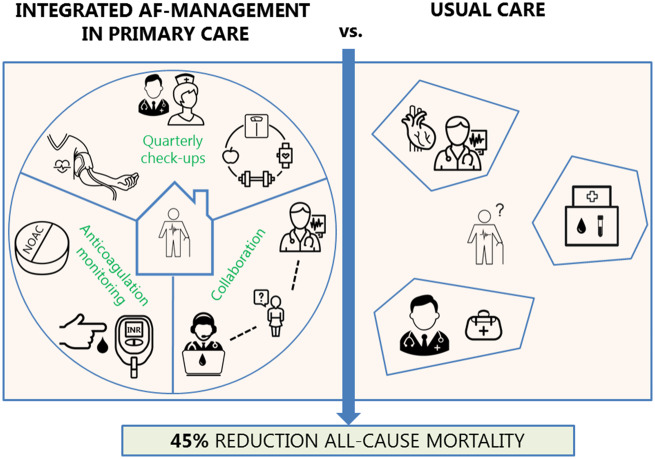
Abstract
Aims
To evaluate whether integrated care for atrial fibrillation (AF) can be safely orchestrated in primary care.
Methods and results
The ALL-IN trial was a cluster randomized, open-label, pragmatic non-inferiority trial performed in primary care practices in the Netherlands. We randomized 26 practices: 15 to the integrated care intervention and 11 to usual care. The integrated care intervention consisted of (i) quarterly AF check-ups by trained nurses in primary care, also focusing on possibly interfering comorbidities, (ii) monitoring of anticoagulation therapy in primary care, and finally (iii) easy-access availability of consultations from cardiologists and anticoagulation clinics. The primary endpoint was all-cause mortality during 2 years of follow-up. In the intervention arm, 527 out of 941 eligible AF patients aged ≥65 years provided informed consent to undergo the intervention. These 527 patients were compared with 713 AF patients in the control arm receiving usual care. Median age was 77 (interquartile range 72–83) years. The all-cause mortality rate was 3.5 per 100 patient-years in the intervention arm vs. 6.7 per 100 patient-years in the control arm [adjusted hazard ratio (HR) 0.55; 95% confidence interval (CI) 0.37–0.82]. For non-cardiovascular mortality, the adjusted HR was 0.47 (95% CI 0.27–0.82). For other adverse events, no statistically significant differences were observed.
Conclusion
In this cluster randomized trial, integrated care for elderly AF patients in primary care showed a 45% reduction in all-cause mortality when compared with usual care.
Keywords: Atrial fibrillation, Integrated care, Primary care, Multimorbidity, Anticoagulation
See page 2845 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa168)
Introduction
Integrated care has been proposed as a solution for the increasing disease burden of atrial fibrillation (AF) and is recommended in the 2016 European Society of Cardiology (ESC) guidelines on the management of AF (Class IIa recommendation, level of evidence B).1 The motive for integrated care is grounded on the view that AF is not merely an isolated heart rhythm disorder with an increased risk of stroke, but more, in general, a ‘hypercoagulable state’ caused by (or associated with) the presence of multiple underlying and interacting comorbidities.2 Consequently, notably for elderly AF patients, management is evolving towards a more integrated care including also management of comorbidities.
A meta-analysis of studies investigating integrated care coordinated by tertiary care hospitals showed a reduction in all-cause mortality and cardiovascular hospitalization.3 More recently, the RACE 4 trial confirmed that integrated, nurse-led care reduced cardiac mortality and hospitalization, yet only when provided in experienced AF clinics.4 Hence, it is yet unknown whether such integrated care could be safely orchestrated in primary care, a setting characterized by non-specialist doctors and nurses and AF patients being typically older, frailer, and suffering from multimorbidity. When proven safe, integrated AF care in primary care can be instrumental in managing the ever-increasing prevalence of AF and the associated burden and mortality, especially among the elderly.5,6
Therefore, the aim of our study was to assess whether integrated care for AF organized in primary care is non-inferior compared to usual care as performed by cardiologists and anticoagulation clinics.
Methods
Setting
This study was conducted in the setting of Dutch primary care, a setting characterized by small teams with one or more general practitioners (GPs), closely working together with practice nurses and assistants, providing care for about 2200 patients enlisted per GP. The coverage of practices across the country is high, with 75% of people living within 1 km of a GP practice.7 Another important characteristic is that the GP serves as a gatekeeper to secondary care.
Trial design
The ALL-IN trial was a cluster randomized, pragmatic, non-inferiority trial in primary care. Full details on the study design and protocol have been previously reported.8 In brief, primary care practices located in the region of three affiliated secondary care hospitals (Zwolle, Deventer, and Hardenberg) in the Netherlands could be included if they were willing and able to provide integrated care. Given the uncertainty of whether primary care could safely orchestrate such integrated care, our aim was to demonstrate that management of AF in primary care was at least as safe and effective as current care provided (mainly) in secondary care. Therefore, this study was designed as a non-inferiority study regarding the primary outcome of all-cause mortality. During the design, conduct, and reporting of this study, we closely adhered to the CONSORT 2010 statement extension for cluster trials.9 The trial was registered at the Netherlands Trial Register (NL5407).
