Abstract
Background
Venous thromboembolism (VTE) may complicate the course of Coronavirus Disease 2019 (COVID-19).
Objectives
To evaluate the incidence of VTE in patients with COVID-19.
Methods
MEDLINE, EMBASE, and PubMed were searched up to 24th June 2020 for studies that evaluated the incidence of VTE, including pulmonary embolism (PE) and/or deep vein thrombosis (DVT), in patients with COVID-19. Pooled proportions with corresponding 95% confidence intervals (CI) and prediction intervals (PI) were calculated by random-effect meta-analysis.
Results
3487 patients from 30 studies were included. Based on very low-quality evidence due to heterogeneity and risk of bias, the incidence of VTE was 26% (95% PI, 6%–66%). PE with or without DVT occurred in 12% of patients (95% PI, 2%–46%) and DVT alone in 14% (95% PI, 1%–75%). Studies using standard algorithms for clinically suspected VTE reported PE in 13% of patients (95% PI, 2%–57%) and DVT in 6% (95% PI, 0%–60%), compared to 11% (95% PI, 2%–46%) and 24% (95% PI, 2%–85%) in studies using other diagnostic strategies or patient sampling. In patients admitted to intensive care units, VTE occurred in 24% (95% PI, 5%–66%), PE in 19% (95% PI, 6%–47%), and DVT alone in 7% (95% PI, 0%–69%). Corresponding values in general wards were respectively 9% (95% PI, 0%–94%), 4% (95% PI, 0%–100%), and 7% (95% CI, 1%–49%).
Conclusions
VTE represents a frequent complication in hospitalized COVID-19 patients and often occurs as PE. The threshold for clinical suspicion should be low to trigger prompt diagnostic testing.
Keywords: Anticoagulants, COVID-19, Pulmonary embolism, SARS virus, Venous thromboembolism
Highlights
-
•
Incidence of venous thromboembolism (VTE) in Coronavirus Disease-2019 (COVID-19) is unclear.
-
•
A total of 3487 patients with COVID-19 were included in 30 observational studies.
-
•
VTE incidence varied due to differences in diagnostic protocols and hospital setting.
-
•
VTE risk was higher in intensive care units, but seemed also substantial in general wards despite prophylaxis.
1. Introduction
Coronavirus disease 2019 (COVID-19) represents an unprecedented threat to global health care systems with more than 4,900,000 cases and over 300,000 deaths worldwide as of 22th May 2020 [1]. The severity of COVID-19 varies between patients ranging from mild respiratory disease to acute respiratory distress syndrome, shock, and multi-organ failure associated with an increased risk of death [2]. The clinical course of COVID-19 is often accompanied by a hyperinflammatory response and systemic coagulation derangement, which may evolve into overt disseminated intravascular coagulopathy (DIC) [3]. The systemic activation of blood coagulation and pulmonary thrombo-inflammation with local vascular damage caused by COVID-19 may increase the risk of venous thromboembolism (VTE) and pulmonary artery thrombosis [4,5].
Since the start of the outbreak, several studies reported on the risk of VTE in hospitalized patients with COVID-19. While initial observations showed a VTE incidence of 15–25%, more recent studies found that VTE risk may be as high as 85% despite all patients receiving pharmacological thromboprophylaxis [[6], [7], [8], [9], [10]]. The large variability may be related to differences in patient sampling, hospital setting, and diagnostic protocols for VTE.
The objectives of this systematic review and meta-analysis were to evaluate the incidence of VTE in patients admitted for COVID-19 and identify subgroups at high risk.
2. Materials and methods
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) guidelines [11].
2.1. Study search and selection
A systematic search of MEDLINE, EMBASE, and PubMed was performed from 1st January 2020 up to 24th June 2020 for studies which reported on VTE in hospitalized adult patients with COVID-19 (Supplementary Tables 1 to 3).
Two authors independently screened title, abstract, and full-text of records identified from the search. The following inclusion criteria were applied: (i) cohort study or RCT evaluating the incidence of VTE in COVID-19; (ii) inclusion of ≥10 hospitalized patients with COVID-19. Case reports and case series including less than 10 patients were excluded as they may report misleading results due to the small sample size. Publications in languages other than English were not considered. Any disagreement was resolved through discussion between the review authors or involving a third review author.
2.2. Data extraction and risk of bias assessment
The following data were independently extracted by two review authors: study characteristics (e.g. study design, country where study was conducted, number of included patients, health-care setting), patients characteristics (e.g. age, sex, presence of comorbidities), anticoagulant treatment during hospitalization (e.g. type, dose, and duration of anticoagulant therapy), number of patients who experienced VTE, type and clinical presentation of VTE (symptomatic or incidentally detected pulmonary embolism [PE] or deep vein thrombosis [DVT]). Any disagreement was resolved by consensus between the two review authors or by involving a third review author.
