Abstract
Aim:
Over the last decades, few significant achievements have been made in tuberculosis (TB) therapy. As a result, there is an urgent need for new anti-TB drugs.
Results:
Two new classes of bis-(imidazole/benzimidazole)-pyridine derivatives were designed, synthesized and evaluated for their antimycobacterial activity.
Conclusion:
The synthesis is efficient and straightforward, involving only two successive N-alkylations. The anti-TB assay reveal that our compounds have an excellent anti-TB activity against both replicating and nonreplicating Mtb, are not cytotoxic, exhibited a very good intracellular activity and are active against drug-resistant Mtb strains, some compounds have a bactericidal mechanism. The absorption, distribution, metabolism, excretion and toxicity studies performed for one compound are promising, indicating that it is a good candidate for a future drug.
Keywords: : ADMET, antimycobacterial, bis-(imidazole/benzimidazole)-pyridine, MBC, MIC
During the last two centuries, tuberculosis (TB) became an extremely aggressive disease responsible for millions of deaths around the world [1]. According to the WHO, TB now remains a leading infectious disease worldwide, almost a third of the world’s population (around 2 billion people) being infected with nonreplicating Mtb [1]. Drug-resistant TB, multidrug-resistant TB, extensively drug-resistant TB and totally drug-resistant TB are present in almost all countries all around the world, and making the situation even more challenging for global TB treatment. In 2017, there were around 700,000 new cases with resistance to rifampicin, the most effective first-line drug, of which 500,000 had multi-drug resistant-TB [1–3]. In addition, association of TB with HIV/AIDS complicates the situation for patients even more, with TB being the leading cause of death among the patients infected with HIV (in 2017, there were an estimated 400,000 TB deaths among HIV-positive people) [1,4,5]. As a result of these considerations, each year (in the last 5), there were an estimated two million TB deaths and about eight million new cases of TB reported [1].
The TB treatment is a challenging, complex and extremely difficult task. In the last 50 years, only two new drugs (bedaquiline [6] and delamanid [7]) have been developed in anti-TB therapies, for drug-resistant strains of Mtb. Therefore, the search for new anti-TB drugs active against Mtb remains one of the priority tasks of medicinal chemistry. There are currently several strategies used for the development of new anti-TB drugs [8–11], one of the most important and used strategy consist of searching for novel structures which the TB organism has never encountered with before. Nitrogen heterocyclic compounds, especially five and six member ring derivatives, represent the most effective and administered class of drugs in TB therapy. Among them, pyridine and imidazole/benzimidazole derivatives play a central role, the most effective and promising anti-TB drugs being related to these classes [8–18].
Encouraged by the above considerations and in continuation of our research in the area of new antimycobacterial compounds with azaheterocyclic skeleton [18–30], we report herein the design, synthesis and antimycobacterial activity of a new class of bis-(imidazole/benzimidazole)-pyridine (BIP) derivatives.
Experimental
Materials & measurements
Melting points were determined using an electrothermal Mel-Temp apparatus and were uncorrected (Barnstead International, IA, USA). The nuclear magnetic experiments (1H-NMR, 13C-NMR, 2D-COSY, 2D-HMQC, 2D-HMBC) were recorded using Bruker Avance III 500/Bruker Avance DRX 400 spectrometers (Bruker, Vienna, Austria) operating at 500/400 and 125/100 MHz for 1H and, respectively, 13C nuclei. The following abbreviations were used to designate chemical shift multiplicities: s = singlet, d = doublet, t = triplet, m = multiplet, brs = broad singlet, ad = apparent doublet, at = apparent triplet. All chemical shifts are reported in δ units (p.p.m.) relative to the residual peak of solvent (ref: DMSO, 1H: 2.50 p.p.m.; 13C: 39.52 p.p.m.). Coupling constants (J) are given in Hz. The IR spectra were recorded on a Fourier transform IR (FTIR) Shimadzu Prestige 8400s spectrophotometer (Shimadzu, Kyoto, Japan). Thin layer chromatography (TLC) was carried out on Merck silica gel 60F254 plates. Visualization of the plates was achieved using a UV lamp (λmax = 254 or 365 nm). The ultrasonic bath Elma Transsonic T310 (power 34.5 W, frequency 35 kHz; Elma Schmidtbauer, Singen, Germany) was used for solubilizing the starting materials. The micro-analyses were in satisfactory agreement with the calculated values: C, ± 0.15; H, ± 0.10; N, ± 0.30.
Compounds 2a and 5 were previously reported [31].
General procedure for synthesis of starting materials 2a,b & 5
To a solution of imidazole/4-nitroimidazole (3 mmol, 3 equivalent, 0.20 g/0.34 g, dissolved in 15 ml tetrahydrofuran dry/35 ml dimethylformamide using US bath), or benzimidazole (2.4 mmol, 2.4 equivalent, 0.28 g, dissolved in 15 tetrahydrofuran dry), was added dropwise, at 0°C (ice), NaH (60% in mineral oil; 3.6 mmol, 3.6 equivalent, 0.14 g, suspended in 15 ml tetrahydrofuran dry). The sodium hydride was firstly washed three times with 5 ml n-hexane. Then, 2,6-bis(halogenomethyl)pyridine 1 (1 mmol, 1 equivalent, 0.18 g (X = -Cl for the solution of imidazole/4-nitroimidazole), 0.26 g (X = -Br for the solution of benzimidazole) dissolved in 15 ml tetrahydrofuran dry), was added dropwise. The resulting solutions were stirred for additional time (72, 24, 96 h) at room temperature, to give the corresponding starting materials 2a,b and 5. The solutions were filtered off, concentrated on rotary evaporator, than precipitated with diethyl ether for compounds 2a and 5, or dimethylformamide/methanol: 3/15 (v/v), for compound 2b. The formed precipitates were collected by filtration and dried in vacuo.
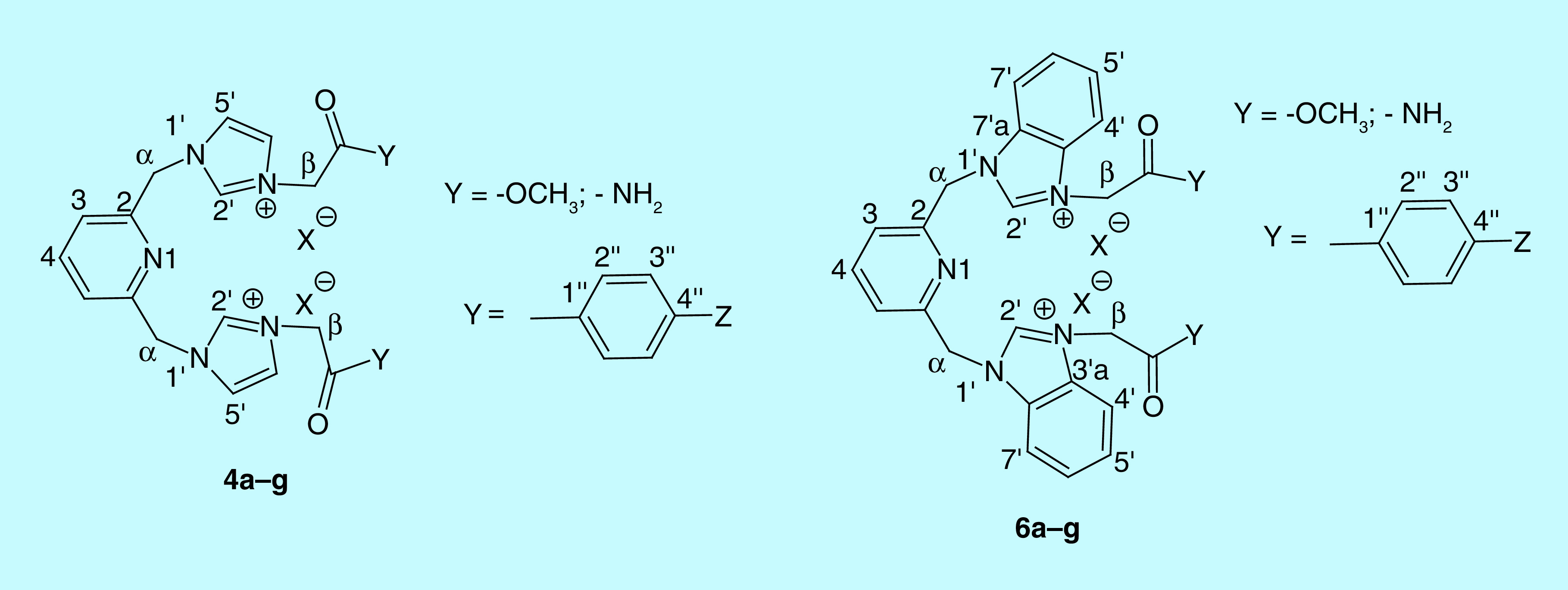
General procedure for synthesis of quaternary salts 4a–g
A solution of 2,6-bis((1H-imidazol-1-yl)methyl)pyridine 2a (1 mmol, 1 equivalent, 0.24 g, dissolved in 40 ml acetone using the ultrasound bath) and the α-halogeno ester and amide (2.4 mmol, 2.4 equivalent, 0.22 ml 3a, 0.44 g 3b) or the bromacetophenone derivatives (2.8 mmol, 2.8 equivalent, 0.56 g 3c, 0.78 g 3d, 0.65 g 3e, 0.68 g 3f, 0.64 g 3g) solubilized in acetone (15 ml), was magnetically stirred for 24 h, to give the corresponding quaternary salts 4a–g. The completion of the reactions was carried out using TLC. The obtained salts were filtered off, washed two times with the same solvent (10 ml) and dried in vacuo. No other purification required.
General procedure for synthesis of quaternary salts 6a–g
To a solution of 2,6-bis((1H-benzo[d]imidazol-1-yl)methyl)pyridine 5 (1 mmol, 1 equivalent, 0.34 g, dissolved in 40 ml acetone using the ultrasound bath) were added the α-halogeno ester and amide (4.8 mmol, 4.8 equivalent, 0.73 ml 3a, 0.89 g 3b) or the bromacetophenone derivatives (4.5 mmol, 4.5 equivalent, 0.89 g 3c, 1.25 g 3d, 1.05 g 3e, 1.1 g 3f, 1.03 g 3g) solubilized in acetone (20 ml). The solutions were refluxed for 12 h and magnetically stirred for another 12 h, resulting the desired salts 6a–g. TLC was used for follow the evolution of chemical reactions. The precipitates were collected by filtration, then washed with acetone (3 times with 10 ml) and dried in vacuum. No other purification required.