Randomization and participants
Randomization occurred at the level of primary care practices (clusters), performed by an independent researcher through off-site computerized block randomization stratified by practice size. Because of cluster randomization, one practice (including all eligible patients within this practice) was allocated to either the intervention arm or the control arm. Randomization at this practice level was necessary to prevent contamination of the intervention and thus dilution of any true effect, as it is practically impossible for a GP and his/her practice nurse to provide integrated care to one AF patient while refraining from doing so to the next.
After randomization, all patients within the participating practices with documented AF and aged 65 years or older were assessed at the practices for eligibility using their electronic medical records. The following exclusion criteria were applied: (i) presence of an internal cardioverter-defibrillator or a cardiac resynchronization therapy device; (ii) cardioversion, cardiac ablation, or cardiac surgery <3 months prior to inclusion or being planned; (iii) heart valve surgery in the past; (iv) a rheumatic mitral valve stenosis; (v) pulmonary vein isolation in the past or being planned; (vi) being legally incapable of providing informed consent; (vii) a life expectancy shorter than 3 months; and finally (viii) participation in another randomized trial on AF.
Informed consent and ethics
All eligible patients from practices randomized to the intervention were informed on study purposes and asked for written informed consent before undergoing the intervention. In the control arm, informed consent was only asked for filling out quality of life questionnaires (secondary outcome, see below). The Medical Ethics Committee provided a waiver of informed consent for the collection of anonymized baseline and outcome data for all eligible patients in both arms, yet all strictly under the auspices of the treating GP. It was decided that to ensure the scientific validity of the trial such a waiver of informed consent for anonymized data collection was necessary, for three reasons: (i) to enable the assessment of otherwise undetectable possible selection bias caused by providing informed consent for participation after randomization, inherent to cluster randomized trials, (ii) to enhance the generalizability of our findings, especially to frail elderly AF patients, and (iii) informing all eligible patients in the control practices would involve providing information and education on AF and its risks, thus inducing a risk of contamination. Moreover, no additional examinations for anonymized data collection were needed and thus no additional risk was imposed to patients. This approach is increasingly applied in cluster randomized trials to ensure its merits to science and society.10–12
Index intervention and usual care
Details of the intervention and a comparison with usual care are shown in the Supplementary material online, Appendix Section A. The aspects included in our intervention largely overlap with the aspects mentioned in the 2016 ESC guidelines for the management of AF (except for the use of decision support software). Additionally, the primary care setting enabled our intervention to be even broader, as it involved also care for non-cardiovascular comorbidities that likely interact with AF, such as diabetes, infectious diseases, and chronic obstructive lung disease.
In short, the intervention consisted of three pivotal items: (i) quarterly AF check-ups by the practice nurse on symptoms and comorbidities, notably assessment of early signs and symptoms of heart failure and also patient education (for checklist, see Supplementary material online, Appendix Section A), (ii) case management of anticoagulant treatment, including international normalized ratio (INR) measurements performed by the intervention practice in those treated with a vitamin K antagonist (VKA), special attention to drug compliance, and monitoring of kidney function in patients using a non-vitamin K antagonist oral anticoagulant (NOAC), and (iii) easy-access consultation of anticoagulation clinics and/or cardiologists, thus truly enabling ‘shared care and responsibility’ between primary care, anticoagulation clinics, and cardiology care. When patients needed to be referred to secondary care or needed additional check-ups by a cardiologist (in case of other cardiac conditions or pacemaker), they continued their participation in the intervention arm. Practice nurses in the intervention practices received a 3 h training at the start of the intervention with education on signs and symptoms of AF and heart failure, rate and rhythm control, anticoagulant treatment, and an explanation of the most important recommendations of the guidelines on AF.1,13 In addition, we organized three meetings throughout the 2-year follow-up period for both practice nurses and GPs to (i) share experiences and ‘best practices’, (ii) discuss complex patients, and (iii) provide additional education on topics based on existing questions of the practice nurses. Decisions regarding pharmacotherapy and referral to cardiology care were left to the GPs, guided by the Dutch College of General Practitioners’ guidelines on AF.13
Usual care could vary per patient, but for most patients, it involved a once yearly consultation of a cardiologist or AF nurse at the outpatient cardiology department of the affiliated hospital. Some patients may already have been discharged from treatment by their cardiologist and for those patients, the GP was the first person to contact in case of signs or symptoms related to AF or other conditions. However, this occurs on an ‘ad hoc basis’, initiated by the patient. For patients using a VKA, anticoagulation clinics affiliated to the local hospital performed the INR measurements and created the dosage calendar, yet without involvement of the GP. For patients using a NOAC, no structured control was in place in the control group.
Data collection and outcomes
Data on comorbid conditions and medication use for all eligible patients were automatically derived at baseline from the electronic medical records using International Classification of Primary Care (ICPC) codes and Anatomical Therapeutic Chemical (ATC) codes, respectively.
