Summary
Uveitis is a serious intra‐ocular inflammatory disease that can lead to visual impairment even blindness worldwide. Notch signaling can regulate the differentiation of naive CD4+ T cells, influencing the development of uveitis. DNA methylation is closely related to the autoimmune diseases. In this study, we measured the Notch1 DNA methylation level, determined the Notch1 and related DNA methylases mRNA expression and evaluated the ratio of T helper type 17 regulatory T cell (Th17/Treg) in peripheral blood mononuclear cells (PBMCs) from uveitis patients and normal control subjects; we also tested the levels of relevant inflammatory cytokines in serum from the participants. Results indicated that compared with those in normal control individuals, the expression of ten–eleven translocation 2 (TET2) and Notch1 mRNA is elevated in uveitis patients, whereas the methylation level in Notch1 DNA promotor region [−842 ~ −646 base pairs (bp)] is down‐regulated, and is unrelated to anatomical location. Moreover, the Th17/Treg ratio is up‐regulated in PBMCs from uveitis patients, accompanied by the elevated levels of proinflammatory cytokines [e.g. interleukin (IL)‐2, IL‐6, IL‐17 and interferon (IFN)‐γ] in serum from uveitis patients. These findings suggest that the over‐expression of TET2 DNA demethylase may lead to hypomethylation of Notch1, activate the Notch1 signaling, induce naive CD4+ T cells to differentiate theTh17 subset and thus disturb the balance of the Th17/Treg ratio in uveitis patients. Overall, hypomethylation of Notch1 DNA is closely associated with the occurrence of uveitis. Our study preliminarily reveals the underlying mechanism for the occurrence of uveitis related to the hypomethylation of Notch1 DNA, providing a novel therapeutic strategy against uveitis in clinical practice.
Keywords: hypomethylation, Notch1, Th17, Treg, uveitis
The hypomethylation of Notch1 produces a high level of Notch1, activates the Notch signaling pathway, and thus stimulates the naïve CD4+ T cells to Th17 differentiation. Further, the elevated Th17 frequency disrupts the Th17/Treg balance and elevates the related inflammatory cytokine levels, finally leading to the occurrence of uveitis.
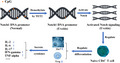
Introduction
Uveitis is a group of potentially blinding intraocular inflammatory diseases; it is mainly a type of autoimmune disease mediated by T lymphocytes and involves the iris, ciliary body and choroid [1, 2, 3]. Endogenous uveitis usually occurs in conjunction with systemic diseases, including Behçet’s disease (BD), sarcoidosis, Vogt–Koyanagi–Harada (VKH) disease and human leukocyte antigen (HLA)‐B27–associated uveitis. It is estimated that uveitis cases are involved in 5–10% of visual impairment worldwide [4].
Although the exact pathogenesis of the occurrence of uveitis is poorly understood, accumulating evidence suggests that the activation of Notch signaling is presumed to play a vital role in uveitis pathogenesis. In mammals, Notch signaling consists of four receptors (Notch1–4) and five ligands of the Jagged family (Jagged1–2) and Delta‐like ligand family (DLL1, DLL3 and DLL4) [5]. It can regulate the differentiation of naive CD4+ T cells towards a given Th cell subset, and thus plays a role in the pathogenesis of autoimmune diseases [6]. It is confirmed that Notch signaling plays a vital role in systemic sclerosis (SSc) and rheumatoid arthritis (RA) [7, 8]. Meanwhile, Notch signaling is also involved in the pathogenesis of uveitis. Qi et al. demonstrates that Notch signaling is enhanced in active BD, which may contribute to the pathogenesis of BD [9]. Moreover, experimental data also show that the activation of Notch1 signaling pathway can aggravate the clinical features of uveitis by suppressing regulatory T (Treg) cell function and boosting the T helper 17 (Th17) cell effect in experimental autoimmune uveitis (EAU) [10, 11]. These findings indicate that the Notch signaling pathway plays a fundamental role in the pathogenesis of uveitis.
DNA methylation is a key mechanism for epigenetic regulation of gene expression related to regulating many cellular processes. It can influence embryogenesis, cellular proliferation and differentiation [12, 13] which is mediated and maintained by DNA methyltransferases (DNMTs), including DNMT1, DNMT3a, DNMT3b, DNMT3L and ten–eleven translocation enzymes (TETs), including TET1, TET2 and TET3. DNA methylation mainly occurs at cytosine residues in cytosine–phosphate–guanosine (CpG) dinucleotides and involves the addition of a methyl group to the 5′ position of the cytosine pyrimidine ring mediated by DNMTs [14].
Recently, studies have shown that aberrant DNA methylation is strongly connected to autoimmune diseases [15, 16]. In an experimental autoimmune encephalomyelitis (EAE) model, transferred TET2‐deficient CD4+ T cells were found to be more pathogenic compared to wild‐type (WT) cells [17]. Several clinical studies have also indicated that abnormal DNA hypomethylation in T cells is an important epigenetic feature in systemic lupus erythematosus (SLE) [18]. Recent studies suggest that the abnormal DNA methylation of a certain gene is not only associated with autoreactivity of immune cells in vitro, but also can result in autoimmunity in vivo [15]. Nevertheless, whether or not DNA methylation of Notch signaling is involved in the occurrence of uveitis has not yet been addressed. In the present study, the methylation level and mRNA expression of Notch1 and the ratio of Th17/Treg in PBMCs were explored in uveitis patients and normal control subjects. In addition, we detected the changes of related inflammatory cytokines in serum from both uveitis patients and normal control subjects. Our investigations will facilitate understanding of the occurrence of uveitis associated with the level of Notch1 DNA methylation.