Spectral data of starting materials 2a,b & 5
2,6-bis((1H-imidazol-1-yl)methyl)pyridine (2a)
Appearance: white powder. Yield, 76%. melting point (mp) 98–106°C. FTIR (KBr) cm-1 = 3101, 2987, 1595, 1506, 1431, 1230. 1H-NMR (400 MHz, DMSO-d6) δ = 7.81–7.77 (t, J = 7.6 Hz, 1H, H4), 7.75 (s, 2H, 2xH2′), 7.20 (s, 2H, 2xH5′), 7.05–7.03 (d, J = 7.6 Hz, 2H, 2xH3), 6.93 (s, 2H, 2xH4′), 5.30 (s, 4H, 2xCH2). 13C-NMR (100 MHz, DMSO-d6) δ = 156.7 2xC2, 138.5 C4, 137.7 2xC2′, 128.6 2xC4′, 120.3 2xC3, 119.8 2xC5′, 51.1 2xCH2. Anal. Calcd. for C13H13N5: C, 65.25; H, 5.48; N, 29.27; found: C, 65.40; H, 5.38; N, 29.57.
2,6-bis((4-nitro-1H-imidazol-1-yl)methyl)pyridine (2b)
Appearance: white powder. Yield, 72%. mp 189–195°C. FTIR (KBr) cm-1 = 3140, 3119, 1595, 1525, 1485, 1330. 1H-NMR (400 MHz, DMSO-d6) δ = 8.36 (ad, 2H, 2xH2′), 7.91–7.87 (at, J = 8.0 Hz, 3H, 2xH5′, H4), 7.34–7.32 (d, J = 7.6 Hz, 2H, 2xH3), 5.42 (s, 4H, 2xCH2). 13C-NMR (100 MHz, DMSO-d6) δ = 154.9 2xC2, 146.9 2xC4′, 138.7 C4, 137.7 2xC5′, 121.8 2xC2′, 121.2 2xC3, 51.6 2xCH2. Anal. Calcd. for C13H11N7O4: C, 47.42; H, 3.37; N, 29.78; found: C, 47.32; H, 3.30; N, 29.98.
2,6-bis((1H-benzo[d]imidazol-1-yl)methyl)pyridine (5)
Appearance: white powder. Yield, 64%. mp 154–160°C. FTIR (KBr) cm-1 = 3120, 2990, 1590, 1526, 1451, 1270. 1H-NMR (500 MHz, DMSO-d6) δ = 8.35 (s, 2H, 2xH2′), 7.74–7.71 (t, J = 8.0 Hz, 1H, H4), 7.66–7.65 (d, J = 8.0 Hz, 2H, 2xH7′), 7.40–7.38 (d, J = 8.0 Hz, 2H, 2xH4′), 7.19–7.11 (m, 6H, 2xH6′, 2xH3, 2xH5′), 5.54 (s, 4H, 2xCH2). 13C- NMR (125 MHz, DMSO-d6) δ = 155.9 2xC2, 144.4 2xC2′, 143.4 2xC3′a, 138.4 C4, 133.7 2xC7′a, 122.4 2xC5′, 121.5 2xC6′, 120.9 2xC3, 119.4 2xC7′, 110.6 2xC4′, 49.4 2xCH2. Anal. Calcd. for C21H17N5: C, 74.32; H, 5.05; N, 20.63; found: C, 74.47; H, 5.15; N, 20.33.
Spectral data of quaternary salts 4a–g & 6a–g
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-methoxy-2-oxoethyl)-1H-imidazol-3-ium) bromide (4a)
Appearance: white powder. Yield, 50%. mp 83–85°C. FTIR (KBr) cm-1 = 3095, 2983, 1730, 1593, 1440, 1240, 1070. 1H-NMR (500 MHz, DMSO-d6) δ = 9.33 (s, 2H, 2xH2′), 8.00–7.97 (t, J = 7.5 Hz, 1H, H4), 7.80 (s, 2H, 2xH4′), 7.75 (s, 2H, 2xH5′), 7.53–7.52 (d, J = 8.0 Hz, 2H, 2xH3), 5.67 (s, 4H, 2xCH2-α), 5.39 (s, 4H, 2xCH2-β), 3.76 (s, 6H, 2xCH3). 13C-NMR (125 MHz, DMSO-d6) δ = 167.4 2xCO, 153.4 2xC2, 138.8 C4, 137.8 2xC2′, 123.6 2xC4′, 123.0 2xC5′, 122.2 2xC3, 52.8 2xCH3, 52.6 2xCH2-α, 49.7 2xCH2-β. Anal. Calcd. for C19H23Br2N5O4: C, 41.85; H, 4.25; N, 12.84; found: C, 41.95; H, 4.34; N, 12.65.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-amino-2-oxoethyl)-1H-imidazol-3-ium) iodide (4b)
Appearance: yellowish powder. Yield, 68%. mp 182–184°C. FTIR (KBr) cm-1 = 3348, 3140, 3079, 2940, 1679, 1584, 1520, 1432. 1H-NMR (500 MHz, DMSO-d6) δ = 9.15 (s, 2H, 2xH2′), 8.00–7.97 (t, J = 7.5 Hz, 1H, H4), 7.88 (brs, 2H, 2x(-NH-)), 7.68 (s, 4H, 2xH4′, 2xH5′), 7.55 (brs, 2H, 2x(-NH-)), 7.51–7.49 (d, J = 7.5 Hz, 2H, 2xH3), 5.59 (s, 4H, 2xCH2-α), 5.01 (s, 4H, 2xCH2-β). 13C-NMR (125 MHz, DMSO-d6) δ = 166.7 2xCO, 153.5 2xC2, 138.9 C4, 137.8 2xC2′, 123.8 2xC4′, 122.5 2xC5′, 122.1 2xC3, 52.6 2xCH2-α, 50.6 2xCH2-β. Anal. Calcd. for C17H21I2N7O2: C, 33.52; H, 3.47; N, 16.09; found: C, 33.67; H, 3.57; N, 15.84.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-oxo-2-phenylethyl)-1H-imidazol-3-ium) bromide (4c)
Appearance: white powder. Yield, 85%. mp 165–167°C. FTIR (KBr) cm-1 = 3080, 2920, 1687, 1583, 1525, 1430. 1H-NMR (500 MHz, DMSO-d6) δ = 9.30 (s, 2H, 2xH2′), 8.06–8.01 (m, 5H, 4xH2′′, H4), 7.82–7.81 (ad, 4H, 2xH4′, 2xH5′), 7.77–7.74 (t, J = 7.5 Hz, 2H, 2xH4′′), 7.62–7.57 (m, 6H, 4xH3′′, 2xH3), 6.21 (s, 4H, 2xCH2-β), 5.74 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 191.4 2xCO, 153.6 2xC2, 138.8 C4, 137.8 2xC2′, 134.5 2xC4′′, 133.6 2xC1′′, 129.0 4xC3′′, 128.1 4xC2′′, 123.9 2xC4′, 122.9 2xC5′, 122.1 2xC3, 55.6 2xCH2-β, 52.6 2xCH2-α. Anal. Calcd. for C29H27Br2N5O2: C, 54.65; H, 4.27; N, 10.99; found: C, 54.55; H, 4.36; N, 10.79.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-bromophenyl)-2-oxoethyl)-1H-imidazol-3-ium) bromide (4d)
Appearance: white powder. Yield, 92%. mp 238–240°C. FTIR (KBr) cm-1 = 3115, 3070, 2960, 1695, 1583, 1560, 610. 1H-NMR (500 MHz, DMSO-d6) δ = 9.26 (s, 2H, 2xH2′), 8.04–8.01 (t, J = 8.0 Hz, 1H, H4), 7.97–7.95 (d, J = 9.0 Hz, 4H, 4xH2′′), 7.83–7.82 (d, J = 8.5 Hz, 4H, 4xH3′′), 7.80 (s, 2H, 2xH4′), 7.78 (s, 2H, 2xH5′), 7.57–7.55 (d, J = 8.0 Hz, 2H, 2xH3), 6.16 (s, 4H, 2xCH2-β), 5.72 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 190.8 2xCO, 153.6 2xC2, 138.9 C4, 137.8 2xC2′, 132.6 2xC1′′, 132.1 4xC3′′, 130.1 4xC2′′, 128.7 2xC4′′, 123.9 2xC5′, 122.9 2xC4′, 122.2 2xC3, 55.6 2xCH2-β, 52.6 2xCH2-α. Anal. Calcd. for C29H25Br4N5O2: C, 43.8; H, 3.17; N, 8.81; found: C, 43.7; H, 3.26; N, 9.01.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-chlorophenyl)-2-oxoethyl)-1H-imidazol-3-ium) bromide (4e)
Appearance: yellowish powder. Yield, 77%. mp 209–212°C. FTIR (KBr) cm-1 = 3126, 3086, 2972, 1697, 1589, 1558, 758. 1H-NMR (500 MHz, DMSO-d6) δ = 9.23 (s, 2H, 2xH2′), 8.05–8.02 (m, 5H, 4xH2′′, H4), 7.78 (s, 2H, 2xH4′), 7.76 (s, 2H, 2xH5′), 7.69–7.67 (d, J = 8.5 Hz, 4H, 4xH3′′), 7.56–7.55 (d, J = 8.0 Hz, 2H, 2xH3), 6.13 (s, 4H, 2xCH2-β), 5.71 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 190.6 2xCO, 153.6 2xC2, 139.4 2xC4′′, 138.9 C4, 137.9 2xC2′, 132.3 2xC1′′, 130.0 4xC2′′, 129.2 4xC3′′, 123.9 2xC5′, 122.9 2xC4′, 122.2 2xC3, 55.6 2xCH2-β, 52.7 2xCH2-α. Anal. Calcd. for C29H25Br2Cl2N5O2: C, 49.32; H, 3.57; N, 9.92; found: C, 49.20; H, 3.67; N, 9.74.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-nitrophenyl)-2-oxoethyl)-1H-imidazol-3-ium) bromide (4f)
Appearance: pink powder. Yield, 81%. mp 233–236°C. FTIR (KBr) cm-1 = 3120, 2935, 1698, 1580, 1523, 1473, 1348. 1H-NMR (500 MHz, DMSO-d6) δ = 9.31 (s, 2H, 2xH2′), 8.40–8.39 (d, J = 9.0 Hz, 4H, 4xH3′′), 8.29–8.28 (d, J = 9.0 Hz, 4H, 4xH2′′), 8.05–8.02 (t, J = 8.0 Hz, 1H, H4), 7.83–7.82 (add, 4H, 2xH4′, 2xH5′), 7.58–7.57 (d, J = 8.0 Hz, 2H, 2xH3), 6.28 (s, 4H, 2xCH2-β), 5.75 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 190.9 2xCO, 153.6 2xC2, 150.4 2xC4′′, 138.9 C4, 138.2 2xC1′′, 137.8 2xC2′, 129.7 4xC2′′, 124.0 4xC3′′, 123.8 2xC4′, 123.0 2xC5′, 122.2 2xC3, 56.0 2xCH2-β, 52.7 2xCH2-α. Anal. Calcd. for C29H25Br2N7O6: C, 47.89; H, 3.46; N, 13.48; found: C, 47.99; H, 3.37; N, 13.65.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-imidazol-3-ium) bromide (4g)