All patients were followed for at least 2 years. The primary outcome was all-cause mortality. Secondary outcomes were cardiovascular and non-cardiovascular mortality, cardiovascular and non-cardiovascular hospitalization, major adverse cardiac events (MACE), stroke, major bleeding, clinically relevant non-major bleeding (CRNMB), health-related quality of life (HrQoL), and cost-effectiveness. For major and clinically relevant non-major bleeding, the definitions of the International Society on Thrombosis and Haemostasis (ISTH) were used.14,15 Definitions of the other outcomes are described in Supplementary material online, Appendix Section B. An independent adjudication committee, blinded for treatment allocation, adjudicated all causes of death. HrQoL was assessed by the 12-item Short-Form Health Survey (SF-12), measured at baseline and after 1 year and 2 years of follow-up.16 The SF-12 consists of a physical health component score (PCS) and a mental health component score (MCS), both ranging from 0 to 100 with higher scores indicating better hrQoL. Results of the cost-effectiveness analyses will be published separately. Except for the SF-12, all follow-up data were manually retrieved by the researchers from the primary care electronic medical records (i.e. from hospital discharge letters and reports from consultations). As all patients participating in the intervention needed to have this clearly noted in their files, researchers could not be blinded for treatment allocation during data collection.
Sample size calculation and statistical analysis
We anticipated that all-cause mortality (primary outcome) would occur more frequently in our older and frailer primary care study population than in the population studied by Hendriks et al.,17 where 2.5% of patients died of a cardiovascular reason. We estimated mortality to occur in 8% of participants in the usual care arm of the trial during 24 months of follow-up. Assuming a 1% margin (absolute risk, one-sided) for non-inferiority and accounting for clustering [with an estimated intra-cluster correlation coefficient (ICC) of 0.005], we (conservatively) needed to include 500 patients in each arm to demonstrate non-inferiority for our primary outcome. In a post hoc analysis, considering the outcome all-cause mortality as a binary event in the absence of appropriate methods to calculate the true ICC with time-to-event data, the estimate of the observed ICC appeared to be 0.008. Like we assumed beforehand, albeit with a slightly smaller ICC of 0.005, this ICC indeed indicates very little clustering. This sample size allowed us to demonstrate superiority if the hazard ratio (HR) of the effect size of the intervention would be 0.60 or lower. A possible superiority analysis was thus pre-planned and described in our protocol paper albeit that at study initiation we expected superiority to occur only for the secondary outcome hospitalization.8
In the main analyses, we compared the outcomes of all eligible patients in the control arm with the outcomes of the patients in the intervention arm who provided informed consent to receive integrated AF care, as described and pre-planned in our protocol.8 Because individual informed consent for participating in the intervention was asked after randomization of practices, differences in baseline characteristics between study arms might still occur. Therefore, we a priori defined to adjust for age, sex, and the frailty index (FI).18 The FI is a validated frailty indicator based on ICPC and ATC codes from routine electronic healthcare data.19 For each patient, the FI score (ranging from 0 to 1, with higher values indicating more frailty) was calculated by dividing the number of health deficits present, by the total fixed number of 36 pre-specified health deficits (including for example heart failure, cancer, renal impairment, and polypharmacy).
For the outcomes mortality, MACE, ischaemic stroke, and major bleeding, we used random effects Cox proportional hazard regression models with the clusters (practices) introduced as a frailty term to account for clustering and adjusting for age, sex, and FI. Scaled Schoenfeld residuals were plotted to visually assess the proportional hazards assumption and Martingale residuals were checked for the continuous covariates age and FI.20,21 As traditional Cox models would analyse only the first event, we used negative binomial regression adjusted for age, sex, FI, and length of follow-up (as an offset variable) for the analyses of the frequently recurrent outcome events hospitalization and CRNMB. Finally, hrQoL and in particular changes in the PCS and MCS of the SF-12 between baseline, 1 year and 2 years of follow-up were analysed using linear mixed models, with a random intercept for the patient level and the practice level, and adjusted for age, sex, FI, and baseline PCS or MCS.
Finally, to assess the robustness of our findings and the impact of potential selection bias due to asking informed consent for the intervention after randomization, we performed a pre-planned, additional analysis for our primary outcome comparing all eligible control patients to all eligible intervention patients, including also those patients who did not sign informed consent to undergo the intervention. Survival analyses were performed in R version 3.4.1.22 with package survival version 2.42-3,23 and quality of life questionnaires were analysed in SAS for Windows, version 9.4.