The primary outcome of interest was VTE which included objectively diagnosed fatal or non-fatal PE and DVT of the upper or lower limbs. Secondary outcomes were PE with or without DVT, and DVT alone.
The risk of bias for observational studies was evaluated with the methodological index for non-randomized studies (MINORS), while the Cochrane tool was used to evaluate the quality of RCTs [12,13]. The MINORS tool considers eight quality items which may be deemed as adequate, inadequate, or unclear [12]. The item related to the endpoint definition was considered appropriate if type of VTE and diagnostic protocol used in the study were clearly specified. Follow-up duration was considered adequate if the occurrence of VTE was assessed in all patients included in the study until hospital discharge or death.
2.3. Statistical analysis
Categorical variables were described as counts and percentages and continuous variables presented as median (interquartile range) or mean (standard deviation), as appropriate. For meta-analytical purposes, single proportions were logit transformed to maintain symmetry. Summary estimates with the corresponding 95% prediction interval (PI) and 95% confidence interval (CI) were calculated by random-effect meta-analysis. The Wilson score was used to estimate CIs for the individual studies. As estimates of I-squared are uninformative in meta-analyses of single proportions, the between-study heterogeneity was evaluated by visual inspection of forest plots. The incidence of VTE was evaluated according to hospital setting (i.e. intensive care unit [ICU] versus general ward) and diagnostic protocol for VTE (standard diagnostic algorithm vs other) in random-effects subgroup meta-analyses. Standard diagnostic algorithm was defined as the use of imaging tests in case of clinical suspicion of VTE and/or sudden increase of D-dimer levels [14,15]. In addition, we planned to analyse the incidence of VTE according to dose of pharmacological prophylaxis, if data were available.
Statistical analyses were performed using R studio version 1.2.5001, “meta” and “forest” packages [16].
3. Results
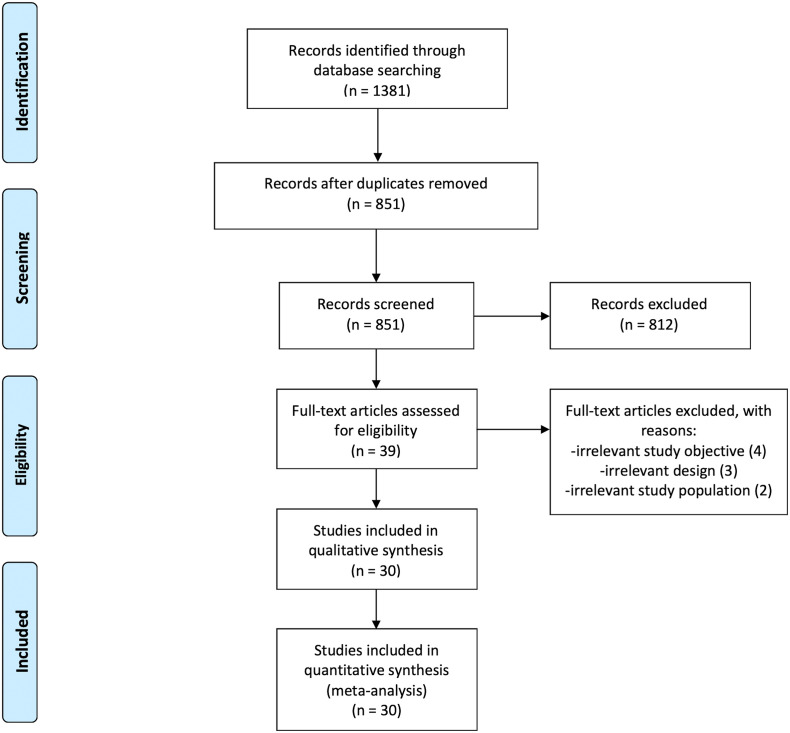
A total of 1381 records were identified from the database search (Fig. 1 ). After removing 530 duplicates, 812 records were excluded by title and abstract screening. Of the remaining 39 studies evaluated as full-text, 30 were included with a total of 3487 patients. The inter-reviewer agreement was excellent with a kappa statistic of 0.90 [17].
Fig. 1.
PRISMA flow diagram.