Materials and methods
Ethics
The present study was approved by the Ethics Committee of Eye Institute of Shandong University of Traditional Chinese Medicine (2018‐XK‐007). All participants signed an informed consent and all procedures adhered to the tenets of the Declaration of Helsinki. Data collection was carried out in a manner compliant with the related legal requirements.
Participants
The standardized diagnostic investigation protocol and classification of anatomy were diagnosed based on the established clinical criteria, the Standardization of Uveitis Nomenclature (SUN) working group grading scheme [19], with supporting laboratory evidence if needed [20]. In the present study, 42 uveitis patients (27 male and 15 female, average age = 49·64 ± 15·28) and 46 normal controls (24 male and 22 female, average age = 42·00 ± 14·30) were enrolled, and all uveitis patients enrolled into the present study showed typical ocular features. Moreover, patients with active and typical ocular inflammation who did not receive treatment or who discontinued medication for more than a week were confirmed before drawing blood. All enrolled participants were diagnosed in our uveitis center from December 2018 to October, 2019. Detailed information of the participants is listed in Table 1.
Table 1.
Demographic and disease characteristics of study subjects
| Normal controls (n = 46) | Uveitis patients (n = 42) | |
|---|---|---|
| Mean age (standard deviation) | 42·00 (14·30) | 49·64 (15·27) |
| Gender (F/M) | 22/24 (0·91) | 15/27 (0·56) |
| Anatomical location of uveitis | n.a. | Anterior uveitis (n = 12) |
| Intermediate uveitis (n = 5) | ||
| Posterior or pan uveitis (n = 25) | ||
| Etiological diagnoses | n.a. | Behcet’s disease (n = 2) |
| Idiopathic uveitis (n = 24) | ||
| VKH (n = 5) | ||
| Others (n = 11) |
VKH = Vogt–Koyanagi–Harada; F/M = female/male; n.a. = not applicable.
Selection of Notch1 DNA methylation fragments
After bioinformatics analysis, we noted that there were two abundant CpG island fragments in the Notch1 DNA promotor region. CpG island 1 fragment was located between −494 and −255 bp from the transcription start site (TSS) and CpG island 2 fragment was located between −842 and −646 bp (Fig. 1). In the present study, we selected these two fragments to be used to detect the Notch1 DNA methylation level.
Fig. 1.

Diagram of Notch1 DNA promoter methylation. The grey parts were the two cytosine–phosphate–guanine (CpG) island regions of the Notch1 DNA promotor. TSS = transcription start site.
Bisulfite sequencing
First, genomic DNA from the uveitis patients (n = 27) and normal control subjects (n = 23) was extracted using the Ezup column animal genomic DNA purification kit following the manufacturer’s protocol [Sangon Biotech (Shanghai) Co., Ltd, Shanghai, China]. Bisulfite conversion was performed by adding 5 mol/l of sodium bisulfite to 800 ng DNA samples. The two selected fragments were employed for amplification by polymerase chain reaction (PCR) and were linked into PMD 19‐T vectors (TaKaRa, Dalian, China) to transform the DH5a strain of Escherichia coli after the PCR productions were gel purified. Ten positive clones were selected by blue/white screening, and plasmid DNA was extracted using the SanPrep column plasmid mini‐preps kit (Sangon Biotech). Subsequently, the sequence was performed to screen the methylation sites. The sequence including CpG islands was marked as methylated if cytosine remained unconverted, and the methylated cytosine level was obtained from the value of the amount of methylated cytosine divided by all numbers of cytosine in the amplified region. In the present study, the first fragment primers were listed as follows: forward: 5′‐GCCCTCG GGAGGGAGTGAAGC‐3′, reverse: 5′‐GGCTCAA ACTTTTGGCC TCTGAA‐3′ and the second fragment primers were listed as follows: forward: 5′‐ GGCATCGTGGTGGAGAAATAAATCAG‐3′, reverse: 5′‐AGGGAGTG AAGCCGCTGGGCTA‐3′.
Methylation‐specific PCR
The collected DNA (1 μg) was repurified and treated with the EZ DNA methylation‐gold kit (Zymo Research, Beijing, China), and 50 ng DNA as a template was used for PCR. After electrophoresis on 1% agarose gel and staining with ethidium bromide (EB), PCR products were semiquantitatively analyzed with SeqMan software (DNAStar, Inc., Madison, WI, USA).
Quantitative PCR (qPCR)
The Superpure blood total RNA kit (Sparkjade Science Co., Ltd., Qingdao City, China) was used to extract the total RNA from PBMCs following the manufacturer’s instructions. Further, complementary DNA (cDNA) was synthesized by the HiScript Ⅱ Q RT SuperMix for qPCR kit (Vazyme, Nanjing, China) and SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used to perform PCR analysis for target genes with the LightCycler® 480 II instrument (Roche Diagnostics, Mannheim, Germany). For detection of the levels of Notch1, TET1, TET2, TET3, DNMPT1 and DNMPT3a genes, the primer sequences were as listed in Table 2. The qPCR conditions were set as follows: one cycle at 95°C for 30 s, followed by a 45 cycle at 95°C for 10 s and 62°C for 30 s. After qPCR detection, the level of each target gene was calculated using the 2−ΔΔCt method.
Table 2.
