Abstract
AIM
To determine the overall rate of chest imaging findings in asymptomatic cases, describe the most common patterns found, and determine the rate of later symptom development in these initially asymptomatic cases.
MATERIALS AND METHODS
The PubMed and EMBASE databases were searched until 1 May 2020, for studies examining the proportion of positive chest imaging findings in asymptomatic cases diagnosed with COVID-19 and a random-effects meta-analysis of proportions was performed. Heterogeneity was assessed using the I2 statistic.
RESULTS
Among 858 non-duplicate studies, seven studies with a total of 231 asymptomatic cases met the inclusion criteria. In the primary analysis, the pooled estimate of the overall rate of positive chest computed tomography (CT) findings among asymptomatic cases was 63% (95% confidence interval [CI]: 44–78%). Among 155/231 cases that were followed up for later symptom development, 90/155 remained asymptomatic and 65/155 developed symptoms during the study period (that ranged between seven and 30 days of follow-up). The pooled estimate of the rate of positive chest CT findings was 62% (95% CI: 38–81%) in cases that remained asymptomatic, while it was 90% (95% CI: 49–99%) in cases that developed symptoms. Among CT findings, the pooled estimate of the overall rate of ground-glass opacities (GGO) at CT alone was 71% (95% CI: 50–86%). Among other CT findings reported, 22/231 patients had GGO with consolidation, 7/231 patients had stripe shadows with or without GGO, and 8/231 patients had GGO with interlobular septal thickening. Among initially asymptomatic cases with positive CT findings, the pooled estimate of the overall rate of later symptom development was 26% (95% CI: 14–43%).
CONCLUSION
In COVID-19, asymptomatic cases can have positive chest CT findings, and COVID-19 should be considered among cases with CT abnormalities even when there are no other symptoms. There is a need for close clinical monitoring of asymptomatic cases with radiographic findings as a significant percentage will develop symptoms.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects human respiratory epithelial cells via interaction of the virus envelope spike (S) protein and the angiotensin-converting enzyme 2 (ACE2) cell receptor.1 SARS-CoV-2 replicates efficiently in the respiratory tract and during the prodromal period, infected individuals produce a large quantity of virus, contributing to the spread of the infection.2 SARS-CoV-2 also causes lower respiratory tract lesions2 and autopsy findings in patients with COVID-19 revealed diffuse alveolar damage and infiltrating perivascular lymphocytes, along with vascular features of microthrombi and angiogenesis.3
Patients with COVID-19 present with fever, dry cough, fatigue, sputum production, shortness of breath, sore throat, headache, nausea, vomiting, and diarrhoea4; however, asymptomatic infected individuals are of great concern as they undermine control interventions that rely on identifying symptomatic cases to contain the pandemic5 and >40% of cases are infected by an asymptomatic or pre-symptomatic carrier.6 Notably, chest imaging findings are very common in COVID-19.7 , 8 Most commonly, patients present with bilateral or multilobar involvement and with ground-glass opacities (GGO).8 The purpose of this systematic review and meta-analysis is to determine the overall rate of chest imaging findings in asymptomatic cases, describe the most common patterns found and determine the rate of later symptom development in these initially asymptomatic cases.
Materials and methods
This review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.9 The PRISMA checklist for this review is provided in Electronic Supplementary Material Table S1.
Literature search strategy
The Pubmed and EMBASE databases were searched until 1 May 2020, for articles reporting chest imaging findings in asymptomatic cases with COVID-19. The following combination of keywords was used for search (all fields): (covid OR sars-cov2 OR 2019-ncov) AND (imaging OR “computed tomography” OR CT OR Xray OR asymptomatic).
Study selection and data extraction
Potentially relevant articles (selected based on their titles and abstracts) were assessed for eligibility by two independent investigators (M.T.V., E.A.). Apart from chest computed tomography (CT) findings, chest radiograph findings were included in the literature search to potentially broaden the analysis; however, only one study reported chest radiograph findings, so chest radiograph findings were not included in the analysis.