Results
Baseline characteristics
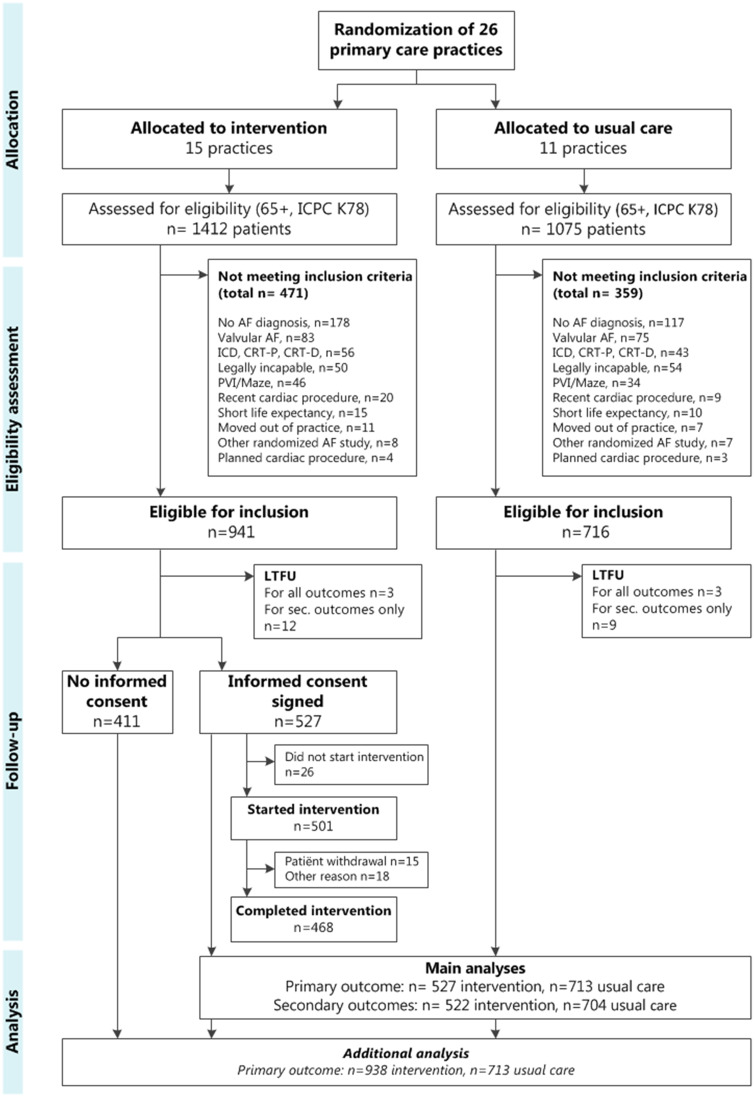
A total of 119 practices were informed about the trial, of which 26 practices decided to participate. Between October 2015 and January 2017, these 26 practices were randomized (see flowchart, Figure 1). Within these 26 practices, 1657 (66.6%) out of 2487 AF patients were eligible for inclusion. Fifteen practices were randomized to the intervention arm, involving 32 GPs and 28 practice nurses. In these intervention practices, 527 (56.0%) of the eligible patients provided informed consent for participation in the intervention and were included in our main analyses. The median cluster size in the intervention arm was 29 patients [interquartile range (IQR) 25–46]. In the control arm, all eligible patients (n = 716) were analysed and median cluster size was 53 patients (IQR 45–75). All practices completed at least 2 years of follow-up, ending between April 2018 and March 2019. The uptake and persistence of performing the intervention were high; there was no drop-out of intervention practices and 93% of the patients who started the intervention completed it.
Figure 1.
A flowchart of the ALL-IN cluster randomized trial. CRT-P/D, cardiac resynchronization therapy pacemaker/defibrillator; ICD, implantable cardioverter-defibrillator; ICPC K78, International Classification of Primary Care code for atrial fibrillation; LTFU, lost to follow-up; PVI, pulmonary vein isolation.
Baseline characteristics are shown in Table 1. Median age was 77 years. Some differences in baseline characteristics between both groups were observed, although not consistently in favour of one of the treatment arms. At baseline, 78% of all included patients used a VKA. The proportion of patients not receiving anticoagulant therapy despite having an indication was low in both arms (8.3% vs. 6.3% in the intervention and control arm, respectively). Baseline characteristics of patients in the intervention arm who did not give informed consent to undergo the intervention are shown in the Supplementary material online, Appendix Section C, as are the baseline characteristics per practice.
Table 1.
Baseline characteristics of included patients
| Integrated care (n = 527) | Usual care (n = 713) | P-value | |
|---|---|---|---|
| Age (years), median (IQR) | 76 (71–81) | 78 (73–84) | <0.001 |
| Female sex | 239 (45.4) | 374 (52.5) | 0.016 |
| Years since AF diagnosis, median (IQR) | 4.3 (2.1–7.4) | 4.0 (2.0–8.4) | 0.177 |
| Quality of life | |||
| Median PCS (IQR) | 42.6 (33.6–50.4) | 40.6 (32.7–48.7) | 0.351 |
| Median MCS (IQR) | 52.8 (45.5–57.4) | 52.3 (44.0–57.4) | 0.376 |
| Hypertension | 311 (59.0) | 389 (54.6) | 0.132 |
| Diabetes mellitus | 131 (24.9) | 185 (25.9) | 0.712 |
| Prior stroke/TIA | 84 (15.9) | 95 (13.3) | 0.225 |
| Coronary artery disease | 93 (17.6) | 120 (16.8) | 0.764 |
| Prior myocardial infarction | 36 (6.8) | 50 (7.0) | 0.991 |
| Heart failure | 72 (13.7) | 136 (19.1) | 0.015 |
| Peripheral vascular disease | 36 (6.8) | 48 (6.7) | 1.000 |
| Prior venous thromboembolism | 25 (4.7) | 30 (4.2) | 0.754 |
| Chronic renal impairment | 59 (11.2) | 110 (15.4) | 0.039 |
| Chronic obstructive pulmonary disease | 73 (13.9) | 99 (13.9) | 1.000 |
| History of cancer | 95 (18.0) | 131 (18.4) | 0.935 |
| Pacemaker | 34 (6.5) | 62 (8.8) | 0.171 |
| Frailty index, median (IQR) | 0.14 (0.11–0.22) | 0.17 (0.11–0.19) | 0.577 |
| Polypharmacy (≥5 chronic drugs) | 134 (25.4) | 140 (19.6) | 0.018 |
| Anticoagulant use | |||
| VKA | 390 (74.0) | 571 (80.1) | 0.014 |
| NOAC | 84 (15.9) | 80 (11.2) | 0.019 |
| None | 53 (10.1) | 62 (8.7) | 0.473 |
| Undertreatmenta | 44 (8.3) | 45 (6.3) | 0.203 |
| Antiplatelet therapy | 48 (9.1) | 51 (7.2) | 0.250 |
| Beta-blockers | 378 (71.7) | 522 (73.2) | 0.606 |
| Calcium channel antagonists | 150 (28.5) | 182 (25.5) | 0.276 |
| Digoxin | 97 (18.4) | 137 (19.2) | 0.775 |
| Classes I and III antiarrhythmic drugs | 32 (6.1) | 52 (7.3) | 0.464 |
| Diuretics | 198 (37.6) | 341 (47.8) | <0.001 |
| RAAS inhibitors | 279 (52.9) | 400 (56.1) | 0.295 |
Numbers are counts (%) unless stated otherwise. The frailty index consists of the presence or absence of 36 health deficit items (scale 0–1, higher value indicating more frailty), see text.