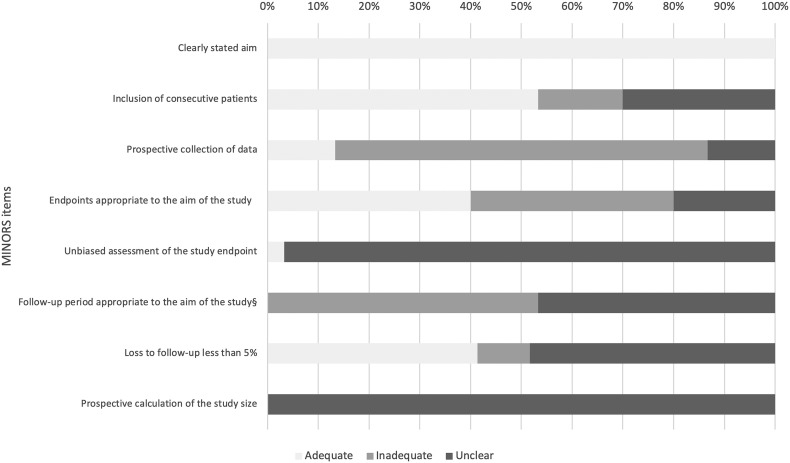
The size of the included studies ranged from 12 to 400 patients (Table 1 ). Four studies were prospective, 22 retrospective, and in 4 the study design was unclear. Twenty-four studies were conducted in Europe, three in China, and three in the United States. Fig. 2 shows the summary risk of bias. Sixteen studies (70.0%) included patients consecutively and none had adequate follow-up duration with 33.7% of patients (499/1480; 11 studies) still hospitalized at the end of the study.
Table 1.
Characteristics of included studies.
| Study | Center | Country | Patients No. |
Age years |
Male No. (%) |
Patients in ICU No. (%) |
Any dose anticoagulant, No. (%) | Prophylactic dose anticoagulant, No. (%) | Therapeutic dose anticoagulant, No. (%) | Diagnostic workup | VTE No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Samkari H [33] | Multicenter | US | 400 | – | 228 (57.0) | – | 390 (97.5) | 355 (88.8) | – | Suspicion of VTE | 20 (5.0) |
| Artifoni M [34] | Multicenter | France | 71 | 64 | 43 (60.6) | 0 | 71 (100) | 70 (98.6) | – | Suspicion of VTE | 16 (22.5) |
| Betoule A [35] | Single center | France | 76 | 62 | – | 0 | – | – | – | Not specified | 2 (2.6) |
| Beun R [36] | Single center | The Netherlands | 75 | – | – | 75 (100) | – | – | – | Suspicion of VTE | 23 (30.7) |
| Beyls C [37] | Single center | France | 12 | 62 | 10 (83.3) | 12 (100) | – | – | – | Not specified | 1 (8.3) |
| Bompard F [38] | Multicenter | France | 135 | 64 | 94 (69.6) | 24 (17.8) | – | 72 (53.3) | – | Suspicion of VTE | 32 (23.7) |
| Cattaneo M [39] | Single center | Italy | 64 | 70 | 35 (54.7) | 0 | 64 (100) | 64 (100) | 0 | Screening CUSb | 0 |
| Criel M [40] | Single center | Belgium | 82 | – | 48 (58.5) | 30 (36.6) | 80 (97.6) | – | – | Not specified | 6 (7.3) |
| Cui S [8] | Single center | China | 81 | 60 | 37 (45.7) | 81 (100) | 0 | 0 | – | Not specified | 20 (24.7) |
| Demelo-Rodrìguez P [18] | Single center | Spain | 156 | 68 | 102 (65.4) | 0 | 153 (98.1) | 153 (98.1) | – | Screening CUSb | 23 (14.7) |
| Desborough M [28] | Single center | UK | 66 | 59 | 48 (72.7) | 66 (100) | 66 (100) | 55 (83.3) | 11 (16.7) | Suspicion of VTE | 10 (15.2) |
| Fraissè M [29] | Single center | France | 92 | 61 | 73 (79.3) | 92 (100) | 92 (100) | 43 (46.7) | 49 (53.3) | Suspicion of VTE | 31 (33.7) |
| Grandmaison G [41] | Single center | Switzerland | 58 | – | – | 29 (50.0) | 49 (84.5) | 42 (72.4) | 7 (12.1) | Suspicion of VTEb | 23 (39.7) |
| Grillet F [42] | Single center | France | 100 | 66 | 70 (70.0) | 39 (39.0) | – | – | – | CT scan in selected patients | 23 (23.0) |
| Helms j [30] | Multicenter | France | 150 | 63 | 122 (81.3) | 150 (100) | 150 (100) | 105(70.0) | 45 (30.0) | Suspicion of VTEa | 28 (18.7) |
| Klok FA [7] | Multicenter | The Netherlands | 184 | 64 | 139 (75.5) | 184 (100) | 184 (100) | 167 (90.8) | 17 (9.2) | Suspicion of VTE | 68 (37.0) |