Primer sequences for relevant target genes
| Genes | Primer sequences |
|---|---|
| GAPDH | Forward: 5′‐CGGGGCTCTCCAGAACATCATCC‐3′ |
| Reverse: 5′‐CCAGCCCCAGCGTCAAAGGTG‐3′ | |
| Notch1 | Forward: 5′‐GAGGGCGCCACGTGTGAGAATGAC‐3′ |
| Reverse: 5′‐GGCGGGGCACAGGCAACGGTAGA‐3′ | |
| TET1 | Forward: 5′‐CACCAGCCCTCCTTCCTCACCTC‐3′, |
| Reverse: 5′‐CGCCGGGCACACTCAATCAA‐3′ | |
| TET2 | Forward: 5′‐TCGGCGAAAAGTCAGGATGTTAG‐3′ |
| Reverse: 5′‐TGGCTTGCGTTTTCAGTTTGTT‐3′ | |
| TET3 | Forward: 5′‐ACGCCACCACCCCACTCCTCAC‐3′, |
| Reverse: 5′‐CTTGCCCATCTTGCCAGCGTCTCT‐3′ | |
| DNMT1 | Forward: 5′‐CCCTCATCGGCTTCAGCACCTCAT‐3′, |
| Reverse: 5′‐CCCCGGCCTCGTCATAACTCTCC‐3′ | |
| DNMT3a | Forward: 5′‐ATCTGCTGTGGGGGCCGTGAGGTG‐3′, |
| Reverse: 5′‐CCGCCGCAGCAGCCCGTAGGTA‐3′ |
GAPDH = glyceraldehyde 3‐phosphate dehydrogenase; Notch1 = Notch homolog 1, translocation‐associated; TET1 = ten–eleven translocation 2; DNMT1 = DNA methyltransferase.
Flow cytometry
In the present study, 2 ml fasting blood was collected from both uveitis patients and normal control subjects with ethylene diamine tetraacetic acid (EDTA) anti‐coagulant tubes. Subsequently, peripheral blood lymphocytes were isolated using human lymphocyte separation medium (Beijing Solarbio Science and Technology Co., Ltd, Beijing, China). To analyze Th17 and Treg subsets, the isolated peripheral blood lymphocytes were divided equally into two tubes. One of them was used to detect the frequency of Th17 cells, which were stained with fluorescein isothiocyanate (FITC)‐CD4 and phycoerythrin (PE)‐IL‐17; the other was used to detect the frequency of Treg cells, which were stained with FITC‐CD4, allophycocyanin (APC)‐CD25 and PE‐forkhead box P3 (FoxP3) (all purchased from eBioscience, San Jose, CA, USA). For Th17 subset analysis, PBMCs were stimulated with cell activation cocktail (Biolegend, Inc., San Diego, CA, USA) in 12‐well plates (Wuxi NEST Biotechnology, Wuxi, China) for 6 h, and were then stained with anti‐CD4 (eBioscience) solution at 4°C for 30 min for surface staining, followed by treatment with the Fix and Perm reagent (BD Bioscience) and PE‐IL‐17A (eBioscience) for intracellular staining; for Treg subset analysis, PBMCs were stained with anti‐CD4 (eBioscience) and anti‐CD25 (eBioscience) solutions in 12‐well plates (Wuxi NEST Biotechnology) at 4°C for 30 min, followed by treatment with Fix and Perm reagent, and PE‐FoxP3 solutions (eBioscience) for intracellular staining. After resuspension with phosphate‐buffered saline (PBS, pH 7.2), the cells were analyzed by flow cytometry (FACSVerse™; BD Bioscience).
Cytokine assays
Moreover, we further investigated the levels of IL‐17, IL‐6, IL‐4, IL‐2, IL‐10, IFN‐γ and TNF‐α in serum from uveitis patients and normal control subjects. After collection of serum from uveitis patients and normal control subjects, the sera were treated with a cytometric bead array human inflammation kit (CBA; BD Bioscience), according to the manufacturer’s instructions, and were analyzed by flow cytometry (Accuri C6; BD Bioscience).
Statistical analysis
The normally distributed continuous variables were assessed by the Shapiro–Wilk test and the data were expressed as mean ± standard error of the mean (s.e.m.). Student’s t‐test was performed to analyze the between‐group differences. The non‐normally distributed data were presented as median and range, and were analyzed by the Mann–Whitney U‐test. P‐values less than 0·05 were regarded as significant differences. The spss version 21.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 software (GraphPad Software, Inc., San Diego, CA, USA) were used to perform the statistical analysis.
Results
Suppressed methylation of Notch1 in uveitis patients
To measure the methylation levels of Notch1 DNA, we selected the abundant CG regions of Notch1 DNA promoter. The results indicated that there was a lower methylation level in the Notch1 DNA promotor region located between −842 and −646 bp (CpG island 2) in uveitis patients compared with that of normal control subjects (Fig. 2b, P < 0·05), whereas the methylation level located between −494 and −255 bp (CpG island 1) had no apparent change (Fig. 2a, P > 0·05), indicating that the hypomethylation level of CpG island 2 may be closely related to the occurrence of uveitis. Importantly, we noted that the hypomethylation level of Notch1 DNA was unrelated to the anatomical location of uveitis, and the location between −842 and −646 bp (CpG island 2) of Notch1 DNA was prone to hypomethylation in both anterior uveitis (AU) and posterior uveitis (PU) patients (Fig. 2c–f, P < 0·05).
Fig. 2.

