Abstract
AIM
To determine whether findings from lung ultrasound and chest high-resolution computed tomography (HRCT) correlate when evaluating COVID-19 pulmonary involvement.
MATERIALS AND METHODS
The present prospective single-centre study included consecutive symptomatic patients with reverse transcription polymerase chain reaction (RT-PCR)-proven COVID-19 who were not in the intensive care unit. All patients were assessed using HRCT and ultrasound of the lungs by distinct operators blinded to each other's findings. The number of areas (0–12) with B-lines and/or consolidations was evaluated using ultrasound and compared to the percentage and classification (absent or limited, <10%; moderate, 10–25%; extensive, 25–50%; severe, 50–75%; critical, >75%) of lung involvement on chest HRCT.
RESULTS
Data were analysed for 21 patients with COVID-19 (median [range] age 65 [37–90] years, 76% male) and excellent correlation was found between the ultrasound score for B-lines and the classification (p<0.01) and percentage of lung involvement on chest HRCT (r=0.935, p<0.001). In addition, the ultrasound score correlated positively with supplemental oxygen therapy (r=0.45, p=0.041) and negatively with minimal oxygen saturation at ambient air (r=–0.652, p<0.01).
CONCLUSION
The present study suggests that among COVID-19 patients, lung ultrasound and HRCT findings agree in quantifying lung involvement and oxygen parameters. In the context of the COVID-19 pandemic, lung ultrasound could be a relevant alternative to chest HRCT.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) is an emergent respiratory viral disease first reported in China that quickly spread worldwide. On 2 July 2020, the global COVID-19 pandemic had resulted in more than 10 billion cases and 512,842 deaths worldwide.1 Similar to other coronavirus pneumonia diseases such as SARS caused by SARS-CoV and Middle East respiratory syndrome coronavirus, COVID-19 can also lead to acute respiratory distress syndrome. Recent studies have demonstrated that chest high-resolution computed tomography (HRCT) is important for screening, primary diagnosis, and evaluation of COVID-19 severity.2 , 3 Typical chest HRCT features are ground-glass opacities, interstitial changes, or multifocal patchy consolidation, with peripheral and posterior distribution.2 The first imaging features are present in the early phase of the disease, whereas consolidations are seen later in the disease course.4
HRCT may have higher sensitivity for COVID-19 diagnosis than reverse transcription polymerase chain reaction (RT-PCR).3 In the context of a pandemic with a high fatality rate, there is an urgent need for a prompt identification of patients with COVID-19, thus limiting the access to chest HRCT. In addition, the need for transporting such contagious patients can be a limitation to wide use of the HRCT.5 In this context of limiting the spread of infection, ultrasonography, with the possibility of sterilising equipment, seems useful. Moreover, ultrasound avoids radiation exposure, allows for repeated procedures, and can be performed at the bedside, which limits the need to transport patients.6, 7, 8
Because pulmonary damage occurs mainly in the periphery of the lungs, these lesions could be accessible to ultrasonography. Lung ultrasound is based on the detection of pleural irregularities, consolidation, and B-line artefacts.9 Among patients with normal aeration of the lung, the only ultrasound-detected structure is the pleura, seen as a hyperechoic line. This pleural line is suggested to represent an artefact secondary to a reflection phenomenon between alveolar air and the thoracic wall.10 Some hyperechoic horizontal lines arising at regular intervals from the pleural line are non-pathological and are called A-lines (Fig 1 a). When the alveolar air disappears due to the presence of any lung liquid or collagen, this acoustic artefact is decreased, and ultrasound can be reflected at deeper zones in the lung. This modification of the propagation of ultrasound waves creates vertical artefact lines called B-lines (Fig 1). B-lines appear as a hyperechoic comet-tail vertical line extending from the pleural line to the bottom of the screen with fading. Ultrasound B-lines evoke lung interstitial syndrome and are different from Kerley B-lines, which are X-ray features of interlobular septum thickening. The number and distance between B-lines is also important for interpreting ultrasound of the lungs. The number increases when alveolar air decreases and the distance between B-lines <3 mm evokes ground-glass opacities.11 Previous studies demonstrated that lung ultrasound is efficient for detecting interstitial lung disease, subpleural consolidations, or acute respiratory syndrome.9 , 12, 13, 14, 15 The detection of consolidation with dynamic air bronchograms may suggest a diagnosis of bacterial pneumonia.16 Although HRCT allows for a better detection of pulmonary tumours, ultrasound is able to detect chest-wall tumours by showing a mass with hypervascularisation on power Doppler.17 Regarding COVID-19, four case studies involving 12 to 30 patients18, 19, 20, 21 and two case reports22 , 23 are available. The proportion of patients with B-lines and consolidations ranged from 90% to 100% and 20%–50%, respectively, in the case studies.