| Leonard-Lorant I [43] | Single center | France | 106 | 63 | 70 (66.0) | 48 (45.3) | 49 (46.2) | 42 (39.6) | 7 (6.6) | CT scan in selected patients | 32 (30.2) |
| Llitjos JF [9] | Multicenter | France | 26 | 68 | 20 (76.9) | 26 (100) | 26 (100) | 8 (30.8) | 18 (69.2) | Suspicion of VTEb | 20 (76.9) |
| Lodigiani C [31] | Single center | Italy | 388 | 66 | 264 (68.0) | 61 (15.7) | 335 (86.3) | 194 (50.0) | 74 (19.1) | Suspicion of VTE | 16 (4.1) |
| Longchamp A [32] | Single center | Switzerland | 25 | 68 | 16 (64.0) | 25 (100) | 25 (100) | 23 (92.0) | 2 (8.0) | Unclear | 8 (32.0) |
| Maatman TK [44] | Multicenter | US | 109 | 61 | 62 (56.9) | 109 (100) | 109 (100) | 109 (100) | 0 | Suspicion of VTEc | 31 (28.4) |
| Middeldorp S [45] | Single center | The Netherlands | 198 | 61 | 130 (65.7) | 74 (37.4) | 186 (93.9) | 167 (84.3) | 19 (9.6) | Suspicion of VTEd | 33 (16.7) |
| Nahum J [46] | Single center | France | 34 | 62 | 25 (73.5) | 34 (100) | 34 (100) | 34 (100) | 0 | Screening CUS | 27 (79.4) |
| Poissy J [47] | Single center | France | 107 | 57 | 13 (12.1) | 107 (100) | 107 (100) | 107 (100) | 0 | Suspicion of VTEa | 24 (22.4) |
| Poyiadji N [48] | Multicenter | US | 328 | – | 150 (45.7) | 82 (25.0) | – | – | – | CT scan in selected patients | 72 (22.0) |
| Ren B [10] | Multicenter | China | 48 | 70 | 26 (54.2) | 48 (100) | 47 (97.9) | 47 (97.9) | – | Screening with CUS | 41 (85.4) |
| Tavazzi G [49] | Single center | Italy | 54 | 68 | 45 (83.3) | 54 (100) | 54 (100) | 54 (100) | 0 | Not specified | 10 (18.5) |
| Thomas W [50] | Single center | UK | 63 | 59 | 44 (78.6) | 63 (100) | – | – | – | Suspicion of VTE | 6 (9.5) |
| Voicu S [51] | Single center | France | 56 | – | 42 (75.0) | 56 (100) | 56 (100) | 49 (87.5) | 7 (12.5) | Screening CUS | 26 (46.4) |
| Zhang L [52] | Single center | China | 143 | 63 | 74 (51.7) | 0 | 53 (37.1) | 53 (37.1) | 0 | Suspicion of VTEb | 66 (46.2) |
CT, computed tomography; CUS, compression ultrasonography; DVT, deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism; UK, United Kingdom; US, United States of America.
Diagnostic workup for DVT was unclear.
Screening for DVT was performed in all patients.
Diagnostic workup for PE was unclear.
Screening for DVT was performed in a part of patients.
Fig. 2.
Summary risk of bias for included studies.
MINORS, methodological index for non-randomized studies.
The mean age ranged between 57 and 70 years and 62.1% were males (1951/3144; 25 studies). Seventy-two percent of patients (1008/1395, 72.3%; 8 studies) were hospitalized in medical wards and 27.7% were admitted to the ICU (387/1395; 8 studies). Eighteen studies provided information on VTE risk factors: the presence of cancer was reported in 8.7% of patients (178/2054; 16 studies) and previous VTE in 4.0% (72/1785; 14 studies). Fifteen studies reported on anticoagulants for VTE prevention which were used at prophylactic doses in 73.2% of patients (2013/2751; 23 studies), intermediate doses in 8.0% (67/837 patients; 7 study), and therapeutic doses in 16.8% (256/1526; 13 studies).
