Abstract
Background
HIV continues to disproportionately affect men who have sex with men (MSM) and transgender women (TW). Undiagnosed HIV is a major driver of HIV transmission rates, and increasing the uptake of regular HIV testing and facilitating timely initiation of HIV treatment is a global HIV prevention priority. However, MSM and TW experience a range of barriers that limit their access to testing and other prevention services. Given their growing ubiquity, digital communication technologies are increasingly being used to support HIV prevention efforts, and a growing number of studies have trialed the use of digital technology to promote HIV testing among MSM and TW.
Objective
We undertook a systematic review and meta-analysis to assess the impact of digital communication technology on HIV testing uptake among MSM and TW. Subanalyses aimed to identify the features and characteristics of digital interventions associated with greater impact.
Methods
A systematic literature review was undertaken using select databases and conference repositories. Studies describing the use of a digital technology—internet-enabled devices, including phones, tablets, and computers—to increase HIV testing uptake among MSM or TW using either randomized or observational cohort design with measurement of HIV testing rates measured pre- and postintervention, and published in English between 2010 and 2018 were included. Pooled effect estimates were calculated using a random effects meta-analysis. Subanalyses calculated effect estimates grouped by selected features of digital interventions.
Results
A total of 13 randomized or observational studies were included in the final review. Digital interventions most commonly used mainstream, existing social media platforms (n=7) or promotion through online peer educators (n=5). Most interventions (n=8) were categorized as interactive and allowed user engagement and most directly facilitated testing (n=7) either by providing self-testing kits or referral to testing services. A total of 1930 participants were included across the 13 studies. HIV testing uptake among MSM and TW exposed to digital interventions was 1.5 times higher than that of unexposed MSM and TW (risk ratio [RR] 1.5; 95% CI 1.3-1.7). Subanalyses suggested an increased impact on HIV testing uptake among interventions that were delivered through mainstream social media–based platforms (RR 1.7; 95% CI 1.3-2.1), included direct facilitation of HIV testing (RR 1.6; 95% CI 1.4-1.9), were interactive (RR 1.6; 95% CI 1.4-1.8), and involved end users in the design process (RR 1.6; 95% CI 1.3-2.0).
Conclusions
These findings provide broad support for the integration of technology with existing approaches to promote and facilitate HIV testing among MSM and TW. Our findings identified key features that may be associated with greater impact on HIV testing uptake and can be used to inform future development efforts given the growing interest and application of digital technologies in HIV prevention.
Trial Registration
PROSPERO International Prospective Register of Systematic Reviews CRD42017070055; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017070055.
Keywords: digital technology, men who have sex with men, transgender women, HIV testing, HIV prevention
Introduction
Globally, men who have sex with men (MSM) and transgender women (TW) are disproportionately affected by HIV [1-4]. Evidence of expanding epidemics among MSM have been noted, with new infections among this group comprising up to half of all incident cases in some regions, including North America [5-9], and up to approximately 70% in specific countries, such as the Philippines [7]. This burden of HIV occurs against a background of expanded access to HIV testing and treatment and the emergence and growing coverage of biomedical HIV prevention strategies [5].
The timely diagnosis of HIV plays an important role in preventing transmission, both by prompting reductions in risk behaviors to prevent onward transmission [10,11] and by facilitating early access to treatment and viral suppression. It is now known that people with suppressed HIV cannot transmit the virus to others [12-15]; promoting regular HIV testing is therefore a key global prevention strategy [16]. However, a range of barriers faced by MSM and TW limits their access to HIV testing and other preventive services. Stigma and discrimination remains a key deterrent to HIV service utilization in many parts of the world, particularly in settings where legislation prohibits same-sex relationships and legitimizes discriminatory behavior toward sexual minorities [17-19]. Other structural barriers, such as accessibility of services, costs, waiting time, and confidentiality concerns [20-24], also limit access to HIV testing among MSM and TW and prevent levels of testing coverage required to impact HIV incidence [25].
The use of digital communication technology—internet-enabled technology such as mobile phones, computers, and tablets that allow access to digital platforms and apps—to promote HIV testing among MSM and TW is an area of growing interest. Digital technology has assumed a prominent role in contemporary gay culture [26], and to a lesser extent in TW communities [27], as a tool to meet sex partners [28-30]. Evidence pointing to higher rates of condomless sex [31-33] and diagnoses of sexually transmitted infections (STI) [27,28,34,35] among MSM and TW who use online platforms to find sex partners suggests that online platforms may be appropriate targets for sexual health promotion. It has been suggested that internet-enabled technology can provide a discrete means for HIV health promotion in locations where same-sex behaviors are highly stigmatized or illegal [36], while the acceptability of HIV prevention interventions delivered through digital communication technologies has been noted among MSM and TW [37-43]. Together, these findings enhance the potential of using digital technology to reach those at high risk of HIV and serve as an important platform for health promotion outreach.
We performed a systematic review and meta-analysis to describe the impact of digital communication technology interventions on the uptake of HIV testing among MSM and TW. Subanalyses were undertaken to identify intervention characteristics associated with increased impact.
Methods
Overview
We systematically searched for current literature that describes the impact of digital communication technology interventions on the uptake of HIV testing among MSM and TW. We defined digital communication technology as technologies that were internet-enabled (through devices such as computers, mobile phones, or tablets) and which provided access to digital platforms such as social media sites, websites, apps, and email. The systematic review was conducted in accordance with the Preferred Reporting for Systematic Reviews and Meta-Analyses guidelines [44] and was registered on PROSPERO (registration number CRD42017070055).
Eligible studies were defined as those that:
Utilized at least one digital communication platform to deliver an intervention to promote HIV testing
Reported uptake of HIV testing as a result of a digital communication intervention for MSM and TW participants
Measured impact either by prospectively comparing testing rates pre- and postintervention exposure within a single cohort or through a randomized study design.
Studies that measured HIV testing outcomes but utilized digital communication technologies primarily for a purpose not directly related to improving HIV testing uptake (eg, to facilitate data collection or recruitment) and those reporting intention to test outcomes only were excluded.
Uptake of HIV testing was defined as any quantitative count of HIV testing events among MSM and TW measured using either self-report or clinic records.
For randomized controlled studies, we report on HIV testing uptake among participants in the intervention and control groups at the study endpoint. For nonrandomized studies, we report on HIV testing uptake at pre-intervention baseline and postintervention study endpoints.
Search Strategy
We conducted a systematic search of the literature published in English using the Medical Literature Analysis and Retrieval System Online, or MEDLARS Online (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINHAL), EMBASE, PubMed, and PsychInfo databases. We limited our search to studies published between January 1, 2010, and May 1, 2018, to account for the redundancy of older platforms and technologies. Our search strategy comprised key terms; Medical Subject Headings (MeSH) terms; and subject headings related to participant (eg, MSM), intervention (eg, internet), and outcome (eg, HIV testing and counseling) variables (Multimedia Appendix 1 shows the illustration of search strategy and MeSH terms). Electronic repositories of the International AIDS Society and the International AIDS Conferences were manually searched for abstracts from January 2010 onward. Reference sections of the identified papers were also searched for additional papers. No restrictions were placed on participant age or other demographic characteristics of the study population or geographical location or setting of the intervention.
Data Extraction
Following the literature search, the first author (VV) removed duplicate records and assessed the remaining abstracts on the basis of the eligibility criteria. The second author (KR) reviewed a random sample (equivalent to 10%) of discarded abstracts to ensure accuracy. Both authors then conducted a full-text review of the remaining abstracts to determine their inclusion in the final analysis.
The following domains were extracted for final analysis by two authors (VV and KR) using a standardized, Excel-based tool: study identification, study design (data collection period, recruitment, and sampling method), intervention characteristics (aim of intervention, mode of intervention delivery, and duration), study population (inclusion and exclusion criteria, sample size, primary HIV testing outcome used and time frame for the outcome, the mean age of sample, proportion reporting previous testing), and results and analysis (number of testing outcomes, effect size measurement, and reported effect size).
Any discrepancies identified between the two authors during the review and data extraction process were discussed with a third author (MS).
Analysis
Qualitative Synthesis of Study Aims, Intervention Characteristics, and Study Outcomes
A qualitative synthesis was undertaken to characterize included studies by their study participants, location of study, study design, digital communication intervention platform, intervention features, length and frequency of exposure, sample size, and rated study quality. For each study, we then described the proportion of MSM and TW reporting previous HIV testing at baseline, how testing uptake was defined and measured during the study period, whether testing was provided or offered as part of the intervention, and the proportion of MSM and TW reporting or receiving HIV testing at the end of the study period.
The primary outcome of this study was uptake of HIV testing among MSM and TW participants, which we defined as the number of individual MSM and TW reporting or receiving an HIV test divided by the total number of MSM and TW exposed to a digital intervention. Intervention effectiveness was determined by manually calculating risk ratios (RRs) comparing testing uptake between exposed groups and unexposed groups for randomized controlled trials (RCTs) or between pre- and postintervention commencement for nonrandomized studies. HIV testing uptake among controls or at preintervention time point was used as the reference group so that an RR greater than one demonstrates a higher chance of HIV testing uptake following exposure to a digital communication intervention.
