Abstract
Objective
To investigate cancer- and noncancer-specific mortality following lobectomy by minimally invasive surgery (MIS) versus open thoracotomy in elderly patients with non-small cell lung cancer (NSCLC).
Background
Two-thirds of patients with NSCLC are ≥65 years of age. As age increases, the risk of competing events, such as noncancer death, also increases.
Methods
Elderly patients (≥65 years of age) who have undergone curative-intent lobectomy for stage I-III NSCLC without induction therapy (2002–2013) were included (n=1 303). Of those, 607 patients had undergone MIS and 696 had undergone thoracotomy. Propensity-score matching was performed to identify pairs of thoracotomy and MIS patients with comparable clinical characteristics (e.g., year of surgery, comorbidities, and pulmonary function). Association between surgical approach (MIS vs. thoracotomy) and lung cancer-specific and noncancer-specific cumulative incidence of death (CID) was analyzed using competing risks approach.
Results
Following propensity score matching of patients who had undergone thoracotomy (n=338) versus MIS (n=338), MIS was associated with shorter length of stay (p <0.001), lower noncancer-specific 1-year mortality (p=0.027), and lower noncancer-specific CID (p=0.014) compared with thoracotomy; there was no difference in lung cancer-specific CID between surgical approaches. On multivariable analysis, thoracotomy was a significant risk factor for noncancer-specific death (subhazard ratio 2.45, 95% CI 1.18–5.06, p=0.016) independent of age, sex, and diffusion capacity of the lungs for carbon monoxide.
Conclusion
In a propensity score-matched cohort, multivariable analysis has indicated that lobectomy performed by MIS is associated with lower incidence of noncancer-specific mortality compared with lobectomy performed by open thoracotomy in elderly patients with NSCLC.
MINI-ABSTRACT
We investigated cancer- and noncancer-specific mortality following lobectomy by minimally invasive surgery versus open thoracotomy in elderly patients (≥65 years of age) with non-small cell lung cancer using competing risks analysis. In a propensity score-matched cohort, minimally invasive surgery was associated with lower incidence of noncancer-specific mortality compared with thoracotomy.
INTRODUCTION
More than two-thirds of lung cancer patients are ≥65 years of age at time of diagnosis, half of whom are ≥75 years of age.1 With the demonstration of reduced lung cancer mortality with low-dose computed tomography (CT) screenings, the detection of early-stage lung cancer in elderly patients is expected to increase.2 A higher proportion of elderly patients results in an increase in the incidence of diseases that are attributable to aging and frailty, thus making the cohort of elderly patients highly susceptible to competing risk events. For example, noncancer specific deaths prelude or “competes with” the occurrence of cancer-specific deaths and vice versa. In studies with multiple endpoints, such as cancer—specific death and noncancer—specific death, conventional statistical approaches evaluate these endpoints using a separate Kaplan-Meier analysis without cons idering whether these endpoints are competing events for each other. When study populations, such as elderly or critic ally-ill patients, are susceptible to competing events a competing risk approach is recommended.3 Recently, we have demonstrated that noncancer-specific death is a significant competing event against lung cancer-specific death in elderly patients following lung resection for early-stage non-small cell lung cancer (NSCLC) using competing risks analysis.4 The standard treatment of early-stage NSCLC is lobectomy with systematic mediastinal lymph node evaluation.5 However, the treatment distribution of lobectomy decreases as age increases because of its higher postoperative risk compared with other treatments including sub lobar resection, irradiation, or observation.4,6,7
The use of minimally invasive surgery (MIS)—which includes video-assisted thoracic surgery (VATS) and robotic-assisted surgery—for lung lobectomy has been increasing, even though thoracotomy remains the mostly commonly used approach.8, 9 A recent randomized trial that compared postoperative pain and quality of life between VATS and anterolateral thoracotomy demonstrated that VATS was associated with less postoperative pain and better quality of life than thoracotomy.10 Other randomized trials have demonstrated that MIS was associated with lower postoperative morbidity,11 lower C-reactive protein, interleukin 6, and equivalent overall survival12 (OS) compared with thoracotomy. Additionally, various non-randomized studies have demonstrated shorter length of hospital stay, lower postoperative morbidity, and lower perioperative mortality after MIS compared with thoracotomy.8, 13–21
We hypothesized that surgical approach for lobectomy will affect postoperative noncancer-specific outcomes in elderly patients and reviewed published studies that have investigated short-and long-term outcomes between MIS and thoracotomy (e-Table 1).8, 13–26 No studies performed noncancer-specific mortality analysis using competing risks approach in a cohort of elderly patients. The aim of our study was to investigate lung cancer-specific and noncancer-specific mortality, as well as postoperative morbidity, in elderly patients who had undergone lobectomy via MIS or open thoracotomy for early-stage NSCLC using propensity score (PS) matching competing risks analysis.