The following eligibility criteria were applied. Inclusion criteria were studies published in English and reporting chest imaging findings (both chest radiograph and CT) in individuals tested positive for SARS-CoV-2, without clinical symptoms at the time of the positive test were included. Exclusion criteria were studies with <10 cases (in order to reduce sampling error); studies including only asymptomatic cases with evident pneumonia on CT; studies including asymptomatic cases that were diagnosed based on chest imaging criteria; studies providing chest imaging findings in cases tested positive for SARS-CoV-2 without available data on the clinical symptoms.
Data extraction was performed independently by two authors (M.T.V., E.A.). Disagreements and inconsistencies were resolved by a third author (M.K.). The following information were extracted from each eligible study: number of asymptomatic cases, number of asymptomatic cases with chest imaging findings, type of imaging findings, and potential development of symptoms among asymptomatic cases.
Terms used in the secondary analysis
Based on the available data in the studies, cases were further divided into two groups (remained asymptomatic and developed symptoms), based on the development of clinical symptoms. The “remained asymptomatic” group included cases that did not have clinical symptoms of infection when tested positive for SARS-CoV-2 and never developed symptoms during the study period. Therefore, this group included individuals with subclinical infection.5 The “developed symptoms” group included cases that did not have clinical symptoms of infection when tested positive for SARS-CoV-2, but developed symptoms later on during the study period. Therefore, these were individuals that when tested for SARS-CoV-2 were in the incubation period.5
Statistical analysis and visualisation tools
In the primary analysis, a random effects meta-analysis of proportions was performed using the DerSimonian–Laird model10 and the logit transformation to determine the overall positive chest imaging findings rate (pooled estimate) among asymptomatic cases with COVID-19. A random effects model was selected, because it was assumed that the rates are heterogeneous due to differences in the study settings as well as differences in the study populations, in terms of age, comorbidities, etc.
In the secondary analysis, only studies reporting later symptom development with associated chest imaging findings for each case were included. Subgroup analysis was performed, including cases that remained asymptomatic and cases that developed symptoms. Common between variance was assumed, to determine the proportion of the heterogeneity that could be explained by the subgroups.
In the tertiary analysis, only initially asymptomatic patients with positive CT were included. Among them, a meta-analysis of proportions was performed to calculate the rate of GGO alone in their CT. Furthermore, in the tertiary analysis, a meta-analysis of proportions was performed to calculate the rate of symptom development among them. Meta-analyses of proportions and subgroup analysis were performed, and forest plots using R statistics software were created.11
R statistics software was also applied to assess heterogeneity between the studies included in the meta-analyses (Cochran Q test)12 and to determine the inconsistency across the studies included (I2 statistic).13 The criterion for statistical significance for the test for heterogeneity was set at alpha =0.05. Although this is a meta-analysis of proportions and the risk of publication bias is limited,14 the latter were included in the analysis to be comprehensive. The risk of publication bias was assessed and visualised using a funnel plot.15
Results
Study selection
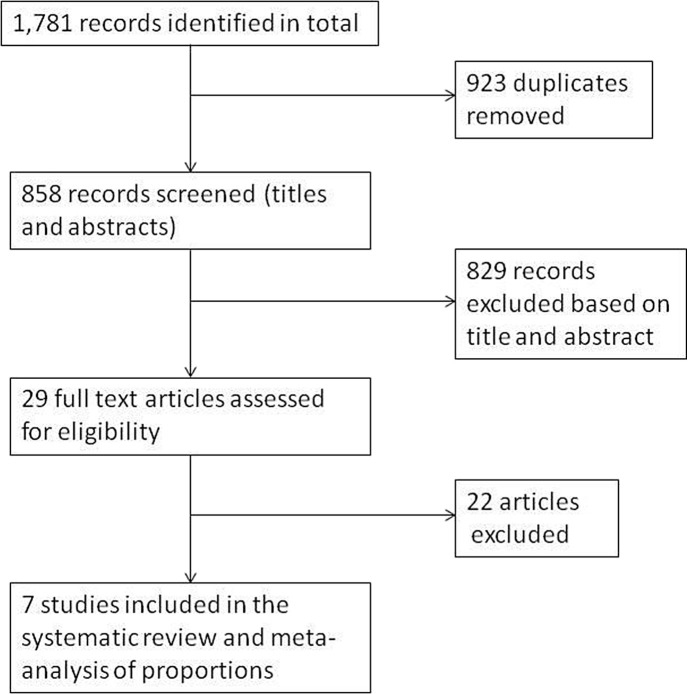
Initially, 1,781 studies were identified. After removing duplicate studies, 858 studies were screened based on title and abstract. After the screening, 29 full-text articles were assessed for eligibility based on the aforementioned inclusion and exclusion criteria. At the end, seven studies fulfilled the criteria and were included in the analysis. Fig 1 outlines the study selection process.