Analysis of Notch1 DNA promotor methylation level. The methylation levels of cytosine–phosphate–guanine (CpG) island 1 located between −494 and −255 and CpG island 2 located between −842 and −646 base pairs (bp) from Notch1 DNA promotor region in uveitis patients and normal control subjects were detected using the bisulfite sequencing method. The result indicated that there was a lower methylation level in Notch1 DNA promotor region that was located between −842 and−646 bp (CpG island 2) in uveitis patients compared with those of normal control subjects (b). However, the methylation level in Notch1 DNA promotor region located between −494 and −255 bp (CpG island 1) showed no apparent change (a). Moreover, the hypomethylation level of Notch1 DNA was unrelated to anatomical location of uveitis (c–f) [normal controls = 7, uveitis patients = 23, AU anterior uveitis (AU) = 8, posterior uveitis (PU) patients = 12; *P < 0·05].
Elevated Notch1 and TET2 mRNA levels in uveitis patients
As shown in Fig. 3, we found that the Notch1 mRNA level in PBMCs from uveitis patients was apparently higher than that of normal control subjects (P < 0·01), indicating that the elevated Notch1 mRNA level in uveitis patients may be correlated with the occurrence of uveitis. For the demethylating enzyme TET1‐3 expression, we observed that only the TET 2 level was increased in uveitis patients (versus normal control subjects, * P < 0·05), demonstrating that the elevated TET2 is associated with the high level of Notch1; in contrast, both TET1 and TET3 expression showed no significant change in uveitis patients (versus normal control subjects, P > 0·05). Regarding DNMT1 and DNMT3A mRNA levels, we found that neither of them showed an apparent change in uveitis patients in comparison to normal control subjects (P > 0·05).
Fig. 3.

Measurement of Notch1, ten–eleven translocation enzymes (TET)1–3, DNA methyltransferase (DNMPT)1 and DNMT3A mRNA levels in peripheral blood mononuclear cells (PBMCs). (a) The Notch1 mRNA expression in PBMCs from uveitis patients was higher than that of normal control subjects (normal controls = 7, uveitis patients = 7, ** P < 0·01); (b) the level of demethylating enzyme TET2 was significantly elevated in uveitis patients versus normal control subjects (normal controls = 8, uveitis patients = 8, * P < 0·05).
Elevated Th17 and down‐regulated Treg cell frequencies in uveitis patients
To investigate the relationship between uveitis patients and Th17, Treg frequencies, we determined both Th17 and Treg frequencies in PBMCs from uveitis patients and normal control subjects. The results showed that, compared with those of normal controls, Th17 cell frequencies were increased whereas Treg cell frequencies were suppressed in PBMCs from uveitis subjects (Fig. 4e,f). In addition, it was noted that the ratio of Th17/Treg was elevated in uveitis patients (Fig. 4i), indicating that the Th17/Treg balance plays a critical role in uveitis; the disrupted Th17/Treg balance is closely correlated with the occurrence of uveitis.
Fig. 4.

Alterations in T helper type 17 (Th17) and regulatory T cell (Treg) frequencies in peripheral blood mononuclear cells (PBMCs) from uveitis patients. Up‐regulated Th17 frequency and down‐regulated Treg frequencies were observed in PBMCs from uveitis patients. (a) T lymphocytes were gated in P1 of (a). (b) T lymphocytes were gated in P1 of (b); (c,d) from P1, CD4+forkhead box protein 3 (Foxp3+) T cells were gated in P2 of (c,d); (f) from P2 of (c,d,), and CD4+ CD25+FoxP3+Treg cells are shown in the right‐upper quadrant of (f). (e) Th17 cell frequencies in PBMCs from normal controls and uveitis patients were detected by flow cytometry. The changes of Treg cells in the two groups were determined by flow cytometry (** P < 0·01). (g) Scatter‐plot analysis of the Th17 frequencies. We found that Th17 frequencies in PBMCs from uveitis patients were significantly higher than those from normal controls (** P < 0.01). (h) Scatter‐plot analysis of the Treg frequencies. We found that Treg frequencies in PBMCs from uveitis patients were significantly lower than those from normal controls (** P < 0·01). (i) Up‐regulated Th17/Treg ratio in uveitis patients (versus normal control subjects; ** P < 0·01); n = 11 for each group).
Elevated cytokine levels in uveitis patients
Given that Th17 and Treg cells are important effector T cells in the pathological process of uveitis [11, 21], we measured the contents of Th17‐ and Treg‐related inflammatory cytokines. The results indicated that Th17‐promoting cytokines such as IL‐17 in uveitis patients were significantly higher than those in normal control subjects (Fig. 5) Moreover, we noted that the Treg‐related cytokines such as IL‐10, a critical cytokine that can suppress Th17 activity [22], was also elevated in uveitis patients (* P < 0·05, ** P < 0·01).
Fig. 5.

Relevant cytokine levels in serum from uveitis patients and normal control subjects. After isolation of the serum from uveitis patients and normal control subjects, the sera were treated with cytometric bead array human inflammation kit and the levels of interleukin (IL)‐17, interferon (IFN)‐γ, tumor necrosis factor (TNF)‐α, IL‐10, IL‐6, IL‐4, and IL‐2 were determined by flow cytometry. Result indicated that the expression of IL‐17, IFN‐γ, TNF‐α, IL‐10, IL‐6, IL‐4 and IL‐2 was significantly elevated in uveitis patients (versus normal control subjects; * P < 0·05, **P<0·01; n = 10 for each group).