PATIENTS AND METHODS
Study cohort
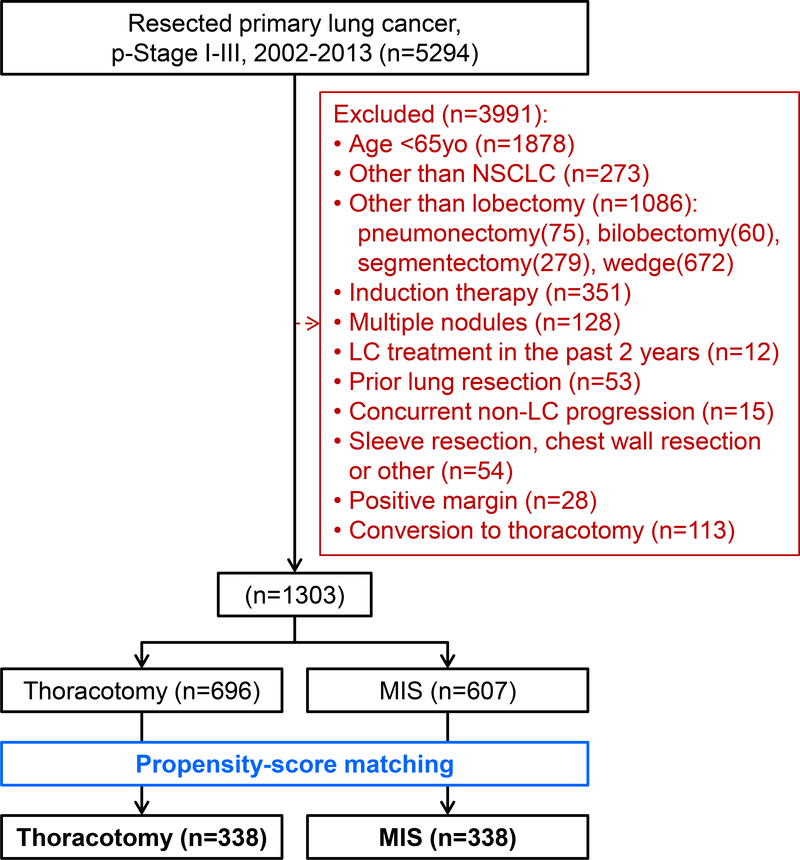
Our retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). The MSK Thoracic Surgery Service’s prospectively maintained lung cancer database was reviewed and we identified consecutive patients who had been treated with surgery for pathologic stage I-III primary lung cancer between January 1, 2002 and December 31, 2013. Pathologic stage was based on the seventh edition of the American Joint Committee on Cancer Staging Manual.27 Our exclusion criteria included: <65 years of age at surgery; diagnosis other than NSCLC; lung resection other than lobectomy; induction therapy; have multiple nodules; lung cancer diagnosis within the past two years; prior lung resection; concurrent other disease progression; a combined resection of the chest wall, pericardium, and/or diaphragm due to disease invasion; sleeve resection; and positive surgical margins (R1 or R2) (Figure 1).
Figure 1. CONSORT diagram.
Abbreviations: LC, lung cancer; MIS, minimally invasive surgery; NSCLC, non-small cell lung cancer; p-Stage, pathologic stage; yo, year old
In order to focus on possible differences in invasiveness based on different approaches, we did not include patients who had undergone a converted thoracotomy in the MIS group because these patients had ultimately undergone open thoracotomy. We performed PS matching to reduce potential functional and pathologic differences between the groups that can affect both selection bias and outcomes.