Figure 1.
Flow chart of the study selection process.
Study characteristics and findings
All seven studies that were included in the meta-analysis described chest CT findings in cases that had no clinical manifestations of viral infection at the time they were tested positive for SARS-CoV-2. In total, 231 asymptomatic cases were included in the seven studies. Table 1 summarises in detail the characteristics of each study.5 , 16, 17, 18, 19, 20, 21 As noted above, only one study reported chest radiograph findings, so chest radiograph findings were not included in the analysis.
Table 1.
Characteristics of eligible studies.
| Study | No. of asymptomatic cases included in the beginning | Type of imaging (CT or radiograph) | Remained asymptomatic or developed symptoms or undetermined course | No. of cases that remained asymptomatic-developed symptoms-undetermined course | No. of cases with positive findings | Findings (n) | Lung findings distribution (n) |
|---|---|---|---|---|---|---|---|
| An et al., 202016 | 25 | CT | Remained asymptomatic | 16 | 24 (undetermined which case did not have imaging findings) | GGO alone (16), GGO + consolidation shadow (5), GGO + stripe shadow (2), GGO + interlobular septal thickening (1) | Single lobe (16), Two lobes (4), >2 lobes (4) Bilateral (6) Peripheral (18) |
| Developed symptoms | 9 | ||||||
| He et al., 202017 | 12 | CT | Remained asymptomatic | 12 | 5 | GGO (5) | Bilateral (4) |
| Hu et al., 202018 | 24 | CT | Remained asymptomatic | 19 | 12 | GGO (8), Stripe shadow (4) | NA |
| Developed symptoms | 5 | 5 | GGO (4), Stripe shadow (1) | NA | |||
| Inui et al., 202019 | 76 | CT | Undetermined | 76 | 41 | GGO alone (17), GGO + intra/inter-lobular septal thickening (7), GGO + consolidation (17) |
Single lobe (10), Two lobes (12), >2 lobes (19) RLL (29), LLL (29) Bilateral (34) Peripheral (24) |
| Wang et al., 202020 | 55 | CT | Remained asymptomatic | 16 | 37 (undetermined which case did not have imaging findings) | Pneumonia (39) | NA |
| Developed symptoms | 39 | ||||||
| Zhou X et al., 20205 | 13 | CT | Remained asymptomatic | 10 | 9 | GGO (12) | NA |
| Developed symptoms | 3 | 3 | |||||
| Zhou J et al., 202021 | 26 | CT | Remained asymptomatic | 17 | 5 (undetermined which case did not have imaging findings) | GGO (5) | Peripheral (5) |
| Developed symptoms | 9 |
CT, computed tomography; GGO, ground glass opacity; NA, not applicable; RLL, right lower lobe; LLL, left lower lobe.