Meta-Analysis
We performed a meta-analysis of intervention effectiveness to generate pooled RR using a DerSimonian and Laird random effects model to account for the anticipated heterogeneity between studies. We identified a range of characteristics common to digital communication interventions or regarded as features that potentially enhance the intervention effectiveness and performed submeta-analyses to generate pooled RR to examine the impact of these characteristics on overall intervention effectiveness:
Intervention interactivity (yes/no): interventions that permitted end users to interact or engage, for example, by chatting with peer educators or other participants, as opposed to passive viewing of an online video
End-user involvement in the design process (yes, no, or not reported): any reported involvement of the intended end users in the intervention design process (eg, consultation and pilot testing)
HIV testing facilitated as part of the intervention (yes/no): interventions that directly provided (eg, provided self-tests) or facilitated (eg, direct referral) HIV testing to participants, as opposed to simply promoting HIV testing
Social media platform (yes/no): interventions implemented through an established social media platform (eg, Facebook) that facilitates social networking among the general population or specifically among gay and other MSM, as opposed to nonsocial media platforms
Single dose exposure (yes/no): interventions that delivered a single, time-bound exposure as opposed to multiple exposures over time.
The presence and magnitude of heterogeneity were assessed in meta-analyses using the χ2 and I2 tests, respectively. All statistical analyses were performed using Stata version 14 (StataCorp LP).
Assessing Study Quality
Study quality was assessed by two authors (VV and KR) using the quality assessment tool for quantitative studies [45]. This tool critiqued studies on the basis of selection bias, study design, confounders, blinding, data collection methods, and withdrawal and dropouts. Each criterion was rated as strong, moderate, or weak. On the basis of the combined scores, studies were given a final, global rating of strong (no weak ratings), moderate (one weak rating), or weak (two or more weak ratings). As the final global rating was determined by the number of subcategories scored as weak, the tool was modified to include an N/A option for blinding and withdrawal and dropout fields to account for nonrandomized studies. The first and second authors (VV and KR) met after completing the first two quality assessments to ensure consistency in the use of the tools. After consistency was confirmed, the two authors completed the remaining quality assessments, and the final results were compared and discussed. Similar to the process for data extraction, any disagreements between the assessments of the first and second author were discussed with a third senior author (MS).
Results
Search Results
The systematic literature search resulted in 1436 identified records, including 8 conference abstracts and 6 papers identified in the references of the included studies. 37.30% (541/1436) were removed as duplicates. The remaining 909 records were reviewed at the abstract level, 4.4% (40/909) of which were retained for full-text review. Thirteen papers were included in the final analysis [46-58] (Figure 1).
Figure 1.
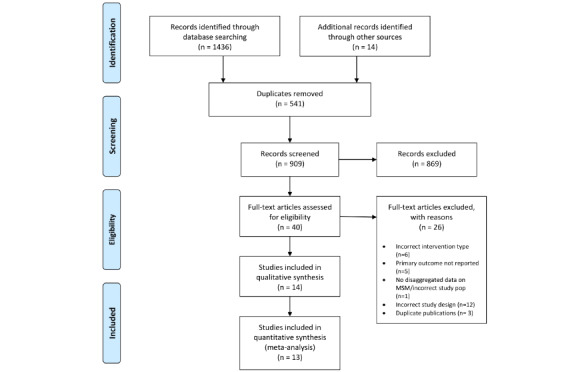
The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram depicting study screening and selection.
Study and Sample Characteristics
Table 1 presents the characteristics of the included studies. All included studies specifically targeted men who identified as gay or self-reported anal sex with a male partner. Two studies included TW [53,54]. Study participants were typically aged in their midtwenties, and the overall level of educational attainment varied substantially across studies. On the basis of the World Bank classifications [59], the majority of studies took place in high-income countries (Hong Kong, n=1; Taiwan, n=1; and United States, n=6), 4 occurred in upper-middle-income countries (China, n=1 and Peru, n=3), and 1 in a low-middle-income country (India). The studies collectively included 8875 participants, 64 (0.70%) of whom were reported as TW, with a mean sample size of 682 participants (SD 808; range 56-3092 participants). Two studies excluded participants with a history of HIV testing, and two studies did not report on participants’ previous testing history. Among the 9 remaining studies, the mean proportion of participants reporting a history of HIV testing was 44.1% (SD 19.0; range 21.3%-73.8%). The recall period for these studies varied, with one study reporting lifetime HIV testing history and the remaining studies using recall periods that ranged from 3 months to 3 years. A total of 10 studies used an RCT design [46-49,51,54-58]. The specific study design used by the remaining 3 studies was a prospective cross-sectional study with nonequivalent control group [50], a prospective single-group cross-sectional study [52], and a prospective cross-sectional matched-pair randomized trial design [53]. Due to the lack of individual-level randomization and nonequivalence of control groups, we calculated relative risk for these 3 studies using reported HIV testing uptake at preintervention baseline and postintervention endpoints in the intervention arm only. One RCT described two intervention arms and did not present disaggregated findings; therefore, this study was treated as a single cohort, and relative risk was calculated based on HIV testing uptake at baseline and endpoint [51].
Table 1.
Overview of included studies.
| Reference | Description of study participants | Location (country of income classificationa) | Total sample size | TWb participants, n (%) | Percentage of participants reporting previous HIV testing preintervention (time frame for testing history) | Study design |
| Bauermeister et al (2015) [46] | Inclusion criteria: Young MSMc aged 15-24 years, self-identified cis male, reported sex with a male partner in the past 6 months; sample characteristics: mean age 21 years, 92.3% educated to high school or GEDe level | Michigan, United States (high) | 130 | 0 (0) | 73.8 (lifetime) | RCTd |
| Blas et al (2010) [47] | Inclusion criteria: MSM aged 18 years and above, reporting lifetime sex with another man, and not reporting testing within the past 12 months; sample characteristics: mean age 26.1 years (range 18-61), 42% educated to university or technical graduate level | Lima, Peru (upper middle) | 459 | 0 (0) | 21.3 (more than 1 year ago) | RCT |
| Blas et al (2014) [48] | NRf | Peru (upper middle) | 400 | 0 (0) | NR | RCT |
| Hirshfield et al (2012) [49] | Inclusion criteria: male aged 18 years or above, reporting oral or anal sex with a current male partner and oral, anal, or vaginal sex with at least one new partner (male or female) in the past 6 months; sample characteristics: median age 39 years (range 18-81), 55% educated to college degree or higher level | United States (high) | 3092 | 0 (0) | 69.0 (past 3 years) | RCT |
| Ko et al (2013) [50] | Inclusion criteria: MSM aged 18 years and above, reported sex with another man in the past 12 months; sample characteristics: mean age 24.8 years, 63% educated to college level | Taiwan (high) | 1037 | 0 (0) | 29.4 (past 6 months) | Prospective cross-sectional study with nonequivalent control |
| Patel (2016) [51] | Inclusion criteria: MSM aged 18 years or older living in Mumbai | Mumbai, India (low middle) | 244 | 0 (0) | 61.5 (past 6 months) | RCT |
| Rhodes et al (2011) [52] | Inclusion criteria: male, registered user of chat room servicing MSM in North Carolina, United States; mean age 37 years; gay-identifying; sample characteristics: mean age 37.1 years (range 18-71 years; education not reported) | North Carolina, United States (high) | 346 | 0 (0) | 44.5 (past 3 years) | Prospective cross-sectional study |
| Rhodes et al (2016) [53] | Inclusion criteria: male, social media user, mean age 40 years, gay-identifying; sample characteristics: mean age 40.9 years (range 18-74 years; education not reported) | United States (high) | 1292 | 28 (2.17) | 36.6 (past 12 months) | Prospective cross-sectional matched-pair randomized trial design |
| Tang et al (2016) [54] | Inclusion criteria: male aged 16 years or above, reporting lifetime anal sex with another man and no HIV testing history; sample characteristics: 37% aged between 21 and 25 years, 65% college educated | China (upper middle) | 721 | 36 (5.0) | N/Ag | RCT |
| Washington et al (2017) [55] | Inclusion criteria: Black or African American male aged 18-30 years reporting sex with another man during the past 3 months and not tested within the past 6 months; sample characteristics: mean age 23.1 years, 52% educated to high school or GED level | Los Angeles, United States (high) | 56 | 0 (0) | 46.5 (past 12 months) | RCT |
| Wang et al (2018) [56] | Inclusion criteria: Chinese-speaking males in Hong Kong aged 18 years or over reporting anal sex with 1 or more male partner in the past 6 months with access to online live chat apps (Line, WhatsApp, and Skype); sample characteristics: 63.1% aged between 18 and 31 years, 81.4% educated to a university level | Hong Kong, China (high) | 430 | 0 (0) | N/A | RCT |
| Young et al (2013) [57] | Inclusion criteria: African American or Latino male aged 18 years or above, registered Facebook users, reporting sex with another man in the past 12 months; sample characteristics: mean age 31.8 years (SD 10.2 years), 36.4% educated to high school level | Los Angeles, United States (high) | 112 | 0 (0) | NR | RCT |
| Young et al (2015) [58] | Inclusion criteria: MSM aged 18 years and over, reporting sex with another man during the past 12 months; sample characteristics: 28.9 mean age (SD 7.9 years), 37.8% educated to vocational school level | Lima, Peru (upper middle) | 556 | 0 (0) | 33.4 (past 3 months) | RCT |
aClassification based on World Bank countries and lending groups [59].
bTW: transgender women.
cMSM: men who have sex with men.
dRCT: randomized controlled trial.
eGED: general education development.
fNR: not reported.
gN/A: not applicable.