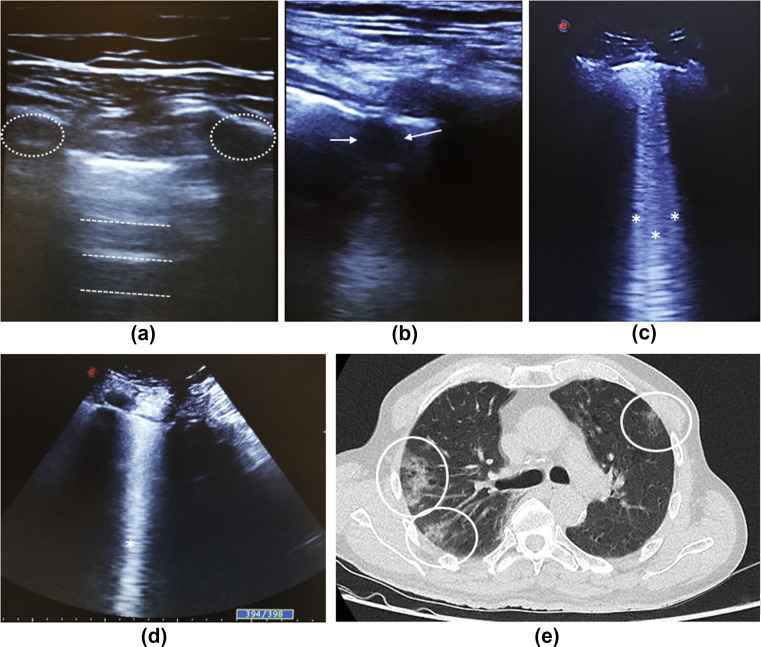
Figure 1.
Imaging features of COVID-19 patients. (a) Ultrasonography of the lung in axial view (linear probe) showing the normal artefact A-lines (white dashed lines) and ribs (white dashed circles). (b) Ultrasound of the lung in axial view (linear probe) showing an irregular pleural line with hypoechoic tissue structure poorly delineated corresponding to a consolidation (white arrow). (c,d) Ultrasound of the lungs with convex probe representing multiple (c) and coalescent (d) B-lines (white asterisks). (e) Chest CT showing multiple ground-glass opacities (white circles) with a peripheral distribution.
The aim of the present study was to determine the usefulness of lung ultrasound for COVID-19 diagnosis by investigating the correlation between ultrasound and chest HRCT findings.
Materials and methods
Patients and study design
This prospective single-centre study included consecutive symptomatic patients, age >18 years, with COVID-19 proven by positive RT-PCR for SARS-CoV2 who were not in the intensive care unit (ICU). All patients were admitted in the unit between 23 March and 14 April 2020. Exclusion criteria were hospitalisation in the ICU during the last month and previous thoracic surgery, acute heart failure, and bacterial pneumonia. All included patients underwent clinical evaluation (respiratory frequency, oxygen saturation measurement, quantification of oxygen supplementation), including disease history (presence of fever, diarrhoea, cough, dyspnoea) and C-reactive protein (CRP) measurement. All patients underwent chest HRCT and lung ultrasound on the same day.
