabolism (Tibbetts and Appling, 2010). Two MTHFD2-specific inhibitors, MIN and NIT (Asai et al., 2018), were used to inhibit MTHFD2 enzymatic activity in mESCs. Both MIN- and MIT-treated mESCs (MI-mESCs) maintained typical stem cell morphology and pluripotency-associated marker gene expression (Figures S3A–S3C). Therefore, we speculated that the MTHFD2 has non-enzymatic functions in mESCs. To characterize the global function of Mthfd2 in mESCs, we analyzed changes in the transcriptome due to suppression of Mthfd2 (Figure S3D and Table S2). Among the differentially expressed genes (DEGs), pluripotency-associated genes were downregulated in Mthfd2 KD mESCs (Figure S3E). Interestingly, gene ontology (GO) terms such as glycolytic process were among the top ten terms enriched in upregulated genes (Figure S3F), and additional GO terms associated with carbohydrate metabolism were among the terms enriched in upregulated genes (p < 0.05) (Figure 3A). Glucose metabolism is a complex and dynamic process and is the most potent modulator of PSC pluripotency states and cell-fate changes (Carbognin et al., 2016; Shyh-Chang and Daley, 2015; Zhou et al., 2012). Subsequently, we evaluated whether the cellular glucose metabolism state was altered in Mthfd2 KD mESCs. Among the DEGs, the levels of key glycolytic genes were increased and those of some key OXPHOS genes were decreased in Mthfd2 KD mESCs (Figures 3B and 3C). Lower intracellular glucose levels and higher intracellular lactate levels were detected in Mthfd2 KD cells than in control cells (Figures 3D and 3E), indicating increased consumption of glucose and production of lactate in Mthfd2 KD mESCs. Therefore, Mthfd2 depletion shifts glucose metabolism from OXPHOS to glycolysis in mESCs.
Figure 3.
MTHFD2 Interacts with Complex III to Regulate Glucose Metabolism in mESCs
(A) Summary of enriched GO terms that were potently upregulated and downregulated by Mthfd2. p < 0.05. For details, see Table S2.
(B and C) Heatmap of DEGs about glycolysis (B) and OXPHOS (C) between control mESCs and Mthfd2 KD mESCs.
(D and E) Examination of intracellular glucose levels (D) and lactate levels (E) in Mthfd2 KD mESCs. Data are pooled from three independent experiments (mean ± SD). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control.
(F and G) CoIP results showing the specific interactions between endogenous MTHFD2 and both UQCRC2 and CYC1 in mESCs (F) and in the cytoplasm fraction of mESCs (G).
(H and I) GST pull-down assays for interaction between UQCRC2-His (H), CYC1-His (I), and MTHFD2-GST fusion proteins at 250 mM NaCl containing GST binding buffer.
(J) Western blot analysis of the levels of the UQCRC2 and CYC1 proteins in Mthfd2 KD mESCs. β-Tubulin was used as a loading control.
(K) Western blot analysis of the levels of UQCRC2 protein in Mthfd2 KD mESCs at indicated times post cycloheximide (CHX) treatment. GAPDH was used as a loading control.
See also Figures S3–S5.
MTHFD2 Interacts with Mitochondrial ETC Complex III in Mitochondria
To understand the mechanism by which Mthfd2 functions in mESCs, we used an immunoprecipitation and mass spectrometry (IP-MS) assay to search for proteins that interact with MTHFD2. MTHFD2 was localized to both the nucleus and mitochondria in mESCs (Figures S4A and S4B), and its function in mESCs, particularly in the nucleus, remains unknown. Hence, we separated nuclear and cytoplasmic fractions from Mthfd2-FLAG mESCs and subjected them to IP-MS (Figures S4C–S4F).
Among the identified MTHFD2 partners were the complex III members UQCRC2 and CYC1 (Figure S4G and Table S3). Complex III is a component of the mitochondrial ETC, via which OXPHOS is mediated to generate ATP in eukaryotic cells (Hatefi, 1985; Mitchell, 1975). Given the upregulation of glycolysis in Mthfd2 KD mESCs, the interaction between MTHFD2 and complex III was particularly interesting. First, the interaction between FLAG-tagged MTHFD2 and hemagglutinin (HA)-tagged UQCRC2 was verified in E14 mESCs by reciprocal coimmunoprecipitation (coIP) experiments (Figure S5A). The specific interactions between endogenously expressed MTHFD2 and both UQCRC2 and CYC1 were then verified in E14 mESCs (Figure 3F). We further verified that MTHFD2 formed protein complexes with UQCRC2 and CYC1 in the cytoplasmic fraction (Figure 3G). To address the questions of whether the interactions between MTHFD2 and those proteins were direct, we conducted glutathione S-transferase (GST) pull-down assays using recombinant proteins. No direct interaction between MTHFD2-GST and either UQCRC2-His or CYC1-His was detected (Figures 3H and 3I). Thus, these results provide evidence for an indirect interaction between MTHFD2 and complex III in mitochondria of mESCs.
The protein level of UQCRC2 were reduced in Mthfd2 KD mESCs compared with control mESCs (Figure S5B) but did not differ between MI-mESCs and control mESCs (Figure S5C). Treatment with a 26S proteasome inhibitor, MG132, efficiently prevented the Mthfd2 depletion-induced reduction in UQCRC2 expression (Figure 3J). By treating cells with the protein synthesis inhibitor cycloheximide, we found that the half-life of UQCRC2 was considerably shortened upon depletion of Mthfd2 (Figure 3K). Thus, Mthfd2 might play a role in maintaining the stability of the UQCRC2 protein.