Aims of Included Studies
Promoting HIV testing uptake was identified as the primary aim of all 13 studies. Five studies had multiple primary aims: mutual disclosure of HIV status with sexual partners (ie, asking and disclosing HIV serostatus [49]), STI testing [46], intention to test [47,51], and reduction of unprotected anal intercourse [49,50] (data not reported).
Intervention Characteristics of the Included Studies
Table 2 presents the characteristics of the digital interventions among the included studies. Studies utilized a variety of digital communication platforms to deliver interventions. Six used only social media platforms, 3 used only online videos, and 1 delivered a tailored online HIV/STI testing intervention through a customized website. The remaining 3 studies used multiple platforms: 1 used online videos in conjunction with motivational messages sent via email or instant messaging, 1 used social media and live chat apps, and 1 used online videos in conjunction with live chat apps.
Table 2.
Characteristics of digital interventions of included studies.
| Reference | Platform | Description | Interactive | Intervention length (frequency of exposure) | HIV testing provided or facilitated | Measurement of HIV testing uptake used | Length of follow-up period for outcome | Comparator | Involvement of end users in intervention design | Theoretical framework |
| Bauermeister et al (2015) [46] | Website | Interactive, customized website (Get Connected!) that delivered HIV/STIa testing and prevention content tailored to specific participant profiles of based on psychosocial data and previous engagement with HIV testing | Yes | One time | No | Self-reported | 30 days | Control group: test-locator website | Yes | Self-determination theory principles and integrated behavioral model |
| Blas et al (2010) [47] | Online video | One 5-min video delivered through existing gay and commercial websites promoting HIV testing customized based on self-identification of participant as either gay or nongay | No | One time (5 min) | Yes—facilitated (referral) | Attendance based | 125 daysb | Control group: standard public health text | Yes | Health belief model |
| Blas et al (2014) [48] | Multiple: online videos, email/instant messaging | Motivational videos and messages about HIV testing sent through email and instant messaging, respectively | No | NRc | Yes—facilitated (referral) | Attendance based | 184 daysb | Control group: health promotion message with invitation for free HIV testing | No or not reported | None reported |
| Hirshfield et al (2012) [49] | Online video | HIV prevention videos in either dramatic or documentary style (or both), accessed via banner ads on gay-oriented sexual networking sites, and designed to promote critical thinking about HIV disclosure, testing, and condom use | No | One time (9 and 5 min) | No | Self-reported | 60 days | Control group: no content | No or not reported | Social learning theory |
| Ko et al (2013) [50] | Social media (Facebook) | Trained internet popular opinion leaders promoting HIV testing and prevention to members of a closed Facebook group | Yes | 6 months (user-dependent) | No | Self-reported | 6 months | Baseline | No or not reported | None reported |
| Patel (2016) [51] | Multiple: social media (Facebook); online live chat apps (WhatsApp); email | 16 health promotion messages promoting HIV testing framed in either approach or avoidance style of messaging sent by trained peers via their preferred modality (private Facebook group, individual WhatsApp messaging, or email) | No | 12 weeks (twice weekly) | Yes—facilitated (test locator) | Self-reported | 12 weeks | Baseline | No or not reported | Information motivation behavioral skills model |
| Rhodes et al (2011) [52] | Social media (MSMd-specific sites) | Trained peer posting regular triggers about HIV and HIV testing in existing chat room used by gay and other MSM and engaging in direct communication about testing services, processes, and locations with chat room users | Yes | 6 months (daily) | No | Self-reported | 6 months | Baseline | Yes | Natural helping |
| Rhodes et al (2016) [53] | Social media (MSM-specific sites) | Trained peer posting regular triggers in four existing social media sites used by gay and other MSM about HIV and HIV testing and engaging in direct communication with users about testing services, processes, and locations | Yes | 12 months (daily) | No | Self-reported | 12 months | Baseline | No or not reported | Empowerment education, social cognitive theory, and natural helping |
| Tang et al (2016) [54] | Online video | Online video promoting HIV testing based on a crowdsourced design accessed via banner ads placed on gay-oriented social networking platforms | No | 4 weeks (one time) | No | Self-reported | 3 weeks | Control group: noncrowd sourced online video (standard public health text) | Yes | None reported |
| Washington et al (2017) [55] | Social media (Facebook) | Five, 1-min long videos promoting HIV testing sent through a private Facebook group to black or African American MSM, with moderated group discussion | Yes | 6 weeks (weekly) | Yes—facilitated (test locator) | Self-reported | 6 weeks | Control group: closed Facebook group receiving generic health information | Yes | Integrative model of behavior change |
| Wang et al (2018) [56] | Multiple: online videos; online live chat apps (Line, WhatsApp, and Skype) | Home-based self-testing service comprising online promotional video about HIV testing, plus additional videos on home-based HIV self-testing and offer of free HIV self-testing kit and online real-time instructions and pre- and posttest counseling provided via live chat apps | Yes | 6 months (one time) | Yes—provided (HIV self-testing) | Self-reported or observed uptake of self-testing | 6 months | Control group: online video about (general) HIV testing only | Yes | Health belief model |
| Young et al (2013) [57] | Social media (Facebook) | Trained peer educators providing HIV prevention and testing messages, including 4 weekly reminders about availability of HIV home testing, to participants of a closed Facebook group | Yes | 12 weeks (user -dependent) | Yes—provided (HIV self-testing) | Requested and returned home-based HIV testing kit and followed-up results | 12 weeks | Control group: closed Facebook group receiving per-delivered generic health information | No or not reported | None reported |
| Young et al (2015) [58] | Social media (Facebook) | Trained peer educators providing HIV prevention and testing messages, including 4 weekly reminders about availability of HIV home testing, to participants of a closed Facebook group | Yes | 12 weeks (user-dependent) | Yes—facilitated (referral) | Attendance based | 12 weeks | Control group: closed Facebook group providing HIV testing information without peer leaders | No or not reported | Diffusions of innovation theory and social normative theory |
aSTI: sexually transmitted infection.
bReported as the average follow-up time.
cNR: not reported.
dMSM: men who have sex with men.
Of the 7 studies that used social media, 5 delivered interventions using Facebook [50,51,55,57,58]. All of these studies used closed or private Facebook groups to promote HIV testing, using a range of accompanying features and modalities using internet popular opinion leaders to disseminate HIV-related information and engage in conversations about HIV testing, prevention, and risk behavior [50]; using trained peer educators to deliver HIV prevention and testing information and promote HIV testing uptake [55,58], including through the provision of HIV self-tests [57]; delivering weekly videos promoting HIV testing alongside moderated group discussion [47]; and sending HIV prevention and HIV testing promotion messages (in conjunction with messages sent by WhatsApp and email) [51].
Two social-media-based interventions were delivered through sites specifically targeting the gay community [52,53]. Both reported on the Cyber-Based Education and Referral/testing (CyBER/testing) intervention, which utilized trained peers to promote HIV testing through existing social and sexual networking sites popular among the gay community. CyBER/testing was implemented through an existing chat room used by MSM [52] and later through four geographically focused social media sites used by MSM and TW [53], both in the United States.
Only one intervention was delivered through a custom-built website (Get Connected!), which aimed to promote and connect HIV and STI testing to young MSM [46]. The website delivered customized content to participants based on sociodemographic, sexual identity, and behavior and previous engagement with HIV testing data provided during a baseline assessment, so that messaging and content were personalized to mirror participant profiles, and experiences with testing, including motivations and perceived barriers.
Four studies used online videos to promote HIV testing [47-49,54]. One used single-session online videos focusing on HIV prevention to motivate HIV testing among MSM in the United States, in which participants received either dramatic- or documentary-style videos, or both [49]. A 2010 intervention delivered one 5 min video promoting HIV testing customized based on self-reported sexual identity (gay or nongay identified) to MSM in Peru [47]. In 2014, this intervention was repeated with the addition of motivational messages sent by email or instant messaging to encourage HIV testing to MSM in Peru [48]. Finally, online videos developed with crowdsourced content depicted two Chinese men falling in love and getting tested together to target MSM and TW naïve testers in China [54]. One study used online videos in combination with live chat apps to deliver a home-based HIV self-testing service to MSM in Hong Kong [56]. In this study, all participants were exposed to an online video promoting HIV testing, and then, the participants in the intervention group were offered a home-based self-testing kit and online, real-time HIV pre- and posttest counseling and instruction through Line, WhatsApp, or Skype.
Regarding the length of intervention and the frequency of exposure, interventions that used online videos were typically shorter in duration and involved a single exposure to a video [47,54,56]. Two studies sent multiple videos [49,55] and one combined videos and motivational messages via text, email, or instant messaging but did not report on the length or number of videos and frequency of motivational messages [48]. The customized website-based intervention [46] also provided a single exposure to the website content, but data on the length of time spent on the website were not reported.
