This quality improvement study assesses aerosol filtration efficiencies for fitted face mask alternatives used during the coronavirus disease 2019 pandemic.
Key Points
Question
How effective are the aerosol filtration efficiencies for fitted face mask alternatives used during the coronavirus disease 2019 pandemic?
Findings
In this quality-improvement study of 29 fitted face mask alternatives, expired N95 respirators with intact elastic bands and masks that had been subjected to ethylene oxide and hydrogen peroxide sterilization had unchanged fitted filtration efficiencies (FFEs) of more than 95%, while the performance of N95 respirators in the wrong size resulted in decreased FFEs between 90% and 95%. As a group, surgical and procedure masks had lower FFEs relative to N95 respirators, with masks secured with elastic ear loops showing the lowest performance.
Meaning
When new N95 respirators are unavailable, N95 respirators past their expiration date; sterilized, used N95 respirators; and other less common respirators can be acceptable alternatives.
Abstract
Importance
Procuring respiratory protection for clinicians and other health care workers has become a major challenge of the coronavirus disease 2019 (COVID-19) pandemic and has resulted in nonstandard practices such as the use of expired respirators and various decontamination processes to prolong the useful life of respirators in health care settings. In addition, imported, non–National Institute for Occupational Safety and Health (NIOSH)-approved respirators have been donated or acquired by hospitals as a potential replacement for limited NIOSH-approved N95 respirators.
Objective
To assess fitted filtration efficiencies (FFEs) for face mask alternatives used during the COVID-19 pandemic.
Design, Setting, and Participants
For this quality-improvement study conducted between April and June 2020, we used the Occupational Safety and Health Administration’s Quantitative Fit Testing Protocol for Filtering Facepiece Respirators in a laboratory atmosphere supplemented with sodium chloride particles to assess the FFEs of a variety of respirators worn by a male volunteer and female volunteer.
Main Outcomes and Measures
The FFEs of respirators commonly worn by clinicians and other health care workers and available respirator alternatives during the COVID-19 pandemic.
Results
Of the 29 different fitted face mask alternatives tested on 1 man and 1 woman, expired N95 respirators with intact elastic straps and respirators subjected to ethylene oxide and hydrogen peroxide sterilization had unchanged FFE (>95%). The performance of N95 respirators in the wrong size had slightly decreased performance (90%-95% FFE). All of the respirators not listed as approved in this evaluation (n = 6) failed to achieve 95% FFE. Neither of the 2 imported respirators authorized for use by the Centers for Disease Control and Prevention that were not NIOSH-approved tested in this study achieved 95% FFE, and the more effective of the 2 functioned at approximately 80% FFE. Surgical and procedural face masks had filtering performance that was lower relative to that of N95 respirators (98.5% overall FFE), with procedural face masks secured with elastic ear loops showing the lowest efficiency (38.1% overall FFE).
Conclusions and Relevance
This quality-improvement study evaluating 29 face mask alternatives for use by clinicians interacting with patients during the COVID-19 pandemic found that expired N95 respirators and sterilized, used N95 respirators can be used when new N95 respirators are not available. Other alternatives may provide less effective filtration.
Introduction
A major concern during the coronavirus disease 2019 (COVID-19) pandemic has been protection of clinicians and other health care workers from severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection by respiratory aerosol and contact transmission. A widespread, acute shortage in personal protective equipment, primarily filtering facepiece respirators (henceforth referred to as respirators), has prompted the implementation of nonstandard practices to fill the need for protection in health care settings. The Centers for Disease Control and Prevention (CDC) has developed contingency and crisis strategies to provide alternatives for respirators, including use beyond expiration dates, decontamination and reuse, and use of non–National Institute for Occupational Safety and Health (NIOSH)-approved respirators.1
Comparative fitted filtration efficiencies (FFEs), combined intrinsic filtering efficiency of material and efficacy of fit to the face, for respirator alternatives have not previously been quantified in a comprehensive manner, and health care facilities are faced with prioritization of their available options without clear data to guide decision-making. To address this need, we have performed a series of FFE evaluations for a wide range of 29 respirators and face masks used by health care facilities, including expired N95 respirators, N95 respirators that have undergone sterilization, CDC-approved imported respirators, respirators not listed as approved, and surgical or procedure masks with ties and ear loops.