Mthfd2 Deficiency Induces Mitochondrial Dysfunction by Regulating the Activity of Complex III
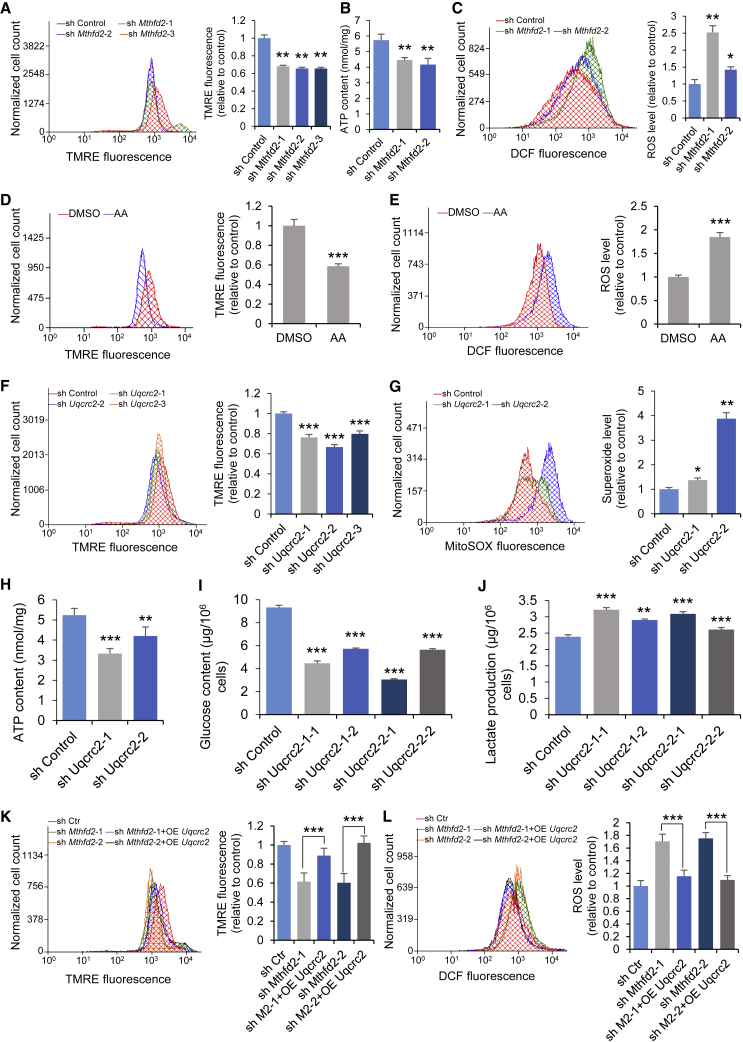
The main function of complex III is to transfer electrons between ubiquinol and cytochrome c, thus generating an electrochemical potential that drives ATP synthesis (Crofts, 2004). In addition, complex III is the major site of ROS generation (Chen et al., 2003). The mitochondrial membrane potential (MMP) and ATP production were decreased (Figures 4A and 4B) and cellular ROS levels were increased in Mthfd2 KD mESCs (Figure 4C). These effects are consistent with those observed in cells treated with antimycin A (a complex III inhibitor), which impairs mitochondrial function (Figures 4D and 4E), and indicated that Mthfd2 deficiency induces mitochondrial dysfunction in mESCs.
Figure 4.
Mthfd2 Deficiency Induces Mitochondrial Dysfunction by Reducing Uqcrc2 Expression
(A) Measurements of the mitochondrial membrane potential (MMP) in Mthfd2 KD mESCs using tetramethylrhodamine methyl ester (TMRE).
(B) Total ATP levels in Mthfd2 KD mESCs.
(C) Flow-cytometry analysis of ROS levels in Mthfd2 KD mESCs.
(D) Measurements of the MMP in antimycin A-treated mESCs (AA-mESCs) using TMRE.
(E) Flow-cytometry analysis of ROS levels in AA-mESCs.
(F) Measurements of the MMP in Uqcrc2 KD mESCs using TMRE.
(G) Flow-cytometry analysis of superoxide levels in Uqcrc2 KD mESCs.
(H) Total ATP levels in Uqcrc2 KD mESCs.
(I and J) Examination of intracellular glucose levels (I) and lactate levels (J) in Uqcrc2 KD mESCs.
(K) Measurements of the MMP in OE Uqcrc2-Mthfd2 KD mESCs using TMRE.
(L) Flow-cytometry analysis of ROS levels in OE Uqcrc2-Mthfd2 KD mESCs.
All data are pooled from three independent experiments (mean ± SD). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control. A representative histogram (left) and quantification of the mean fluorescence intensity (right) are presented for (A), (C) to (G), (K), and (L). See also Figure S5.
The mitochondrial dysfunction induced by Mthfd2 depletion resembled the phenotype observed in cells with Uqcrc2 deficiency, which leads to complex III deficiency (Aguilera-Aguirre et al., 2009; Miyake et al., 2013). Uqcrc2 KD cells exhibited reduced MMP and ATP production, increased levels of superoxide (a major ROS generated in mitochondria), and a shift of glucose metabolism to glycolysis (Figures 4F–4J). Moreover, forced expression of Uqcrc2 effectively rescued the Mthfd2 KD-induced changes in the MMP (Figure 4K) and the cellular ROS level (Figure 4L). In addition, the cellular ROS level (Figure S5D) and the MMP (Figure S5E) did not differ between MI-mESCs and control mESCs. Therefore, Mthfd2 depletion induces mitochondrial dysfunction through inhibition of complex III activity caused by a decrease in UQCRC2 expression.