Most interventions (n=8) were categorized as interactive for their ability to allow user engagement, for example, through social media platforms, responses on messaging platforms or through email, or via a website. Interventions involving online peer educators interacting with participants through social media platforms were typically longer in duration, ranging from 12 weeks [51,57,58] to 12 months [53]. The frequency of exposure to online peer educators was user-determined, that is, participants were free to choose how often, if at all, they interacted with online peer educators. The exception involved participants receiving twice weekly messages through Facebook, email, or instant messaging, over a 12-week period [51].
Seven studies directly facilitated HIV testing, through the provision of HIV home or self-testing kits [56,57]; referrals to specific, local HIV testing clinics [47,48,58]; or providing location details of free, local HIV testing sites [51,55]. The remaining six studies provided general promotion of HIV testing only.
Five studies specifically mentioned the involvement of intended end users in the implementation design process [46,47,52,55,56]. Theoretical underpinning to intervention development was described by 9 studies [46,47,49,51-53,55,56,58] (Table 2).
Outcomes of the Included Studies
Table 3 presents the reported outcomes of studies using the RCT study design. Most RCT studies measured testing uptake through self-reports [46,49,54,55]. The length of follow-up over which testing was measured varied greatly from 3 weeks [54] to 6 months [56].
Table 3.
Reported HIV testing outcomes in included randomized controlled trial studies.
| Reference | Control group | Intervention group | Risk ratio (95% CI) | |||
|
|
Total number of participants | Participants tested, n (%) | Total number of participants | Participants tested, n (%) |
|
|
| Bauermeister et al (2015) [46] | 36 | 4 (11) | 68 | 18 (26) | 2.4 (0.9-6.5) | |
| Blas et al (2010) [47] | 220 | 10 (4.5) | 239 | 19 (7.9) | 1.7 (0.8-3.7) | |
| Blas et al (2014) [48] | 200 | 3 (1.5) | 200 | 2 (1.0) | 0.7 (0.1-3.9) | |
| Hirshfield et al (2012) [49] | 240 | 48 (20.0) | 676 | 142 (21.0) | 1.1 (0.8-1.4) | |
| Tang et al (2016) [54] | 317 | 111 (35.0) | 307 | 114 (37.1) | 1.1 (0.9-1.3) | |
| Wang et al (2018) [56] | 215 | 109 (50.7) | 215 | 193 (89.8) | 1.8 (1.5-2.0) | |
| Washington et al (2017) [55] | 22 | 8 (36) | 20 | 16 (80) | 2.2 (1.2-4.0) | |
| Young et al (2013) [57] | 55 | 0 (0) | 57 | 8 (14) | N/Aa | |
| Young et al (2015) [58] | 246 | 16 (6.5) | 252 | 43 (17.1) | 2.6 (1.5-4.5) | |
aN/A: not applicable.
On the basis of the calculated RRs, 3 of 9 RCTs demonstrated a significant improvement in HIV testing uptake [55,56,58], whereas 4 demonstrated nonsignificant improvements [46,47,49,54]. The two remaining RCTs did not demonstrate any impact; in one RCT, testing uptake was extremely low in both intervention and control arms [48], and in another, no members of the control group tested for HIV during follow-up [57].
Table 4 presents the outcomes from nonrandomized studies. All of the 4 nonrandomized studies relied on self-reported HIV testing uptake. The length of follow-up ranged from 12 weeks [51] to 12 months [53], and all 4 studies demonstrated significant improvements in testing uptake based on calculated RRs (Table 4).
Table 4.
Reported HIV testing outcomes in included quasi-experimental studies.
| Reference | Baseline | End line | Risk ratio (95% CI) | ||
|
|
Total number of participants | Participants tested, n (%) | Total number of participants | Participants tested, n (%) |
|
| Ko et al (2013) [50] | 501 | 150 (29.9) | 499 | 219 (43.9) | 1.5 (1.2-1.7) |
| Patel (2016) [51] | 130 | 42 (32.3) | 130 | 57 (43.8) | 1.4 (1.0-1.9) |
| Rhodes et al (2011) [52] | 346 | 154 (44.5) | 315 | 187 (59.4) | 1.3 (1.4-1.5) |
| Rhodes et al (2016) [53] | 353 | 122 (34.6) | 399 | 216 (54.1) | 1.6 (1.3-1.9) |
Across all 13 studies included in this review, 21.75% participants (1930/8875) received an HIV test during a cumulative 3.6 years of study follow-up. Three studies reported on HIV diagnoses (all RCTs) [47,54,56], with 75 new HIV infections detected across these studies (56% in the intervention arm; data not reported).
Meta-Analysis
Primary Outcome
The pooled RR across 12 studies (RR could not be calculated for one study because no tests were recorded in the control arm) [57] indicated a significant increase in the uptake of HIV testing following exposure to digital interventions (RR 1.5; 95% CI 1.3-1.7). Statistical heterogeneity was high (χ211=31.7; I2=65.2%; Figure 2).
Figure 2.
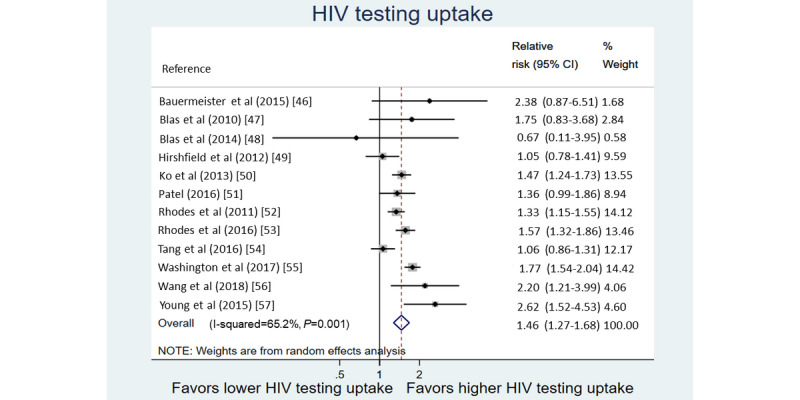
Forest plot of relative risk of HIV testing uptake among MSM and TW following digital intervention exposure.
Subanalysis of the Study Characteristics
A positive impact was seen on the HIV testing uptake across all intervention type subanalyses. The highest pooled RR was seen for interventions that were delivered through the mainstream social media–based platforms (RR 1.7; 95% CI 1.3-2.1), interventions that included direct facilitation of HIV testing (RR 1.6; 95% CI 1.4-1.9), interventions that were interactive (RR 1.6; 95% CI 1.4-1.8), and interventions that involved end users in the design process (RR 1.6; 95% CI 1.3-2.0; Table 5).
Table 5.
Subanalyses by selected study and intervention characteristics.
| Intervention characteristic | ka | Risk ratio (95% CI) | χ2 (df) | I2 | |
| Overall effect size | 12 | 1.5 (1.3-1.7) | 31.7 (11) | 65.2 | |
| Randomized controlled trials only | 8 | 1.6 (1.2-2.1) | 29.1 (7) | 76.0 | |
| Quasi-experimental | 4 | 1.4 (1.3-1.6) | 2.1 (3) | 0 | |
| Direct facilitation of HIV testing | |||||
|
|
Yes | 7 | 1.6 (1.4-1.9) | 13.8 (6) | 65.2 |
|
|
No | 5 | 1.3 (1.0-1.6) | 9.4 (4) | 58.1 |
| Interactive intervention | |||||
|
|
Yes | 7 | 1.6 (1.4-1.8) | 15.0 (6) | 46.7 |
|
|
No | 5 | 1.1 (1.0-1.3) | 1.7 (4) | 65.2 |
| User involvement in design | |||||
|
|
Yes | 5 | 1.6 (1.3-2.0) | 9.4 (4) | 57.6 |
|
|
No or not reported | 6 | 1.4 (1.1-1.6) | 18.0 (5) | 66.7 |
| Theoretical basis to intervention | |||||
|
|
Yes | 9 | 1.6 (1.3-1.8) | 20.5 (8) | 61.0 |
|
|
No or not reported | 3 | 1.2 (0.9-1.7) | 6.2 (2) | 67.5 |
| Social media–based intervention | |||||
|
|
Yes—general | 4 | 1.7 (1.3-2.1) | 6.0 (3) | 49.8 |
|
|
Yes—gay oriented | 2 | 1.4 (1.2-1.7) | 2.0 (1) | 48.7 |
|
|
No | 6 | 1.4 (1.0-1.9) | 23.1 (5) | 78.4 |
| Single-dose intervention | |||||
|
|
Yes | 5 | 1.4 (1.0-1.9) | 22.4 (4) | 82.1 |
|
|
No | 6 | 1.5 (1.3-1.7) | 8.5 (5) | 41.2 |
ak: number of studies included in the subcategory.
Study Quality
The majority of studies (n=8) were classified as moderate quality [46,47,52-56,58], 4 were classified as weak [48-51], and 1 was classified as strong [57]. The most common limitation across studies was insufficient description of blinding procedures (rated as weak in 9 studies), whereas controlling for confounding in either study design or analyses was a common strength (rated as strong in 9 studies; Multimedia Appendix 2).
The observed asymmetry in the study funnel plot (Figure 3) may be explained by the heterogeneity between studies, given the variability in the intervention design. The attribution of asymmetry to heterogeneity is also supported by the high level of variance between studies (I2=65.2%).