Informed consent was obtained for all patients. Because the study was observational without an invasive procedure, no ethics committee approval was required.
Chest HRCT
HRCT was performed by the local senior radiologist (>20 years of experience) who was blinded to clinical and ultrasound findings. The following characteristics were assessed2 , 1: presence of ground-glass opacities,2 presence of consolidation,3 severity classified in five grades (grade 1, absent or limited, with <10% of the involved lung; grade 2, moderate [10–25%]; grade 3, extensive [25–50%]; grade 4, severe [50–75%] and grade 5, critical [>75%]), as recommended by the European Society of Radiology and European Society of Thoracic Imaging.24
Lung ultrasound assessment
Ultrasound assessment of the lung was performed at the bedside of all patients by one trained senior ultrasound operator (S.O. with >10 years of experience) and involved a portable Esaote MyLabFive echograph (Genoa, Italy) dedicated to exclusive use for COVID-19 patients. The operator was blinded to clinical and HRCT findings. According to the body size of patients, a linear or a convex probe (5–18 MHz) was used as suggested.25
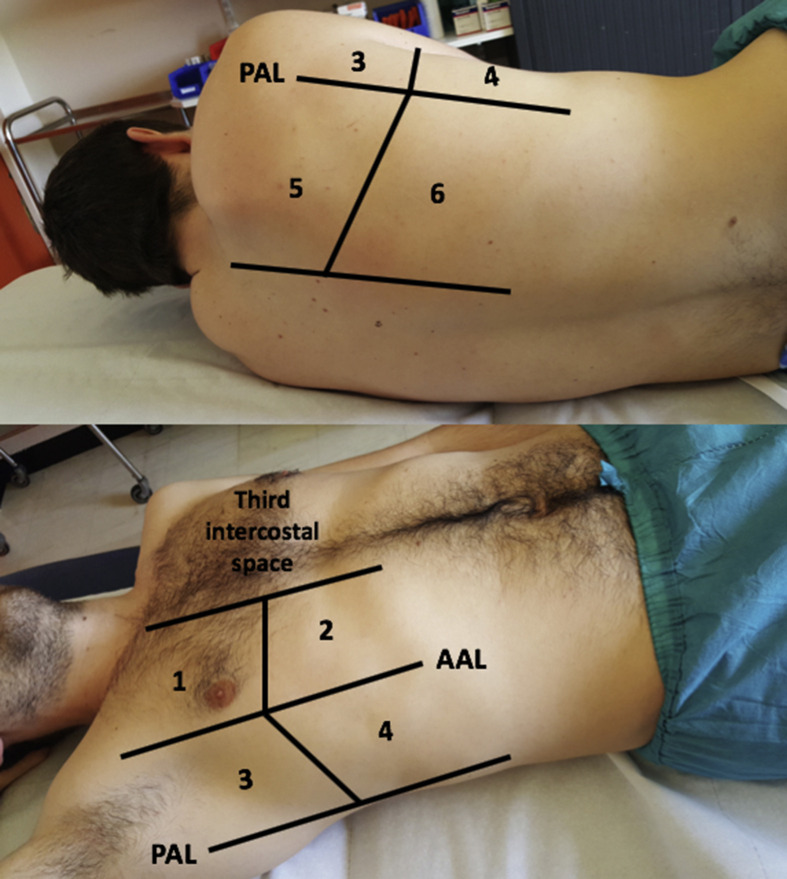
As previously described,26 a simplified 12-zone lung ultrasound score was used: six lung regions delineated by a parasternal line, anterior axillary line, posterior axillary line, and paravertebral line were assessed on each side (Electronic Supplementary Material Fig. S1). The following ultrasound features of lung involvement were assessed (Fig 1)1: presence of B-lines defined as a comet tail artefact fanning out from the lung–wall interface and spreading up to the edge of the screen15 and2 presence of consolidation defined as a poorly delineated hypoechoic tissue structure. The presence of multiple (at least three B-lines) or confluent comet tails in each analysed area was graded B-score 1. At least one consolidation in the examined area was graded C-score 1. Thus, for each patient, the sum of the total B-score and total C-score was from 0 to 12.