Discussion
Human uveitis is characterized as intraocular inflammation and is a major outcome in sight loss in the working population. Clinical data and experimental models indicate that uveitis may be the result of inappropriate activation of the immune system, which is generally related to either systemic autoimmune or autoinflammatory diseases [23].
Human data and experimental models have demonstrated that uveitis is associated with parallel alterations in the inflammatory milieu, especially in the intraocular microenvironment, and is also involved in autoreactive T cells and the regulation of multiple cell signaling pathways [24, 25, 26]. In our previous experimental studies, we found that the activation of Notch signaling can lead to the disturbed Th17/Treg balance and aggravate the severity of EAU. However, the inhibition of Notch signaling can reduce Th17 levels, restore the Th17/Treg balance and ameliorate the inflammatory response. Thus, the Notch signaling pathway plays a critical role in EAU [11, 27].
Notch signaling is an evolutionary ancient cell‐to‐cell interaction mechanism which plays an important role in multi‐cellular development. The Notch receptor signal exchange between adjacent cells can determine cell fate, especially in cell development and tissue homeostasis [28]. Freddy et al. found that Notch1 is involved in T cell development and is essential in lymphopoiesis and T cell neoplasia [29]. Increasing clinical data show that the Notch signaling pathway is fundamental in the pathogenesis of several clinical autoimmune diseases [8, 30]. Qi et al. found that activating Notch signaling can enhance the pathogenic Th17 response, whereas blocking Notch signaling can preferentially attenuate the Th17 response in active BD patients [9]. Similarly, Notch1 can also aggravate psoriasis vulgaris, an autoimmune cutaneous disease that is involved in the activation of CD4+ T cells by regulating the Th17/Treg imbalance [31]. However, Rauen et al. found that promoter of Notch1 bonded to cAMP‐responsive element modulator α (CREMα) remained at a high methylation level, accompanied by a decreased Notch1 level in patients with active SLE [32]. Apparently, this result is paradoxical to our study, which may be attributed to the different methylation levels of Notch1 DNA varying from these diseases. Overall, Notch signaling plays a key role in inflammatory diseases, and investigation into epigenetics that are related to the Notch signaling pathway may pave a way towards the understanding of uveitis.
Our study indicated that Notch1 was demethylated in uveitis patients. Hence, our attention focused on the expression of the DNMT and TET family enzymes involved in DNA methylation/demethylation. The result showed that TET2 expression was significantly up‐regulated in uveitis patients, whereas the DNMPT levels showed no difference between the two groups. We speculate that up‐regulation of TET2 could cause the change of DNA methylation patterns with dramatic consequences, which may determine the level of Notch1 methylation and even influence uveitis risk and progression. Currently, accumulating evidence demonstrates that abnormal DNA demethylation modulated by TET enzymes promotes an imbalanced immune response. Li et al. found elevated TET1 expression in dendritic cells (DCs) from allergic rhinitis (AR) patients, and further investigation has indicated that TET‐mediated DNA demethylation is involved in the pathogenesis of AR [33]. In multiple sclerosis (MS) patients, the expression of TET2 and DNMT1 is down‐regulated at both mRNA and protein levels in PBMCs. Calabrese et al. revealed that those two genes coding for enzymes are important players in determining DNA methylation patterns, and this down‐regulation could be associated with significant differences in the methylation of specific CpGs present in their promoters [16]. In an in‐vitro experiment, TET2 can decrease the DNA methylation level of signature cytokines to regulate their expression, which boosts the development of Th1 and Th17 cells. In addition, TET2 can also change the chromatin remodeling to influence the target gene recruitment [17]. Thus, activation of Notch signaling may be involved in the decreased methylation level of the Notch‐related gene via regulation by TET2. Together, this evidence suggests that epigenetic regulation may play a vital role in pathogenesis and immunological mechanisms of autoimmune system disease.
Dysregulation of the Th17/Treg balance can cause several autoimmune diseases, such as RA [34], MS [35] and inflammatory bowel disease [36]. As the Notch signaling pathway can regulate naive CD4+ T cell differentiation and thus influence the Th17/Treg balance in uveitis, we further investigated the changes of Th17 and Treg levels, Th17/Treg ratio and Th17‐ and Treg‐related cytokine levels in uveitis patients [28]. We noted that, compared to normal controls, there was a Th17/Treg imbalance drifting in the direction of Th17 cells in uveitis patients. To date, accumulated evidence suggests that the Th17/Treg balance play an essential role in the pathogenic mechanisms driving autoimmune diseases. RA has been extensively studied from the perspective of the Th17/Treg balance. Samson et al. showed that Th17 levels were increased and Treg levels were decreased in PBMCs in patients with active RA [37]. Consistent with the outcome of Samson et al.’s study, we demonstrated that the level of Th17 cell lineage and the Th17/Treg ratio were elevated and the disturbed Th17/Treg immune balance is closely associated with the pathogenesis of uveitis. In addition, we also noted that the Th17/Treg ratios of PU and AU patients were higher than those of normal controls. However, we found that there was no difference in Th17/Treg ratio between PU and AU patients (Supporting information, Fig. S1). This result indicated that the occurrence of uveitis will disrupt the Th17/Treg balance.