Appearance: white powder. Yield, 92%. mp 210–213°C. FTIR (KBr) cm-1 = 3076, 2933, 1683, 1600, 1244, 1168. 1H-NMR (500 MHz, DMSO-d6) δ = 9.26 (s, 2H, 2xH2′), 8.07–7.98 (m, 5H, H4, 4xH2′′), 7.79–7.78 (ad, 4H, 2xH4′, 2xH5′), 7.57–7.56 (d, J = 7.5 Hz, 2H, 2xH3), 7.11–7.09 (d, J = 8.5 Hz, 4H, 4xH3′′), 6.10 (s, 4H, 2xCH2-β), 5.72 (s, 4H, 2xCH2-α), 3.87 (s, 6H, 2xCH3). 13C-NMR (125 MHz, DMSO-d6) δ = 189.7 2xCO, 164.1 2xC4′′, 153.6 2xC2, 138.9 C4, 137.9 2xC2′, 130.6 4xC2′′, 126.4 2xC1′′, 123.9 2xC4′, 122.8 2xC5′, 122.2 2xC3, 114.3 4xC3′′, 55.7 2xCH3, 55.2 2xCH2-β, 52.6 2xCH2-α. Anal. Calcd. for C31H31Br2N5O4: C, 53.39; H, 4.48; N, 10.04; found: C, 53.26; H, 4.58; N, 9.86.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-methoxy-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) bromide (6a)
Appearance: white powder. Yield, 39%. mp 184–188°C. FTIR (KBr) cm-1 = 3135, 3080, 2933, 1729, 1600, 1431, 1234, 1069. 1H-NMR (500 MHz, DMSO-d6) δ = 9.93 (s, 2H, 2xH2′), 8.05–8.03 (m, 3H, 2xH4′, H4), 7.70–7.68 (d, J = 8.0 Hz, 2H, 2xH3), 7.65–7.61 (t, J = 8.0 Hz, 4H, 2xH7′, 2xH5′), 7.44–7.41 (t, J = 8.0 Hz, 2H, 2xH6′), 5.96 (s, 4H, 2xCH2-α), 5.68 (s, 4H, 2xCH2-β), 3.77 (s, 6H, 2xCH3). 13C-NMR (125 MHz, DMSO-d6) δ = 167.1 2xCO, 152.9 2xC2, 143.7 2xC2′, 139.0 C4, 131.1 2xC3′a, 130.5 2xC7′a, 126.8 2xC5′, 126.6 2xC6′, 122.7 2xC3, 113.9 2xC4′, 113.5 2xC7′, 52.9 2xCH3, 50.7 2xCH2-α, 47.5 2xCH2-β. Anal. Calcd. for C27H27Br2N5O4: C, 50.25; H, 4.22; N, 10.85; found: C, 50.10; H, 4.13; N, 11.04.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-amino-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) iodide (6b)
Appearance: yellow powder. Yield, 74%. mp 123–126°C. FTIR (KBr) cm-1 = 3349, 3168, 3040, 2915, 1680, 1598, 1420. 1H-NMR (500 MHz, DMSO-d6) δ = 9.70 (s, 2H, 2xH2′), 8.05–8.02 (m, 3H, H4, 2x(-NH-)), 7.90–7.88 (d, J = 8.5 Hz, 2H, 2xH4′), 7.72 (brs, 2H, 2x(-NH-)), 7.69–7.68 (d, J = 7.5 Hz, 2H, 2xH3), 7.64–7.61 (t, J = 8.0 Hz, 2H, 2xH5′), 7.60–7.59 (d, J = 8.0 Hz, 2H, 2xH7′), 7.42–7.38 (t, J = 8.0 Hz, 2H, 2xH6′), 5.89 (s, 4H, 2xCH2-α), 5.26 (s, 4H, 2xCH2-β). 13C-NMR (125 MHz, DMSO-d6) δ = 166.4 2xCO, 152.9 2xC2, 143.8 2xC2′, 138.9 C4, 131.2 2xC3′a, 130.5 2xC7′a, 126.6 2xC5′, 126.4 2xC6′, 122.6 2xC3, 113.4 2xC4′, 113.4 2xC7′, 50.5 2xCH2-α, 48.4 2xCH2-β. Anal. Calcd. for C25H25I2N7O2: C, 42.33; H, 3.55; N, 13.82; found: C, 42.43; H, 3.50; N, 13.97.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-oxo-2-phenylethyl)-1H-benzo[d]imidazol-3-ium) bromide (6c)
Appearance: white powder. Yield, 50%. mp 203–205°C. FTIR (KBr) cm-1 = 3139, 2973, 1697, 1586, 1543, 1480. 1H-NMR (500 MHz, DMSO-d6) δ = 9.88 (s, 2H, 2xH2′), 8.12–8.11 (d, J = 8.0 Hz, 4H, 4xH2′′), 8.09–8.06 (m, 3H, H4, 2xH4′), 7.81–7.78 (t, J = 7.5 Hz, 2H, 2xH4′′), 7.75–7.73 (d, J = 8.0 Hz, 2H, 2xH3), 7.68–7.59 (m, 8H, 2xH7′, 4xH3′′, 2xH5′), 7.45–7.42 (t, J = 8.0 Hz, 2H, 2xH6′), 6.49 (s, 4H, 2xCH2-β), 6.02 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 191.3 2xCO, 153.0 2xC2, 143.8 2xC2′, 139.0 C4, 134.6 2xC4′′, 133.6 2xC1′′, 131.6 2xC3′a, 130.5 2xC7′a, 129.0 4xC3′′, 128.5 4xC2′′, 126.7 2xC5′, 126.5 2xC6′, 122.7 2xC3, 113.9 2xC4′, 113.4 2xC7′, 53.4 2xCH2-β, 50.7 2xCH2-α. Anal. Calcd. for C37H31Br2N5O2: C, 60.26; H, 4.24; N, 9.50; found: C, 60.16; H, 4.33; N, 9.35.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-bromophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) bromide (6d)
Appearance: white powder. Yield, 77%. mp 193–195°C. FTIR (KBr) cm-1 = 3140, 3020, 2943, 1694, 1585, 1550, 1470, 620. 1H-NMR (500 MHz, DMSO-d6) δ = 9.83 (s, 2H, 2xH2′), 8.08–8.05 (m, 3H, H4, 2xH4′), 8.02–8.01 (d, J = 9.0 Hz, 4H, 4xH2′′), 7.87–7.85 (d, J = 8.5 Hz, 4H, 4xH3′′), 7.74–7.72 (d, J = 7.5 Hz, 2H, 2xH3), 7.68–7.66 (d, J = 8.5 Hz, 2H, 2xH7′), 7.62–7.59 (t, J = 7.5 Hz, 2H, 2xH5′), 7.46–7.43 (t, J = 7.5 Hz, J = 8.0 Hz, 2H, 2xH6′), 6.43 (s, 4H, 2xCH2-β), 6.00 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 190.7 2xCO, 153.0 2xC2, 143.8 2xC2′, 139.0 C4, 132.6 2xC1′′, 132.1 4xC3′′, 131.6 2xC3′a, 130.5 2xC7′a, 130.4 4xC2′′, 128.8 2xC4′′, 126.7 2xC5′, 126.5 2xC6′, 122.7 2xC3, 114.0 2xC4′, 113.4 2xC7′, 53.3 2xCH2-β, 50.7 2xCH2-α. Anal. Calcd. for C37H29Br4N5O2: C, 49.64; H, 3.26; N, 7.82; found: C, 49.74; H, 3.17; N, 7.98.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-chlorophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) bromide (6e)
Appearance: white powder. Yield, 83%. mp 175–178°C. FTIR (KBr) cm-1 = 3136, 3068, 2970, 1695, 1589, 1564, 1483, 765. 1H-NMR (400 MHz, DMSO-d6) δ = 9.84 (2H, s, 2xH2′), 8.11–8.09 (d, J = 8.4 Hz, 4H, 4xH2′′), 8.07–8.05 (d, J = 8.0 Hz, 3H, 2xH4′, H4), 7.74–7.67 (m, 8H, 2xH3, 4xH3′′, 2xH7′), 7.63–7.59 (t, J = 7.6 Hz, 2H, 2xH5′), 7.46–7.42 (t, J = 7.6 Hz, 2H, 2xH6′), 6.44 (s, 4H, 2xCH2-β), 6.00 (s, 4H, 2xCH2-α). 13C-NMR (100 MHz, DMSO-d6) δ = 190.4 2xCO, 153.0 2xC2, 143.8 2xC2′, 139.5 2xC4′′, 139.0 C4, 132.3 2xC1′′, 131.6 2xC3′a, 130.5 2xC7′a, 130.3 4xC2′′, 129.1 4xC3′′, 126.7 2xC5′, 126.5 2xC6′, 122.7 2xC3, 113.9 2xC4′, 113.4 2xC7′, 53.4 2xCH2-β, 50.7 2xCH2-α. Anal. Calcd. for C37H29Br2Cl2N5O2: C, 55.11; H, 3.62; N, 8.69; found: C, 54.96; H, 3.57; N, 8.85.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-nitrophenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) bromide (6f)
Appearance: yellowish powder. Yield, 48%. mp 235–238°C. FTIR (KBr) cm-1 = 3135, 3018, 2955, 1685, 1583, 1525, 1488, 1348. 1H-NMR (500 MHz, DMSO-d6) δ = 9.88 (s, 2H, 2xH2′), 8.43–8.42 (d, J = 8.0 Hz, 4H, 4xH3′′), 8.35–8.33 (d, J = 8.5 Hz, 4H, 4xH2′′), 8.12–8.06 (m, 3H, 2xH4′, H4), 7.75–7.73 (d, J = 7.5 Hz, 2H, 2xH3), 7.71–7.70 (d, J = 8.5 Hz, 2H, 2xH7′), 7.64–7.61 (t, J = 7.5 Hz, 2H, 2xH5′), 7.48–7.45 (t, J = 7.5 Hz, 2H, 2xH6′), 6.55 (s, 4H, 2xCH2-β), 6.03 (s, 4H, 2xCH2-α). 13C-NMR (125 MHz, DMSO-d6) δ = 190.7 2xCO, 153.0 2xC2, 150.5 2xC4′′, 143.7 2xC2′, 139.0 C4, 138.3 2xC1′′, 131.6 2xC3′a, 130.5 2xC7′a, 130.0 4xC2′′, 126.7 2xC5′, 126.6 2xC6′, 123.9 4xC3′′, 122.7 2xC3, 114.1 2xC4′, 113.5 2xC7′, 53.9 2xCH2-β, 50.7 2xCH2-α. Anal. Calcd. for C37H29Br2N7O6: C, 53.70; H, 3.53; N, 11.85; found: C, 53.80; H, 3.62; N, 11.70.