To evaluate the interobserver variability of lung ultrasound, a second ultrasound trainee operator (M.F. with <1 year of experience) performed a second ultrasound assessment of the lung on the same day as the first ultrasound evaluation. This second ultrasound evaluation involved 10 patients, and the operator was blinded to the first ultrasound evaluation and HRCT findings. Where results differed between the two operators, results obtained by the senior operator (S.O.) were chosen.
Statistical analysis
Data are presented as median (range) or number (%). Wilcoxon's test was used to analyse quantitative variables, chi-square test for categorical data, and Kruskal–Wallis test for comparing more than two groups. Two-sided p < 0.05 was considered statistically significant.
Interobserver agreement was estimated by the κ coefficient, with agreement >0.8, almost perfect; 0.6–0.8, substantial; 0.4–0.6, moderate, 0.2–0.4, fair, ≤0.2, slight; and <0, poor beyond chance. Correlation between ultrasound and chest HRCT findings was estimated by the Spearman correlation coefficient.
Results
Twenty-one non-ICU patients (16 men, 76%) with RT-PCR–diagnosed COVID-19 were included in the study. The characteristics of patients are summarised in Table 1 . The median (range) age was 65 (37–90) years; the delay between the first COVID-19 symptoms and ultrasound assessment was 71, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 days. Symptoms related to COVID-19 were fever (n=15, 71%), dyspnoea (n=12, 57%), dry cough (n=12, 57%), and diarrhoea (n=3, 14%). Median CRP level was 71 (3–300) mg/l. The minimal median oxygen saturation at ambient air was 93% (75–100) and respiratory frequency 2214, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 cycles/min. For patients requiring oxygen supplementation, the median oxygen requirement was 2 (0–15) l/min.
Table 1.
Demographic and clinical characteristics of COVID-19 patients (n = 21)
| Sex, n (%) males | 16 (76) |
|---|---|
| Age at diagnosis, years, median (range) | 65 (37–90) |
| Delay between first symptoms and ultrasound, days, median (range) | 7 (1–16) |
| Symptoms of COVID-19, n (%) | |
| Fever | 15 (71) |
| Dyspnoea | 12 (57) |
| Cough | 12 (57) |
| Diarrhoea | 3 (14) |
| C-reactive protein level, mg/l, median (range) | 71 (3–300) |
| O2 saturation at ambient air, %, median (range) | 93 (75–100) |
| Supplemental O2 therapy, l/min. median (range) | 2 (0–15) |
| Respiratory frequency, cycles/min, median (range) | 22 (14–28) |
Data are n (%) of patients unless indicated.
Lung imaging assessment (Table 2)
Table 2.
Characteristics of COVID-19 patients (n = 21) by chest high-resolution CT (HRCT) and lung ultrasound
| Chest HRCT findings | |
|---|---|
| Chest HRCT classification | |
| Absent or limited (<10%) | 2 (9.5) |
| Moderate (10–25%) | 10 (48) |
| Extensive (25–50%) | 5 (24) |
| Severe (50–75%) | 4 (19) |
| Critical (>75%) | 0 (0) |
| Lung involvement at HRCT, %, median (range) | 20 (0–70) |
| Lung ultrasound findings | |
| Number of patients with B-lines | 19 (90.5) |
| Number of patients with consolidation | 13 (61.9) |
| B-Score, median (range) | 6 (0–11) |
| C-Score, median (range) | 1 (0–4) |
Data are n (%) of patients unless indicated.
For chest HRCT, the classification of lung involvement for COVID-19 was as follows: absent or limited (n=2, 9.5%), moderate (n=10, 48%), extensive (n=5, 24%), severe (n=4, 19%) and critical (none). The median percentage of lung involvement was 20% (0–70).