Figure 3.
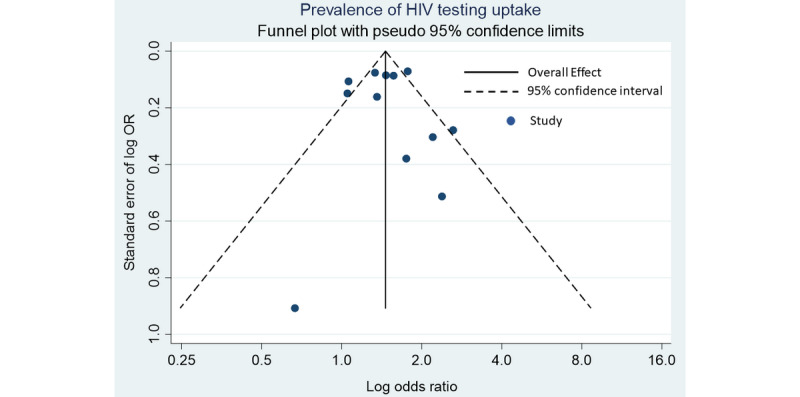
Funnel plot for estimating publication bias and precision of estimate.
Discussion
In this systematic review, exposure to digital communication interventions was associated with greater HIV testing uptake among MSM and TW compared with those unexposed to digital communication interventions. Our findings provide broad support for the integration of technology into existing tools and approaches to HIV prevention among MSM and TW. We extend the current state of evidence regarding the impact of digital interventions on HIV testing by examining the role of key intervention features on effectiveness. Our findings identified key features that were associated with greater uptake for HIV testing for MSM and TW—specifically, interventions that facilitate HIV testing through provision or direct referral to HIV testing services, the use of mainstream social media platforms to engage the target population, interactivity, and involvement of end users in design processes.
Global guidance has recommended the integration of digital technology as a strategy to enhance the reach and effectiveness of HIV prevention efforts among MSM and TW [36,60]. Numerous acceptability studies have identified that MSM would be willing to use phone- and web-based technologies for HIV prevention [39,40,61,62]. Recent systematic reviews have explored the potential of digital technology to advance various HIV priorities among MSM and other key populations [63,64]. One systematic review to date has attempted to quantify the role of digital communication technologies in increasing engagement among key populations across the care continuum through meta-analysis [65]; this study specifically looked at randomized and observational studies describing the impact of social media–based interventions on HIV testing uptake among key populations. All 9 studies included in this meta-analysis targeted MSM and were collectively associated with an approximately 50% increase in HIV testing uptake. Compared with this review, our study adopted a broader definition of digital interventions that encompassed websites, online videos, instant messaging, and live chat apps, resulting in the inclusion of 6 additional studies. Overall, we found that exposure to digital interventions was associated with an approximately 50% increase in HIV testing uptake, in line with the previous study’s estimates, supporting the conclusion that digital communication technologies are effective in promoting HIV testing among MSM and TW. Interventions included in this review invariably took advantage of various online engagement tools such as videos, digital messages tailored to participant profiles, and online social networking and compared the outcomes with more generic and less interactive online messaging. The impact of digital communication technology to increase testing uptake may be even greater when compared with more traditional health promotion mediums such as messaging through public media (such as posters and billboards). In addition, our study identified specific features of digital interventions associated with greater impact on HIV testing uptake among MSM and TW.
First, interventions that went beyond creating general demand for testing, either through health promotion or providing educational content, and instead directly facilitated HIV testing through service referrals or the provision of self-tests demonstrated greater impact on HIV testing uptake compared with the overall estimate. Direct facilitation of HIV testing may potentially address some of the more structural barriers MSM and TW face to HIV testing [17,66,67]. In particular, HIV self-testing has emerged as a strategy to mitigate barriers related to HIV service access [68,69]; however, some MSM and TW populations have expressed concerns about the limited availability of support during the testing process [70,71]. The study by Wang et al [56] included in this review suggests that digital interventions can play a role in promoting HIV self-testing by mitigating user concerns through the provision of real-time, online counseling. It should also be noted that the majority of studies included in this review recruited participants with generally high levels of previous HIV testing behaviors, which may be critical to the success of this approach. Past research has shown that a history of testing is a strong predictor of future testing behaviors among MSM [72-74], suggesting that the direct facilitation of testing through digital communication interventions may work best for those who are experienced in HIV testing and may enable participants to access more frequent testing.
Second, interactive interventions—those that allowed participants to engage directly with online content or other users—demonstrated greater impact on HIV testing uptake compared with the overall pooled estimate. Interactivity in digital interventions has been associated with achieving a greater impact on behavior change across a range of health areas [75-77] and has been identified as a desired feature of digital HIV prevention interventions among MSM and TW [43,78]. Third, our subanalysis also identified that interventions that used mainstream social media platforms, such as Facebook, were also associated with greater uptake of HIV testing. However, all interventions that utilized social media platforms were also categorized as interactive, making it difficult to isolate the source of the enhanced effect. Social networking–based interventions are commonly used for sexual health promotion [79], and the use of existing social media platforms has been identified as a way to enhance retention among young MSM and TW in online HIV prevention activities [43]. Using existing and well-utilized social media platforms may also enhance the reach of digital HIV prevention interventions [80] compared with those that are delivered through new or separate platforms. Interestingly, interventions that used social media sites specifically for gay and other MSM were less effective in increasing HIV testing uptake than interventions delivered through general social media. Others have noted the reluctance of users of gay social networking sites to receive health promotion messages, which are often seen as an intrusion or surveillance and may limit user engagement [81].
Fourth, evidence of enhanced impact was found among digital interventions that reported involvement of end users in the design process. This involvement is a key component of user-centered design, an approach that prioritizes user needs and experiences to maximize functionality and increase engagement and relevance to the target population [82-84]. User involvement is particularly important when developing digital interventions tailored to specific target populations to ensure that such interventions appropriately reflect group priorities, preferences, and culture [83]. Although the literature confirms the value of user-centered design, our review only assessed studies on whether any involvement of end users in the design process was reported; however, it is probable that the quality and depth of this involvement may be a stronger determinant of overall effectiveness. In addition, grounding in theoretical frameworks may also be another indicator of effectiveness, as suggested by the greater uptake of HIV testing reported by theoretically based interventions included in this review. Although the majority of studies in this review reported a theoretical basis for their intervention, the limited number of theory-based, HIV-focused digital interventions have been noted by others [85,86]; this may be attributed to the speed of development and proliferation of digital approaches to improving HIV outcomes. The findings presented here suggest that theoretical grounding is an important component of effective interventions and should be prioritized in future development.
The findings of this review should be considered with the following limitations. First, due to the restrictions we placed on study design, the interventions included in this review reflect only those conducted as research projects and may not reflect the real-world application of digital technology, including interventions that were not formally evaluated or represented in the published literature. Second, despite our finding that the digital interventions included in this review had a positive overall effect on HIV testing uptake among MSM and TW, the findings do not necessarily reflect the actual quality of the content delivered or levels of end-user acceptability, which are likely to interact in important ways with intervention impact. Third, TW participants were underrepresented in the included studies. TW may use social and sexual networking apps less frequently than MSM, which may reflect the limited number of social and sexual networking sites specifically catering to TW relative to MSM [87]. However, factors such as TW’s reported reliance on online sources of sexual health information [88], perceived acceptability of digital approaches to HIV prevention [43], and examples of real-world applications of digital communication technologies to HIV prevention among TW [87] suggest that TW also stand to benefit from digital approaches to HIV prevention and their inclusion in future trials should be prioritized. Finally, the majority of studies were conducted in high- or middle-income settings. Although the use of digital technology to advance HIV prevention priorities in low-resource settings has been both recommended and applied [64,89], further research is warranted to assess the impact of digital interventions of HIV testing among MSM and TW in these settings.
HIV testing is a key focus of global HIV prevention efforts among MSM and TW, yet multiple barriers continue to prevent levels and frequency of testing required to facilitate the early detection of undiagnosed HIV and initiation of treatment. Digital communication technologies are now an accepted medium for HIV prevention efforts; this review provides further evidence of the role of such technologies in increasing HIV testing uptake among MSM and TW. The inclusion of intervention features such as direct facilitation of HIV testing, involvement of end users in the design process, interactivity, and delivery through the existing mainstream social media platforms may enhance the overall impact and maximize the contribution of digital communication technologies to advancing HIV prevention priorities among MSM and TW.
Acknowledgments
This study formed part of the PhD of VV, who was funded through an National Health and Medical Research Council post graduate scholarship.
Abbreviations
- MeSH
Medical Subject Headings
- MSM
men who have sex with men
- RCTs
randomized controlled trials
- RR
risk ratio
- STI
sexually transmitted infection
- TW
transgender women
Appendix
Ovid Medical Literature Analysis and Retrieval System Online, or MEDLARS Online search strategy.
Study quality appraisal.
Footnotes
Authors' Contributions: VV, MS, ML, and MP were responsible for study design; VV and KR conducted the literature review and data extraction; and VV analyzed data and developed the manuscript. KR, ML, AP, CH, and MS provided input into manuscript development. All authors have read and approved the final manuscript before submission.