1,1′-(pyridine-2,6-diylbis(methylene))bis(3-(2-(4-methoxyphenyl)-2-oxoethyl)-1H-benzo[d]imidazol-3-ium) bromide (6g)
Appearance: white powder. Yield, 71%. mp 183–186°C. FTIR (KBr) cm-1 = 3138, 3016, 2931, 1683, 1599, 1566, 1242, 1172. 1H-NMR (500 MHz, DMSO-d6) δ = 9.89 (s, 2H, 2xH2′), 8.08–8.04 (m, 7H, 4xH2′′, 2xH4′, H4), 7.76–7.75 (d, J = 7.5 Hz, 2H, 2xH3), 7.70–7.68 (d, J = 8.0 Hz, 2H, 2xH7′), 7.62–7.58 (t, J = 8.0 Hz, 2H, 2xH5′), 7.45–7.42 (t, J = 8.0 Hz, 2H, 2xH6′), 7.14–7.12 (d, J = 8.5 Hz, 4H, 4xH3′′), 6.42 (s, 4H, 2xCH2-β), 6.01 (s, 4H, 2xCH2-α), 3.90 (6H, s, 2xCH3). 13C-NMR (125 MHz, DMSO-d6) δ = 189.5 2xCO, 164.2 2xC4′′, 153.0 2xC2, 143.8 2xC2′, 139.0 C4, 131.6 2xC3′a, 130.9 4xC2′′, 130.5 2xC7′a, 126.6 2xC5′, 126.5 2xC6′, 126.4 C1′′, 122.7 2xC3, 114.2 4xC3′′, 113.9 2xC4′, 113.4 2xC7′, 55.8 2xCH3, 53.0 2xCH2-β, 50.6 2xCH2-α. Anal. Calcd. for C39H35Br2N5O4: C, 58.73; H, 4.42; N, 8.78; found: C, 58.63; H, 4.47; N, 8.63.
Microbiology
Compounds were evaluated for antimycobacterial activity against Mycobacterium tuberculosis, as a part of the Center of Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) TB screening program under direction of the US National Institute of Health, the NIAID division. Antimycobacterial activities of the compounds were performed by TAACF at Southern Research Institute. All protocols concerning the antimycobacterial evaluation of tested compounds can be found in the Supplementary Figure 1.
Figure 1. . Atom identification in the spectral data of quaternary salts 4a–g and 6a–g.
Results & discussion
Design & synthesis
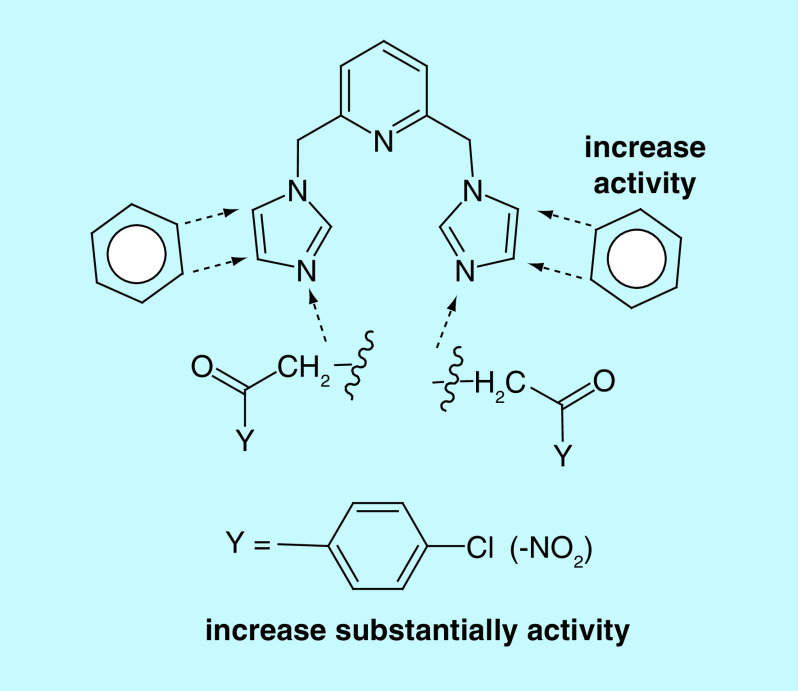
One of the most useful strategies in drug discovery is the pharmacophore combination of well defined scaffolds. Bearing in mind this strategy, we decided to bring new contributions in the anti-TB fighting and, pursuing to achieve products with better activity, better pharmacological properties and alternative mechanisms of action, the present study is aimed to combine three pharmacophores: pyridine, imidazole/benzimidazole and p-chlorobenzoyl moieties. Structural necessities around pyridine and imidazole/benzimidazole moieties, for an anti-TB activity, are depicted in Figure 2.
Figure 2. . Design in the class of bis-(imidazole/benzimidazole)-pyridine derivatives.
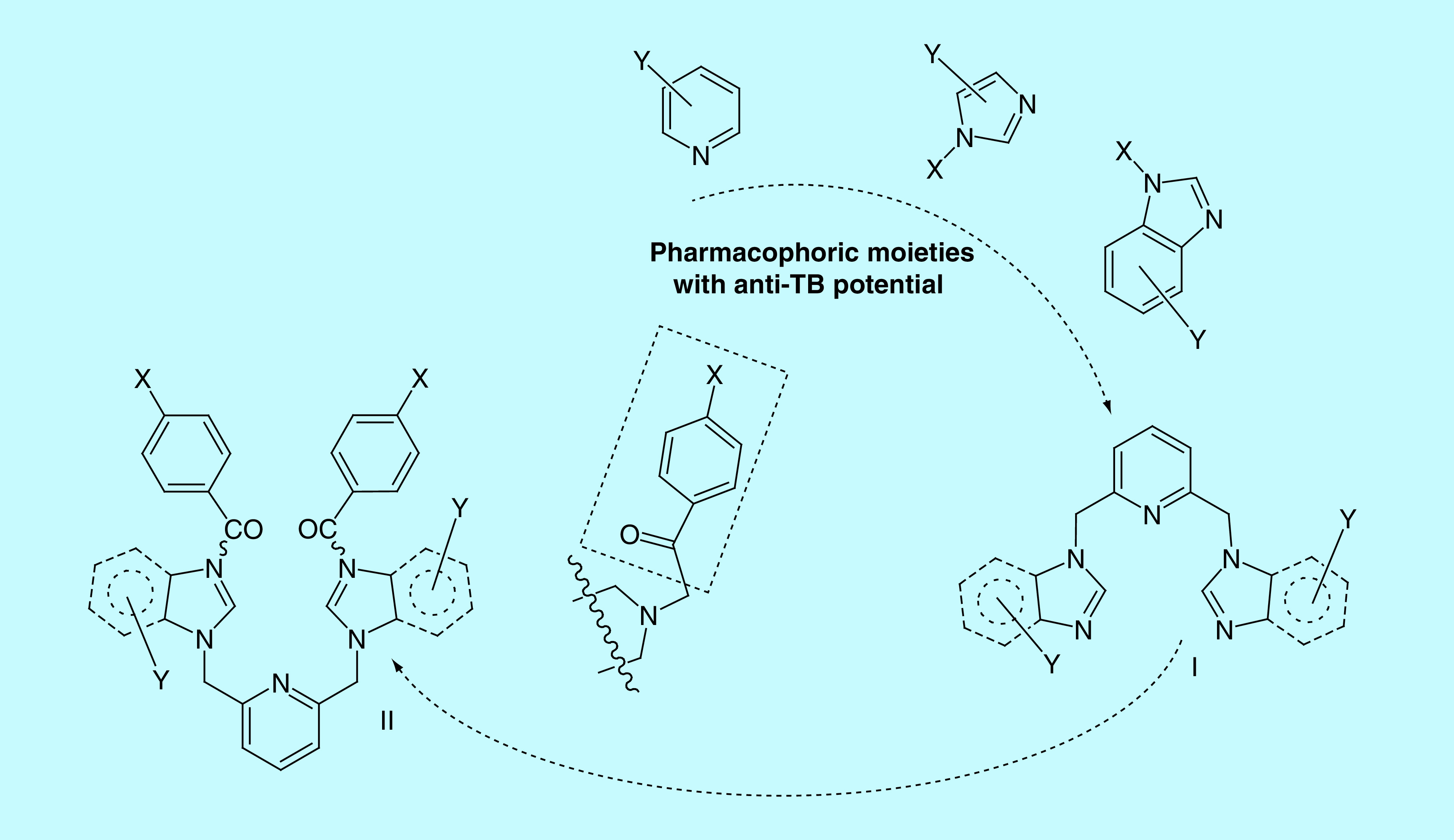
The first two pharmacophores units, pyridine and imidazole/benzimidazole moieties, are connected via a methylene (-CH2-) linker in 2,6- position of pyridine, respectively 1-position of imidazole/benzimidazole, the BIP derivatives type I being obtained. The p-chlorobenzoyl moiety was found to be a potent pharmacophoric unit for the antimycobacterial activity and also a useful antibacterial pharmacophore (due to its high lipophilicity) [19,24,25]. Consequently, we decide to anchor the p-chlorobenzoyl moiety in the 3-position of imidazole/benzimidazole, a second class of BIP derivatives, type II being obtained. We also decided to study the influence of other p-substituted (-H, -NO2, -OMe)-benzoyl moieties and nonbenzoyl moiety (-carbalkoxy, -acetamide) anchored to imidazole/benzimidazole, in order to allow structure–activity relationship (SAR) comparisons with p-chlorobenzoyl moiety.