For lung ultrasound assessment, 19 (90.5%) and 13 (61.9%) patients had at least one area with B-lines and consolidations, respectively. The median B-score and C-score was 6 (0–11) and 1 (0–4), respectively.
The interobserver reliability was substantial for lung ultrasound for B-lines, with κ coefficient 0.65 (95% confidence interval [CI]: 0.47–0.83), and moderate for consolidation (0.41 [95% CI: 0.25–0.57]).
Lung ultrasound and correlation with chest HRCT and clinical variables (Fig 2 ).
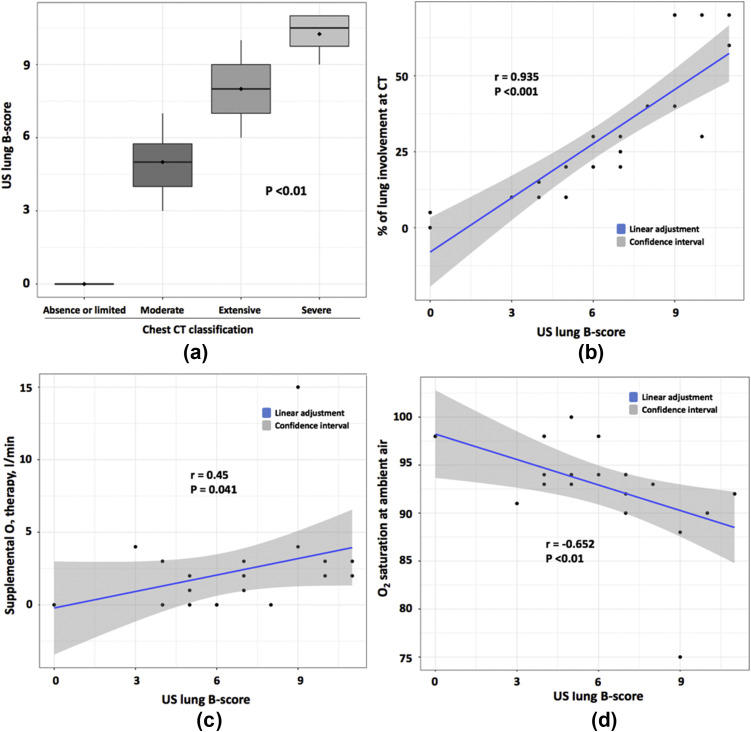
Figure 2.
Chest CT and clinical variables associated with lung ultrasound. (a) Box plot showing a significant association (Kruskal–Wallis test) between ultrasound lung B-score and chest CT classification. (b–d) Correlation between ultrasound lung B-score and percentage of lung involvement using CT (b), oxygen supplementation (c), and oxygen saturation at ambient air (d).
On comparing ultrasound and chest HRCT findings, excellent correlation was found between the ultrasound score for B-lines and classification (p<0.01) and percentage lung involvement on chest HRCT (r=0.935, p<0.001; Fig 2). Ultrasound B-lines score correlated positively with supplemental oxygen therapy (r=0.45, p=0.041) and negatively with oxygen saturation at ambient air (r=–0.652, p<0.01). Ultrasound consolidations correlated only with percentage lung involvement on chest HRCT (r=0.452, p=0.04), but not oxygen parameters (supplemental oxygen therapy, r=0.227, p=0.32; oxygen saturation at ambient air, r=0.338, p=0.13). Chest HRCT also correlated negatively with oxygen saturation at ambient air (p=0.016).
Discussion
The recent outbreak of SARS-CoV-2 infection has resulted in numerous patients with suspected disease waiting for a COVID-19 diagnosis. Chest imaging plays a pivotal role in the diagnostic workflow of patients with suspected virus pneumonia,27 notably for those with very early disease, when viral replication might not be detected by RT-PCR.3 The present study reports the usefulness and relevance of lung ultrasound as a screening tool for identifying COVID-19 features and correlation of ultrasound and chest HRCT findings. The sum of chest–wall areas with ultrasound B-lines (B-score) correlated with the extent of lung involvement when using HRCT as the reference standard. The present results suggest that lung ultrasound could represent a relevant alternative to chest HRCT for screening patients with suspicion of COVID-19 and to assess the severity of pneumonia.