Mthfd2 Depletion Hinders DNA Repair in mESCs
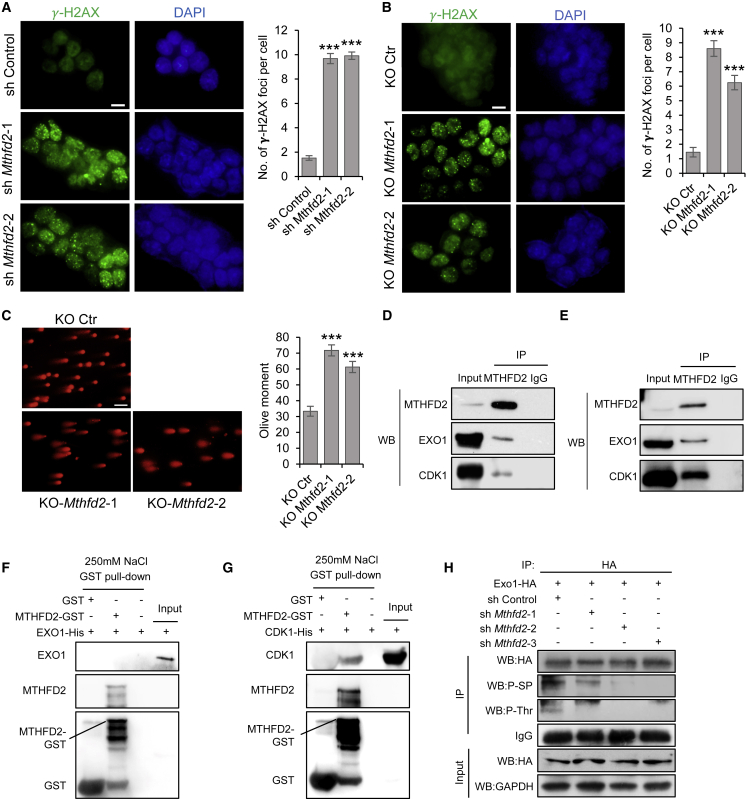
In addition to GO terms related to glycolysis, GO terms such as DNA repair were among the top ten terms enriched in downregulated genes (Figure S6A), and additional terms such as double-strand break repair via HR were enriched in downregulated genes (p < 0.05) (Figure 3A). PSCs rely on a very robust DNA repair response to control DNA damage, and if DNA damage is unresolvable, PSCs prevent the propagation of genetic lesions by undergoing regulated cell death (Liu et al., 2013) or losing pluripotency (Li et al., 2012; Lin et al., 2005). Therefore, we examined whether Mthfd2 deficiency altered DNA damage levels in mESCs. Among the DEGs, DNA repair-associated genes exhibited decreased expression in Mthfd2 KD mESCs (Figure S6B). Phosphorylation of H2AX (termed γ-H2AX) is one of the earliest events to occur following DNA DSB induction and is critical for protecting the genome from DSBs (Rogakou et al., 1998). Interestingly, the number of γ-H2AX foci increased substantially in Mthfd2 KD mESCs (Figure 5A) and Mthfd2 KO mESCs (Figure 5B) with increased passaging. The increase in DNA damage in Mthfd2 KO mESCs was further validated by comet assay, a method that measures the extent of DNA damage on a single-cell basis (Figure 5C). These increases suggested that DNA damage accumulates following Mthfd2 depletion.
Figure 5.
MTHFD2 Interacts with CDK1 and EXO1 to Regulate DNA Damage Level in mESCs
(A and B) IF staining for γ-H2AX in Mthfd2 KD mESCs (A) and Mthfd2 KO mESCs (B). Representative images (left) and quantification of the average number of γ-H2AX foci per cell (right) (n = 50 nuclei) are shown. DAPI was used to indicate the nuclei. Scale bar, 10 μm.
(C) DNA damage levels of Mthfd2 KO mESCs were evaluated by the comet assay. Representative images (left) and quantification of the mean olive tail moment (right) (n = 50 nuclei) are shown. Scale bar, 100 μm.
(D and E) CoIP results showing the specific interactions between endogenous MTHFD2 and both CDK1 and EXO1 in mESCs (D) and in the nuclear fraction of mESCs (E).
(F and G) GST pull-down assays for interaction between EXO1-His (F), CDK1-His (G), and MTHFD2-GST fusion proteins at 250 mM NaCl containing GST binding buffer.
(H) Representative results showing the EXO1 phosphorylation status in Mthfd2 KD mESCs.
Data in (A) to (C) are pooled from three independent experiments (mean ± SEM). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control. See also Figure S6.
MTHFD2 Interacts with CDK1 and EXO1 in the Nucleus
Under normal conditions, PSCs accurately repair DSBs mostly through the HR pathway (Tichy et al., 2010). EXO1 and CDK1, which play key roles in DNA HR repair (Tomimatsu et al., 2014), were detected among the identified MTHFD2 partners (Figure S4G and Table S3). Thus, we explored the interactions between MTHFD2 and both EXO1 and CDK1. The interactions between endogenously expressed MTHFD2 and both CDK1 and EXO1 were confirmed by coIP experiments (Figure 5D). We further verified that MTHFD2 interacts with both EXO1 and CDK1 in the nuclear fraction (Figures 5E and S6C). Moreover, a direct interaction between MTHFD2-GST and CDK1-His was detected by GST pull-down assays (Figures 5F and 5G). These results provide evidence for the interaction of CDK1 and EXO1 with MTHFD2 in the nucleus of mESCs. In addition, the interactions between MTHFD2 and UQCRC2, CYC1, CDK1, and EXO1 remained detectable in MI-mESCs (Figure S6D), emphasizing that the MTHFD2's interactions are not affected by its enzymatic activity.
During HR repair, EXO1 is primed to function in resection by CDK-mediated phosphorylation (Tomimatsu et al., 2014). We assessed the level of CDK1-induced EXO1 phosphorylation in Mthfd2 KD mESCs. EXO1 phosphorylation was significantly reduced in Mthfd2 KD mESCs compared with that in control mESCs (Figure 5H). Collectively, these results indicate that Mthfd2 plays an important role in maintaining the phosphorylation of EXO1 in mESCs.