Methods
Fitted filtration efficiency tests were conducted between April and June 2020 in a custom-built exposure chamber (US Environmental Protection Agency Human Studies Facility in Chapel Hill, North Carolina). The institutional review board at the University of North Carolina at Chapel Hill waived the need for study approval as well as individual consent needed for device testing. Face masks available by purchase or contribution to the local UNC Health Care facility were fitted on an adult male volunteer (weight, 75 kg; height, 178 cm; head circumference, 58.5 cm) with no beard. For sex comparison of small and regular-sized face masks commonly used in health care facilities, an adult female volunteer was also tested (weight, 53 kg; height, 160 cm; head circumference, 56.0 cm). Fitted filtration efficiency tests were conducted as prescribed by the Occupational Safety and Health Administration’s Modified Ambient Aerosol CNC Quantitative Fit Testing Protocol For Filtering Facepiece, Table A-2.2
Accordingly, FFE was measured during a series of repeated movements of the torso, head, and facial muscles to simulate typical occupational activities experienced by a mask wearer. A Particle Generator 8026 (TSI) was used to supplement ambient particle counts in the chamber, with sodium chloride particles having a count median diameter of 0.05 μm, as measured by a scanning mobility particle sizer. Particle concentration in the chamber was allowed to stabilize for 30 minutes prior to testing. All face masks were fitted with sampling probes using a Fit Test Probe Kit for Disposable Facepieces 8025-N95 (TSI) to allow sampling of aerosol inside of the face mask. Face masks fitted with sampling probes can be seen in the Figure. A pair of Condensation Particle Counters 3775 (TSI) was run in single particle analysis mode to continuously monitor particles (0.02-3.00 μm) in the chamber just outside of the face mask (ambient) and behind the face mask at a sampling rate of 1 second. Ambient particle counts/cc were typically in the range of 2000 to 5000. Ten feet of 0.25-inch conductive rubber tubing was used for each sampling line, and a small piece of nonconductive tubing and stopcock served as a connector between the sampling port and the conductive tubing sampling line. The ambient sampling line and masks sampling line were made identical to reduce variability in the system.
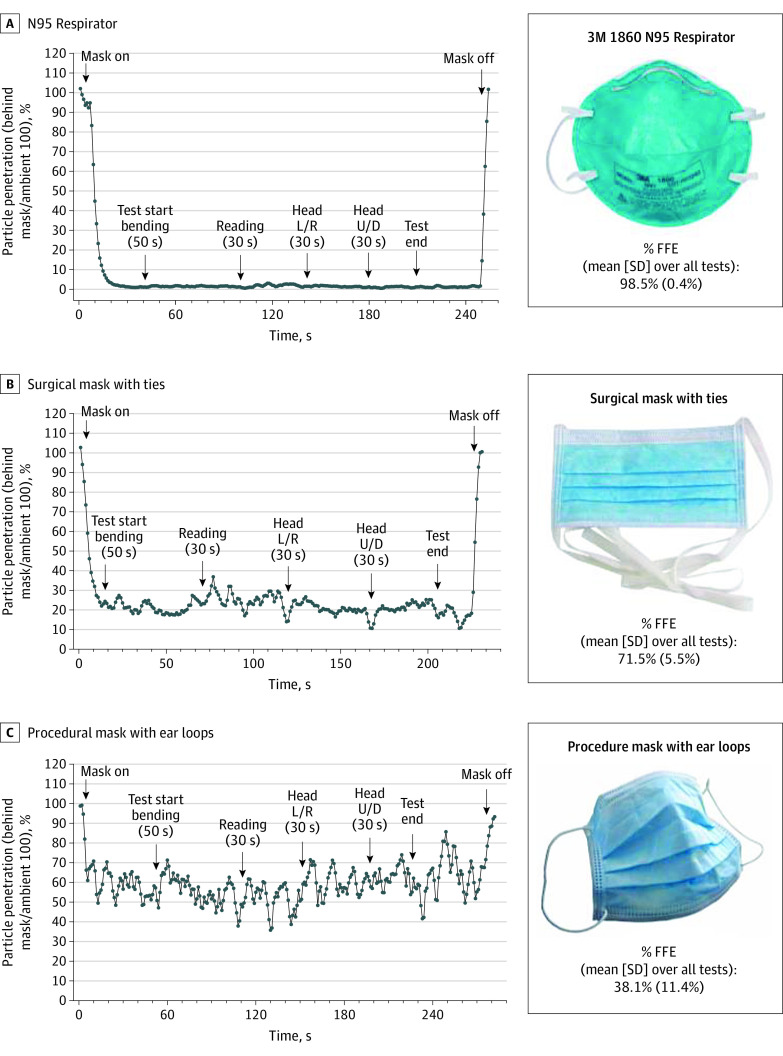
Figure. Evaluation of Fitted Filtration Efficiency (FFE) Using the Occupational Safety and Health Administration Modified Ambient Aerosol CNC Quantitative Fit Testing Protocol for Filtering Facepiece.