A recent study suggested that agreement between lung ultrasound and chest HRCT findings might vary depending on the severity of lung involvement, with diagnostic accuracy from 77% (mild involvement) to 93% (severe).19 The prevalence of B-lines (90%) in the present COVID-19 patients agrees with that in previous case studies.18, 19, 20, 21 In those studies, ultrasound analysis of 12–22 COVID-19 patients revealed a prevalence of B-lines ranging from 90% to 100% and the proportion of consolidations ranged from 20% to 25%. A larger proportion (>60%) with consolidations was observed in the present study. This discrepancy could be explained by distinct populations or by the fact that the number of consolidations observed by ultrasound is low (median = 1), thus leading to increased difficulty to find them. The ability of ultrasound to detect COVID-19-related lung damage could be explained by a specific distribution of mainly peripheral and posterior lung lesions.18
Several arguments strengthen the relevance of lung ultrasound for COVID-19 evaluation. First, chest ultrasound can be easily performed at the bedside,26 requiring a mean time for global assessment of 15 minutes (data not shown). Second, lung ultrasound does not require mobilising radiologist specialist staff and is less time-consuming than chest HRCT. Third, in the context of the pandemic and highly contagious patients, which limits wide access to chest HRCT,5 lung ultrasound could be an acceptable alternative for detecting COVID-19-related lung involvement. Fourth, ultrasound assessment of the lung could be a good alternative in countries with limited access to chest HRCT.
Regarding ultrasound detection of consolidations, the reproducibility was moderate, and ultrasound consolidations were not correlated with any oxygen parameters. Only the percentage of lung involvement on HRCT was slightly correlated with the consolidation score. Moreover, the interobserver reliability for ultrasound consolidation was moderate. This low reproducibility could be explained by a low number of consolidations observed by ultrasound, thus leading to increased difficulty to find them. These data suggest that using ultrasound to search for consolidations has limited use in clinical practice.
The lung ultrasound B-lines score correlated with oxygen parameters (Fig 2). The present prospective study suggests that ultrasound could help estimate the severity of COVID-19. Thus, ultrasound could help clinicians in emergency units determine the prognostic factors for patients at risk of rapid decrease in respiratory function; however, this impact on prognosis should be investigated in future studies.
The present study has some limitations. First, a relatively small number of patients were analysed, and only half were assessed by the two ultrasound operators. Regarding the low number of patients, other studies analysed the same range of patients. Despite the relatively low number of patients, the results were clearly significant, which suggests the robustness of the present findings. Concerning ultrasound assessment by both operators, the second examination by the trainee operator was performed only to evaluate interobserver reliability. When comparing lung ultrasound assessment between experienced and trainee ultrasound operators, the interobserver reproducibility was substantial and was slightly higher than that observed a previous study (kappa = 0.53).19 Another limitation was the use of a non-validated ultrasound score for lung assessment of COVID-19 patients. A simplified score for B-lines was used to maximise the feasibility in the context of the urgent need for alternative screening tools for COVID-19 pulmonary disease. A proposal for standardising the use of lung ultrasound was published after the the present study commenced.25 Using the present score, good agreement between both imaging procedures emphasises the relevance of using lung ultrasound as an alternative rapid screening tool for COVID-19. Nonetheless, HRCT should be considered the reference standard for both the diagnosis and extent of SARS-CoV2 infection allowing for elimination of the differential diagnoses, such as pulmonary embolism, considering that thrombotic events are rapidly emerging as a key threat in COVID-19.28 Finally, ultrasound of the lung was easy to perform in non-ICU patients. Indeed, the patients did not have severe tachypnoea, pulmonary drains, or lobar collapse, which could limit the interpretation on ultrasound.