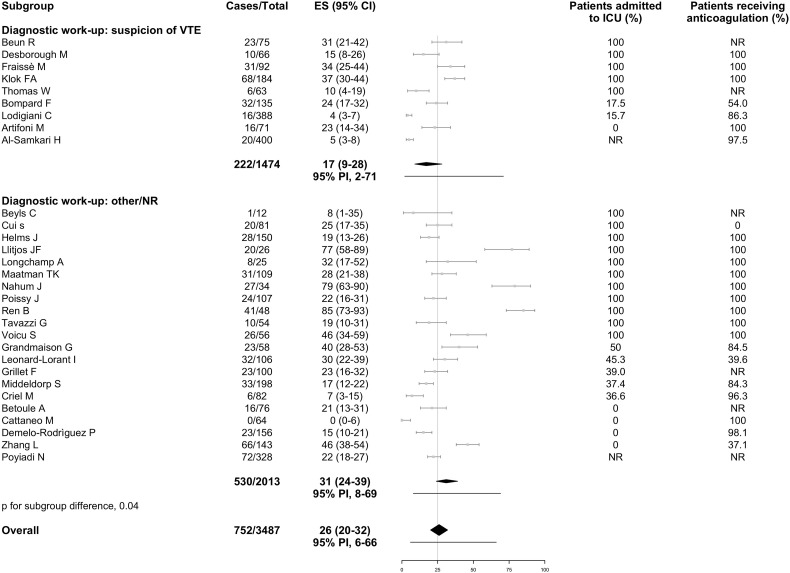
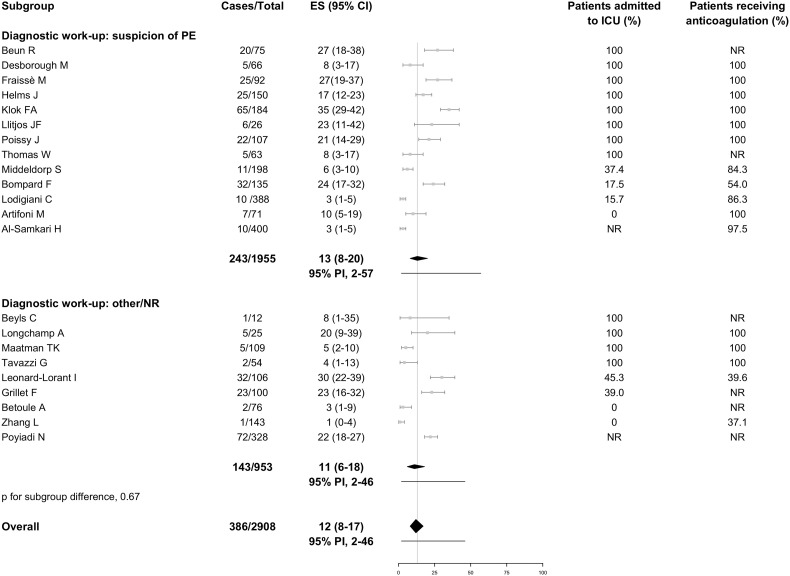
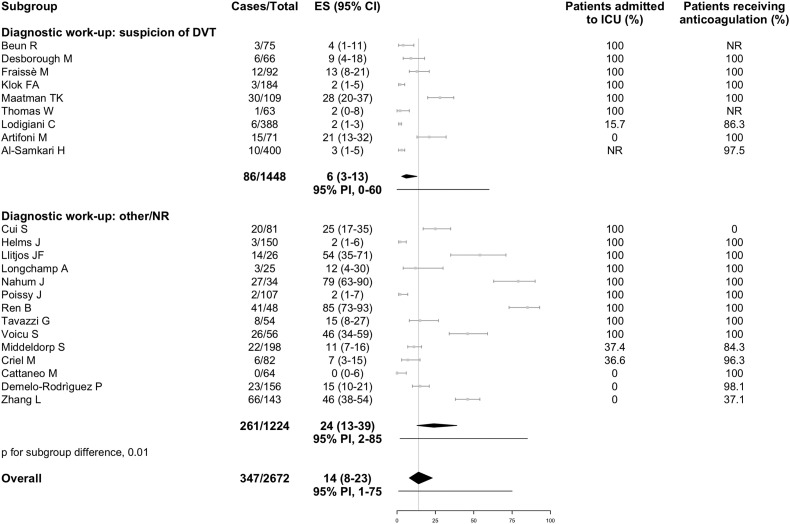
Data for both PE and DVT were available from 16 studies, whereas 6 reported only on PE and 7 only on DVT. Overall, the incidence of VTE was 26% (95% PI, 6% to 66%; 752/3487 patients, Fig. 3 ), including PE with or without DVT in 12% (95% PI, 2% to 46%; 386/2908 patients; 22 studies, Fig. 4 ) and DVT alone in 14% (95% PI, 1% to 75%; 347/2672 patients; 23 studies, Fig. 5 ). Fourteen studies reported information on the extension of PE which involved the main trunk or lobar pulmonary arteries in 37.8% of cases (91/241; 12 studies), segmental arteries in 37.9% (114/301; 12 studies), and subsegmental arteries in 19.0% (59/311; 13 studies). Thirteen studies reported on the extension of DVT which involved the proximal veins in 32.2% of cases (88/273; 13 studies) and distal veins in 67.0% (136/203; 9 studies). Nine studies failed to describe the diagnostic work-up for VTE, 9 studies used imaging tests in case of clinically suspected VTE, and the remaining 12 studies used various approaches which included (serial) screening ultrasonography or patient sampling based on the availability of chest computed tomography independently of VTE suspicion. In studies which applied diagnostic tests only if thrombosis was clinically suspected, VTE was diagnosed in 17% (95% PI, 2% to 71%), PE with or without DVT in 13% (95% PI, 2% to 57%), and DVT alone in 6% (95% PI, 0% to 60%). Higher estimates for VTE and DVT alone were reported in studies using other diagnostic work-up or patient sampling (Fig. 3, Fig. 5). Ten studies which performed screening ultrasonography for DVT in all patients found a DVT incidence that varied widely between 0% and 85% and seemed to be largely accounted by asymptomatic distal DVT [10,18].