When used on a man, the overall FFE for a National Institute for Occupational Safety and Health–approved N95 respirator was 98.5% (A), the overall FFE of a surgical mask with ties was 71.5% (B), and the overall FFE of a procedural mask with elastic ear loops was 38.1% (C). Data correspond to particle penetration into the face mask as expressed as the percentage of total particle/cc measured simultaneously outside of the face mask.
The temperature during testing ranged from 23 °C to 29.5 °C, and the relative humidity was 10% to 50%. The overall FFE was averaged from start to end of the testing period, and the average standard deviation over the period of sampling was computed (total testing time was about 3 minutes). Three respirator sterilization methods were tested on used masks: ethylene oxide (EtO) (500 mg/L-hours at 50 °C, 16-hour cycle), steam (121 °C, 15 minutes), and vaporized hydrogen peroxide (8 g/min, 260 PPM, 100-minute cycle). Each sterilization load was monitored with mechanical, chemical, and biological indicators specific to the sterilizer manufacturer’s instructions. The FFE of these sterilized, used masks was measured after a single sterilization cycle as described above.
Results
Table 13,4 shows FFEs for all face masks tested. A Controlled Air Purifying Respirator system (MAXAIR) fitted with a face shield supplied by the device manufacturer prevented more than 99% of particles from entering the test individual’s breathing space. N95 respirators up to 11 years past their expiration (expired in 2009 and 2011) and N95 respirators subjected to EtO and vaporized hydrogen peroxide sterilization retained FFEs more than 95%. Although N95 respirators with exhalation valves (Particulate Respirator 8511 [3M]) are not generally used in health care settings because the expired air is not filtered by the face mask, their FFE was also more than 95%. All of the respirators not listed as approved (eg, KN95 [Guangdong Fei Fan]) and the 2 CDC-approved, imported, non–NIOSH-approved respirators (DTC-3X-1 and DTC-3X-2 [Dasheng]) in this evaluation failed to achieve 95% FFE. As expected, surgical and procedure masks had substantially lower average FFEs than the N95 respirators, and the variability in their performances was observed to be largely dependent on the tightness of the contact between the material and the test individual’s facial skin. In all tests, the FFE of masks with ties outperformed those with ear loops.
Table 1. Face Mask Fitted Filtration Efficiency (FFE) Against Submicron Particle Penetration.
| Face mask | Condition | Approved | % FFE (SD)a | No. of masks tested on male test individual |
|---|---|---|---|---|
| Commonly used | ||||
| MAXAIR Controlled Air Purifying Respirator systemb | New | NA | 99.6 (0.1) | 1 |
| 3M 8210 N95b | New | Yesc | 97.9 (0.5) | 2 |
| 3M 8210 N95b | Expired in 2011 | NA | 98.5 (0.4) | 3 |
| 3M 1860 N95b | New | Yesc | 98.5 (0.4) | 1 |
| 3M 1860 N95b | Expired in 2009 | NA | 97.0 (1.0) | 3 |
| 3M 1860 N95b | EtO sterilized | NA | 98.1 (0.5) | 3 |
| 3M 1860 N95b | H2O2 sterilized | NA | 96.8 (0.7) | 1 |
| 3M 1870+ Aura N95b | New | Yesc | 99.2 (0.3) | 1 |
| 3M 1870+ Aura N95b | Autoclaved | NA | 98.0 (0.4) | 3 |
| Halyard Health 46827 N95b | New | Yesc | 99.5 (0.1) | 1 |
| Surgical mask | ||||
| With ties | New | NA | 71.5 (5.5) | 4 |
| With ear loops | New | NA | 38.1 (11.4) | 3 |
| Less commonly used | ||||
| Dasheng DTC-3Z with head strapsb | New | Yesc | 99.2 (0.3) | 1 |
| 3M 8511 N95 with exhaust valveb | New | Yesc | 98.0 (0.5) | 1 |
| Moldex 2200 N95b | New | Yesc | 97.8 (0.5) | 1 |
| One Sperian HC-NB295F Duckbillb | New | Yesc | 97.7 (0.7) | 1 |
| 3M 9010 CN N95b | New | Yesc | 97.6 (0.8) | 1 |
| Dasheng DTC-3W with head strapsb | New | Yesc | 95.5 (1.2) | 1 |
| Safemark Magic City 6950 Duckbillb | New | Yesc | 95.2 (1.3) | 1 |
| U-Line S-9632 | New | Yesc | 94.2 (1.4) | 1 |