Regarding symptom development, after the diagnosis and isolation of the asymptomatic cases, six studies reported the clinical course of included cases.5 , 16, 17, 18 , 20 , 21 When reported, the follow-up in the studies ranged from 7–30 days. Among the cases included in these studies, 90 cases remained asymptomatic during the study period, whereas 65 cases developed symptoms at some point during the study period. Notably in three studies, imaging findings were not linked to specific cases, so they were not included in the secondary analysis.16 , 20 , 21
CT findings are described in detail in Table 1. The most common CT finding in all subgroup categories was GGO and was present alone (in 67/231 patients) or together with other findings including consolidation shadow (22/231 patients), stripe shadows (7/231 patients) and interlobular septal thickening (8/231 patients).5 , 16, 17, 18, 19 , 21 The findings were most commonly peripheral and were bilateral or unilateral (mostly involving a single lobe).16 , 17 , 19 , 21
Pooled estimates
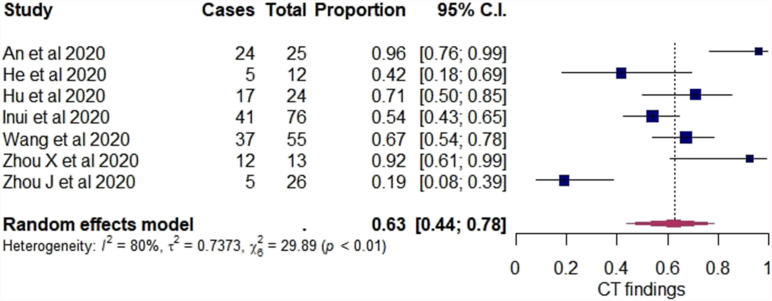
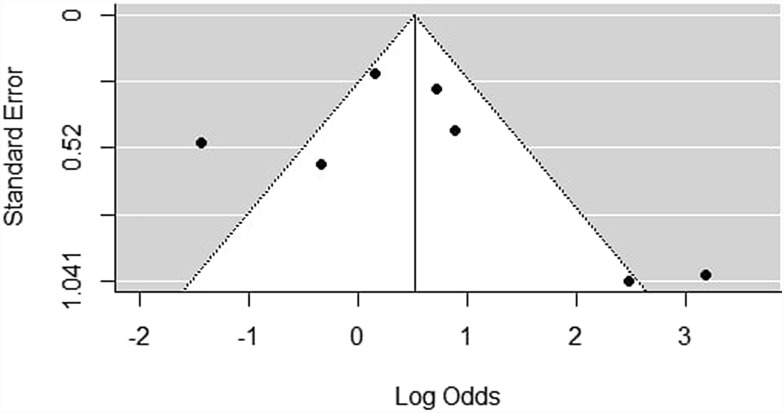
Studies included in all of the proportional meta-analyses and the meta-analysis of odds ratios were heterogeneous, as determined by the Cochrane Q test and I2 statistic. Using the random-effects model, the pooled estimate of the overall rate of initially asymptomatic cases with positive chest CT findings was 63% (95% CI: 44–78%) and the heterogeneity was substantial (I2 80%, p<0.01). In addition, a forest plot was constructed to visualise the pooled estimates with their confidence intervals (Fig 2 ) and a funnel plot for the assessment of publication bias (Fig 3 ); however, no statistical test to assess this bias was conducted, as <10 studies were included in the analysis and, in this case, the power of the tests was too low to distinguish chance from real asymmetry.22
Figure 2.
Forest plot of proportional meta-analysis on proportion of initially asymptomatic cases with positive chest CT findings.
Figure 3.
Bias assessment (funnel) plot for studies assessing the proportion of asymptomatic cases with positive chest CT findings.