Fig. 3.
Incidence of venous thromboembolism in hospitalized patients with COVID-19.
Grey squares indicate individual study estimates of the proportion of the outcome, grey horizontal lines indicate 95% confidence intervals of the individual studies, diamonds indicate summary estimates with 95% confidence intervals, and horizontal black lines represent 95% prediction intervals.
CI, confidence intervals; COVID-19, Coronavirus disease 2019; ES, estimates; ICU, intensive care unit; NR, not reported; PI, prediction intervals; VTE, venous thromboembolism.
Fig. 4.
Incidence of pulmonary embolism in hospitalized patients with COVID-19.
Grey squares indicate individual study estimates of the proportion of the outcome, grey horizontal lines indicate 95% confidence intervals of individual studies, diamonds indicate summary estimates with 95% confidence intervals, and horizontal black lines represent 95% prediction intervals.
CI, confidence intervals; COVID-19, Coronavirus disease 2019; ES, estimates; ICU, intensive care unit; NR, not reported; PE, pulmonary embolism; PI, prediction intervals.
Fig. 5.
Incidence of deep vein thrombosis in hospitalized patients with COVID-19.
Grey squares indicate individual study estimates of the proportion of the outcome, grey horizontal lines indicate 95% confidence intervals of the individual studies, diamonds indicate summary estimates with 95% confidence intervals, and horizontal black lines represent 95% prediction intervals.
CI, confidence intervals; COVID-19, Coronavirus disease 2019; DVT, deep vein thrombosis; ES, estimates; ICU, intensive care unit; NR, not reported; PI, prediction intervals.
In the analysis restricted to studies in ICU patients where referral to imaging was based on clinical suspicion, VTE was diagnosed in 24% (95% PI, 5% to 66%; 7 studies), PE with or without DVT in 19% (95% PI, 6% to 47%; 10 studies), and DVT alone in 7% (95% PI, 0% to 69%; 6 studies, Supplementary Fig. 1). The corresponding values in patients admitted to general wards were 9% (95% PI, 0% to 94%; 4 studies), 4% (95% PI, 0% to 100%; 3 studies), and 7% (95% CI, 1% to 49%; 2 studies), respectively (Supplementary Fig. 2).
4. Discussion
This meta-analysis found that VTE occurs in a relevant proportion of patients hospitalized for COVID-19. The incidence is higher in patients admitted to ICU, but seems also substantial in patients of general wards despite use of thromboprophylaxis. All prediction intervals were wide indicating low confidence in the summary estimates.
The incidence of VTE in hospitalized COVID-19 patients appears to be considerably higher compared to the incidence reported in previous studies on acute medical patients and critically-ill patients with sepsis or septic shock [[19], [20], [21]]. Most cases of VTE were represented by PE with or without concomitant DVT. The combination of systemic activation of coagulation, extensive pulmonary endotheliopathy and thrombo-inflammatory process caused by SARS-CoV2 may result in diffuse pulmonary intravascular coagulopathy and pulmonary arterial thrombosis [[22], [23], [24]]. The clinical presentation of PE may overlap with that of COVID-19 pneumonia which may hindrance the recognition of PE symptoms in patients who are already complaining of dyspnoea. Therefore, current estimates may even represent an underestimation of the actual PE incidence in COVID-19 as also suggested by autopsy studies [25]. Nine studies followed standard diagnostic protocols for suspected VTE, which included imaging tests performed only in cases of clinical suspicion or sudden increase in D-dimer levels. Although the incidence of VTE appeared to be lower compared to studies using other diagnostic strategies or patient sampling, the risk of VTE remained substantial with an average PE incidence that was as high as 19% in patients admitted to ICU.