In order to synthesize the BIP derivatives a direct approach was used, involving only two successive N-alkylations (Figure 3). A first N-alkylation of NH- imidazolic unit (from imidazole, 4-nitroimidazole and benzimidazole) is leading to the corresponding BIP derivatives type I, 2a, b and 5. The second alkylation consists in a quaternization reaction of N3-nitrogen atom from the imidazolic moiety with variously activated halogenated derivatives type 3 (2–bromo/iodo-alkyl esters/amide 3a, b or ϖ–bromo p-Z-substituted-acetophenones 3c–g), the BIP derivatives type II, 4a–g and 6a–g, being obtained.
Figure 3. . Reaction pathway to obtain bis-imidazole/benzimidazole-pyridine derivatives.
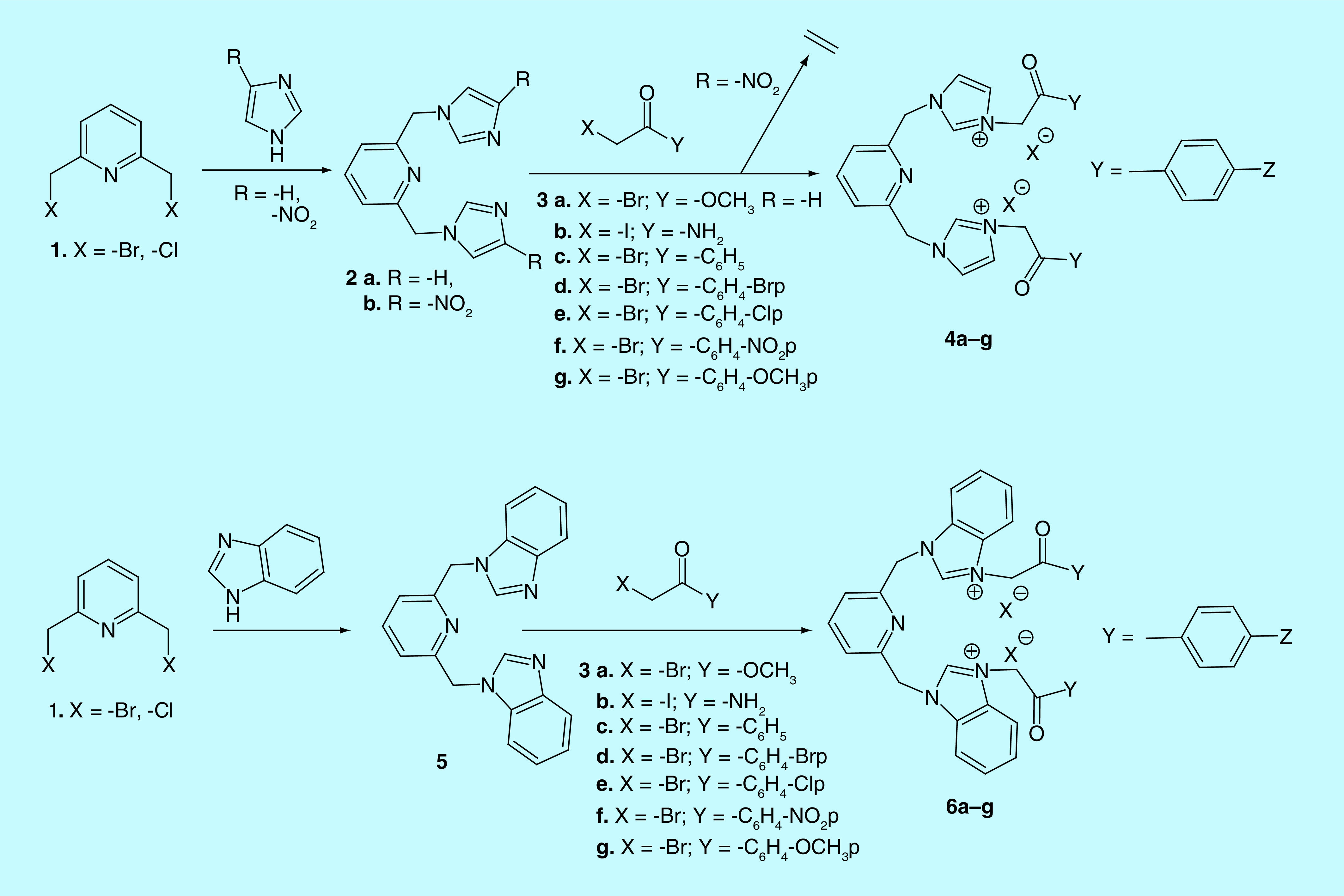
For the BIP compound 2b, the quaternization reaction of N3-nitrogen atom from imidazole does not occur, the most probable because the imidazole ring is too deactivated (Figure 4).
Figure 4. . Structure–activity relationship correlations in the class of bis-(imidazole/benzimidazole)-pyridine derivatives.
Using the modern tools of structural organic analysis (IR, 1H-NMR, 13C-NMR, 2D-COSY, 2D-HMQC, 2D-HMBC, and elemental analysis: C, H, N), we proved equivocally the structure of our compounds. In our structural organic analysis, we considered compound 6e as representative for the BIP derivatives (is the most active and promising anti-TB compound). The most informative signals furnished by 1H-NMR spectrum of 6e are those one of the aromatic protons H2′ (from imidazole ring) and H2′′ (from benzoyl), respectively the methylene protons: 2H (CH2-α pyridine) and 2H (CH2-β benzoyl). The most deshielded protons are H2′ (9.84 p.p.m., singlet) from benzimidazole moiety, due to the powerful deshielding effect of the positive nitrogen atom N3′ and N1′ nitrogen. The next deshielded protons are H2′′ from benzoyl moiety (8.11–8.09 p.p.m., doublet, J = 8.4 Hz), being deshielded by the electron withdrawing effect of the adjacent carbonyl ketone group and p-chlorophenyl moiety. The signals of the two methylene groups appear as singlet, at a very low field, unusual for this type of protons: 6.44 p.p.m. (CH2-β benzoyl) and 6.00 p.p.m. (CH2-α pyridine). This situation could be explained by the powerful electron withdrawing effect exerted by the CO carbonyl group and positive nitrogen atom N3′ from imidazole moiety (for CH2-β) from proximity, respectively nitrogen atom N1′ from imidazole and α-pyridine ring (for CH2-α).
In the 13C-NMR spectrum, the most important data are furnished by the signals corresponding to the carbonyl group, C2 (from pyridine), C2′ (from imidazole ring) and the two carbons from the methylene groups (CH2-α pyridine and CH2-β benzoyl). The signals for carbonyl group appear at 190.4 p.p.m., typical for a C = O carbonyl ketone group. The C2 carbon is strongly deshielded (appears at 153.0 p.p.m.), being a α-endocyclic carbon from pyridine moiety. The C2′ carbon is also strongly deshielded (appears at 143.8 p.p.m.), in accordance with the powerful deshielding effect of the two adjacent nitrogen atoms from imidazole ring. The carbons from methylene groups appear at unusual low field: 53.4 p.p.m. (carbon from CH2-β benzoyl) and 50.7 p.p.m. (carbon from CH2-α pyridine). The same explications as far for methylene protons remain valid. The other remaining peaks form NMR spectra fully confirm the structure of compounds. Additional information concerning the 1H- and 13C-NMR spectra of compound 6e is shown in the Supplementary Information.
Antimycobacterial assay against Mycobacterium tuberculosis
The obtained compounds have been evaluated for their in vitro antimycobacterial activity against Mtb H37Rv (grown under aerobic conditions), on TAACF, US NIH, the NIAID division, at Southern Research Institute. The first step of assay consisted in the determination of relative solubility of our products in microbiological medium using turbidity as a standard parameter [32]. The results listed in Table 1 show that all the BIP compounds are soluble at the highest concentration (200 μM). For a future drug candidate, this excellent solubility microbiological medium is an excellent strength.
Table 1. . Solubility in microbiological medium and antimycobacterial activity of bis-(imidazole/benzimidazole)-pyridine compounds against Mtb H37Rv under aerobic conditions.
| Compound | MIC (μM) | IC50 (μM) | IC90 (μM) | Compound solubility | |
|---|---|---|---|---|---|
| Starting concentration (μM) | Lowest insoluble concentration (μM) | ||||
| 2a | >200 | >200 | >200 | 200 | N/A |
| 2b† | 92 | 77 | 100 | 200 | N/A |
| 4a | >200 | >200 | >200 | 200 | N/A |
| 4b | >200 | >200 | >200 | 200 | N/A |
| 4c | >200 | >200 | >200 | 200 | N/A |
| 4e† | 51 | 34 | 63 | 200 | N/A |
| 4f† | 58 | 38 | 71 | 200 | N/A |
| 4g | >200 | 160 | >200 | 200 | N/A |
| 5† | >50 | 43 | >50 | 200 | N/A |
| 6a | >200 | >200 | >200 | 200 | N/A |
| 6b | >200 | 180 | >200 | 200 | N/A |
| 6c† | 56 | 25 | 60 | 200 | N/A |
| 6e† | 17 | 7.9 | 18 | 200 | N/A |
| 6f† | 24 | 14 | 21 | 200 | N/A |
| 6g† | 58 | 28 | 6 | 200 | N/A |
| Rifam picin | 0.0060 | 0.0036 | 0.070 | 0.04 | N/A |
Values indicate that compounds have a very good activity.
IC50: 50% inhibition of growth; IC90: 90% inhibition of growth; N/A: Compound was soluble at the highest concentration; MIC: Minimum inhibitory concentration.