| SAS Safetycorp 8617 Duckbill | New | Not listed | 93.2 (1.4) | 1 |
| Willson Saf-T Fit N1105 medium/large (Honeywell) | New | Yesc | 93.0 (1.8) | 1 |
| Fangtian Duckbill FT-032 with exhaust valve | New | Not listed | 86.2 (2.8) | 1 |
| Safe-Life N95 B150 | New | Not listed | 85.9 (2.0) | 1 |
| Jia Hu Kang KN95 mask with ear loops | New | Not listed | 85.1 (2.2) | 1 |
| Dasheng DTC-3X1 with ear loops | New | Yes (CDC only)d | 79.7 (4.4) | 1 |
| Zhongshan Dongfeng Huangshang GM700 | New | Not listed | 79.2 (6.8) | 1 |
| Dasheng DTC-3X2 with ear loops | New | Yes (CDC only)d | 76.8 (5.5) | 1 |
| Guangdong Fei Fan KN95 | New | Not listed | 53.2 (6.8) | 1 |
Abbreviations: CDC, Centers for Disease Control and Prevention; EtO, ethylene oxide; H2O2, vaporized hydrogen peroxide; NA, not applicable; NIOSH, National Institute of Occupational Safety and Health.
The FFE percentage corresponds to the mask condensation particle counter counts/ambient condensation particle counter counts × 100. The FFE percentage and SD were calculated across the length of the test.
Mask functioned at or above 95% FFE.
Denotes NIOSH-approved N95 particulate filtering facepiece respirators.3
Denotes CDC-approved, imported, non–NIOSH-approved respirators manufactured in China.4
Finally, Table 2 compares respirator and face mask FFEs for the man and woman. The small 1860 N95 respirator (3M) on the man and the regular 1860 N95 respirator on the woman failed to achieve an FFE more than 95%; however, both were more than 90% efficient. All other single-sized N95 face masks reached more than 95% FFE on both the man and woman. The mask with ear loops was less efficient on the woman’s face relative to the man’s face.
Table 2. Face Mask Fitted Filtration Efficiency (FFE) Against Submicron Particle Penetration.
| Face mask | % FFE (SD)a | |
|---|---|---|
| Man | Woman | |
| 3M 1860 N95 | ||
| Small | 91.1 (4.0) | 98.6 (0.4) |
| Regular | 98.4 (0.5) | 93.1 (1.6) |
| 3M 8210 N95 (one size only)b | 99.4 (0.3) | 98.2 (0.7) |
| 3M 8511 N95 with exhalation valveb | 98.1 (0.7) | 98.3 (0.9) |
| Surgical mask | ||
| With ties | 69.8 (5.1) | 68.9 (10.9) |
| With ear loops | 39.7 (10.5) | 26.5 (14.0) |
The FFE percentage corresponds to the mask condensation particle counter counts/ambient condensation particle counter counts × 100. The FFE percentage and SD were calculated across the length of the test.
Mask functioned at or above 95% FFE.
Discussion
While Controlled Air Purifying Respirator systems and new NIOSH-approved N95 respirators fitted to the face are clearly the preferred choice of protection from bioaerosols, the availability of these items may be compromised during periods of high demand, such as a pandemic. The current study provides a comparative evaluation of particle penetration for commonly available face masks and alternatives while fitted to the face at baseline and under nonstandard conditions (eg, expired, sterilized) using a worst-case scenario of exposure to very small aerosols. Results of this study show that, despite an 11-year expiration, N95 respirators with intact headbands exceeded 95% FFE. Recently, sterilization and decontamination of face masks has emerged as a novel method to prolong the limited supply of existing respirators.5 Both EtO and vaporized hydrogen peroxide, which are effective sterilization agents and well known for material compatibility, had no deleterious effect on FFE after a single sterilization. A potential disadvantage of EtO sterilization is that the wearer may be exposed to residual EtO within the face mask. We also evaluated steam sterilization for 2 respirator models. Steam visibly distorted the 1860 N95 respirators, making them unsuitable for reuse. However, 1870+ Aura face masks (3M) were not visibly altered and maintained more than 95% FFE when subjected to a single cycle of steam autoclaving.