IQR, interquartile range; MCS, mental health component score (scale 0–100, higher score indicating better hrQoL); NOAC, non-vitamin K antagonist oral anticoagulant; PCS, physical health component score (scale 0–100, higher score indicating better hrQoL); RAAS, renin–angiotensin–aldosterone system; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
aUndertreatment was defined as no oral anticoagulant prescription in the 12 months prior to baseline, despite a CHA2DS2-VASc score of 2 or more and in the absence of only a single AF episode following cardiac surgery.
Primary outcome
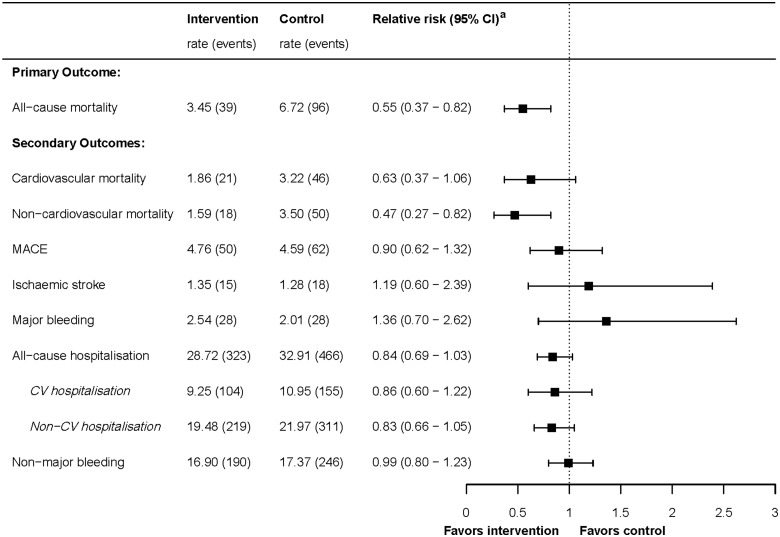
During a median follow-up time of 2.3 years in the intervention arm and 2.2 years in the control arm, 39 patients in the intervention arm and 96 patients in the control arm died (7.4% and 13.5%, respectively). Incidence rates and crude and adjusted HRs are presented in the Supplementary material online, Appendix Section D. The HR for all-cause mortality, after adjustment for age, sex, and FI, was 0.55 (0.37–0.82, Figure 2). The cumulative event plot is shown in Figure 3. When we repeated the analysis including the 411 patients who did not sign informed consent for participation in the intervention arm, the effect was attenuated but a reduction in all-cause mortality was still observed [adjusted HR 0.81 (0.61–1.07)], though the confidence interval (CI) overlapped with 1.0.
Figure 2.
Forest plot of Cox regression analyses of primary and secondary outcomes, and recurrent events analyses of hospitalization and clinically relevant non-major bleeding. Rates are incidence rates per 100 person-years. aRelative risks are adjusted hazard ratios, except for the recurrent events hospitalizations and clinically relevant non-major bleeding which are adjusted incidence rate ratios (adjusted for age, sex, frailty index, and accounted for clustering). CV, cardiovascular.
Figure 3.
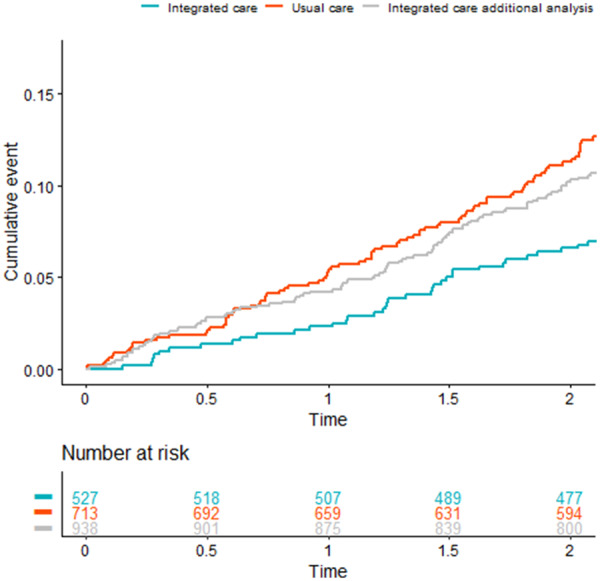
Cumulative event plot all-cause mortality. The red and blue lines represent the cumulative events for all-cause mortality of the 713 patients in the usual care arm and the 527 patients who gave informed consent in the intervention arm, respectively (main analysis). The grey line represents the integrated care arm when including also the 411 patients who did not sign informed consent to participate in the intervention (additional analysis).