Further, the in vitro screening assay allowed to determine the minimum inhibitory concentration (MIC), IC50 and IC90 (the concentrations that resulted in 50% and 90% inhibition of growth; Table 1) [33–37].
As can be seen, eight (2b, 5, 4e, 4f, 6c, 6e, 6f and 6g) from the 15 tested compounds had activity against Mtb H37Rv under aerobic conditions, with a MIC in the range of 17– 92 μM, and an IC50 in the range of 7.9 – 77 μM. From SAR point of view, the following remarks could be done: the BIP II compounds are more active than BIP I, which means that anchoring of a p-substituted-benzoyl moiety in the 3-position of imidazole/benzimidazole moiety is favorable for activity; the compounds bearing a benzimidazole moiety are more active than those one bearing an imidazole moiety. Compound 6e (with a p-chlorobenzoyl moiety anchored to benzimidazole) have shown the most pronounced antimycobacterial activity, which confirms our initial design considerations; and the p-substituted-benzoyl compounds are more much active than nonbenzoyl substituted (-carbalkoxy, -acetamide). Also, the active compounds are those one having in the para position of benzoyl moiety a chloride or nitro group, while replacing them with other atoms or group of atoms is leading to a dropping of antimycobacterial activity.
Five BIP derivatives, namely 2b, 5, 4f, 6c and 6f, with showed the best anti-TB profile in the primary assay, was passed into the secondary assays for evaluation of antimcyobacterial activity in a low throughput format. These assays include a revaluation of MIC, IC50 and IC90 against Mtb H37Rv grown under aerobic conditions, MIC under low oxygen, minimal bactericidal concentration (MBC), testing on drug-resistant Mtb, intracellular activity and cytotoxicity.
Initially, MIC, IC50 and IC90 were repeated at lower starting concentrations (Table 2). As we may notice from Table 2, the results of assay are reproducible, the obtained values being comparable with the previous ones listed in Table 1.
Table 2. . Activity against Mtb H37Rv at lower starting concentrations for bis-(imidazole/benzimidazole)-pyridine derivatives 2b, 5, 4f, 6c and 6f.
| Compound | MIC aerobic condition (μM) | IC50 (μM) | IC90 (μM) |
|---|---|---|---|
| 2b† | 101 | 93 | 100 |
| 5 | >50 | >50 | >50 |
| 4f† | 104 | 45 | 140 |
| 6c† | 87 | 34 | 110 |
| 6f† | 16 | 11 | 20 |
| Rifampicin | 0.0063 | 0.0040 | 0.0077 |
Values indicate that compounds have a very good activity.
IC50: 50% inhibition of growth; IC90: 90% inhibition of growth; MIC: Minimum inhibitory concentration.
The bactericidal activity of BIP derivatives was determined subsequent to MIC assay. The MBC assay determines whether a drug's mechanism of action is bacteriostatic or bactericidal. A bactericidal mechanism of action is a truly advantage of drug candidates, because could shorten the standard treatment of TB with a minimum of 6 months. The MBC was determined against Mtb H37Rv grown in aerobic conditions, and indicate that three of the tested compounds (4f, 6c, 6f) have a bactericidal mechanism of action, Table 3. We may also notice from Table 3 that the activity of compounds 6c and 6f, are concentration dependent, which is associated with an optimal free drug maximum concentration to MIC ratio [38]. In the case of 4f molecule, a time dependent effect is observed, which is usually linked with a free drug concentrations existing above the MIC for a certain section of the dosing interval [38].
Table 3. . Minimal bactericidal concentration assay for compounds 2b, 5, 4f, 6c and 6f.
| Compound | MIC (μM) | MBC (μM) | Concentration dependent | Time dependent |
|---|---|---|---|---|
| 2b† | 92 | >200 | ND | ND |
| 4f† | 58 | 100 | N | Y |
| 5 | >50 | >50 | ND | ND |
| 6c† | 56 | 100 | Y | N |
| 6f† | 18 | 50 | Y | N |
Values indicate that compounds have a very good activity.
MBC: Minimal bactericidal concentration; MIC: Minimum inhibitory concentration; N: No; ND: Not determined; Y: Yes.
Low oxygen recovery assay are furnishing essential information concerning activity against Mtb in a state of nonreplicating persistence (NRP). Nonreplicating state of Mtb is responsible for antimicrobial tolerance found in more and more cases of TB infections. As a result, the antimycobacterial activity against NRP Mtb H37Rv for compounds 2b, 5, 4f, 6c and 6f, were determined [39–41]. Results for the low oxygen recovery assay in terms of MIC, IC50 and IC90, are listed in Table 4.
Table 4. . Low oxygen recovery assay for compounds 2b, 5, 4f, 6c and 6f.
| Compound | Low oxygen | Normal oxygen | ||||
|---|---|---|---|---|---|---|
| MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | IC50 (μM) | IC90 (μM) | |
| 2b† | 200 | 26 | 91 | >200 | >200 | >200 |
| 5 | >50 | >50 | >50 | >50 | >50 | >50 |
| 4f† | 183 | 25 | 65 | 129 | 49 | 78 |
| 6c† | 200 | 16 | 130 | >200 | 17 | 60 |
| 6f† | 120 | 9.7 | 33 | 71 | 9.9 | 25 |
| Rifampicin | 0.13 | 0.0041 | 0.0065 | 0.096 | 0.0072 | 0.0025 |
| Metronidazole | 200 | 29 | 110 | >200 | >200 | >200 |
Values indicate that compounds have a very good activity.
IC50: 50% inhibition of growth; IC90: 90% inhibition of growth; MIC: Minimum inhibitory concentration.
As can be seen in Table 4, our compounds (except 5) had activity against NRP Mtb H37Rv superior to control metronidazole, with an IC50 in the range of 9.7– 26 μM, and a MIC in the range of 120–200 μM.
Next, MIC, IC50 and IC90 of compounds 2b, 5, 4f, 6c and 6f against five resistant isolates of Mtb strains [34–37] and nontuberculous mycobacteria (NTM) [34,37,42] under aerobic conditions were determined, Table 5. The NTM were Mycobacterium avium and Mycobacterium abscessus, while the resistant isolates were INH-R1 and INH-R2 (strains resistant to isoniazid), RIF-R1 and RIF-R2 (strains resistant to rifampicin), and FQ-R1 (strain resistant to fluoroquinolone).
Table 5. . MIC, IC50 and IC90 of compounds 2b, 5, 4f, 6c and 6f against Mtb resistant at different treatment and nontuberculous mycobacteria.
| Compound | INH-R1 | INH-R2 | RIF-R1 |
Mycobacterium avium |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | |
| 2b† | 98 | 68 | 90 | 28 | 24 | 28 | 111 | 60 | 160 | – |
| 5 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | – |
| 4f† | 55 | 46 | 52 | 65 | 39 | 75 | 67 | 35 | 100 | >200 |
| 6c† | 120 | 42 | 170 | 110 | 42 | 150 | 87 | 24 | 110 | >200 |
| 6f† | 24 | 13 | 21 | 25 | 13 | 22 | 25 | 15 | 24 | 200 |
| C1 | 0.018 | 0.0084 | 0.022 | 0.065 | 0.0047 | 0.012 | 2 | 1.2 | 2.3 | >200 |
| C2 | >200 | >200 | >200 | >200 | >200 | >200 | 0.17 | 0.15 | 0.21 | – |
| C3 | 1.2 | 0.64 | 1.4 | 1.4 | 0.84 | 1.4 | 0.76 | 0.59 | 0.91 | – |
Values indicate that compounds have a very good activity.
C1: Rifampicin control; C2: Isoniazid control; C3: Levofloxacin control; FQ: Fluoroquinolone resistant strain; IC50: 50% inhibition of growth; IC90: 90% inhibition of growth; INH: Isoniazid resistant strain; MIC: Minimum inhibitory concentration; RIF: Rifampicin resistant strain.
Table 6. . MIC, IC50 and IC90 of compounds 2b, 5, 4f, 6c and 6f against Mtb resistant at different treatment and nontuberculous mycobacteria.
| Compound | RIF-R2 | FQ-R1 | Mycobacterium abscessus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | IC50 (μM) | IC90 (μM) | MIC (μM) | IC50 (μM) | IC90 (μM) | |
| 2b | 68 | 38 | 61 | 102 | 66 | 92 | – | – | – |
| 5 | >50 | >50 | >50 | >50 | >50 | >50 | – | – | – |
| 4f† | >200 | 40 | 120 | 87 | 49 | 91 | 110 | 89 | 97 |
| 6c† | 100 | 44 | 120 | 200 | 40 | >200 | >200 | 120 | 200 |
| 6f† | 30 | 17 | 31 | 38 | 18 | 38 | 88 | 56 | 68 |
| C1 | >50 | >50 | >50 | 0.027 | 0.013 | 0.039 | 3.3 | 2.1 | 3.1 |
| C2 | 0.62 | 0.54 | 0.60 | 0.35 | 0.36 | 0.47 | – | – | – |
| C3 | 1.1 | 0.60 | 1.2 | 20 | 12 | 22 | – | – | – |
Values indicate that compounds have a very good activity.
C1: Rifampicin control; C2: Isoniazid control; C3: Levofloxacin control; FQ: Fluoroquinolone resistant strain; INH: Isoniazid resistant strain; MIC: Minimum inhibitory concentration; RIF: Rifampicin resistant strain.
The data from Table 5 indicate that Mtb monoresistant isolates were not resistant to our compounds (except 5) and, compounds 4f, 6c and 6f showed activity against M. abscessus, while against M. avium only compound 6f showed a moderate activity.
The cytotoxicity of BIP compounds were established by using the THP-1 human monocytic cell line [40], and measuring the IC50 values. Similar, the activity of compounds against intracellular Mtb H37Rv were determined by measuring viability in infected THP-1 cell line [40], and expressed in terms of IC50 and IC90, values. The obtained results are listed in Table 7.
Table 7. . Intracellular activity and cytotoxicity evaluation.
| Compound | Cytotoxicity IC50 (μM) | IC50 intracell (μM) | IC90 intracell (μM) |
|---|---|---|---|
| 2b† | >50 | >200 | >200 |
| 5† | >50 | >200 | >200 |
| 4f† | >50 | 26 | 48 |
| 6c† | >50 | 37 | >50 |
| 6f† | >50 | 14 | 26 |
| Control compound‡,§ | 0.018‡ | 0.23§ | 0.29§ |
Bold and italic values indicate that compounds have a very good activity.