Neither of the CDC-approved, imported respirators lacking NIOSH certification functioned at or above 95% efficiency, and the most effective face mask achieved only 79.7% FFE. These respirators, which have elastic ear loops and a vertical fold design, were least effective when the test individual bent at the waist and looked up and down. Procedure masks with ear loops performed at 38.1% FFE and were the least effective when moving the head left and right (21.2% FFE), and created visible gaps between the face mask and the wearer. Taken together, these data suggest that elastic ear loops may not provide adequate tension to maintain a tight fit during a typical range of motions. Moreover, these findings illustrate the importance of fit for maximizing the overall effectiveness of both respirators and masks.
Clinicians and other health care workers are exposed to polydispersed aerosols when caring for patients, particularly during aerosol-generating procedures. SARS-CoV-2 virions are 50 to 200 nm in diameter6 but can be transported and transmitted on much larger droplets, which may be the major source of transmission. The count median diameter of particles in the test atmosphere was measured at 50 nm, likely smaller than SARS-CoV-2 virions or droplets containing the virus. However, the particle size of the test atmosphere was very similar to the sodium chloride particle size used to test and certify N95 respirators (75 ± 20 nm), which is deemed appropriate by the US Code of Federal Regulations (42 CFR §84, subpart K). Based on the mechanisms of particle deposition that govern filtration by face masks (ie, diffusion, impaction, interception, and sedimentation), it is clear that protection against aerosols with a count median diameter of 50 nm would also confer similar or better protection against much larger aerosols or droplets larger than 3 μm.7 In fact, for masks with an electric charge (such as those manufactured by 3M), the most penetrating particle size was found to be 30 to 60 nm,8 which is similar in size to those used for measurements in this study.
Limitations
A limitation of this study is the decision to test each mask on a single man (and woman for a few comparisons) rather than a large number of individuals with a full range of facial configurations. On the other hand, the use of 1 common individual allowed for testing of a larger quantity of masks in a short period of time, which addressed the urgent need for hospital infection prevention decision-making.
Conclusions
Evidence from previous studies suggests that even face masks with less than 95% FFE (eg, surgical masks) are effective in preventing acquisition of epidemic coronaviruses (ie, severe acute respiratory syndrome coronavirus 1, SARS-CoV-2) by clinicians and other health care workers except possibly during aerosol-generating procedures.9,10,11 For prevention of a related coronavirus, severe acute respiratory syndrome coronavirus 1, N95 respirators had no increased prevention benefit over surgical masks.10 However, the CDC and Infectious Diseases Society of America has recommended the use of N95 respirators especially during aerosol-generating procedures as long as the supplies are available. This evaluation provides quantitative results on which health care administrators, supply chain leaders, and hospital epidemiologists can make evidence-based decisions to protect clinicians and other health care workers during a pandemic or long-term mask shortage.
References
- 1.Strategies for optimizing the supply of N95 respirators. Centers for Disease Control and Prevention. April 2, 2020. Updated June 28, 2020. Accessed July 24, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html
- 2.Additional ambient aerosol CNC quantitative fit testing protocols: respiratory protection standard. Occupational Safety and Health Administration. September 26, 2019. Accessed July 30, 2020. https://www.osha.gov/laws-regs/federalregister/2019-09-26
- 3.NIOSH-approved N95 particulate filtering facepiece respirators. Centers for Disease Control and Prevention. Updated June 15, 2020. Accessed July 24, 2020. https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/N95list1sect3.html
- 4.Appendix A: authorized imported, non-NIOSH approved respirators manufactured in China. US Food and Drug Administration. Updated June 24, 2020. Accessed July 24, 2020. https://www.fda.gov/media/136663/download
- 5.Decontamination and reuse of filtering facepiece respirators. Centers for Disease Control and Prevention. April 29, 2020. Updated April 30, 2020. Accessed July 24, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- 6.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinds, WC. Filter efficiency. In: Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. 2nd ed. Wiley;1999:196-199. [Google Scholar]
- 8.Rengasamy S, Eimer BC, Shaffer RE. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hyg. 2009;53(2):117-128. doi: 10.1093/annhyg/men086 [DOI] [PubMed] [Google Scholar]
- 9.Wong SCY, Kwong RT, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119-127. doi: 10.1016/j.jhin.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(11):1934-1942. doi: 10.1093/cid/cix681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch JB, Davitkov P, Anderson DJ, et al. Infectious Diseases Society of America guidelines on infection prevention in patients with suspected or known COVID-19. Infectious Diseases Society of America. April 27, 2020. Updated April 30, 2020. Accessed July 24, 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/