Data collection
Data on clinicopathologic variables were obtained by reviewing patient medical records to determine patient characteristics: year of surgery; age at surgery; sex; smoking status; history of chronic obstructive pulmonary disease (COPD); history of cardiovascular disease (CVD; which includes myocardial infarction, congestive heart failure, and peripheral vascular disease); history of diabetes mellitus (DM); body mass index (BMI); serum creatinine levels; predicted postoperative forced expiratory volume in one second (ppoFEV1)4, 28, 29; predicted postoperative diffusion capacity of the lung for carbon monoxide (ppoDLCO)4, 28, 29; resected lobe; histologic subtype (e.g., adenocarcinoma); pathologic tumor size; pathologic stage (p-Stage); status of adjuvant chemotherapy after lung resection; and follow-up status. All preoperative variables were evaluated by at most three months prior to surgery. Patient follow-up status was updated as of September 2016.
We also obtained operative data from operation records including type of lobectomy, surgical approach (MIS, thoracotomy, or conversion to thoracotomy from MIS), and reason for conversion. MIS included both VATS and robot-assisted surgery. Our definition of MIS was consistent with the consensus definition used in the Cancer and Leukemia Group B 39802 study.30 Conversion was defined as the use of a rib-spreading thoracotomy at any point after initiation of MIS. The reasons for conversion were classified into following categories: (1) difficulty with single-lung ventilation; (2) bleeding; (3) pleural adhesion; and (4) other technical or anatomic considerations such as lymph nodal anthracosis and vascular anomaly.
Endpoints and cause of death
The endpoints of this study were length of stay, severe morbidity, 1-year lung cancer-specific and noncancer-specific mortality, lung cancer-specific cumulative incidence of death (LC-CID), and noncancer-specific cumulative incidence of death (NC-CID). Severe morbidities were defined as those grade ≥3 (in accordance with Common Terminology Criteria for Adverse Events [CTCAE] guidelines31) within 30 days after surgery. The cause of death was classified as lung cancer-specific, other cancer-specific, noncancer-specific, or unknown.4 Lung cancer—specific mortality was defined as death due to recurrent disease associated with resected lung cancer. Patients who had progressive recurrent disease at the last follow-up and death without a documented specific reason were included in the lung cancer—specific group. Death due to second primary lung cancer or other malignancies was regarded as “other cancer-specific.” Noncancer-specific mortality was defined as death due to specific causes other than malignant disease, including death without a documented specific reason within 6 months of the last follow-up in the absence of lung cancer recurrence or progressive malignant diseases.
Statistical analysis
We presented results from PS-matching analysis between the MIS and thoracotomy groups and recorded their comparable characteristics. Propensity scores were computed as the conditional probability of receiving MIS using a logistic regression model that included baseline demographic and clinical characteristics—year of surgery, age, sex, smoking status, COPD, CVD, DM, serum creatinine, BMI, ppoFEV1, ppoDLCO, resected lobe, histologic subtypes, pathologic tumor size, p-Stage, adjuvant chemotherapy—to achieve balance in covariates between the two groups. The PS-matching procedure selects matched pairs with similar baseline probabilities of being in either the MIS or the thoracotomy group.32, 33 Propensity score matching pairs were identified without replacement using a 1:1 nearest neighbor matching algorithm with caliper width determined by the recommendation from Austin (0.2 of the standard deviation of the logit of the PSs).34 Balance of covariates between the groups was assessed by the absolute standardized mean difference (ASMD) before and after the matching procedure. An ASMD <0.1 indicated balance in the covariate between the two groups.35 After the matching procedure, 338 matched pairs were generated with comparable patient characteristics. Subsequent analyses accounted for the matched pairs of data as clusters.
Length of stay was compared between both groups using linear regression with clustered standard errors after log transformation of the outcome. Comparisons between groups for severe morbidity and 1-year mortality were performed using logistic regression with clustered standard errors.
The associations between factors and the hazard of each cause of death were evaluated using competing risks analysis. Patients were censored if they were alive at the time of last follow-up. The hazard of death was analyzed using competing risks method, grouped by matched pair identifiers after PS matching. Cumulative incidence of death (CID) was estimated from time of surgery using a cumulative incidence function that accounted for death due to lung cancer or noncancer causes as competing events.36 Differences in CID between groups were assessed using Gray’s method for univariable analyses. The association between morbidity (grades 0/1, 2, and 3) and noncancer-specific death was identified by CID curves for each morbidity grade and analyzed separately by surgical approach (MIS or thoracotomy). Fine and Gray’s competing risk regression analysis was used to estimate the subhazard ratio37 in order to evaluate the association between clinicopathologic variables and hazard of noncancer-specific death. Factors that yielded p <0.1 on univariable analysis were considered candidates in the multivariable model. Statistical analyses were conducted using R v3.3.1 (R Development Core Team, Austria, Vienna), including the “survival,” “cmprsk,” “crrSC,” and “rms” packages that were downloaded in January 2017.