Staurosporine control.
Isoniazid control.
IC50: 50% inhibition of growth; IC90: 90% inhibition of growth.
As can be seen in Table 7, all our compounds are not cytotoxic and all tested compounds exhibited a very good intracellular activity, those one of compound 6f being remarkable. The data presented before indicate that compound 6f have a promising anti-TB profile and, as a result, a study concerning absorption, distribution, metabolism, excretion and toxicity (ADMET) have been done. The ADMET study begin with plasma–protein binding assay of compound 6f, using equilibrium dialysis method with a semipermeable membrane, which separates two compartments containing protein (human plasma) and buffer [43,44]. The obtained results are listed in Table 8.
Table 8. . Results of plasma–protein binding for compound 6f.
| Compound | Test species | Mean plasma fraction unbound (%) | Mean plasma fraction bound (%) | Recovery |
|---|---|---|---|---|
| 6f | Human | 7.8 | 92.2 | 24.5 |
| Propranolol† | Human | 20.7 | 79.3 | 93 |
| Warfarin† | Human | 0.51 | 99.5 | 96.7 |
Control compounds: propranolol (normal binding) and warfarin (high-binding).
As it could be seen in Table 8, compound 6f have a value of plasma fraction bound lesser than 95% (92.2%), which means a lower clearance rate and a greater half-time in vivo and, consequently a more efficacy because of higher free drug concentration. The absorption properties of compound 6f through the intestinal epithelium were studied using the Caco-2 cell monolayer permeability assay, measuring in both directions (apical to basal, respectively basal to apical) the permeability of compound 6f [45–49]. The obtained results are listed in Table 9.
Table 9. . Results of Caco-2 assay of compound 6f.
| Compound | Mean A→B Papp† (10-6cm/s) | Mean B→A Papp† (10-6cm/s) | Efflux ratio‡ |
|---|---|---|---|
| 6f | 0.47 | 0.095 | 0.20 |
| Atenolol§ | 0.13 | 0.37 | 2.8 |
| Propranolol§ | 12.7 | 25 | 2 |
| Talinolol§ | 0.077 | 3.3 | 43 |
Papp is the apparent permeability;
Efflux ratio (Re) is Papp (B→A)/Papp (A→B);
Control compounds.
The low active efflux (Re = 0.20) and low permeability (A→B Papp <2), indicate that compound 6f is poorly permeable and suggest a mechanism of absorption almost paracellular, with basically no involvement of transporter proteins (P-glycoprotein or others).
The early discovery of the potential drug–drug interactions is a necessary clue to increase the safety of pharmacotherapy, the drug–drug interactions being associated with the increasing of toxicity, decrease of pharmacological effect and adverse drug reactions [50]. One of the best methods used for detection of drug–drug interactions consist in drug metabolism determination via the cytochrome P450 system. In this respect, compound 6f was tested on while the resistant isolates were INH-R1 and INH-R2 (strains resistant to isoniazid), RIF-R1 and RIF-R2 (strains resistant to rifampicin), and FQ-R1 (strain resistant to fluoroquinolone; Table 10) [50–52 ].
Table 10. . Results of cytochrome P450 inhibition for compound 6f.
| Compound | IC50(μM) | ||||||
|---|---|---|---|---|---|---|---|
| CYP3A4 – midazolam | CYP3A4 – testosterone | CYP2C9 | CYP2D6 | CYP2C8 | CYP2B6 | CYP2C19 | |
| 6f | >20 | 6.6 | >20 | 0.92 | >20 | >20 | 8.8 |
| Ketoconazole† | 0.033 | 0.022 | – | – | – | – | – |
| Sulfaphenazole† | – | – | 0.16 | – | – | – | – |
| Quinidine† | – | – | – | 0.032 | – | – | – |
| Montelukast† | – | – | – | – | 0.14 | – | – |
| Tranylcypromine† | – | – | – | – | – | – | 7.5 |
| Ticlopidine† | – | – | – | – | – | 0.72 | – |
Control compounds.
IC50: 50% inhibition of growth.
Compound 6f did not show inhibition (IC50 >20) on CYP3A4 – midazolam, CYP2C9, CYP2C8, CYP2B6 and have a low to moderate inhibition (IC50: 6.6 and 8.8) on CYP3A4 – testosterone and CYP2C19, these results indicating a low potential for drug–drug interactions. The microsomal binding assay is another important parameter in the prediction of in vivo pharmacokinetics from in vitro drug metabolism data. In order to determine microsomal stability of compounds, the assay is using pooled human liver S9 microsomes in the presence of the co-factor NADPH [53–55]. The obtained results are listed in Table 11.
Table 11. . In vitro microsomal stability assay of compound 6f.
| Compound | C (μM) | Test species | NADPH-dependent CLint† (μl/min/mg) | NADPH-dependent T1/2‡ (min) | NADPH-free CLint† (μl/min/mg) | NADPH-free T1/2‡ (min) |
|---|---|---|---|---|---|---|
| 6f | 1 | Human | 21.7 | 106 | <12.8 | >180 |
| Verapamil | 1 | Human | 123 | 18.7 | <12.8 | >180 |
| Dextromethorphan | 1 | Human | 24.3 | 94.9 | <12.8 | >180 |
Microsomal intrinsic clearance = ln(2)/(T1/2[microsomal protein]).
Half-life = 0.693/-k, where k is the rate constant.
The data from Table 11 indicate that compound 6f is a low cleared compound, having a CLint<50 μl/min/mg. These indicate that compound 6f is likely to be slowly cleared in vivo, resulting in a higher duration of action. Finally, the cytotoxicity of compound 6f was determined on human liver cells (HepG2), using staurosporine as control (IC50 = 0.0086 μM) [56–59]. Compound 6f showed an IC50 >100 μM, having no cytotoxicity.
Conclusion
In summary, we report herein the design, synthesis and antimycobacterial activity of two new classes of BIP derivatives. The reaction pathway is advantageous and straight applicable, with two steps only: a N1-alkylations followed by a quaternization reaction of N3-nitrogen atom from the imidazolic moiety. 15 of the newly synthesized compounds were subject to the primary antimycobacterial assay against M. tuberculosis H37Rv under aerobic conditions, and reveal that eight (2b, 5, 4e, 4f, 6c, 6e, 6f and 6g) from the tested compounds had activity against Mtb H37Rv. SAR correlations indicate that the most valuable compounds are belong to the BIP II class, which prove that anchoring of a p-substituted-benzoyl moiety in the 3-position of imidazole/benzimidazole moiety is favorable for activity. From these, the most active compounds are those one having in the para position of benzoyl moiety a chloride or nitro group. All BIP compounds proved to be with excellent solubility in microbiological medium, which is an excellent strength for a future drug candidate. The best anti-TB five compounds (2b, 5, 4f, 6c and 6f) was subject to the secondary antimycobacterial screening, these assay indicating that our compounds are potent against both replicating and nonreplicating Mtb (superior to control metronidazole), posses a very good intracellular activity (those one of compound 6f being the highest), are active against drug-resistant Mtb strains, and have no cytotoxicity (on eukaryotic THP-1 human monocytic cells). Three of the tested compounds (4f, 6c, 6f) have a bactericidal mechanism of action and present a moderate to good activity against NTM. The pharmacokinetic ADMET screening of 6f, indicate a lower clearance rate, a great half-time in vivo, a low potential for drug–drug interactions with a high duration of action and no cytotoxicity (on HepG2 cells). We believe that, all these excellent assets make from compound 6f a good candidate for a future drug.
Future perspective
Considering the high versatility and aggressively of Mtb bacillus, the great and urgent demand of pharmaceutical industry, as well as enormous amount of effort for discovery of new anti-TB entities, we believe that substantially improvements in drug discovery of new antitubercular agents will be performed in the next decade. A special attention will be paid further to pyridine and imidazole scaffolds, which have an enormous potential of growth.
Summary points.
Design, synthesis and antimycobacterial activity of two new classes of bis-(imidazole/benzimidazole)-pyridine derivatives.
The reaction pathway is advantageous and straight applicable and, the newly 15 synthesized compounds was subject to antimycobacterial assay.
Interesting structure–activity relationship correlations have been done. Anchoring of a p-chloro-benzoyl moiety in the 3-position of imidazole/benzimidazole moiety is favorable for activity.
The anti-TB assay reveal that our compounds possess an excellent solubility in microbiological medium, have an excellent anti-TB activity against both replicating and nonreplicating Mtb, are not cytotoxic, exhibited a very good intracellular activity and are active against drug-resistant Mtb strains, some compounds have a bactericidal mechanism.
The pharmacokinetic absorption, distribution, metabolism, excretion and toxicity screening performed for one compound, indicating a lower clearance rate, a great half-time in vivo, a low potential for drug–drug interactions with a high duration of action and no cytotoxicity. We believe that, all these excellent assets make from this compound a good candidate for a future drug.
Supplementary Material
Acknowledgments
The authors are thankful to JP Boyce, the Senior Officer to Division of Microbiology and Infectious Diseases, NIAID. The authors also thank to CERNESIM Research Centre from Alexandru Ioan Cuza University of Iasi, for the NMR experiments.
Footnotes
Supplementary data
Supplementary data related to this article can be found in the online version, and contain NMR spectra of compound 6e and all protocols related to the antimycobacterial evaluation of tested compounds. To view the supplementary data that accompany this paper please visit the journal website at: www.future-science/doi/suppl/10.4155/fmc-2019-0063
Financial & competing interests disclosure
The authors are thankful for financial support to Romanian Ministry of Research and Innovation, Program 1 – Development of the national R&D system, Subprogram 1.2 – Institutional performance – RDI excellence financing projects, grant number 34PFE. Part of this work (biological tests) was supported by National Institutes of Health and the National Institute of Allergy and Infectious Diseases, contract no. HHSN27220110009I/HHSN27200004A19. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WHO, Global tuberculosis report (2017). www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Sacks LV, Behrman RE. Developing new drugs for the treatment of drug-resistant tuberculosis: a regulatory perspective. Tuberculosis 88(Suppl. 1), S93–S100 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Nguyen L, Pieters J. Mycobacterial subversion of chemotherapeutic reagents and host defense tactics: challenges in tuberculosis drug development. Annu. Rev. Pharmacol. Toxicol. 49, 427–453 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Burman WJ, Jones BE. Treatment of HIV-related tuberculosis in the era of effective antiretroviral therapy. Am. J. Respir. Crit. Care Med. 164(1), 7–12 (2001). [DOI] [PubMed] [Google Scholar]; •• An informative article containing recommendations for the use of antiretroviral therapy among patients with HIV-related tuberculosis (TB).