The risk of VTE seemed to remain considerable despite most patients received pharmacological thromboprophylaxis [7]. In all studies the decision on the intensity and duration of anticoagulation for VTE prevention was left to treating physicians limiting any conclusion about optimal dosing in these patients. Whether higher doses of thromboprophylaxis may reduce VTE without significantly increase the risk of major bleeding is currently under evaluation in several RCTs (NCT04345848, NCT04366960, NCT04416048, NCT04360824, NCT04345848, NCT04445623, NCT04427098, NCT04373707). Anti-inflammatory treatment may represent a potentially promising alternative to control inflammation-induced endotheliopathy and local pulmonary thrombotic microangiopathy associated with COVID-19 [26,27].
The current meta-analysis has some limitations which warrant discussion. Most studies included a relatively small number of cases with poor description of patient characteristics, which limited the possibility to explore the effects of concomitant risk factors on the incidence of VTE. The lack of randomization and the little data provided on the incidence of VTE stratified by use, type, dose, and duration of anticoagulation (i.e. prophylactic, intermediate, or therapeutic dose) precluded subgroup analysis on the effects of pharmacological prophylaxis. Based on scanty information available, we could only describe the incidence of VTE according to the percentage of patients receiving anticoagulation in each study (Fig. 3, Fig. 4, Fig. 5 and Supplementary Figures). Five studies reported the incidence of bleeding complications which ranged between 0% and 10.6% [[28], [29], [30], [31], [32]]. Two studies specified the criteria used to define bleeding [28,29], and only two described whether bleeding events occurred on- or off-anticoagulation without providing information on the dose and type of pharmacological prophylaxis at the time of events [28,32]. One fifth of the studies provided no information on the diagnostic work-up for VTE and about half failed to report on both PE and DVT. The proportion of patients who were still in hospital at the end of the study varied between 8% and 75.5%. As the risk of VTE may persist beyond the first week of hospitalization, inadequate follow-up duration may have contributed to underestimating VTE incidence.
5. Conclusion
VTE represents a frequent complication in patients admitted for COVID-19 and often occurs as PE. Pooled incidences are based on very low-quality evidence due to heterogeneity and risk of bias which reduces the confidence in the size of the estimates. Nevertheless, VTE incidence appears to be high, and referral to imaging may be advisable at a low threshold for VTE suspicion.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Study conception and design, A. Porfidia, E. Valeriani, M. Di Nisio; Bibliographic searches: A. Rutjes; Data acquisition: A. Porfidia, E. Valeriani; Statistical analysis: E. Valeriani; Interpretation of the data: All authors; Drafting of the manuscript: All authors; Critical revision of the manuscript for important intellectual content: All authors; Final approval of the manuscript: All authors.
Declaration of competing interest
E. Valeriani, E. Porreca, A. Rutjes has nothing to disclose; A. Porfidia received personal fees and non-financial support from Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Aspen, Novartis outside the submitted work; R. Pola received personal fees and non-financial support from Boehringer Ingelheim, Bayer, Daiichi Sankyo, Pfizer, Aspen, Novartis outside the submitted work; M. Di Nisio received personal fees from Bayer, Daiichi Sankyo, Pfizer, Leo Pharma, and Sanofi outside the submitted work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.08.020.
Appendix A. Supplementary data
Supplementary material 1
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report – 101. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., Brown T.S., Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., Tang C., Zhang N., Zhong N., Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7 doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potere N., Valeriani E., Candeloro M., Tana M., Porreca E., Abbate A., Spoto S., Rutjes A.W.S., Di Nisio M. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit. Care. 2020;24:389. doi: 10.1186/s13054-020-03022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren B., Yan F., Deng Z., Zhang S., Xiao L., Wu M., Cai L. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047407. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J., Altman D., Sterne J. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions version. 2017;520 [Google Scholar]
- 14.Revel M.P., Parkar A.P., Prosch H., Silva M., Sverzellati N., Gleeson F., Brady A., European Society of Radiology, the European Society of Thoracic Imaging COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur. Radiol. 2020 doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Society of Cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. 2020. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance Accessed 20th May 2020 at.