RESULTS
Patient characteristics and comparison between MIS and thoracotomy before/after matching
Patient characteristics and comparison between MIS and thoracotomy groups, before and after propensity score matching, are shown in Table 1. Before matching, 12 out of 16 covariates were unbalanced between the MIS and thoracotomy groups (ASMD ≥0.1). Compared with thoracotomy, MIS is associated with a more recent period of surgery, a greater number of female patients, a greater number of never smokers, fewer patients with a history of COPD, lower serum creatinine levels, higher ppoFEV1, higher ppoDLCO, a greater number of upper lobectomies, a greater number of adenocarcinoma diagnoses, smaller tumor size, lower pathologic stage, and less adjuvant chemotherapy.
Table 1:
Patient characteristics and outcomes before and after propensity score matching – MIS vs. Thoracotomy
| Before matching (n = 1 303) |
After matching (n = 676) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIS | Thoracotomy | MIS | Thoracotomy | |||||||
| n = 607 (%) | n = 696 (%) | n = 338 (%) | n = 338 (%) | |||||||
| Clinicopathologic variables | ASMD | ASMD | ||||||||
| Period of surgery | 0.702 | 0.091 | ||||||||
| 2002–2005 | 120 | (20) | 323 | (46) | 100 | (30) | 104 | (31) | ||
| 2006–2009 | 210 | (35) | 243 | (35) | 126 | (37) | 136 | (40) | ||
| 2010–2013 | 277 | (46) | 130 | (19) | 112 | (33) | 98 | (29) | ||
| Age at surgery (year) | 73 | (69–78) | 72 | (68–77) | 0.079 | 72 | (68–77) | 72 | (68–78) | 0.012 |
| Sex | 0.134 | 0.048 | ||||||||
| Female | 363 | (60) | 370 | (53) | 185 | (55) | 193 | (57) | ||
| Male | 244 | (40) | 326 | (47) | 153 | (45) | 145 | (43) | ||
| Smoking | 0.116 | 0.040 | ||||||||
| Never | 112 | (18) | 101 | (15) | 53 | (16) | 58 | (17) | ||
| Former | 435 | (72) | 513 | (74) | 247 | (73) | 243 | (72) | ||
| Current | 60 | (10) | 82 | (12) | 38 | (11) | 37 | (11) | ||
| COPD history | 109 | (18) | 170 | (24) | 0.156 | 66 | (20) | 73 | (22) | 0.051 |
| CVD history | 116 | (19) | 152 | (22) | 0.068 | 75 | (22) | 71 | (21) | 0.029 |
| DM history | 80 | (13) | 88 | (13) | 0.016 | 49 | (14) | 42 | (12) | 0.061 |
| BMI | 27 | (23–30) | 27 | (24–30) | 0.066 | 27 | (24–30) | 27 | (24–30) | 0.029 |
| Serum creatinine (mg/dL) | 1.0 | (0.9–1.2) | 1.1 | (0.9–1.2) | 0.107 | 1.1 | (0.9–1.3) | 1.1 | (0.9–1.2) | 0.017 |
| ppoFEV1 (%) (N =1 281) | 73 | (63–85) | 68 | (55–80) | 0.360 | 70 | (61–82) | 72 | (59–81) | 0.039 |
| ppoDLCO (%) (N=1 240) | 65 | (55–76) | 61 | (50–73) | 0.268 | 63 | (53–74) | 62 | (52–77) | 0.034 |
| Resected lobe | 0.199 | 0.093 | ||||||||
| RUL | 251 | (41) | 244 | (35) | 124 | (37) | 128 | (38) | ||
| RML | 35 | (6) | 44 | (6) | 18 | (5) | 17 | (5) | ||
| RLL | 92 | (15) | 139 | (20) | 56 | (17) | 64 | (19) | ||
| LUL | 154 | (25) | 155 | (22) | 95 | (28) | 83 | (25) | ||
| LLL | 75 | (12) | 114 | (16) | 45 | (13) | 46 | (14) | ||
| Histologic subtypes | 0.426 | 0.063 | ||||||||
| Adenocarcinoma | 513 | (85) | 466 | (67) | 270 | (80) | 275 | (81) | ||
| Squamous | 72 | (12) | 182 | (26) | 53 | (16) | 46 | (14) | ||
| Adenosquamous | 12 | (2) | 18 | (3) | 8 | (2) | 9 | (3) | ||