Conflicts of Interest: None declared.
References
- 1.Beyrer C, Sullivan P, Sanchez J, Baral SD, Collins C, Wirtz AL, Altman D, Trapence G, Mayer K. The increase in global HIV epidemics in MSM. AIDS. 2013 Nov 13;27(17):2665–78. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 2.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, Brookmeyer R. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012 Jul 28;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. http://europepmc.org/abstract/MED/22819660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000-2006: a systematic review. PLoS Med. 2007 Dec;4(12):e339. doi: 10.1371/journal.pmed.0040339. http://dx.plos.org/10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013 Mar;13(3):214–22. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 5.Global AIDS Update. UNAIDS. 2016. [2020-06-02]. https://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 6.Stahlman S, Lyons C, Sullivan PS, Mayer KH, Hosein S, Beyrer C, Baral SD. HIV incidence among gay men and other men who have sex with men in 2020: where is the epidemic heading? Sex Health. 2017 Feb;14(1):5–17. doi: 10.1071/SH16070. [DOI] [PubMed] [Google Scholar]
- 7.Ross AG, Ditangco RA, Belimac JG, Olveda RM, Mercado ES, Rogers GD, Cripps AW, Lam A, Crowe SM. HIV epidemic in men who have sex with men in Philippines. Lancet Infect Dis. 2013 Jun;13(6):472–3. doi: 10.1016/S1473-3099(13)70129-4. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan PS, Hamouda O, Delpech V, Geduld JE, Prejean J, Semaille C, Kaldor J, Folch C, Op de Coul E, Marcus U, Hughes G, Archibald CP, Cazein F, McDonald A, Casabona J, van Sighem A, Fenton KA, Annecy MSM Epidemiology Study Group Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996-2005. Ann Epidemiol. 2009 Jun;19(6):423–31. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Hu H, Liu X, Zhang Z, Xu X, Shi L, Fu G, Huan X, Zhou Y. Increasing HIV incidence among men who have sex with men in Jiangsu province, China: results from five consecutive surveys, 2011-2015. Int J Environ Res Public Health. 2016 Aug 6;13(8):795. doi: 10.3390/ijerph13080795. http://www.mdpi.com/resolver?pii=ijerph13080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon CM, Wong NS, Kwan TH, Wong HT, Chan KC, Lee SS. Changes of sexual risk behaviors and sexual connections among HIV-positive men who have sex with men along their HIV care continuum. PLoS One. 2018;13(12):e0209008. doi: 10.1371/journal.pone.0209008. http://dx.plos.org/10.1371/journal.pone.0209008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosropour CM, Dombrowski JC, Kerani RP, Katz DA, Barbee LA, Golden MR. Changes in condomless sex and serosorting among men who have sex with men after HIV diagnosis. J Acquir Immune Defic Syndr. 2016 Dec 1;73(4):475–81. doi: 10.1097/QAI.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. http://europepmc.org/abstract/MED/21767103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.INSIGHT START Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015 Aug 27;373(9):795–807. doi: 10.1056/NEJMoa1506816. http://europepmc.org/abstract/MED/26192873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Lancet HIV U=U taking off in 2017. Lancet HIV. 2017 Nov;4(11):e475. doi: 10.1016/S2352-3018(17)30183-2. [DOI] [PubMed] [Google Scholar]
- 16.90-90-90: an Ambitious Treatment Target to Help End the Aids Epidemic. UNAIDS. 2014. [2020-06-02]. https://www.unaids.org/en/resources/909090.
- 17.Arreola S, Santos G, Beck J, Sundararaj M, Wilson PA, Hebert P, Makofane K, Do TD, Ayala G. Sexual stigma, criminalization, investment, and access to HIV services among men who have sex with men worldwide. AIDS Behav. 2015 Feb;19(2):227–34. doi: 10.1007/s10461-014-0869-x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz SR, Nowak RG, Orazulike I, Keshinro B, Ake J, Kennedy S, Njoku O, Blattner WA, Charurat ME, Baral SD, TRUST Study Group The immediate eff ect of the same-sex marriage prohibition act on stigma, discrimination, and engagement on HIV prevention and treatment services in men who have sex with men in Nigeria: analysis of prospective data from the TRUST cohort. Lancet HIV. 2015 Jul;2(7):e299–306. doi: 10.1016/S2352-3018(15)00078-8. http://europepmc.org/abstract/MED/26125047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevention Gap Report. UNAIDS. 2016. [2020-06-02]. https://www.unaids.org/en/resources/documents/2016/prevention-gap.
- 20.Nelson KM, Pantalone DW, Gamarel KE, Carey MP, Simoni JM. Correlates of never testing for HIV among sexually active internet-recruited gay, bisexual, and other men who have sex with men in the United States. AIDS Patient Care STDS. 2018 Jan;32(1):9–15. doi: 10.1089/apc.2017.0244. http://europepmc.org/abstract/MED/29232170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus U, Gassowski M, Drewes J. HIV risk perception and testing behaviours among men having sex with men (MSM) reporting potential transmission risks in the previous 12 months from a large online sample of MSM living in Germany. BMC Public Health. 2016 Oct 22;16(1):1111. doi: 10.1186/s12889-016-3759-5. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolsewicz K, Vallely A, Debattista J, Whittaker A, Fitzgerald L. Factors impacting HIV testing: a review-perspectives from Australia, Canada, and the UK. AIDS Care. 2015;27(5):570–80. doi: 10.1080/09540121.2014.986050. [DOI] [PubMed] [Google Scholar]
- 23.Berg RC. Predictors of never testing for HIV among a national online sample of men who have sex with men in Norway. Scand J Public Health. 2013 Jun;41(4):398–404. doi: 10.1177/1403494813483216. [DOI] [PubMed] [Google Scholar]
- 24.Conway DP, Holt M, Couldwell DL, Smith DE, Davies SC, McNulty A, Keen P, Cunningham P, Guy R, Sydney Rapid HIV Test Study Barriers to HIV testing and characteristics associated with never testing among gay and bisexual men attending sexual health clinics in Sydney. J Int AIDS Soc. 2015;18:20221. doi: 10.7448/IAS.18.1.20221. http://europepmc.org/abstract/MED/26318960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirtz AL, Walker DG, Bollinger L, Sifakis F, Baral S, Johns B, Oelrichs R, Beyrer C. Modelling the impact of HIV prevention and treatment for men who have sex with men on HIV epidemic trajectories in low- and middle-income countries. Int J STD AIDS. 2013 Jan;24(1):18–30. doi: 10.1177/0956462412472291. [DOI] [PubMed] [Google Scholar]
- 26.Race K. Speculative pragmatism and intimate arrangements: online hook-up devices in gay life. Cult Health Sex. 2015;17(4):496–511. doi: 10.1080/13691058.2014.930181. [DOI] [PubMed] [Google Scholar]
- 27.Sun CJ, Reboussin B, Mann L, Garcia M, Rhodes SD. The HIV risk profiles of Latino sexual minorities and transgender persons who use websites or apps designed for social and sexual networking. Health Educ Behav. 2016 Feb;43(1):86–93. doi: 10.1177/1090198115596735. http://europepmc.org/abstract/MED/26272786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou H, Fan S. Characteristics of men who have sex with men who use smartphone geosocial networking applications and implications for HIV interventions: a systematic review and meta-analysis. Arch Sex Behav. 2017 May;46(4):885–94. doi: 10.1007/s10508-016-0709-3. [DOI] [PubMed] [Google Scholar]
- 29.Garofalo R, Herrick A, Mustanski BS, Donenberg GR. Tip of the iceberg: young men who have sex with men, the internet, and HIV risk. Am J Public Health. 2007 Jun;97(6):1113–7. doi: 10.2105/AJPH.2005.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips G, Magnus M, Kuo I, Rawls A, Peterson J, Jia Y, Opoku J, Greenberg AE. Use of geosocial networking (GSN) mobile phone applications to find men for sex by men who have sex with men (MSM) in Washington, DC. AIDS Behav. 2014 Sep;18(9):1630–7. doi: 10.1007/s10461-014-0760-9. [DOI] [PubMed] [Google Scholar]
- 31.Liau A, Millett G, Marks G. Meta-analytic examination of online sex-seeking and sexual risk behavior among men who have sex with men. Sex Transm Dis. 2006 Sep;33(9):576–84. doi: 10.1097/01.olq.0000204710.35332.c5. [DOI] [PubMed] [Google Scholar]
- 32.Landovitz RJ, Tseng C, Weissman M, Haymer M, Mendenhall B, Rogers K, Veniegas R, Gorbach PM, Reback CJ, Shoptaw S. Epidemiology, sexual risk behavior, and HIV prevention practices of men who have sex with men using GRINDR in Los Angeles, California. J Urban Health. 2013 Aug;90(4):729–39. doi: 10.1007/s11524-012-9766-7. http://europepmc.org/abstract/MED/22983721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao B, Liu C, Stein G, Tang W, Best J, Zhang Y, Yang B, Huang S, Wei C, Tucker JD. Faster and riskier? Online context of sex seeking among men who have sex with men in China. Sex Transm Dis. 2017 Apr;44(4):239–44. doi: 10.1097/OLQ.0000000000000575. http://europepmc.org/abstract/MED/28282651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beymer MR, Weiss RE, Bolan RK, Rudy ET, Bourque LB, Rodriguez JP, Morisky DE. Sex on demand: geosocial networking phone apps and risk of sexually transmitted infections among a cross-sectional sample of men who have sex with men in Los Angeles County. Sex Transm Infect. 2014 Nov;90(7):567–72. doi: 10.1136/sextrans-2013-051494. http://europepmc.org/abstract/MED/24926041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmiller JJ, Ioerger M. Social networking smartphone applications and sexual health outcomes among men who have sex with men. PLoS One. 2014;9(1):e86603. doi: 10.1371/journal.pone.0086603. http://dx.plos.org/10.1371/journal.pone.0086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Implementing Comprehensive HIV and STI Programmes with Men Who Have Sex with Men. UNFPA - United Nations Population Fund. 2015. [2020-06-02]. https://www.unfpa.org/publications/implementing-comprehensive-hiv-and-sti-programmes-men-who-have-sex-men.