Given that the Th17/Treg balance plays an important role in many autoimmune diseases, they can convert to the other in certain inflammatory conditions through their shared and different cytokines. Th17‐secreted cytokines such as IL‐17 and IL‐6 are regulated by transcription factor RAR‐related orphan receptor gamma (RORγt) [38]. IL‐17 is the hallmark signature proinflammatory cytokine of Th17 cells. In our study, we found there was a significantly elevated IL‐17 level in uveitis patients compared to those in normal controls; this result was also in agreement with other investigations [39, 40, 41]. IL‐6 is a necessary factor for the early stage of Th17 cell differentiation. During the priming stage of Th17 cell differentiation, IL‐6 is able to stimulate a naive T cell to differentiate into an effector cell with cognate antigen; the expression level of ROR γt, IL‐17 and IL‐23R is then promoted, and the direction towards a Th17 cell is decided [42]. Therefore, during the inflammatory phase, the IL‐6 level was higher in uveitis patients compared to normal controls.
IL‐2 is not only produced primarily by CD4+ T cells following antigen stimulation, but is also produced to a lesser extent by CD8+ T cells [43]. IL‐2 is a critical role for Treg cell development and function. Vang et al. found that in IL‐2‐ or IL‐2Rα‐deficient mice, the number and proportion of Treg cells decrease drastically; in contrast, loss of IL‐15 or IL‐7 alone was unable to impair the Treg cell generation [44]. Thus, IL‐2 plays an essential role in controlling immune homeostasis. However, we found that the expression of IL‐2 is significantly elevated in uveitis patients. We speculate that during inflammation the related immune cells will secrete more IL‐2 to antagonize proinflammatory factors to help it recover due to the self‐protection mechanism. IL‐10 plays an important role in repressing the immune response and controlling inflammation, which is synthesized by B cells, Th2 and Treg cells, not a single subset [45, 46]. Hui et al. showed that the differentiation of CD4+ T cells towards Treg cells and IL‐10 is elevated after collagen‐induced arthritis (CIA) mouse treatment [47]. Therefore, IL‐10, similar to IL‐2, is an anti‐inflammatory cytokine and is elevated in inflammatory stage.
IFN‐γ is mainly secreted by the Th1 cell subset [48]. TNF‐α is produced by several proinflammatory cells such as Th17/Th1 subsets [48, 49]. In their study of psoriasis, Furiati et al. found that treatment with anti‐TNF can down‐regulate the production of IL‐17A, IFN‐γ, TNF‐α and IL‐10 [50]. Consistent with the former result, we found that the expression levels of IFN‐γ and TNF‐α were elevated in uveitis patients. Overall, the dynamic alterations of cytokines in serum are closely related to the inflammatory change; therefore, detecting the level of cytokines will facilitate understanding of the progress of the disease and contribute to diagnosis.
Nevertheless, limitations should be mentioned in the present study. First, some patients (five of 23, 21·74%) received immunosuppressants such as cyclosporin A or dexamethasone prior to the determination of Notch1 DNA methylation levels. Although we noted that there was no statistical difference in Notch1 DNA methylation levels in both CpG island 1 and CpG island 2 regions between normal control individuals and the uveitis patients who received immunosuppressants (Supporting information, Fig. S2), indicating that immunosuppressant administration may enhance the methylation level of Notch1 DNA, much more investigation should be carried out to evaluate the effect of immunosuppressants on the Notch1 DNA methylation level. Secondly, only 42 uveitis patients were enrolled into the present study; more uveitis patients should be recruited for detailed investigations.
As illustrated in Fig. 6, we presume that hypomethylation of Notch1 will generate a high level of Notch1, activate the Notch signaling pathway and thus stimulate the naive CD4+ T cells to Th17 differentiation. Further, the elevated Th17 frequency disrupts the Th17/Treg balance and elevates the related inflammatory cytokine levels, finally leading to the occurrence of uveitis.
Fig. 6.

Illustration of the relationship between hypomethylation of Notch1 DNA and the occurrence of uveitis.
Conclusion
In summary, the present study shows that the hypomethylation of Notch1 contributes to elevated Notch signaling, increases the Th17 frequency and enhances the related inflammatory cytokine expression, contributing to the disrupted Th17/Treg balance in uveitis patients. The occurrence of uveitis may be related to the abnormal DNA hypomethylation of Notch1 and the consequent activation of Notch signaling and elevated Th17 frequency. Based on our findings, it is suggested that passive, rather than active, demethylation plays a key role in the pathogenic hypomethylation in uveitis. In light of the reversibility of epigenetic modifications, specific epigenetic drugs targeting the aberrant DNA hypomethylation of Notch1 may provide a promising protect against uveitis.
Disclosures
The authors declare that they have no conflict of interests.
Author contributions
D. G. conceived and designed the study; H. W., X. Y. and B. L. performed the experiments; H. W., X. Y., H. T. and Y. H. analyzed the data; H. B., Y. G., Q. W., Q. T. and D. G. contributed reagents/materials/analysis tools; H. W. and D. G. wrote the paper.
Supporting information
Fig. S1. Comparison of Th17/Treg ratio between PU and AU patients. SFig. 1A‐C demonstrated the Th17 cell subset, Treg cell subset, and Th17/Treg ratio in normal controls and PU patients, respectively. SFig. 1D‐F showed the Th17 cell subset, Treg cell subset, and Th17/Treg ratio in normal controls and AU patients, respectively. We found that the Th17/Treg ratio of PU patients was higher than that of normal controls (P < 0·01). Similarly, Th17/Treg ratio of AU patients was higher than that of normal controls (P < 0·01). Moreover, we also noted that there was no difference in Th17/Treg ratio between PU and AU patients. (normal controls = 11, PU patients = 6, AU patients = 4, **P < 0·01).