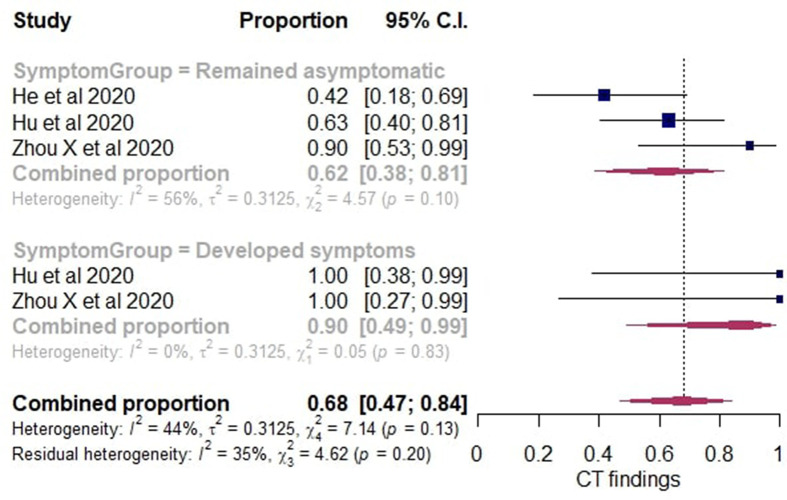
In the secondary analysis, only studies that reported later symptom development with associated chest imaging findings for each case were included. A subgroup analysis was performed and the cases were divided into those who remained asymptomatic and those who developed symptoms. Using the random-effects model, among the cases that remained asymptomatic the pooled estimate of the rate positive chest CT findings was 62% (95% CI: 38–81%). Among the individuals who developed symptoms, the pooled estimate of the rate of positive chest CT findings was 90% (95% CI: 49–99%). The pooled estimate of the overall rate of initially asymptomatic cases included in the secondary analysis with positive chest CT findings was 68% (95% CI: 47–84%). A forest plot was constructed (Fig 4 ).
Figure 4.
Forest plot of proportional meta-analysis with subgroup analysis on proportion of asymptomatic cases with positive chest CT findings.
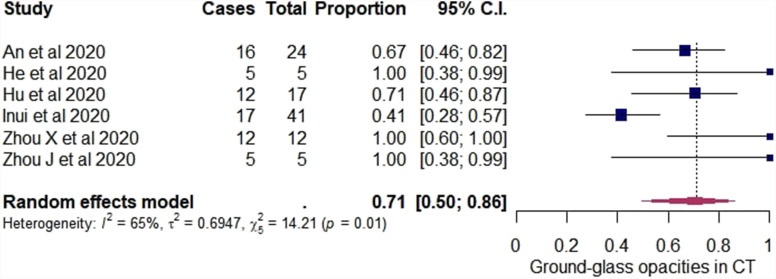
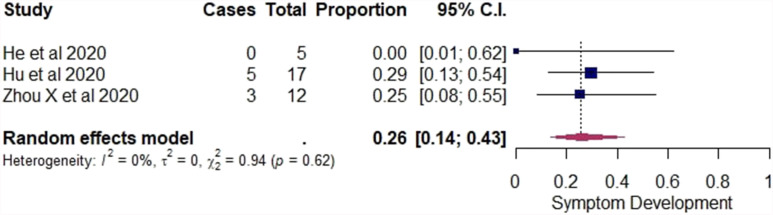
In the tertiary analysis, initially asymptomatic cases with normal CT were excluded and only initially asymptomatic cases with positive chest CT findings were included. Using the random-effects model among these cases, the pooled estimate of the overall rate of GGO alone in the CT was 71% (95% CI: 50–86%). A forest plot was constructed (Fig 5 ). For each study, the “cases” column presents the number of asymptomatic cases with GGO in their CT and the “total” column presents the number of initially asymptomatic cases with positive chest CT findings in the study. Among the cases included in the tertiary analysis, the pooled estimate of the overall rate of symptom development was 26% (95% CI: 14–43%). A forest plot was constructed (Fig 6 ) and the “cases” column presents the number of cases that developed symptoms and the “total” column presents the number of initially asymptomatic cases with positive chest CT findings. In both the forest plots in Figure 5, Figure 6, the “proportion” column presents the proportion for each study with their 95% CI and in bold are presented the pooled estimates of the overall rates with their 95% CI.
Figure 5.
Forest plot of proportional meta-analysis on proportion of ground-glass opacities in CT of initially asymptomatic cases with positive chest CT.
Figure 6.
Forest plot of proportional meta-analysis on proportion of symptom development in initially asymptomatic cases with positive chest CT.