- 37.Lelutiu-Weinberger C, Pachankis JE, Gamarel KE, Surace A, Golub SA, Parsons JT. Feasibility, acceptability, and preliminary efficacy of a live-chat social media intervention to reduce HIV risk among young men who have sex with men. AIDS Behav. 2015 Jul;19(7):1214–27. doi: 10.1007/s10461-014-0911-z. http://europepmc.org/abstract/MED/25256808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pachankis JE, Lelutiu-Weinberger C, Golub SA, Parsons JT. Developing an online health intervention for young gay and bisexual men. AIDS Behav. 2013 Nov;17(9):2986–98. doi: 10.1007/s10461-013-0499-8. http://europepmc.org/abstract/MED/23673791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muessig KE, Pike EC, Fowler B, LeGrand S, Parsons JT, Bull SS, Wilson PA, Wohl DA, Hightow-Weidman LB. Putting prevention in their pockets: developing mobile phone-based HIV interventions for black men who have sex with men. AIDS Patient Care STDS. 2013 Apr;27(4):211–22. doi: 10.1089/apc.2012.0404. http://europepmc.org/abstract/MED/23565925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holloway IW, Rice E, Gibbs J, Winetrobe H, Dunlap S, Rhoades H. Acceptability of smartphone application-based HIV prevention among young men who have sex with men. AIDS Behav. 2014 Feb;18(2):285–96. doi: 10.1007/s10461-013-0671-1. http://europepmc.org/abstract/MED/24292281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowen AM, Williams ML, Daniel CM, Clayton S. Internet based HIV prevention research targeting rural MSM: feasibility, acceptability, and preliminary efficacy. J Behav Med. 2008 Dec;31(6):463–77. doi: 10.1007/s10865-008-9171-6. http://europepmc.org/abstract/MED/18770021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young SD, Jaganath D. Online social networking for HIV education and prevention: a mixed-methods analysis. Sex Transm Dis. 2013 Feb;40(2):162–7. doi: 10.1097/OLQ.0b013e318278bd12. http://europepmc.org/abstract/MED/23324979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawat S, Wilkerson JM, Lawler SM, Patankar P, Rosser BS, Shukla K, Butame S, Ekstrand ML. Recommendations for the development of a mobile HIV prevention intervention for men who have sex with men and hijras in Mumbai: qualitative study. JMIR Public Health Surveill. 2018 May 3;4(2):e46. doi: 10.2196/publichealth.9088. https://publichealth.jmir.org/2018/2/e46/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. http://dx.plos.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quality Assessment Tool for Quantitative Studies. Effective Public Healthcare Panacea Project. 1998. [2017-03-02]. https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/
- 46.Bauermeister JA, Pingel ES, Jadwin-Cakmak L, Harper GW, Horvath K, Weiss G, Dittus P. Acceptability and preliminary efficacy of a tailored online HIV/STI testing intervention for young men who have sex with men: the Get Connected! program. AIDS Behav. 2015 Oct;19(10):1860–74. doi: 10.1007/s10461-015-1009-y. http://europepmc.org/abstract/MED/25638038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blas MM, Alva IE, Carcamo CP, Cabello R, Goodreau SM, Kimball AM, Kurth AE. Effect of an online video-based intervention to increase HIV testing in men who have sex with men in Peru. PLoS One. 2010 May 3;5(5):e10448. doi: 10.1371/journal.pone.0010448. http://dx.plos.org/10.1371/journal.pone.0010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blas M, Menacho L, Alva I. Randomized Controlled Trial to Evaluate the Effect of a Novel Web-based Intervention to Increase HIV Testing in Men Who Have Sex With Men in Lima-Peru. ICH GCP: Good Clinical Practice. 2014. [2020-06-02]. https://ichgcp.net/clinical-trials-registry/NCT01760057.
- 49.Hirshfield S, Chiasson MA, Joseph H, Scheinmann R, Johnson WD, Remien RH, Shaw FS, Emmons R, Yu G, Margolis AD. An online randomized controlled trial evaluating HIV prevention digital media interventions for men who have sex with men. PLoS One. 2012;7(10):e46252. doi: 10.1371/journal.pone.0046252. http://dx.plos.org/10.1371/journal.pone.0046252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko N, Hsieh C, Wang M, Lee C, Chen C, Chung A, Hsu S. Effects of internet popular opinion leaders (iPOL) among internet-using men who have sex with men. J Med Internet Res. 2013 Feb 25;15(2):e40. doi: 10.2196/jmir.2264. https://www.jmir.org/2013/2/e40/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel V, Rawat S, Dange A, Lelutiu-Weinberger C, Golub SA. An internet-based, peer-delivered messaging intervention for hiv testing and condom use among men who have sex with men in India (CHALO!): pilot randomized comparative trial. JMIR Public Health Surveill. 2020 Apr 16;6(2):e16494. doi: 10.2196/16494. https://publichealth.jmir.org/2020/2/e16494/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhodes SD, Vissman AT, Stowers J, Miller C, McCoy TP, Hergenrather KC, Wilkin AM, Reece M, Bachmann LH, Ore A, Ross MW, Hendrix E, Eng E. A CBPR partnership increases HIV testing among men who have sex with men (MSM): outcome findings from a pilot test of the CyBER/testing internet intervention. Health Educ Behav. 2011 Jun;38(3):311–20. doi: 10.1177/1090198110379572. http://europepmc.org/abstract/MED/21393625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhodes SD, McCoy TP, Tanner AE, Stowers J, Bachmann LH, Nguyen AL, Ross MW. Using social media to increase HIV testing among gay and bisexual men, other men who have sex with men, and transgender persons: outcomes from a randomized community trial. Clin Infect Dis. 2016 Jun 1;62(11):1450–3. doi: 10.1093/cid/ciw127. http://europepmc.org/abstract/MED/26980878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W, Han L, Best J, Zhang Y, Mollan K, Kim J, Liu F, Hudgens M, Bayus B, Terris-Prestholt F, Galler S, Yang L, Peeling R, Volberding P, Ma B, Xu H, Yang B, Huang S, Fenton K, Wei C, Tucker JD. Crowdsourcing HIV test promotion videos: a noninferiority randomized controlled trial in China. Clin Infect Dis. 2016 Jun 1;62(11):1436–42. doi: 10.1093/cid/ciw171. http://europepmc.org/abstract/MED/27129465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Washington TA, Applewhite S, Glenn W. Using Facebook as a platform to direct young black men who have sex with men to a video-based HIV testing intervention: a feasibility study. Urban Soc Work. 2017;1(1):36–52. doi: 10.1891/2474-8684.1.1.36. http://europepmc.org/abstract/MED/29276800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Lau JT, Ip M, Ho SP, Mo PK, Latkin C, Ma YL, Kim Y. A randomized controlled trial evaluating efficacy of promoting a home-based HIV self-testing with online counseling on increasing HIV testing among men who have sex with men. AIDS Behav. 2018 Jan;22(1):190–201. doi: 10.1007/s10461-017-1887-2. [DOI] [PubMed] [Google Scholar]
- 57.Young SD, Cumberland WG, Lee S, Jaganath D, Szekeres G, Coates T. Social networking technologies as an emerging tool for HIV prevention: a cluster randomized trial. Ann Intern Med. 2013 Sep 3;159(5):318–24. doi: 10.7326/0003-4819-159-5-201309030-00005. http://europepmc.org/abstract/MED/24026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young SD, Cumberland WG, Nianogo R, Menacho LA, Galea JT, Coates T. The HOPE social media intervention for global HIV prevention in Peru: a cluster randomised controlled trial. Lancet HIV. 2015 Jan;2(1):e27–32. doi: 10.1016/S2352-3018(14)00006-X. http://europepmc.org/abstract/MED/26236767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Bank Country and Lending Groups. The World Bank. 2018. [2020-06-02]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 60.World Health Organization . Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. Geneva, Switzerland: World Health Organization; 2014. [PubMed] [Google Scholar]
- 61.Khosropour CM, Lake JG, Sullivan PS. Are MSM willing to SMS for HIV prevention? J Health Commun. 2014;19(1):57–66. doi: 10.1080/10810730.2013.798373. [DOI] [PubMed] [Google Scholar]