In conclusion, the present findings suggest that lung ultrasound and chest HRCT findings are well correlated in patients with COVID-19, and ultrasound could be helpful for screening patients with suspected COVID-19.
Funding source
None.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Laura Smales (BioMedEditing) for copyediting.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crad.2020.07.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
References
- 1.World Health Association. WHO coronavirus disease dashboard Update of 2020/8/17. Available at: https://covid19.who.int/.
- 2.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M.J., Hayward S.A., Innes S.M. Point-of-care lung ultrasound in patients with COVID-19—a narrative review. Anaesthesia. 2020 Aug;75(8):1096–1104. doi: 10.1111/anae.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Convissar D., Gibson L.E., Berra L. Application of lung ultrasound during the COVID-19 pandemic: a narrative review. Anesth Analg. 2020 Aug;131(2):345–350. doi: 10.1213/ANE.0000000000004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiala M.J. A brief review of lung ultrasonography in COVID-19: is it useful? Ann Emerg Med. 2020;75(6):784–785. doi: 10.1016/j.annemergmed.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofia S., Boccatonda A., Montanari M. Thoracic ultrasound and SARS-COVID-19: a pictorial essay. J Ultrasound. 2020;23(2):217–221. doi: 10.1007/s40477-020-00458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo P.H., Copetti R., Feller-Kopman D. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200–1211. doi: 10.1007/s00134-019-05725-8. [DOI] [PubMed] [Google Scholar]
- 10.Gargani L., Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. doi: 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich C.F., Mathis G., Blaivas M. Lung B-line artefacts and their use. J Thorac Dis. 2016;8(6):1356–1365. doi: 10.21037/jtd.2016.04.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demi L., Demi M., Smargiassi A. Ultrasonography in lung pathologies: new perspectives. Multidiscip Respir Med. 2014;9(1):27. doi: 10.1186/2049-6958-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demi L., van Hoeve W., van Sloun R.J.G. Determination of a potential quantitative measure of the state of the lung using lung ultrasound spectroscopy. Sci Rep. 2017;7(1):12746. doi: 10.1038/s41598-017-13078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mojoli F., Bouhemad B., Mongodi S. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701–714. doi: 10.1164/rccm.201802-0236CI. [DOI] [PubMed] [Google Scholar]
- 15.Volpicelli G., Mussa A., Garofalo G. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24(6):689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Alzahrani S.A., Al-Salamah M.A., Al-Madani W.H. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. doi: 10.1186/s13089-017-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandi V., Lunn W., Ernst A. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest. 2008;133(4):881–886. doi: 10.1378/chest.07-1656. [DOI] [PubMed] [Google Scholar]
- 18.Lomoro P., Verde F., Zerboni F. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu W., Zhang S., Chen B. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease-19 (COVID-19) by bedside ultrasound. Ultraschall Med. 2020 Jun;41(3):300–307. doi: 10.1055/a-1154-8795. [DOI] [PubMed] [Google Scholar]
- 20.Poggiali E., Dacrema A., Bastoni D. Can lung ultrasound help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;295(3):E6. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing C., Li Q., Du H. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):174. doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buonsenso D., Piano A., Raffaelli F. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24(5):2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A., Haljan G., Mitra A. Lung ultrasound findings in a 64-year-old woman with COVID-19. CMAJ. 2020 doi: 10.1503/cmaj.200414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revel M.P., Parkar A.P., Prosch H. COVID-19 patients and the radiology department — advice from the European society of radiology (ESR) and the European society of thoracic imaging (ESTI) Eur Radiol. 2020 Apr 20:1–7. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldati G., Smargiassi A., Inchingolo R. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020 Jul;39(7):1413–1419. doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouhemad B., Brisson H., Le-Guen M. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183(3):341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 27.Koo H.J., Lim S., Choe J. Radiographic and CT features of viral pneumonia. RadioGraphics. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 28.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]