- 16.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org/2019 URL. [Google Scholar]
- 17.Higgins J.P.T., Green S. Cochrane handbook for systematic reviews of interventions version 5.0.2 [updated September 2009] The Cochrane Collaboration. 20082008 https://training.cochrane.org/handbook URL. [Google Scholar]
- 18.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macias M., Toledo-Samaniego N., Garcia-Garcia A., Garcia-Fernandez-Bravo I., Ji Z., de-Miguel-Diez J., Alvarez-Sala-Walther L.A., Del-Toro-Cervera J., Galeano-Valle F. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zusman O., Paul M., Farbman L., Daitch V., Akayzen Y., Witberg G., Avni T., Gafter-Gvili A., Leibovici L. Venous thromboembolism prophylaxis with anticoagulation in septic patients: a prospective cohort study. QJM. 2015;108:197–204. doi: 10.1093/qjmed/hcu183. [DOI] [PubMed] [Google Scholar]
- 20.Protect Investigators for the Canadian Critical Care Trials Group, the Australian New Zealand Intensive Care Society Clinical Trials Group, Cook D., Meade M., Guyatt G., Walter S., Heels-Ansdell D., Warkentin T.E., Zytaruk N., Crowther M., Geerts W., Cooper D.J., Vallance S., Qushmaq I., Rocha M., Berwanger O., Vlahakis N.E. Dalteparin versus unfractionated heparin in critically ill patients. N. Engl. J. Med. 2011;364:1305–1314. doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd N.S., Douketis J.D., Moinuddin I., Lim W., Crowther M.A. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J. Thromb. Haemost. 2008;6:405–414. doi: 10.1111/j.1538-7836.2007.02847.x. [DOI] [PubMed] [Google Scholar]
- 22.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGonagle D., O’Donnell J., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schroder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Puschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7 doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O’Donnell J.S. Endothelial cells orchestrate COVID-19 coagulopathy. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb. Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit. Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Angles-Cano E., Sattler L., Mertes P.M., Meziani F., Crics Triggersep Group High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., Sandri M.T., Barco S., Humanitas, Covid-Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longchamp A., Longchamp J., Manzocchi-Besson S., Whiting L., Haller C., Jeanneret S., Godio M., Garcia Martinez J., Bonjour T., Caillat M., Maitre G., Matthias Thaler J., Pantet R., Donner V., Dumoulin A., Emonet S., Greub G., Friolet R., Robert-Ebadi H., Righini M., Sanchez B., Delaloye J. 2020. Venous Thromboembolism in Critically Ill Patients With Covid-19: Results of a Screening Study for Deep Vein Thrombosis. rpth doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P., Bornikova L., Gupta S., Leaf D., Kuter D.J., Rosovsky R.P. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020 doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artifoni M., Danic G., Gautier G., Gicquel P., Boutoille D., Raffi F., Néel A., Lecomte R. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J. Thromb. Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betoule A., Martinet C., Gasperini G., Muller P., Foucher S., Benner P., Renard A. Diagnosis of venous and arterial thromboembolic events in COVID-19 virus-infected patients. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beun R., Kusadasi N., Sikma M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020 doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyls C., Huette P., Abou-Arab O., Berna P., Mahjoub Y. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome and risk of thrombosis. Br. J. Anaesth. 2020 doi: 10.1016/j.bja.2020.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L., Sanchez O., Lorut C., Chassagnon G., Revel M.P. Pulmonary embolism in patients with Covid-19 pneumonia. Eur. Respir. J. 2020 doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., Muscarella G., Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemost. 2020 doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Criel M., Falter M., Jaeken J., Van Kerrebroeck M., Lefere I., Meylaerts L., Mesotten D., Vander Laenen M., Fivez T., Thomeer M., David R. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur. Respir. J. 2020 doi: 10.1183/13993003.01201-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grandmaison G., Andrey A., Périard D., Engelberger R.P., Carrel G., Doll S., Dexpert J.B., Krieger C., Ksouri H., Hayoz D., Sridharan G. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;4 doi: 10.1055/s-0040-1713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., Schneider F., Labani A., Bilbault P., Moliere S., Leyendecker P., Roy C., Ohana M. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maatman T.K., Jalali F., Feizpour C., Douglas A., McGuire S.P., Kinnaman G., Hartwell J.L., Maatman B.T., Kreutz R.P., Kapoor R., Rahman O., Zyromski N.J., Meagher A.D. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit. Care Med. 2020 doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., Smits L.P., Bonta P.I., van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M., Thierry S. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., Lille I.C.U. Haemostasis Covid-group. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 48.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K., Nadig J., Keimig T., Spizarny D., Reeser N., Klochko C., Peterson E.L., Song T. Acute pulmonary embolism and COVID-19. Radiology. 2020:201955. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavazzi G., Civardi L., Caneva L., Mongodi S., Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., Lavinio A., Besser M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voicu S., Bonnin P., Stépanian A., Chousterman B.G., Le Gall A., Malissin I., Deye N., Siguret V., Mebazaa A., Mégarbane B. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., Zhang C., Li H., Xia X., Kong S., Liao J., Jia H., Pang X., Song Y., Tian Y., Wang B., Wu C., Yuan H., Zhang Y., Li Y., Sun W., Zhu S., Wang S., Xie Y., Ge S., Hu Y., Xie M. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1