Fig. S2. Comparison of Notch1 methylation level between normal controls and the uveitis patients who received immunosuppressants. We found that there was no statistical difference in Notch1 DNA methylation level in both CpG island 1 and CpG island 2 regions between the two groups (normal controls = 6, uveitis patients received immunosuppressants = 5, P > 0·05).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81873163), the Natural Science Foundation of Shandong Province (ZR2017LH042), the Development Project of Science and Technology of Traditional Chinese Medicine of Shandong Province (2015‐145), and the Development Project of Medicine and Health Science Technology of Shandong Province (2013WS0251).
References
- 1. Touhami S, Diwo E, Sève P et al Expert opinion on the use of biological therapy in non‐infectious uveitis. Expert Opin Biol Ther 2019; 19:477–90. [DOI] [PubMed] [Google Scholar]
- 2. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest 2010; 120:3073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yehuda SB, Luger D, Ochaion A et al Inhibition of experimental auto‐immune uveitis by the A3 adenosine receptor agonist CF101. Int J Mol Med 2011; 28:727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsirouki T, Dastiridou A, Symeonidis C et al A focus on the epidemiology of uveitis. Ocul Immunol Inflamm 2018; 26:2–16. [DOI] [PubMed] [Google Scholar]
- 5. Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity 2010; 32:14–27. [DOI] [PubMed] [Google Scholar]
- 6. Auderset F, Coutaz M, Cottier FT. The role of Notch in the differentiation of CD4+ T helper cells. Curr Top Microbiol Immunol 2012; 360:115–34. [DOI] [PubMed] [Google Scholar]
- 7. Dees C, Zerr P, Tomcik M et al Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum 2011; 63:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiao Z, Wang W, Guo M et al Expression analysis of Notch‐related molecules in peripheral blood T helper cells of patients with rheumatoid arthritis. Scand J Rheumatol 2010; 39:26–32. [DOI] [PubMed] [Google Scholar]
- 9. Qi J, Yang Y, Hou SP et al Increased Notch pathway activation in Behcet’s disease. Rheumatology (Oxf) 2014; 53:810–20. [DOI] [PubMed] [Google Scholar]
- 10. Rong H, Shen HJ, Xu YL, Yang H. Notch signalling suppresses regulatory T‐cell function in murine experimental autoimmune uveitis. Immunology 2016; 149:447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin XW, Liu B, Wei HX et al Activation of the Notch signaling pathway disturbs the CD4+/CD8+, Th17/Treg balance in rats with experimental autoimmune uveitis. Inflamm Res 2019; 68:761–74. [DOI] [PubMed] [Google Scholar]
- 12. Guo HS, Zhu P, Yan LY et al The DNA methylation landscape of human early embryos. Nature 2014; 511:606–10. [DOI] [PubMed] [Google Scholar]
- 13. Ehrlich M, Lacey M. DNA methylation and differentiation: silencing, upregulation and modulation of gene expression. Epigenomics 2013; 5:553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hedrich CM, Mäbert K, Raue T, Tsoko GC. DNA methylation in systemic lupus erythematosus. Epigenomics 2017; 9:505–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun B, Hu L, Luo ZY, Chen XP, Zhou HH, Zhang W. DNA methylation perspectives in the pathogenesis of autoimmune diseases. Clin Immunol 2016; 164:21–7. [DOI] [PubMed] [Google Scholar]
- 16. Calabrese R, Valentini E, Ciccarone F et al TET2 gene expression and 5‐hydroxymethylcytosine level in multiple sclerosis peripheral blood cells. Biochim Biophys Acta 2014; 1842:1130–6. [DOI] [PubMed] [Google Scholar]
- 17. Ichiyama K, Chen TT, Wang XH et al The mehylcytosine dioxygenase TET2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 2015; 42:613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang YQ, Zhao M, Sawalha AH, Richardson B, Lu QJ. Impaired DNA methylation and its mechanisms in CD4+ T cells of systemic lupus erythematosus. J Autoimmun 2013; 41:92–9. [DOI] [PubMed] [Google Scholar]
- 19. Trusko B, Thorne J, Jabs D et al The Standardization of Uveitis Nomenclature (SUN) project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med 2013; 52:S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El‐Asrar AMA, Berghmans N, Al‐Obeidan SA et al Differential CXC and CX3C chemokine expression profiles in aqueous humor of patients with specific endogenous uveitic entities. Invest Ophthalmol Vis Sci 2018; 59:2222–8. [DOI] [PubMed] [Google Scholar]
- 21. Zhuang ZC, Wang YQ, Zhu GJ et al Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci Rep 2017; 7:40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao W, Lin JX, Leonard WJ. Interleukin‐2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol 2014; 8:1891–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gocho K, Kondo I, Yamaki K. Identification of autoreactive T‐cells in Vogt‐Koyanagi‐Harada disease. Invest Ophthalmol Vis Sci 2001; 42:2004–9. [PubMed] [Google Scholar]
- 25. Tripathi P, Saxena S, Yadav VS, Naik S, Singh VK. Human S‐antigen determinant recognition in uveitis. Exp Mol Pathol 2004; 76:122–8. [DOI] [PubMed] [Google Scholar]
- 26. Guo DD, Hu B, Tang HY et al Proteomic profiling analysis reveals a link between experimental autoimmune uveitis and complement activation in rats. Scand J Immunol 2017; 85:331–42. [DOI] [PubMed] [Google Scholar]
- 27. Yin XW, Wei HX, Wu SS et al DAPT reverses the Th17/Treg imbalance in experimental autoimmune uveitis in vitro via inhibiting Notch signaling pathway. Int Immunopharmacol 2020; 79:106107. [DOI] [PubMed] [Google Scholar]