| Large | 8 | (1) | 23 | (3) | 7 | (2) | 8 | (2) | ||
| Pleomorphic | 2 | (<1) | 7 | (1) | 0 | (0) | 0 | (0) | ||
| Pathologic size (cm) | 2.0 | (1.5–2.8) | 3.0 | (2.0–4.2) | 0.639 | 2.2 | (1.7–3.0) | 2.3 | (1.6–3.3) | 0.090 |
| p-Stage | 0.400 | 0.034 | ||||||||
| I | 491 | (81) | 441 | (63) | 257 | (76) | 252 | (75) | ||
| II | 77 | (13) | 162 | (23) | 53 | (16) | 56 | (17) | ||
| III | 39 | (6) | 93 | (13) | 28 | (8) | 30 | (9) | ||
| Adjuvant therapy (N = 1 275) | 0.173 | 0.047 | ||||||||
| No | 521 | (87) | 549 | (81) | 283 | (84) | 277 | (82) | ||
| Yes | 76 | (13) | 129 | (19) | 55 | (16) | 61 | (18) | ||
| Outcomes | P | |||||||||
| Length of stay (day) | 4 | (3–6) | 5 | (4–8) | 4 | (3–5) | 5 | (4–7) | <0.001 | |
| Postoperative morbidity | ||||||||||
| None/CTCAE grade 1 | 481 | (79) | 468 | (67) | 271 | (80) | 242 | (72) | ||
| CTCAE grade 2 | 91 | (15) | 158 | (23) | 50 | (15) | 69 | (20) | ||
| CTCAE grade ≥3 | 35 | (6) | 70 | (10) | 17 | (5) | 27 | (8) | 0.12# | |
| 30-day mortality | 1 | (0.2) | 10 | (1) | 0 | (0) | 2 | (0.6) | ¶ | |
| 90-day mortality | 3 | (0.5) | 18 | (3) | 1 | (0.3) | 7 | (2) | ¶ | |
| 1-year LC mortality | 11 | (2) | 28 | (4) | 8 | (2) | 6 | (2) | 0.8 | |
| 1-year NC mortality | 3 | (0) | 31 | (4) | 1 | (0.3) | 10 | (3) | 0.027 | |
| 5-year LC-CID (%)* | 11 | (8–14) | 19 | (17–23) | 15 | (12–20) | 14 | (10–19) | 0.9 | |
| 5-year NC-CID (%)* | 3 | (1–5) | 8 | (6–10) | 2 | (1–5) | 7 | (5–11) | 0.014 | |
Data are number (%) or median (25th–75th percentile).
Data are shown as estimated CID or survival probability (95% CI).
Comparison of CTCAE grade ≥3 between MIS vs. thoracotomy.
No statistical comparison due to small number of events.
Abbreviations: ASMD, absolute standardized mean difference; BMI, body mass index; CI, confidence interval; CID, cumulative incidence of death; COPD, chronic obstructive pulmonary disease; CTCAE, Common Terminology Criteria for Adverse Events; CVD, cardiovascular disease; DLCO, diffusion capacity of the lungs for carbon monoxide; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; LC, lung cancer; LLL, left lower lobe; LUL, left upper lobe; NC noncancer; MIS, minimally invasive surgery; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; ppo, predictive postoperative; p-Stage, pathologic stage.
The 1:1 matching for MIS versus thoracotomy resulted in 338 matched pairs (n=676) with balanced covariates between the MIS and thoracotomy groups (ASMD <0.1). The distributions of PS before and after matching are shown in e-Figure 1.
Length of stay, postoperative morbidity, 30-day, 90-day, and 1-year mortality
Table 1 reports the outcomes between MIS and thoracotomy after matching. MIS was associated with shorter length of stay than thoracotomy (MIS vs. thoracotomy, 4 vs. 5 days, respectively; p <0.001). There was no significant difference in the incidence of severe postoperative morbidity and 1-year lung cancer-specific mortality between MIS and thoracotomy. However, MIS was associated with lower 1-year noncancer-specific mortality than thoracotomy (0.3% vs. 3%, respectively; p=0.027). We did not evaluate 30-day and 90-day mortalities statistically due to small number of events.