Take home figure.
ALL-IN trial.
Secondary outcomes
Figure 2 shows the results of Cox survival analyses for the secondary outcomes cardiovascular mortality, non-cardiovascular mortality, MACE, ischaemic stroke, and major bleeding, and the results of the negative binomial regression analyses for the recurrent events hospitalization and CRNMB. Event rates, crude and adjusted HRs, and incidence rate ratios (IRR) are displayed in the Supplementary material online, Appendix Section D. As for cause-specific mortality, risk reduction of non-cardiovascular mortality in intervention practices was more pronounced than risk reduction of cardiovascular mortality [adjusted HR for non-cardiovascular mortality 0.47 (95% CI 0.27–0.82), compared to adjusted HR 0.63 (95% CI 0.37–1.06) for cardiovascular mortality]. A table with the occurrence of the different cardiovascular and non-cardiovascular causes of death is shown in the Supplementary material online, Appendix Section E.
Hospitalizations occurred frequently in both treatment arms: during follow-up, in total 38% of patients had at least one hospital admission and 16% had at least two hospital admissions. Non-cardiovascular hospitalization occurred twice as frequently as cardiovascular hospitalization. The number of all-cause hospital admissions was 16% lower in the intervention arm, albeit the 95% CI overlapped with 1.0 (adjusted IRR 0.84; 95% CI 0.69–1.03). This effect was similar for cardiovascular and non-cardiovascular hospitalizations.
No statistically significant differences were observed for the outcomes MACE, CRNMB, ischaemic stroke, and major bleeding. However, numbers of events for ischaemic stroke and major bleeding were particularly small. Changes between baseline and follow-up in hrQoL were minimal in both arms. The intervention arm experienced a 0.95 point decrease on the physical health component score (ranging from 0 to 100) over 2 years of follow-up vs. a 1.51 point decrease in the control arm (P = 0.130). For the mental health component score, this was a 2.04 vs. 0.75 points decrease (P = 0.517).
Although we did not have complete data on number of consultations and medication changes, we observed that the mean number of GP consultations per patient during 2.2 years of follow-up was 24.2 in the intervention arm and 15.2 in the usual care arm (data available from 19 out of 26 practices). In the intervention arm, 10.2% of patients switched from VKA to NOAC, compared to 5.9% in the usual care arm (data available from 11 out of 26 practices).
Discussion
Statement of principal findings
In this large cluster randomized trial, we studied the effect of integrated care for AF patients in primary care. Compared to usual care, this integrated care approach delivered by GPs and practice nurses significantly reduced all-cause mortality by 45% (95% CI 0.37–0.82).
Strengths and weaknesses of the study
This is the first study showing effectiveness of structured AF management in primary care. Our results are generalizable to the large majority of AF patients who are on average of high age and preferably managed close to their homes. Our intervention may also be implemented in more rural areas, as it is predominantly provided by nurses and offers a more accessible alternative to the often greater travel distance to the nearest hospital. Other strengths are the low number of patients lost to follow-up and the high compliance rate in the intervention arm. For full appreciation, however, the following issues and limitations need to be discussed.
First, selection bias could have occurred, which is inherent to using a cluster randomized design with interventions delivered on an individual patient level.18,24 As such, we anticipated the potential of such bias and performed pre-specified, adjusted analyses for age, sex, and frailty, which did not substantially change the effect estimate [crude vs. adjusted HR 0.51 (95% CI 0.33–0.76) and 0.55 (95% CI 0.37–0.82), respectively]. Moreover, a cluster randomized design is the best option to assess the effects of integrated care interventions, because randomization at the patient level would have led to considerable contamination. Finally, in the additional analysis free from any potential selection bias and including the 411 patients who did not sign informed consent for participation in the intervention, the effect on mortality was attenuated (as expected, as almost half of these patients did not participate in the intervention) but remained in favour of the intervention (HR 0.81, 95% CI 0.61–1.07).
Second, we did not have information on echocardiographic parameters, NT-proBNP levels, and type of AF (paroxysmal, persistent, or permanent). This information could be informative in understanding why and in whom integrated AF care is most beneficial and should be incorporated in future studies. Finally, the substitution of care from cardiologist to primary care was less than expected: 41% of intervention patients and 48% of the control patients had routine cardiologist control visits during follow-up. Thus, it is likely that many intervention patients received extra care due to the intervention, on top of care from cardiologists or instead of no previous AF care. This could explain part of the observed effect and also exemplifies that modern, integrated AF management should be shared care between primary care, cardiologists, and coagulation experts, across clinical boundaries.