- 28. Artavanis‐Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999; 284:770–6. [DOI] [PubMed] [Google Scholar]
- 29. Radtke F, Wilson A, Mancini SJC, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol 2004; 5:247–53. [DOI] [PubMed] [Google Scholar]
- 30. Piggott K, Deng JS, Warrington K et al Blocking the NOTCH pathway inhibits vascular inflammation in large vessel vasculitis. Circulation 2011; 123:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma L, Xue H, Gao T, Gao M, Zhang Y. Notch1 signaling regulates the Th17/Treg immune imbalance in patients with psoriasis vulgaris. Mediators Inflamm 2018; 2018:3069521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rauen T, Grammatikos AP, Hedrich CM et al cAMP‐responsive eElement modulator α (CREMα) contributes to decreased notch‐1 expression in T cells from patients with active systemic lupus erythematosus (SLE). J Biol Chem 2012; 287:42525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Lu T, Sun W et al Ten‐eleven translocation (TET) enzymes modulate the activation of dendritic cells in allergic rhinitis. Front Immunol 2019; 10:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chabaud M, Durand JM, Buchs N et al Human interleukin‐17: A T cell‐derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 1999; 42:963–70. [DOI] [PubMed] [Google Scholar]
- 35. Lock C, Hermans G, Pedotti R et al Gene‐microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002; 8:500–8. [DOI] [PubMed] [Google Scholar]
- 36. Fujino S, Andoh A, Bamba S et al Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003; 52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samson M, Audia S, Janikashvili N et al Brief report: inhibition of interleukin‐6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum 2012; 64:2499–503. [DOI] [PubMed] [Google Scholar]
- 38. Miller SA, Weinmann AS. Common themes emerge in the transcriptional control of T helper and developmental cell fate decisions regulated by the T‐box. GATA and ROR families. Immunology 2009; 126:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amadi‐Obi A, Yu CR, Liu XB et al TH17 cells contribute to uveitis and scleritis and are expanded by IL‐2 and inhibited by IL‐27/STAT1. Nat Med 2007; 13:711–8. [DOI] [PubMed] [Google Scholar]
- 40. Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, Huang X, Kijlstra A. Upregulated IL‐23 and IL‐17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci 2008; 49:3058–64. [DOI] [PubMed] [Google Scholar]
- 41. Kuiper JJ, Mutis T, de Jager W, de Groot‐Mijnes JD, Rothova A. Intraocular interleukin‐17 and proinflammatory cytokines in HLA‐A29‐associated birdshot chorioretinopathy. Am J Ophthalmol 2011; 152:177–82. [DOI] [PubMed] [Google Scholar]
- 42. Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The role of IL‐17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm 2017; 2017:3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paliard X, deWaalMalefijt R, Yssel H et al Simultaneous production of IL‐2, IL‐4, and IFN‐gamma by activated human CD4+ and CD8+ T cell clones. J Immunol 1988; 141:849–55. [PubMed] [Google Scholar]
- 44. Vang KB, Yang JY, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL‐2, ‐7, and‐15, but not thymic stromal lymphopoeitin, redundantly govern CD4+ Foxp3+ regulatory T cell development. J Immunol 2008; 181:3285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keyston E, Wherry J, Grint P. IL‐10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am 1998; 24:629–39. [DOI] [PubMed] [Google Scholar]
- 46. Mellor‐Pita S, Citores MJ, Castejon R et al Monocytes and T lymphocytes contribute to a predominance of interleukin 6 and interleukin 10 in systemic lupus erythematosus. Cytometry B Clin Cytom 2009; 76:261–70. [DOI] [PubMed] [Google Scholar]
- 47. Hui WP, Yu DP, Cao Z, Zhao XW. Butyrate inhibit collagen‐induced arthritis via Treg/IL‐10/Th17 axis. Int Immunopharmacol 2019; 68:226–33. [DOI] [PubMed] [Google Scholar]
- 48. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 Cells. Annu Rev Immunol 2009; 27:485–517. [DOI] [PubMed] [Google Scholar]
- 49. Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon‐gamma, interleukin‐2, and tumor necrosis factor‐alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol 1999; 113:752–9. [DOI] [PubMed] [Google Scholar]
- 50. Furiati SC, Catarino JS, Silva MV et al Th1, Th17, and Treg responses are differently modulated by TNF‐α inhibitors and methotrexate in psoriasis patients. Sci Rep 2019; 9:7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of Th17/Treg ratio between PU and AU patients. SFig. 1A‐C demonstrated the Th17 cell subset, Treg cell subset, and Th17/Treg ratio in normal controls and PU patients, respectively. SFig. 1D‐F showed the Th17 cell subset, Treg cell subset, and Th17/Treg ratio in normal controls and AU patients, respectively. We found that the Th17/Treg ratio of PU patients was higher than that of normal controls (P < 0·01). Similarly, Th17/Treg ratio of AU patients was higher than that of normal controls (P < 0·01). Moreover, we also noted that there was no difference in Th17/Treg ratio between PU and AU patients. (normal controls = 11, PU patients = 6, AU patients = 4, **P < 0·01).
Fig. S2. Comparison of Notch1 methylation level between normal controls and the uveitis patients who received immunosuppressants. We found that there was no statistical difference in Notch1 DNA methylation level in both CpG island 1 and CpG island 2 regions between the two groups (normal controls = 6, uveitis patients received immunosuppressants = 5, P > 0·05).