Mthfd2 Modulates HR Repair by Regulating EXO1 Phosphorylation
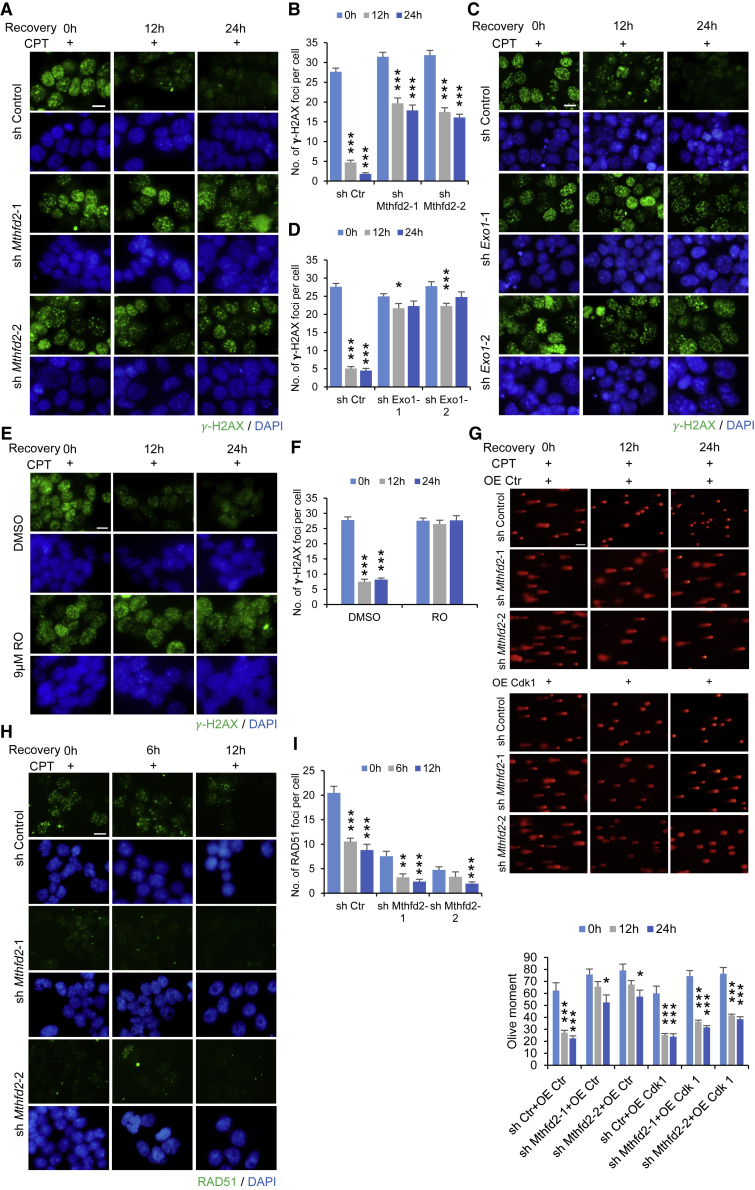
To understand whether the increased DNA damage in Mthfd2 KD mESCs was caused by defects in DNA repair, we quantified and compared γ-H2AX foci formation after treatment with camptothecin (CPT), which induces DSBs (Hsiang et al., 1989). A significant reduction in DNA repair efficiency was observed in Mthfd2 KD mESCs (Figures 6A, 6B, S6E, and S6F) and Exo1 KD mESCs (Figures 6C, 6D, and S6G) after CPT treatment, consistent with a previous report that Exo1 deletion impairs HR repair (Schaetzlein et al., 2007). This pattern was consistent with that observed in cells treated with RO-3306 (a specific CDK1 inhibitor), which hinders DNA repair and increases DNA damage (Figures 6E and 6F). Moreover, the comet assay results showed that forced expression of Cdk1 effectively rescued the Mthfd2 KD-induced impairment in DNA repair (Figure 6G). However, DNA damage level did not differ between MI-mESCs and control mESCs (Figures S6H and S6I), highlighting a key role of EXO1 phosphorylation in Mthfd2-mediated functions during DNA repair. Moreover, Mthfd2 KD mESCs exhibited fewer Rad51 foci, a key component of HR repair, than control cells after CPT treatment, suggesting attenuation of HR repair (Figures 6H and 6I). These results revealed that Mthfd2 regulates EXO1 phosphorylation by affecting the kinase activity of CDK1 to modulate HR repair and protect genomic integrity.
Figure 6.
Mthfd2 Modulates HR Repair by Regulating EXO1 Phosphorylation
(A and B) IF staining for γ-H2AX in Mthfd2 KD mESCs at indicated times post CPT treatment. Representative images (A) and quantification of the average number of γ-H2AX foci per cell (B) (n = 50 nuclei) are shown. DAPI was used to indicate the nuclei. Scale bar, 50 μm.
(C and D) IF staining for γ-H2AX in Exo1 KD mESCs at indicated times post CPT treatment. Representative images (C) and quantification of the average number of γ-H2AX foci per cell (D) (n = 50 nuclei) are shown. DAPI was used to indicate the nuclei. Scale bar, 50 μm.
(E and F) Immunofluorescence staining for γ-H2AX in RO-3306 (RO)-treated mESCs at indicated times post CPT treatment. Representative images (E) and quantification of the average number of γ-H2AX foci per cell (F) (n = 50 nuclei) are shown. DAPI was used to indicate the nuclei. Scale bar, 50 μm.
(G) DNA damage levels in OE Cdk1-Mthfd2 KD mESCs were evaluated by the comet assay at indicated times post CPT treatment. Representative images (upper panels) and quantification of the mean olive tail moment (lower panel) (n = 50 nuclei) are shown. Scale bar, 100 μm.
(H and I) IF staining for RAD51 in Mthfd2 KD mESCs at indicated times post CPT treatment. Representative images (H) and quantification of the average number of Rad51 foci per cell (I) (n = 50 nuclei) are shown. DAPI was used to indicate the nuclei. Scale bar, 20 μm.
All data are pooled from three independent experiments (mean ± SEM). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control. See also Figure S6.
Mthfd2 Regulates Pluripotency of PSCs by Mediating Both Mitochondrial Function and HR Repair
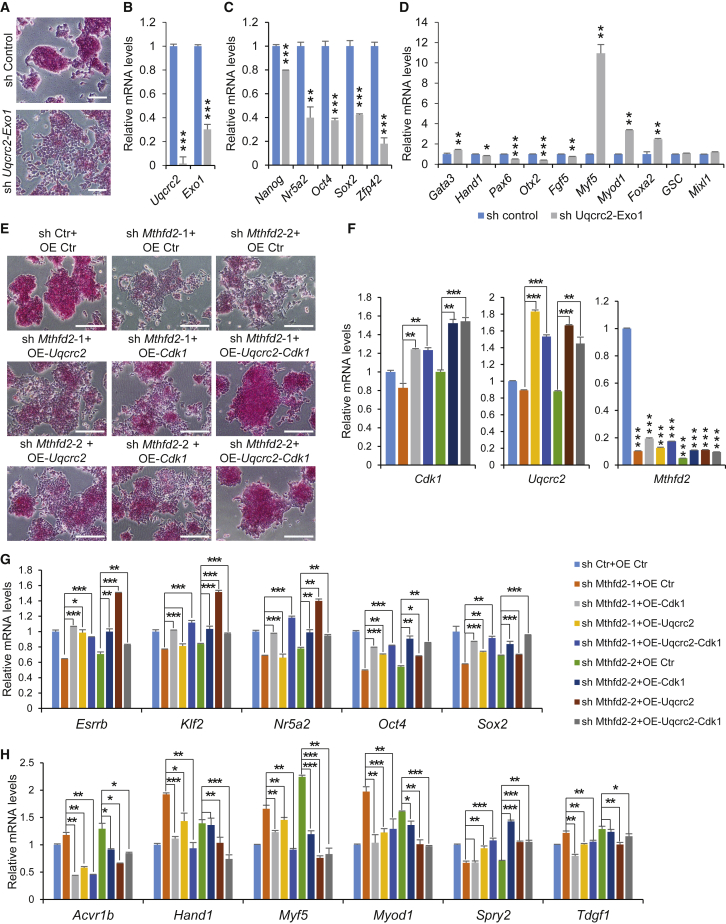
To further confirm whether Mthfd2 regulates pluripotency of PSCs by mediating both mitochondrial function and HR repair, on the one hand we transfected mESCs with two sets of shRNAs against Uqcrc2 or Exo1. Depletion of Uqcrc2 or Exo1 led to the differentiation of mESCs (Figures S7A–S7G). Moreover, cosuppression of Uqcrc2 and Exo1 led to the differentiation of mESCs with reduced AP staining (Figures 7A and 7B), and the expression pattern of marker genes in these cells was more consistent with that in Mthfd2 KD mESCs (Figures 7C and 7D). On the other hand, we forced the expression either of Uqcrc2 or Cdk1 alone or of Uqcrc2 and Cdk1 together in Mthfd2 KD mESCs (Figures 7E and 7F). Forced co-expression of Uqcrc2 and Cdk1 rescued the Mthfd2 KD-induced changes in cell morphology (Figure 7E) and marker gene expression (Figures 7G and 7H), highlighting that Mthfd2 regulates pluripotency of PSCs by mediating both mitochondrial function and HR repair.