Comparison with existing literature
Previous research, notably the RACE 4 study and the study by Hendriks et al.,3,4,17 studied the effect of integrated care in secondary or tertiary care. In hospital care, patients typically differ from those managed in primary care, as is reflected in the baseline characteristics. For instance, our primary care study population was on average 10 years older than the population studied in a systematic review on integrated AF care in tertiary care (mean age 77.4 vs. 66.9 years)3 and more often suffered from comorbidities. Furthermore, contrary to many younger patients in the hospital setting who receive rhythm control therapy (e.g. ablation procedures), treatment in our study population was typically focused on chronic disease management. Such differences notwithstanding, Hendriks et al.17 found a similar reduction in their primary outcome, i.e. a 35% reduction of the risk of the composite outcome of cardiovascular hospitalization and cardiovascular death. Our findings are also in line with the exploratory analysis of the RACE 4 trial showing a favourable effect of nurse-led care, albeit only in experienced centres (HR 0.52; 95% CI 0.37–0.71). As the authors state, this emphasizes the importance of training and a focus on team-based integrated care approaches.4
Clinical implications
As with any so-called ‘complex intervention’, an interesting question is which aspect of the intervention mostly explains the reduction in mortality. While our study was not set-up to address this question, we can hypothesize about the main drivers of the effect. In general, we believe that the protocolled primary care approach including training of practice nurses in AF management and early recognition of clinical deterioration or complications, such as heart failure, was paramount for the observed effect on all-cause mortality. This is exemplified by the fact that urgent hospitalization occurred less frequently in our intervention arm, which was shown in a post hoc exploratory analysis (adjusted IRR 0.79; 95% CI 0.63–1.00). Additionally, repeated focus on cardiovascular risk management, including management of hypertension and lipid levels, may have contributed. Comprehensive and structured management seem to be important drivers of the beneficial effects, as these were common aspects of both our study and the previously mentioned studies on integrated AF care that also showed positive results.4,17 The RACE 3 trial showed, as a proof of concept, that addressing classical cardiovascular risk factors has beneficial effects on ‘AF progression’, defined as the proportion of time in sinus rhythm.25 Furthermore, the beneficial effect in our study was more pronounced for non-cardiovascular mortality, likely because cardiovascular and non-cardiovascular causes of death are often interrelated. For example, a patient dying from pneumonia might have survived if his underlying heart failure or AF had been better controlled. Moreover, during the INR check-ups, patients were routinely asked about factors like pain or fever. Therefore, it can be hypothesized that during the INR check-ups, patients also mentioned symptoms of non-cardiovascular nature that might have led to, for instance, earlier detection of cancer or pneumonia. As can be seen in the Supplementary material online, Appendix E, the benefit was evenly distributed across the different causes of death (except for death from major bleeding, for which no benefit was observed). Finally, the easy accessibility of the primary care practice for patients26 and the integrated anticoagulation monitoring with direct feedback of the INR value, all contributing to patient education and adherence, could have been beneficial. The absence of an effect on ischaemic stroke and bleeding outcomes suggests that the reduction in all-cause mortality is unlikely to be explained by better anticoagulation management in the intervention arm. Prompted by this observation, the largest of the three involved anticoagulation clinics performed a post hoc analysis of the time in therapeutic range of patients using a VKA in their region, which was similar between intervention and control patients (68.2% vs. 68.1%, respectively), thus strengthening this conclusion. Of note, the relatively low proportion of patients receiving NOAC treatment is at least partly due to the fact that GPs in the Netherlands were not allowed to initiate a NOAC before August 2016. In addition, the 2017 Dutch College of General Practitioners’ guidelines on AF does not encourage to switch stable AF patients from VKA to NOAC and recommend to be reticent in prescribing NOACs to frail elderly patients given the lack of evidence from randomized trials for this population.13 However, background information on NOAC use was provided to the intervention practices as part of the education and more patients switched to NOAC treatment in the index arm compared to the control arm (10.2% vs. 5.9%, respectively, based on data of 11 out of 26 practices). Further research is needed to explore the specific and relative components of integrated AF care that contribute most in reducing clinical adverse outcomes. Also, a thorough cost-effectiveness analysis is warranted, which we plan to publish separately.
Currently, no benefit on mortality has yet been shown in AF trials investigating single-faceted interventions, like anti-arrhythmic drugs or ablation techniques (except in AF patients with severe heart failure).27–29 Although these interventions importantly do impact hrQoL, our findings offer extra arguments for the view that AF is not merely a hearth rhythm disorder, but rather part of a systemic condition.2 Although the exact underlying substrate and pathophysiology are currently being unravelled, a focus on integrated care with treatment of underlying comorbidities like obesity and hypertension seems beneficial, especially in elderly patients.30–32
Conclusion
In this cluster randomized pragmatic trial, we observed a reduction of 45% on all-cause mortality by providing integrated care for elderly AF patients primarily in primary care, compared to usual care.
Supplementary Material
Acknowledgements
The authors would like to thank the Data Safety Monitoring Board (Prof. Dr F. Verheugt, Prof. Dr H. ten Cate, and Dr H. Burger) for monitoring the safety of the trial, and the adjudication committee (Dr M. Hemels, Dr M. Nijkeuter, and Dr R. Venekamp) for their efforts in adjudicating the causes of death. Also, they thank the participating practices and patients for their time and dedication to the implementation of the intervention.
Funding
The ALL-IN trial was funded with an unrestricted grant from the Stichting Achmea Gezondheidszorg (SAG number Z646), the Hein Hogerzeil Stichting, and Roche Diagnostics Nederland B.V. There were no restrictions to the execution of the study or the publication process by any of the subsiding parties of this study.