Lung cancer- and noncancer-specific cumulative incidence of death analysis
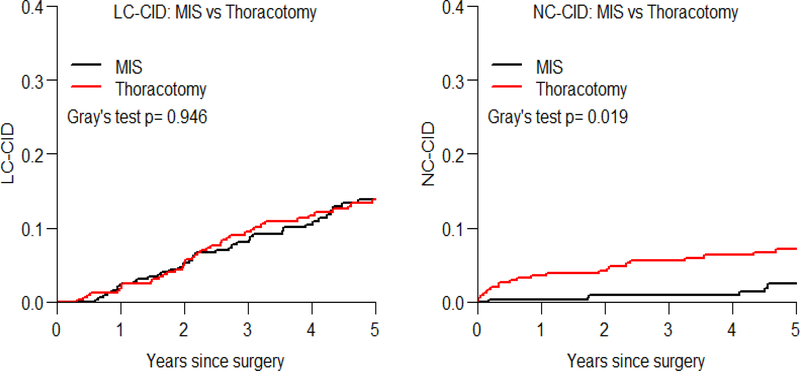
Table 1 reports the estimated 5-year LC-CID and NC-CID, and a comparison between MIS vs. thoracotomy after matching. Figure 2 shows LC-CID and NC-CID curves. The patients in the MIS group were associated with lower NC-CID than patients in the thoracotomy group (5-year NC-CID in MIS vs. thoracotomy, 2% vs. 7%, respectively; p=0.019), whereas there was no statistically significant difference in LC-CID between MIS vs. thoracotomy.
Figure 2. Lung cancer- and noncancer-specific cumulative incidence of death curves by surgical approach.
The patients in the MIS group were associated with lower NC-CID than patients in the thoracotomy group (5-year NC-CID in MIS vs. thoracotomy, 2% vs. 7%, respectively; p=0.019), whereas there was no statistically significant difference in LC-CID between MIS vs. thoracotomy (p=0.946).
Abbreviations: LC-CID, lung cancer-specific cumulative incidence of death; MIS, minimally invasive surgery; NC-CID, noncancer-specific cumulative incidence of death
Univariable and multivariable competing risk regression for noncancer-specific death
Table 2 demonstrates the results of univariable and multivariable competing risks regression for noncancer-specific death after PS matching. On univariable analysis, older age, male sex, CVD history, higher serum creatinine levels, thoracotomy (vs. MIS), squamous cell carcinoma (vs. adenocarcinoma), and p-Stage II (vs. I) were significantly associated with higher risk of noncancer-specific death (p <0.05). After consideration of association and collinearity between variables, the final multivariable model included three variables in addition to surgical approach: age, sex, ppoDLCO, and p-Stage. In this model, older age, male sex (vs. female), lower ppoDLCO, p-Stage II (vs. I), and thoracotomy (vs. MIS) were independently associated with higher risk of noncancer-specific death. Variables that are associated with lower ppoDLCO, such as CVD history and ppoFEV1, were not included in the final model; however, the model that included these variables is shown in e-Table 2. Specifically, thoracotomy was a significant risk factor for noncancer-specific death (subhazard 2.43, 95% confidence interval 1.18–5.01, p=0.017).