Figure 7.
Mthfd2 Regulates Pluripotency of mESCs by Modulating both Mitochondrial Function and DNA Repair
(A) Representative results of Uqcrc2-Exo1 KD mESCs with AP staining. Scale bars, 100 μm.
(B) qRT-PCR analysis of mRNA levels of Uqcrc2 and Exo1 in Uqcrc2-Exo1 KD mESCs.
(C and D) qRT-PCR analysis of mRNA levels of pluripotency marker genes (C) and lineage marker genes (D) in Uqcrc2-Exo1 KD mESCs.
(E) Representative results of OE Uqcrc2-, OE Cdk1- or co-OE Uqcrc2, and Cdk1-Mthfd2 KD mESCs with AP staining. Scale bars, 200 μm.
(F) qRT-PCR analysis of mRNA levels of Uqcrc2, Cdk1, and Mthfd2 in OE Uqcrc2-, OE Cdk1- or co-OE Uqcrc2, and Cdk1-Mthfd2 KD mESCs.
(G and H) qRT-PCR analysis of mRNA levels of pluripotency marker genes (G) and lineage marker genes (H) in OE Uqcrc2-, OE Cdk1- or co-OE Uqcrc2, and Cdk1-Mthfd2 KD mESCs.
Data in (B) to (D) and (F) to (H) are pooled from three independent experiments (mean ± SD) relative to EF1-α and the control. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student's t test) compared with the control. See also Figure S7.
Discussion
High-quality PSCs can differentiate into various types of cells more stably and efficiently, ensuring application security. Many cellular processes, such as pluripotent gene transcription, epigenetic modification, metabolic remodeling, and genomic integrity were involved in somatic cell reprogramming and cell-fate determination (Doege et al., 2012; Graf et al., 2017; Mathieu and Ruohola-Baker, 2017; Silva et al., 2009), but the mechanisms determining the quality of PSCs remain unclear. In our study, we performed transcriptome analysis combined with an IP-MS assay to reveal that Mthfd2 regulates both mitochondrial function and HR repair and that Mthfd2 is a powerful bifunctional regulator for quality of PSCs.
We found that mESCs with inhibited MTHFD2 enzymatic activity retained typical stem cell characteristics. Moreover, the expression of key genes in folate metabolism did not differ between Mthfd2 KD mESCs and control mESCs (Table S2). These results revealed that Mthfd2 has non-enzymatic functions in mESCs. MTHFD2 depletion resulted in an obvious suppression of proliferation and cell death in diverse cancer cell types (Nilsson et al., 2014). In our study, homozygous Mthfd2 KO mESCs showed compromised proliferation, but there was no significant change in cell proliferation in Mthfd2 KD mESCs (Table S7). Whether this difference was due to residual MTHFD2 activity in Mthfd2 KD cells, the activity of other enzymes such as MTHFD2L, or the presence of alternative metabolic pathways that are active in mESCs, is unclear. MTHFD2 depletion resulted in increased glycolysis in breast cancer cells and lung cancer cells (Koufaris et al., 2016; Nishimura et al., 2019). Our results showed that Mthfd2 KD induces mitochondrial dysfunction, which suggests that in Mthfd2-depleted cells mitochondrial dysfunction may force cells to rely mainly on glycolysis to support the cellular ATP demand. However, we cannot rule out the possibility that increased glycolysis is related to mESC differentiation.
Numerous metabolic enzymes, which are often highly expressed by embryonic or undifferentiated cell types, have previously been found to have non-enzymatic functions in the nucleus (Lincet and Icard, 2015). In mESCs, we identified a role of nuclear Mthfd2 in preserving HR repair. FILIA physically interacts with PARP1 and stimulates PARP1's enzymatic activity to regulate the DDR (Zhao et al., 2015). Our study indicated that Mthfd2 plays a role similar to that of Filia in the DDR. MTHFD2 directly interacts with CDK1 and affects CDK1's activity to regulate EXO1 phosphorylation, thereby promoting HR repair. The most dangerous form of DNA damage is DSBs that can arise from ROS and others. Mthfd2 overexpression in the Drosophila abdominal fat body significantly reduced ROS levels (Yu et al., 2015), and ROS levels were increased in Mthfd2 KD mESCs. Hence, Mthfd2 can maintain genomic integrity by both reducing the levels of ROS and promoting HR repair. Genomic stability is necessary for the survival and function of PSCs (Blanpain et al., 2011) and is also a key aspect of improving the quality of iPSCs (Gonzalez et al., 2013; Jiang et al., 2013). Therefore, Mthfd2 improves the quality of iPSCs by maintaining genomic stability, thereby ensuring the safety of iPSC applications.