Discussion
Asymptomatic cases with COVID-19 are of great concern. They contribute to the spread of SARS-CoV-2,23 with similar transmission rates as symptomatic patients,24 while the virus replicates in their lower respiratory tract resulting in radiological evidence of infection.25 Reports show that asymptomatic cases with normal chest CT have shorter periods from diagnosis to being SARS-CoV-2 negative than asymptomatic cases with positive chest CT.26 COVID-19 should be considered among cases with CT abnormalities even when there are no other symptoms.
Interestingly, the present study found that the rate of symptom development in initially asymptomatic cases with chest CT findings is substantial. This should raise concern among clinicians towards close monitoring of patients that test positive for SARS-CoV-2 and have chest imaging findings, as some will eventually become symptomatic. Xiao et al., have reported COVID-19 cases without respiratory symptoms at presentation with incidentally positive chest CT findings, who eventually developed respiratory symptoms warranting critical care and even leading to death27; however, the median age of the patients studied by Xiao et al. was 64 years, whereas in the three studies included in the analysis the median ages of patient populations were 31, 32, and 52 years. In the study, all cases that developed symptoms eventually recovered, indicating a good prognosis of this population. The aforementioned age differences raise concerns about whether older age should prompt more careful monitoring of asymptomatic cases with chest CT findings for development of severe disease.
COVID-19 can result in radiographic lung injury abnormalities even in cases without clinical symptoms. The pooled estimate of the overall rate of positive chest CT findings in asymptomatic individuals was significantly high. This is important and supports that clinicians should be vigilant and suspect COVID-19 among cases with CT abnormalities even when there are no other symptoms. When subgroup analysis based on the later appearance of clinical symptoms was performed, cases with eventual symptom development had higher rates of positive chest CT findings than cases that remained asymptomatic throughout the study period; however, that difference did not reach statistical significance and given the small number of studies included in this analysis, further studies are needed to determine whether such a difference exists.
Peripheral, bilateral GGO with or without consolidation or visible intralobular lines (“crazy-paving”) is a typical CT appearance in COVID-19 pneumonia, according to the Radiological Society of North America Expert Consensus Statement.28 Inui et al., have reported that GGO findings predominate over consolidation in asymptomatic cases, whereas the opposite was observed in the majority of symptomatic patients.19 Shi et al., have outlined the progression of COVID-19 lesions and reported that the predominant pattern in early stages is unilateral and multifocal GGO. These lesions evolve to bilateral, diffuse GGO and are later replaced by consolidation and mixed patterns as the disease progresses.29 In the analysis, most of the initially asymptomatic cases demonstrated GGO in their chest CT. This is congruent with early chest imaging findings as reported by Shi et al., as well as other studies reporting the evolution of CT findings in individuals with COVID-19 pneumonia.29 , 30 Asymptomatic cases with consolidation changes existed in the study, showing that asymptomatic cases can demonstrate even advanced-stage changes. Radiologists have an important role in disease progression monitoring, by identifying the various lung injury patterns.
In the present study, a significant proportion of asymptomatic cases had positive chest CT findings; however, there were still asymptomatic cases with negative chest CT, which would be missed if CT was the only screening method. Chest CT should not replace reverse transcription polymerase chain reaction (RT-PCR) for screening in asymptomatic cases,31 as the diagnostic sensitivity of chest CT at detecting COVID-19 in asymptomatic individuals is lower than detecting COVID-19 in symptomatic patients, and is <60%.32 Moreover, the radiation exposure risk associated with CT is not negligible.33
To the authors' knowledge, this is the first meta-analysis that estimates the proportion of asymptomatic individuals with positive chest CT findings; however some limitations should be considered. First, there was considerable heterogeneity. When only studies that reported later symptom development were considered, the subgroup analysis accounted for some of the heterogeneity, but there was still residual heterogeneity left. This could be due to different study designs, as well as different characteristics of the populations included in the study, such as viral load exposure, or comorbidities. In addition, no conclusions can be drawn regarding the presence of publication bias. Although the funnel plot was asymmetric, the urgency of new evidence during the pandemic facilitates publication of very small studies. Furthermore, the study is geographically limited to Asia. As a result, studies from other continents, including Europe and USA, are needed.