- 62.Czarny HN, Broaddus MR. Acceptability of HIV prevention information delivered through established geosocial networking mobile applications to men who have sex with men. AIDS Behav. 2017 Nov;21(11):3122–8. doi: 10.1007/s10461-017-1743-4. http://europepmc.org/abstract/MED/28260137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnall R, Travers J, Rojas M, Carballo-Diéguez A. eHealth interventions for HIV prevention in high-risk men who have sex with men: a systematic review. J Med Internet Res. 2014 May 26;16(5):e134. doi: 10.2196/jmir.3393. https://www.jmir.org/2014/5/e134/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purnomo J, Coote K, Mao L, Fan L, Gold J, Ahmad R, Zhang L. Using ehealth to engage and retain priority populations in the HIV treatment and care cascade in the Asia-Pacific region: a systematic review of literature. BMC Infect Dis. 2018 Feb 17;18(1):82. doi: 10.1186/s12879-018-2972-5. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-2972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao B, Gupta S, Wang J, Hightow-Weidman LB, Muessig KE, Tang W, Pan S, Pendse R, Tucker JD. Social media interventions to promote HIV testing, linkage, adherence, and retention: systematic review and meta-analysis. J Med Internet Res. 2017 Nov 24;19(11):e394. doi: 10.2196/jmir.7997. https://www.jmir.org/2017/11/e394/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ayala G, Makofane K, Santos G, Beck J, Do TD, Hebert P, Wilson PA, Pyun T, Arreola S. Access to basic HIV-related services and PReP acceptability among men who have sex with men worldwide: barriers, facilitators, and implications for combination prevention. J Sex Transm Dis. 2013;2013:953123. doi: 10.1155/2013/953123. doi: 10.1155/2013/953123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiao S, Zhou G, Li X. Disclosure of same-sex behaviors to health-care providers and uptake of HIV testing for men who have sex with men: a systematic review. Am J Mens Health. 2018 Sep;12(5):1197–214. doi: 10.1177/1557988318784149. http://europepmc.org/abstract/MED/29947563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson C, Baggaley R, Forsythe S, van Rooyen H, Ford N, Mavedzenge SN, Corbett E, Natarajan P, Taegtmeyer M. Realizing the potential for HIV self-testing. AIDS Behav. 2014 Jul;18(Suppl 4):S391–5. doi: 10.1007/s10461-014-0832-x. [DOI] [PubMed] [Google Scholar]
- 69.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav. 2015 Nov;19(11):1949–65. doi: 10.1007/s10461-015-1097-8. http://europepmc.org/abstract/MED/26054390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pal K, Ngin C, Tuot S, Chhoun P, Ly C, Chhim S, Luong M, Tatomir B, Yi S. Acceptability study on HIV self-testing among transgender women, men who have sex with men, and female entertainment workers in Cambodia: a qualitative analysis. PLoS One. 2016;11(11):e0166129. doi: 10.1371/journal.pone.0166129. http://dx.plos.org/10.1371/journal.pone.0166129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirtz AL, Clouse E, Veronese V, Thu KH, Naing S, Baral SD, Beyrer C. New HIV testing technologies in the context of a concentrated epidemic and evolving HIV prevention: qualitative research on HIV self-testing among men who have sex with men and transgender women in Yangon, Myanmar. J Int AIDS Soc. 2017 Apr 25;20(1):21796. doi: 10.7448/IAS.20.01.21796. http://europepmc.org/abstract/MED/28453242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan KE, Wilkinson AL, Leitinger D, El-Hayek C, Ryan C, Pedrana A, Hellard M, Stoové M. Characteristics of gay, bisexual and other men who have sex with men testing and retesting at Australia's first shop-front rapid point-of-care HIV testing service. Sex Health. 2016 Nov;13(6):560–7. doi: 10.1071/SH16027. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson AL, Pedrana AE, El-Hayek C, Vella AM, Asselin J, Batrouney C, Fairley CK, Read TR, Hellard M, Stoové M. The impact of a social marketing campaign on HIV and sexually transmissible infection testing among men who have sex with men in Australia. Sex Transm Dis. 2016 Jan;43(1):49–56. doi: 10.1097/OLQ.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 74.Katz DA, Dombrowski JC, Swanson F, Buskin SE, Golden MR, Stekler JD. HIV intertest interval among MSM in King County, Washington. Sex Transm Infect. 2013 Feb;89(1):32–7. doi: 10.1136/sextrans-2011-050470. http://europepmc.org/abstract/MED/22563016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strecher V, McClure J, Alexander G, Chakraborty B, Nair V, Konkel J, Greene S, Couper M, Carlier C, Wiese C, Little R, Pomerleau C, Pomerleau O. The role of engagement in a tailored web-based smoking cessation program: randomized controlled trial. J Med Internet Res. 2008 Nov 4;10(5):e36. doi: 10.2196/jmir.1002. https://www.jmir.org/2008/5/e36/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neiger BL, Thackeray R, Burton SH, Giraud-Carrier CG, Fagen MC. Evaluating social media's capacity to develop engaged audiences in health promotion settings: use of Twitter metrics as a case study. Health Promot Pract. 2013 Mar;14(2):157–62. doi: 10.1177/1524839912469378. [DOI] [PubMed] [Google Scholar]
- 77.Bailey J, Murray E, Rait G, Mercer CH, Morris RW, Peacock R, Cassell J, Nazareth I. Interactive computer-based interventions for sexual health promotion. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD006483. doi: 10.1002/14651858.CD006483.pub2. [DOI] [PubMed] [Google Scholar]
- 78.Goldenberg T, McDougal SJ, Sullivan PS, Stekler JD, Stephenson R. Preferences for a mobile HIV prevention app for men who have sex with men. JMIR Mhealth Uhealth. 2014 Oct 29;2(4):e47. doi: 10.2196/mhealth.3745. https://mhealth.jmir.org/2014/4/e47/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gold J, Pedrana AE, Sacks-Davis R, Hellard ME, Chang S, Howard S, Keogh L, Hocking JS, Stoove MA. A systematic examination of the use of online social networking sites for sexual health promotion. BMC Public Health. 2011 Jul 21;11:583. doi: 10.1186/1471-2458-11-583. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-11-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones J, Salazar LF. A review of HIV prevention studies that use social networking sites: implications for recruitment, health promotion campaigns, and efficacy trials. AIDS Behav. 2016 Nov;20(11):2772–81. doi: 10.1007/s10461-016-1342-9. [DOI] [PubMed] [Google Scholar]
- 81.Mowlabocus S, Haslop C, Dasgupta RK. From scene to screen: the challenges and opportunities of commercial digital platforms for HIV community outreach. Social Media Soc. 2016 Oct 20;2(4):205630511667288. doi: 10.1177/2056305116672886. [DOI] [Google Scholar]
- 82.Norman D. The Design of Everyday Things. New York, USA: Basic Books; 1988. [Google Scholar]
- 83.Thomas K, Linderoth C, Bendtsen M, Bendtsen P, Müssener U. Text message-based intervention targeting alcohol consumption among university students: findings from a formative development study. JMIR Mhealth Uhealth. 2016 Oct 20;4(4):e119. doi: 10.2196/mhealth.5863. https://mhealth.jmir.org/2016/4/e119/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duff O, Walsh D, Malone S, McDermott L, Furlong B, O'Connor N, Moran K, Woods C. MedFit app, a behavior-changing, theoretically informed mobile app for patient self-management of cardiovascular disease: user-centered development. JMIR Form Res. 2018 Apr 27;2(1):e8. doi: 10.2196/formative.9550. https://formative.jmir.org/2018/1/e8/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henny KD, Wilkes AL, McDonald CM, Denson DJ, Neumann MS. A rapid review of ehealth interventions addressing the continuum of HIV care (2007-2017) AIDS Behav. 2018 Jan;22(1):43–63. doi: 10.1007/s10461-017-1923-2. http://europepmc.org/abstract/MED/28983684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simoni JM, Ronen K, Aunon FM. Health behavior theory to enhance ehealth intervention research in HIV: rationale and review. Curr HIV/AIDS Rep. 2018 Dec;15(6):423–30. doi: 10.1007/s11904-018-0418-8. http://europepmc.org/abstract/MED/30511186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allison SM, Adams D, Klindera KC, Poteat T, Wolf RC. Innovative uses of communication technology for HIV programming for men who have sex with men and transgender persons. J Int AIDS Soc. 2014;17:19041. doi: 10.7448/IAS.17.1.19041. http://europepmc.org/abstract/MED/25280864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magee JC, Bigelow L, Dehaan S, Mustanski BS. Sexual health information seeking online: a mixed-methods study among lesbian, gay, bisexual, and transgender young people. Health Educ Behav. 2012 Jun;39(3):276–89. doi: 10.1177/1090198111401384. [DOI] [PubMed] [Google Scholar]
- 89.Expanding Access to HIV Services for Men Who Have Sex With Men Through Mobile Phone and Web-based Platforms in Myanmar. MEDBOX. 2017. [2020-06-02]. https://www.medbox.org/document/expanding-access-to-hiv-services-for-men-who-have-sex-with-men-through-mobile-phone-and-web-based-platforms-in-myanmar#GO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ovid Medical Literature Analysis and Retrieval System Online, or MEDLARS Online search strategy.
Study quality appraisal.