Metabolic transition from OXPHOS to glycolysis is a necessary process for successful reprogramming (Folmes et al., 2011; Prigione et al., 2014). However, OXPHOS is also induced and activated at different stages of reprogramming to jointly promote reprogramming (Hawkins et al., 2016; Kida et al., 2015). In our study, overexpression of Mthfd2 facilitated iPSC induction, suggesting that maintaining active mitochondrial function during reprogramming is beneficial for improving reprogramming efficiency. In PSCs, the metabolic transition from glycolysis to OXPHOS promotes the primed-to-naive state transition (Carbognin et al., 2016). Therefore, in addition to maintaining genomic stability, Mthfd2 also improves the quality of PSCs by maintaining active OXPHOS. Primed PSCs have lower mitochondrial respiration rates than naive PSCs, which is attributable to a deficiency in ETC complex IV (Zhou et al., 2012). We found that deficiency in ETC complex III also affects the pluripotency state of mESCs. Complex III has two sites, Qo and Qi, to produce ROS. Antimycin A inhibits complex III at the Qi site and increases ROS generation from the Qo site, while it inhibits the Qo site and reduces ROS production (Chen et al., 2003; Demin et al., 1998; Muller et al., 2002). Therefore, Mthfd2 or Uqcrc2 depletion may lead to increased ROS production by inhibiting the Qi site instead of the Qo site. MTHFD2 interacts with UQCRC2 to maintain active mitochondrial function, but the interaction is not direct and is mediated by other factors that need further study.
Finally, Mthfd2 was highly expressed in high-quality iPSCs, and OSKM2 iPSCs were able to develop into viable offspring via tetraploid complementation. The tetraploid complementation assay is the most stringent criterion for assessing the developmental potential of non-human PSCs. Therefore, establishing a fast and effective method to evaluate the pluripotent state of human PSCs is necessary. Moreover, the PSCs of domestic animals such as porcine, bovine, and ovine are still unable to produce chimeras. MTHFD2 is a highly conserved protein between mice and human or domestic animals. Hence, our study should provide new insights into cell pluripotency of other species and provide additional methods to enhance and optimize the acquisition and maintenance of high-quality PSCs.
Experimental Procedures
Co-immunoprecipitation
Cell extracts were prepared in lysis buffer (Beyotime) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Cell Signaling Technology). To reduce the background, we cleaned the cell extracts with mouse or rabbit immunoglobulin G beads before conducting coIP assays. Antibodies were incubated overnight with cell extracts and then bound to Protein-G beads (Roche) following the manufacturer's protocol. Beads were washed extensively in lysis buffer and then boiled in 2× SDS loading buffer before western blotting.
Data and Code Availability
The accession numbers for the RNA-seq data reported in this paper is SRA: SRP149554.
Author Contributions
J.H. conceived and supervised the project. J.H. and L.Y. designed the study and wrote the manuscript. L.Y. performed most experiments and result analyses. Y.P. conducted iPSC induction and results analysis. L.Z. and H.Y. performed bioinformatics analysis. M.Z., S.Z., B.W., J.X., and S.C. performed the iPSC chimeric embryo experiment. Y.W., W.Z., and Q.W. designed the primers. Q.Z., J.G., H.L., and Z.C. helped with the GST pull-down assay, N.C. helped with molecular vector construction. H.L. performed the fluorescence-activated cell sorting experiment.
Acknowledgments
We thank Andras Nagy, Kristina Vintersten, and Marina Gertsenstein (Mount Sinai Hospital) for their support of G4 ESCs; Dr. Shaorong Gao (Tongji University; National Institute of Biological Sciences) for providing the Oct4-GFP mice, and Dr. Shuai Gao (Tongji University; National Institute of Biological Sciences) for Rosa26-M2rtTA Col1a1-tetO-Pou5f1 (TF4) mice. This work was supported by China National Basic Research Program (2016YFA0100202), National Natural Science Foundation of China (31571497, 31601941, 31772601), Plan 111 (B12008), and Research Programs from the State Key Laboratories for Agrobiotechnology and College of Biological Sciences (2020SKLAB1-3, 31051378, 10052521-01).
Published: July 16, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.06.018.
Supplemental Information
References
- Aguilera-Aguirre L., Bacsi A., Saavedra-Molina A., Kurosky A., Sur S., Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A., Koseki J., Konno M., Nishimura T., Gotoh N., Satoh T., Doki Y., Mori M., Ishii H. Drug discovery of anticancer drugs targeting methylenetetrahydrofolate dehydrogenase 2. Heliyon. 2018;4:e01021. doi: 10.1016/j.heliyon.2018.e01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Mohrin M., Sotiropoulou P.A., Passegue E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Boroviak T., Loos R., Lombard P., Okahara J., Behr R., Sasaki E., Nichols J., Smith A., Bertone P. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev. Cell. 2015;35:366–382. doi: 10.1016/j.devcel.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbognin E., Betto R.M., Soriano M.E., Smith A.G., Martello G. Stat3 promotes mitochondrial transcription and oxidative respiration during maintenance and induction of naive pluripotency. EMBO J. 2016;35:618–634. doi: 10.15252/embj.201592629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Long Q., Wang T., Zhao D., Zhou Y., Qi J., Wu Y., Li S., Chen C., Zeng X. Gadd45a is a heterochromatin relaxer that enhances iPS cell generation. EMBO Rep. 2016;17:1641–1656. doi: 10.15252/embr.201642402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Vazquez E.J., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Crofts A.R. The cytochrome bc1 complex: function in the context of structure. Annu. Rev. Physiol. 2004;66:689–733. doi: 10.1146/annurev.physiol.66.032102.150251. [DOI] [PubMed] [Google Scholar]
- Demin O.V., Kholodenko B.N., Skulachev V.P. A model of O2.-generation in the complex III of the electron transport chain. Mol. Cell. Biochem. 1998;184:21–33. [PubMed] [Google Scholar]