In conclusion, the study demonstrates that CT chest can be abnormal in asymptomatic COVID-19 cases, although it should not be used as a screening tool. Clinicians should consider COVID-19 in cases with positive chest CT findings, even without clinical symptoms. Radiologists can greatly help clinicians by identifying the various patterns of disease progression. Finally, asymptomatic individuals with positive chest CT findings should be followed closely, as a significant proportion will eventually develop symptoms.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crad.2020.07.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymann D.L., SNWS and TAG for IH COVID-19: what is next for public health? Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X., Li Y., Li T. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;S1198–743X(20):30169–30175. doi: 10.1016/j.cmi.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J., Zhong Z., Li H. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J Med Virol. 2020:1–12. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration; 2011. Random-effects (DerSimonian and Laird) method for meta-analysis.https://handbook-5-1.cochrane.org/chapter_9/9_4_3_1_random_effects_dersimonian_and_laird_method_for.htm Available at: (accessed May 1st 2020) [Google Scholar]
- 11.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing.https://www.R-project.org/ Available online at: [Google Scholar]
- 12.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 13.Higgins J.P.T., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang N. 2018. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial conducting meta-analyses of proportions in R. [DOI] [Google Scholar]
- 15.Egger M., Smith G.D., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An P., Song P., Wang Y. Asymptomatic patients with novel coronavirus disease (COVID-19) Balk Med J. 2020:19–20. doi: 10.4274/balkanmedj.galenos.2020.2020.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He G., Sun W., Fang P. The clinical feature of silent infections of novel coronavirus infection (COVID-19) in Wenzhou. J Med Virol. 2020 doi: 10.1002/jmv.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z., Song C., Xu C. Clinical characteristics of 24 asymptomatic infections with COVID- 19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inui S., Fujikawa A., Jitsu M. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imag. 2020;2(2) doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Liu Y., Liu L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221(11):1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J Tan Y., Li D., He X. Observation and analysis of 26 cases of asymptomatic SARS-COV2 infection. J Infect. 2020;S0163–4453(20):30156–30160. doi: 10.1016/j.jinf.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration; 2011. Recommendations on testing for funnel plot asymmetry.https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed May 1st 2020) [Google Scholar]
- 23.Aguirre-Duarte N. Can people with asymptomatic or pre-symptomatic COVID-19 infect others: a systematic review of primary data? medRxiv. January 2020 doi: 10.1101/2020.04.08.20054023. [DOI] [Google Scholar]
- 24.Yin G., Jin H. Comparison of transmissibility of coronavirus between symptomatic and asymptomatic patients: reanalysis of the Ningbo Covid-19 data. medRxiv. January 2020 doi: 10.1101/2020.04.02.20050740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Tong J., Qin Y. Characterization of an asymptomatic cohort of SARS-COV-2 infected individuals outside of Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y., Yu X., Du X. Epidemiological and clinical characteristics of 26 asymptomatic SARS-CoV-2 carriers. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao N., Abboud S., McCarthy D.M. Incidentally discovered COVID-19 in low-suspicion patients-a threat to front line health care workers. Emerg Radiol. 2020:1–7. doi: 10.1007/s10140-020-01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson S., Kay F.U., Abbara S. Radiological society of North America Expert Consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. J Thorac Imag. 2020 doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W.H., Wang X.W., Cai Z.Q. Chest CT as a screening tool for COVID-19 in unrelated patients and asymptomatic subjects without contact history is unjustified. Quant Imag Med Surg. 2020;10(4):876–877. doi: 10.21037/qims.2020.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai K., Tabata S., Ikeda M. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J Clin Virol. 2020;128:104393. doi: 10.1016/j.jcv.2020.104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y.X.J., Liu W.H., Yang M.C.W. The role of CT for Covid-19 patient’s management remains poorly defined. Ann Transl Med. 2020;8(4):145. doi: 10.21037/atm.2020.02.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.