Conflict of interest: G.J.G. and F.H.R. report unrestricted institutional grants for performing research in the field of atrial fibrillation from Boehringer-Ingelheim, Bayer Healthcare, BMS Pfizer, and Daiichi Sankyo. All other authors report no competing interests.
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 2. van Doorn S, Tavenier A, Rutten FH, Hoes AW, Moons KGM, Geersing GJ.. Risk of cardiac and non-cardiac adverse events in community-dwelling older patients with atrial fibrillation: a prospective cohort study in the Netherlands. BMJ Open 2018;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P, Hendriks J.. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Wijtvliet EPJP, Tieleman RG, van Gelder IC, Pluymaekers NAHA, Rienstra M, Folkeringa RJ, Bronzwaer P, Elvan A, Elders J, Tukkie R, Luermans JGLM, Van Asselt A, Van Kuijk SMJ, Tijssen JG, Crijns HJGM; RACE 4 Investigators. Nurse-led vs. usual-care for atrial fibrillation. Eur Heart J 2020;41:634–641. [DOI] [PubMed] [Google Scholar]
- 5. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A, Witteman JCM, Stricker BH, Heeringa J.. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray C.. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Leeuwen N, Zuurmond M, Melser C.. Most People Have Their GP Close By Data Stat. Netherlands. 2009. https://www.cbs.nl/en-gb/news/2009/22/most-people-have-their-gp-close-by (22 August 2019).
- 8. van den Dries CJ, Oudega R, Elvan A, Rutten FH, van de Leur JJCM, Bilo HJG, Hoes AW, Moons KGM, Geersing GJ.. Integrated management of atrial fibrillation including tailoring of anticoagulation in primary care: study design of the ALL-IN cluster randomised trial. BMJ Open 2017;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell MK, Piaggio G, Elbourne DR, Altman DG; for the CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 10. Weijer C, Grimshaw JM, Eccles MP, McRae AD, White A, Brehaut JC, Taljaard M, Althabe F, Binik A, Brown JB, Boruch R, Brehaut JC, Chaudhry S, Donner A, Dubois-Flynn G, Eccles MP, Edwards S, Elbourne D, Eldridge S, Forster D, Gallo A, Grimshaw JM, Kiefe C, Kimmelman J, Lin M, Loder E, Lohr K, McRae AD, Naughton ES, Polson RJ; Ottawa Ethics of Cluster Randomized Trials Consensus Group. The Ottawa Statement on the ethical design and conduct of cluster randomized trials. PLoS Med 2012;9:e1001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Graaf R, Koffijberg H, Grobbee DE, de Hoop E, Moons KGM, van Thiel GJMW, de Wit GA, van Delden JJM.. The ethics of cluster-randomized trials requires further evaluation: a refinement of the Ottawa Statement. J Clin Epidemiol 2015;68:1108–1114. [DOI] [PubMed] [Google Scholar]
- 12.Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-related Research Involving Humans 2016.
- 13.NHG-werkgroep Atriumfibrilleren. NHG-Standaard Atriumfibrilleren (derde partiële herziening). Huisarts Wet 2017;60:460. [Google Scholar]
- 14. Schulman S, Kearon C, the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 15. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, the Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119–2126. [DOI] [PubMed] [Google Scholar]
- 16. Ware JE, Kosinski M, Keller SD.. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 17. Hendriks JML, De Wit R, Crijns H, Vrijhoef HJM, Prins MH, Pisters R, Pison L. A F, Blaauw Y, Tieleman RG.. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J 2012;33:2692–2699. [DOI] [PubMed] [Google Scholar]
- 18. Leyrat C, Caille A, Donner A, Giraudeau B.. Propensity scores used for analysis of cluster randomized trials with selection bias: a simulation study. Stat Med 2013;32:3357–3372. [DOI] [PubMed] [Google Scholar]
- 19. Drubbel I, De Wit N, Bleijenberg N, Eijkemans RJC, Schuurmans MJ, Numans ME.. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. Journals Gerontol A Biol Sci Med Sci 2013;68:301–308. [DOI] [PubMed] [Google Scholar]
- 20. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995;14:1707–1723. [DOI] [PubMed] [Google Scholar]
- 21. Therneau TM, Grambsch PM, Fleming TR.. Martingale-based residuals for survival models. Biometrika 1990;77:147–160. [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2018.
- 23. Therneau TM. A Package for Survival Analysis in S Version 2.38. 2015.
- 24. Hahn S, Puffer S, Torgerson DJ, Watson J.. Methodological bias in cluster randomised trials. BMC Med Res Methodol 2005;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, Veldhuisen DV, Crijns H, Gelder IV; for the RACE 3 Investigators. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 26. Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS.. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med 2019;179:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 28. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, Flaker GC, Pokushalov E, Romanov A, Bunch TJ, Noelker G, Ardashev A, Revishvili A, Wilber DJ, Cappato R, Kuck K-H, Hindricks G, Davies DW, Kowey PR, Naccarelli GV, Reiffel JA, Piccini JP, Silverstein AP, Al-Khalidi HR, Lee KL.. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D.. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 30. Kottkamp H. Catheter ablation of atrial fibrillation: on the pathophysiology of the arrhythmia and the impact of cardiac risk factor management. J Am Coll Cardiol 2014;64:2232–2234. [DOI] [PubMed] [Google Scholar]
- 31. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic N. A, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P.. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 32. Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet 2017;390:1873–1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.