- Desmarais J.A., Unger C., Damjanov I., Meuth M., Andrews P. Apoptosis and failure of checkpoint kinase 1 activation in human induced pluripotent stem cells under replication stress. Stem Cell Res. Ther. 2016;7:17. doi: 10.1186/s13287-016-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro E., Sirois J., Tremblay M.L., MacKenzie R.E. Mitochondrial NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is essential for embryonic development. Mol. Cell. Biol. 2002;22:4158–4166. doi: 10.1128/MCB.22.12.4158-4166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege C.A., Inoue K., Yamashita T., Rhee D.B., Travis S., Fujita R., Guarnieri P., Bhagat G., Vanti W.B., Shih A. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Feng B., Jiang J., Kraus P., Ng J.-H., Heng J.-C.D., Chan Y.-S., Yaw L.-P., Zhang W., Loh Y.-H., Han J. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat. Cell Biol. 2009;11:197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- Folmes C.D., Nelson T.J., Martinez-Fernandez A., Arrell D.K., Lindor J.Z., Dzeja P.P., Ikeda Y., Perez-Terzic C., Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Wang Z.L., Di K.Q., Chang G., Tao L., An L., Wu F.J., Xu J.Q., Liu Y.W., Wu Z.H. Melatonin improves the reprogramming efficiency of murine-induced pluripotent stem cells using a secondary inducible system. J. Pineal Res. 2013;55:31–39. doi: 10.1111/jpi.12047. [DOI] [PubMed] [Google Scholar]
- Gonzalez F., Georgieva D., Vanoli F., Shi Z.D., Stadtfeld M., Ludwig T., Jasin M., Huangfu D. Homologous recombination DNA repair genes play a critical role in reprogramming to a pluripotent state. Cell Rep. 2013;3:651–660. doi: 10.1016/j.celrep.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf U., Casanova E.A., Wyck S., Dalcher D., Gatti M., Vollenweider E., Okoniewski M.J., Weber F.A., Patel S.S., Schmid M.W. Pramel7 mediates ground-state pluripotency through proteasomal-epigenetic combined pathways. Nat. Cell Biol. 2017;19:763–773. doi: 10.1038/ncb3554. [DOI] [PubMed] [Google Scholar]
- Gustafsson Sheppard N., Jarl L., Mahadessian D., Strittmatter L., Schmidt A., Madhusudan N., Tegner J., Lundberg E.K., Asplund A., Jain M. The folate-coupled enzyme MTHFD2 is a nuclear protein and promotes cell proliferation. Sci. Rep. 2015;5:15029. doi: 10.1038/srep15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Yuan P., Yang H., Zhang J., Soh B.S., Li P., Lim S.L., Cao S., Tay J., Orlov Y.L. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hawkins K.E., Joy S., Delhove J.M., Kotiadis V.N., Fernandez E., Fitzpatrick L.M., Whiteford J.R., King P.J., Bolanos J.P., Duchen M.R. NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming. Cell Rep. 2016;14:1883–1891. doi: 10.1016/j.celrep.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng J.C., Feng B., Han J., Jiang J., Kraus P., Ng J.H., Orlov Y.L., Huss M., Yang L., Lufkin T. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Hsiang Y.H., Lihou M.G., Liu L.F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- Huang Y., Osorno R., Tsakiridis A., Wilson V. In vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep. 2012;2:1571–1578. doi: 10.1016/j.celrep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Jackson S.P. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- Jiang J., Lv W., Ye X., Wang L., Zhang M., Yang H., Okuka M., Zhou C., Zhang X., Liu L. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res. 2013;23:92–106. doi: 10.1038/cr.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khacho M., Clark A., Svoboda D.S., Azzi J., MacLaurin J.G., Meghaizel C., Sesaki H., Lagace D.C., Germain M., Harper M.E. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional Program. Cell Stem Cell. 2016;19:232–247. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Kida Y.S., Kawamura T., Wei Z., Sogo T., Jacinto S., Shigeno A., Kushige H., Yoshihara E., Liddle C., Ecker J.R. ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell. 2015;16:547–555. doi: 10.1016/j.stem.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufaris C., Gallage S., Yang T., Lau C.H., Valbuena G.N., Keun H.C. Suppression of MTHFD2 in MCF-7 breast cancer cells increases glycolysis, dependency on exogenous Glycine, and sensitivity to folate depletion. J. Proteome Res. 2016;15:2618–2625. doi: 10.1021/acs.jproteome.6b00188. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Kim D.K., Ko J.J., Kim K.P., Park K.S. Rad51 regulates reprogramming efficiency through DNA repair pathway. Dev. Reprod. 2016;20:163–169. doi: 10.12717/DR.2016.20.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., He Y., Dubois W., Wu X., Shi J., Huang J. Distinct regulatory mechanisms and functions for p53-activated and p53-repressed DNA damage response genes in embryonic stem cells. Mol. Cell. 2012;46:30–42. doi: 10.1016/j.molcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Huang B., Wang H., Liu X., Hong Y., Qiu S., Zheng J. MTHFD2 overexpression predicts poor prognosis in renal cell carcinoma and is associated with cell proliferation and vimentin-modulated migration and invasion. Cell. Physiol. Biochem. 2018;51:991–1000. doi: 10.1159/000495402. [DOI] [PubMed] [Google Scholar]
- Lin T., Chao C., Saito S., Mazur S.J., Murphy M.E., Appella E., Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Lincet H., Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene. 2015;34:3751–3759. doi: 10.1038/onc.2014.320. [DOI] [PubMed] [Google Scholar]
- Liu F., Liu Y., He C., Tao L., He X., Song H., Zhang G. Increased MTHFD2 expression is associated with poor prognosis in breast cancer. Tumour Biol. 2014;35:8685–8690. doi: 10.1007/s13277-014-2111-x. [DOI] [PubMed] [Google Scholar]
- Liu J.C., Guan X., Ryan J.A., Rivera A.G., Mock C., Agrawal V., Letai A., Lerou P.H., Lahav G. High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell. 2013;13:483–491. doi: 10.1016/j.stem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Wang F., Ye X., Wang L., Yang J., Zhang J., Liu L. KSR-based medium improves the generation of high-quality mouse iPS cells. PLoS One. 2014;9:e105309. doi: 10.1371/journal.pone.0105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J., Ruohola-Baker H. Metabolic remodeling during the loss and acquisition of pluripotency. Development. 2017;144:541–551. doi: 10.1242/dev.128389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975;56:1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Miyake N., Yano S., Sakai C., Hatakeyama H., Matsushima Y., Shiina M., Watanabe Y., Bartley J., Abdenur J.E., Wang R.Y. Mitochondrial complex III deficiency caused by a homozygous UQCRC2 mutation presenting with neonatal-onset recurrent metabolic decompensation. Hum. Mutat. 2013;34:446–452. doi: 10.1002/humu.22257. [DOI] [PubMed] [Google Scholar]