- 5.Lawn SD, Zumla AI. Tuberculosis. Lancet 378(9785), 57–72 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Kakkar AK, Dahiya N. Bedaquiline for the treatment of resistant tuberculosis: promises and pitfalls. Tuberculosis 94(4), 357–362 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Gler MT, Skripconoka V, Sanchez-Garavito E. et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N. Engl. J. Med. 366(23), 2151–2160 (2012). [DOI] [PubMed] [Google Scholar]; • The results of the presented study suggest that Delamanid, a nitro-dihydro-imidazooxazole derivative, could enhance treatment options for multidrug-resistant TB.
- 8.Beena RDS. Antituberculosis drug research: a critical overview. Med. Res. Rev. 33(4), 693–693 (2013). [DOI] [PubMed] [Google Scholar]; •• A review article outlines various classes of compounds (such as quinolines, diamines, quinolones, pyrimidines and purines) as future drug candidates for anti-TB.
- 9.Dover LG, Coxon GD. Status and research strategies in tuberculosis drug development. J. Med. Chem. 54(18), 6157–6165 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Ma Z, Lienhardt C, McLleron H. et al. Global tuberculosis drug development pipeline: the need and the reality. Lancet 375(9731), 2100–2109 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Post-Martens K, Denkin S. New drug candidates and therapeutic targets for tuberculosis therapy. Drug Discov. Today 11(1–2), 21–27 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Keri RS, Rajappa CK, Patil SA. et al. Benzimidazole-core as an antimycobacterial agent. Pharmacol. Rep. 68(6), 1254–1265 (2016). [DOI] [PubMed] [Google Scholar]; •• A review article details the recent advancements on benzimidazole derivatives for anti-TB applications.
- 13.Akhtar W, Khan MF, Verma G. et al. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem. 126, 705–753 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Jeon AB, Ackart DF, Li W. et al. 2-Aminoimidazoles collapse mycobacterial proton motive force and block the electron transport chain. Sci. Rep. 9, 1513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu X, Williams Z, Hards K. et al. Pyrazolo[1,5- a]pyridine inhibitor of the respiratory cytochrome bcc complex for the treatment of drug-resistant tuberculosis. ACS Infect. Dis. 5(2), 239–249 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Fan YL, Jin XH, Huang ZP. et al. Recent advances of imidazole-containing derivatives as anti tubercular agents. Eur. J. Med. Chem. 150, 347–365 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Campaniço A, Moreira R, Lopes F. Drug discovery in tuberculosis. New drug targets and antimycobacterial agents. Eur. J. Med. Chem. 150, 525–545 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Al Matarneh CM, Ciobanu, Apostu M, Mangalagiu II, Danac R. Cycloaddition versus amidation in reactions of 2-amino-2-oxoethyl-phenanthrolinium ylides to activated alkynes and alkenes. CR Chim. 21(1), 1–8 (2018). [Google Scholar]
- 19.Olaru A, Vasilache V, Danac R. et al. Antimycobacterial activity of nitrogen heterocycles derivatives: 7-(pyridine-4-yl)-indolizine derivatives. Part VII. J. Enzyme Inhib. Med. Chem. 32(1), 1291–1298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the synthesis of some indolizinyl-pyridinium quaternary salts and the evaluation of antimicrobial activity against Mycobacterium tuberculosis.
- 20.Mantu D, Antoci V, Nicolescu A. et al. Synthesis, stereochemical studies and antimycobacterial activity of new acetylhydrazines pyridazinone. Curr. Org. Synth. 14(1), 112–119 (2017). [Google Scholar]
- 21.Al Matarneh CM, Ciobanu CI, Mangalagiu II. et al. Design, synthesis and antimycobacterial evaluation of some new azaheterocycles with 4,7-phenanthroline skeleton. Part VI. J. Serb. Chem. Soc. 81(2), 133–140 (2016). [Google Scholar]
- 22.Mantu D, Antoci V, Moldoveanu C. et al. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzyme Inhib. Med. Chem. 31(Suppl. 2), 96–103 (2016). [DOI] [PubMed] [Google Scholar]; • Describes the synthesis, structure and in vitro anticancer and antimycobacterial activity of new hybrid imidazol(benzimidazol)/2-aminopyridine(8-aminoquinoline) derivatives.
- 23.Al Matarneh CM, Mangalagiu II, Shova S. et al. Synthesis, structure, antimycobacterial and anticancer evaluation of new pyrrolo-phenanthroline derivatives. J. Enzyme Inhib. Med. Chem. 31(3), 470–480 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Danac R, Al Matarneh CM, Shova S. et al. New indolizines with phenanthroline skeleton: synthesis, structure, antimycobacterial and anticancer evaluation. Bioorg. Med. Chem. 23(10), 2318–2327 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Danac R, Daniloaia T, Antoci V. et al. Design, synthesis and antimycobacterial activity of some new azaheterocycles: phenanthroline with p-halo-benzoyl skeleton. Part V. Lett. Drug Des. Discov. 12(1), 14–17 (2015). [Google Scholar]
- 26.Danac R, Mangalagiu II. Antimycobacterial activity of nitrogen heterocycles derivatives: bipyridine derivatives. Part III. Eur. J. Med. Chem. 74, 664–670 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Mantu D, Antoci V, Mangalagiu II. Design, synthesis and antituberculosis activity of some new pyridazine derivatives: bis-pyridazine. Part IV. Infect. Dis. Drug Targets 13(5), 344–351 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Mantu D, Luca C, Moldoveanu C. et al. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur. J. Med. Chem. 45(11), 5164–5168 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Moldoveanu C, Mangalagiu G, Drochioiu G. et al. New antituberculosis compounds derived from diazine. An. Stiint. Univ. “Al.I. Cuza” Iasi 11, 367–374 (2003). [Google Scholar]
- 30.Lungu CN, Bratanovici BI, Grigore MM. et al. Hybrid imidazole-pyridine derivatives: an approach to novel anticancer DNA intercalators. Curr. Med. Chem. (2018) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]; • Investigates the antimicrobial and antitumoral properties of some imidazole-pyridine derivatives by experimental and computational methods.
- 31.Haque RA, Asekunowo PO, Razali MR. Dinuclear silver(I)-N-heterocyclic carbene complexes of N-allyl substituted (benz)imidazol-2-ylidenes with pyridine spacers: synthesis, crystal structures, nuclease and antibacterial studies. Transition Met. Chem. 39(3), 281–290 (2014). [Google Scholar]
- 32.Bevan CD, Lloyd RS. A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal. Chem. 72(8), 1781–1787 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Castagnolo D, De Logu A, Radi M. et al. Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis. Bioorg. Med. Chem. 16(18), 8587–8591 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Ollinger J, Bailey MA, Moraski GC. et al. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS ONE 8(4), e60531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelmer A, Carroll P, Andreu N. et al. A new in vivo model to test anti-tuberculosis drugs using fluorescent imaging. J. Antimicrob. Chemother. 67(8), 1948–1960 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll P, Schreuder LJ, Muwanguzi-Karugaba J. et al. Sensitive detection of gene expression in mycobacteria under replicating and non-replicating conditions using optimized far-red reporters. PLoS ONE 5(3), e9823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert RJ, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl Microbiol. 88(5), 784–790 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Kuti JL. Optimizing antimicrobial pharmacodynamics: a guide for stewardship program. Rev. Med. Clin. Condes. 27(5), 615–624 (2016). [Google Scholar]
- 39.Cho SH, Warit S, Wan B. et al. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Ag. Chemother. 51(4), 1380–1385 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreu N, Zelmer A, Fletcher T. et al. Optimisation of bioluminescent reporters for use with mycobacteria. PLoS ONE 5(5), e10777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wayne LG. In vitro model of hypoxically induced nonreplicating persistence of Mycobacterium tuberculosis. : Mycobacterium Tuberculosis Protocols. Parish T, Stoker NG (). Humana Press, NJ, USA, 247–270 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Franzblau SG, Witzig RS, McLaughlin JC. et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue Assay. J. Clin. Microbiol. 36(2), 362–366 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banker MJ, Clark TH, Williams JA. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J. Pharm. Sci. 92(5), 967–974 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat. Rev. Drug. Discov. 9(12), 929–939 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Stewart BH, Chan OH, Lu RH. et al. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm. Res. 12(5), 693–699 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46(1–3), 27–43 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man – fact or myth. Pharm. Res. 14(6), 763–766 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Endres CJ, Hsiao P, Chung FS. et al. The role of transporters in drug interactions. Eur. J. Pharm. Sci. 27(5), 501–517 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Balimane PV, Han YH, Chong S. Current industrial practices of assessing permeability and P-glycoprotein interaction. AAPS J. 8(1), E1–E13 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsky RL, Obach RS. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 32(6), 647–660 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Kim MJ, Kim H, Cha IJ. et al. High-throughput screening of inhibitory potential of nine cytochrome P450 enzymes in vitro using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 19(18), 2651–2658 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Fowler S, Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug–drug interactions. AAPS J. 10(2), 410–424 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem. Pharmacol. 47(9), 1469–1479 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and non-specific binding to microsomes. Drug Metab. Dispos. 27(11), 1350–1359 (1999). [PubMed] [Google Scholar]
- 55.Di L, Kerns EH, Ma XJ. et al. Applications of high throughput microsomal stability assay in drug discovery. Comb. Chem. High Throughput Screen. 11(6), 469–476 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Crouch SP, Kozlowski R, Slater KJ. et al. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160(1), 81–88 (1993). [DOI] [PubMed] [Google Scholar]
- 57.Lundin A, Hasenson M, Persson J. et al. Estimation of biomass in growing cell lines by ATP assay. Methods Enzymol. 133, 27–42 (1986). [DOI] [PubMed] [Google Scholar]
- 58.Maehara Y, Anai H, Tamada R. et al. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur. J. Cancer Clin. Oncol. 23(3), 273–276 (1987). [DOI] [PubMed] [Google Scholar]
- 59.Slater K. Cytotoxicity tests for high-throughput drug discovery. Curr. Opin. Biotechnol. 12(1), 70–74 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.