- Muller F., Crofts A.R., Kramer D.M. Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry. 2002;41:7866–7874. doi: 10.1021/bi025581e. [DOI] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Jain M., Madhusudhan N., Sheppard N.G., Strittmatter L., Kampf C., Huang J., Asplund A., Mootha V.K. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Nakata A., Chen X., Nishi K., Meguro-Horike M., Sasaki S., Kita K., Horike S.I., Saitoh K., Kato K. Cancer stem-like properties and gefitinib resistance are dependent on purine synthetic metabolism mediated by the mitochondrial enzyme MTHFD2. Oncogene. 2019;38:2464–2481. doi: 10.1038/s41388-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Yue L., Zhang W., Wang Y., Wen B., Zhong L., Xiang J., Li J., Zhang S., Wang H. Improvement in mouse iPSC induction by Rab32 reveals the importance of lipid metabolism during reprogramming. Sci. Rep. 2015;5:16539. doi: 10.1038/srep16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikman Y., Puissant A., Alexe G., Furman A., Chen L.M., Frumm S.M., Ross L., Fenouille N., Bassil C.F., Lewis C.A. Targeting MTHFD2 in acute myeloid leukemia. J. Exp. Med. 2016;213:1285–1306. doi: 10.1084/jem.20151574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione A., Rohwer N., Hoffmann S., Mlody B., Drews K., Bukowiecki R., Blumlein K., Wanker E.E., Ralser M., Cramer T. HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. 2014;32:364–376. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Ryu J.M., Lee H.J., Jung Y.H., Lee K.H., Kim D.I., Kim J.Y., Ko S.H., Choi G.E., Chai, Song E.J. Regulation of stem cell fate by ROS-mediated alteration of metabolism. Int. J. Stem Cells. 2015;8:24–35. doi: 10.15283/ijsc.2015.8.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaetzlein S., Kodandaramireddy N.R., Ju Z., Lechel A., Stepczynska A., Lilli D.R., Clark A.B., Rudolph C., Kuhnel F., Wei K. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130:863–877. doi: 10.1016/j.cell.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N., Daley G.Q. Metabolic switches linked to pluripotency and embryonic stem cell differentiation. Cell Metab. 2015;21:349–350. doi: 10.1016/j.cmet.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Silva J., Nichols J., Theunissen T.W., Guo G., van Oosten A.L., Barrandon O., Wray J., Yamanaka S., Chambers I., Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M., Liu J., Mehta G.U., Patel S.J., Roychoudhuri R., Crompton J.G., Klebanoff C.A., Ji Y., Li P., Yu Z. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 2016;23:63–76. doi: 10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Krueger F., Oxley D., Santos F., Clarke J., Mansfield W. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Tibbetts A.S., Appling D.R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Tichy E.D., Pillai R., Deng L., Liang L., Tischfield J., Schwemberger S.J., Babcock G.F., Stambrook P.J. Mouse embryonic stem cells, but not somatic cells, predominantly use homologous recombination to repair double-strand DNA breaks. Stem Cells Dev. 2010;19:1699–1711. doi: 10.1089/scd.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimatsu N., Mukherjee B., Catherine Hardebeck M., Ilcheva M., Vanessa Camacho C., Louise Harris J., Porteus M., Llorente B., Khanna K.K., Burma S. Phosphorylation of EXO1 by CDKs 1 and 2 regulates DNA end resection and repair pathway choice. Nat. Commun. 2014;5:3561. doi: 10.1038/ncomms4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbein U., Benvenisty N., Ben-David U. Quality control: genome maintenance in pluripotent stem cells. J. Cell Biol. 2014;204:153–163. doi: 10.1083/jcb.201310135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Cao S., Zhong L., Wang H., Pei Y., Wei Q., Wen B., Mu H., Zhang S., Yue L. Pluripotent stem cells secrete Activin A to improve their epiblast competency after injection into recipient embryos. Protein Cell. 2018;9:717–728. doi: 10.1007/s13238-017-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M., Hayashizaki Y., Murakawa Y. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev. Rep. 2017;13:7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Jang Y., Paik D., Lee E., Park J.J. Nmdmc overexpression extends Drosophila lifespan and reduces levels of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2015;465:845–850. doi: 10.1016/j.bbrc.2015.08.098. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ratanasirintrawoot S., Chandrasekaran S., Wu Z., Ficarro S.B., Yu C., Ross C.A., Cacchiarelli D., Xia Q., Seligson M. LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell. 2016;19:66–80. doi: 10.1016/j.stem.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang J., Tam W.L., Tong G.Q., Wu Q., Chan H.Y., Soh B.S., Lou Y., Yang J., Ma Y., Chai L. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cui P., Li Y., Feng G., Tong M., Guo L., Li T., Liu L., Li W., Zhou Q. Mitochondrially produced ATP affects stem cell pluripotency via Actl6a-mediated histone acetylation. FASEB J. 2018;32:1891–1902. doi: 10.1096/fj.201700626RR. [DOI] [PubMed] [Google Scholar]
- Zhao B., Zhang W.D., Duan Y.L., Lu Y.Q., Cun Y.X., Li C.H., Guo K., Nie W.H., Li L., Zhang R. Filia is an ESC-specific regulator of DNA damage response and safeguards genomic stability. Cell Stem Cell. 2015;16:684–698. doi: 10.1016/j.stem.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.Y., Li W., Lv Z., Liu L., Tong M., Hai T., Hao J., Guo C.L., Ma Q.W., Wang L. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- Zhong X., Cui P., Cai Y., Wang L., He X., Long P., Lu K., Yan R., Zhang Y., Pan X. Mitochondrial dynamics is critical for the Full pluripotency and embryonic developmental potential of pluripotent stem cells. Cell Metab. 2019;29:979–992.e4. doi: 10.1016/j.cmet.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Zhou G., Meng S., Li Y., Ghebre Y.T., Cooke J.P. Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 2016;15:919–925. doi: 10.1016/j.celrep.2016.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Choi M., Margineantu D., Margaretha L., Hesson J., Cavanaugh C., Blau C.A., Horwitz M.S., Hockenbery D., Ware C. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012;31:2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession numbers for the RNA-seq data reported in this paper is SRA: SRP149554.