Table 2:
Univariable and multivariable competing risk regression for noncancer-specific death after propensity score matching
| Univariable analysis |
Final multivariable model |
|||||
|---|---|---|---|---|---|---|
| Variables | SHR | 95% CI | P | SHR | 95% CI | P |
| Year of surgery (per 1 year increase) | 0.97 | 0.88–1.08 | 0.6 | |||
| Age at surgery (per 1 year increase) | 1.06 | 1.00–1.12 | 0.04 | 1.07 | 1.01–1.14 | 0.030 |
| Male (vs. female) | 3.22 | 1.57–6.60 | 0.001 | 3.88 | 1.78–8.44 | <0.001 |
| Smoking history (vs. never) | ||||||
| Former | 0.95 | 0.39–2.33 | 0.9 | |||
| Current | 1.41 | 0.45–4.42 | 0.6 | |||
| COPD history (vs. none) | 1.08 | 0.50–2.32 | 0.9 | |||
| CVD history (vs. none) | 2.32 | 1.2–4.5 | 0.012 | |||
| DM history (vs. none) | 1.56 | 0.68–3.55 | 0.3 | |||
| BMI (per 1 index increase) | 1.04 | 0.98–1.09 | 0.2 | |||
| Serum creatinine (per 1mg/dL increase) | 4.35 | 2.07–9.13 | <0.001 | |||
| ppoFEV1 (per 1% increase) | 0.99 | 0.97–1.01 | 0.2 | |||
| ppoDLCO (per 1% increase) | 0.98 | 0.96–0.99 | 0.013 | 0.98 | 0.96–1.00 | 0.022 |
| Resected lobe (vs. RUL) | ||||||
| RML | 1.02 | 0.23–4.55 | 1.0 | |||
| RLL | 0.88 | 0.32–2.42 | 0.8 | |||
| LUL | 1.37 | 0.63–3.00 | 0.4 | |||
| LLL | 1.17 | 0.42–3.31 | 0.8 | |||
| Thoracotomy (vs. MIS) | 2.35 | 1.15–4.81 | 0.020 | 2.43 | 1.18–5.01 | 0.017 |
| Histologic subtypes (vs. adenocarcinoma) | ||||||
| Squamous | 2.15 | 1.0–4.6 | 0.049 | |||
| Adenosquamous | 2.7 | 0.65–11.18 | 0.17 | |||
| Large | 1.22 | 0.19–7.71 | 0.8 | |||
| p-Stage (vs. I) | ||||||
| II | 2.18 | 1.05–4.53 | 0.037 | 2.47 | 1.16–5.27 | 0.019 |
| III | 1.53 | 0.53–4.41 | 0.4 | 1.55 | 0.53–4.57 | 0.4 |
| Adjuvant chemotherapy (vs. none) | 0.94 | 0.39–2.30 | 0.18 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DLCO, diffusion capacity of the lungs for carbon monoxide; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; ppo, predictive postoperative; p-Stage, pathologic stage; SHR, subhazard ratio
Noncancer-specific cumulative incidence of death curves by morbidity status and surgical approach
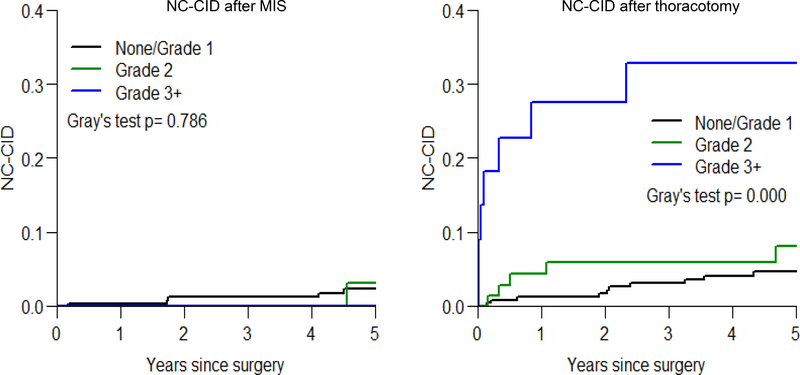
To assess prognostic impact of the severity of postoperative morbidity on noncancer-specific death in relation to the type of surgical approach received, NC-CID by morbidity status was investigated separately in patients who had undergone MIS and patients who had undergone thoracotomy after matching. When dividing patients into three morbidity grade-based groups (no morbidity or CTCAE grade 1 morbidity; grade 2 morbidity; and grade ≥3 morbidity) for those who have undergone MIS, there was no difference in NC-CID curves between the three groups; however, among patients who had undergone thoracotomy, there were significant difference between the groups (p <0.001), specifically, the grade ≥3 morbidity group had greater risk of non-cancer death.
Cause of postoperative severe morbidity and death
Table 3 shows the cause of postoperative severe morbidity (CTCAE grade ≥3) before and after matching. In both the MIS and thoracotomy groups, the majority of postoperative severe morbidity was categorized in respiratory, thoracic, and mediastinum disorders, followed by cardiac and vascular disorders, according to the CTCAE definition.
Table 3:
Cause of postoperative severe morbidity (CTCAE grade ≥ 3) before/after propensity score matching
| Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MIS | Thoracotomy | MIS | Thoracotomy | ||||||
| System organ class and adverse events | n = 607 | n = 696 | n = 338 | n = 338 | P | ||||
| Respiratory, thoracic and mediastinal disorders | 20 | (3.3) | 56 | (8.0) | 9 | (2.7) | 20 | (5.9) | 0.056 |
| Adult respiratory distress syndrome/respiratory failure | 6 | (1.0) | 24 | (3.4) | 4 | (1.2) | 8 | (2.4) | |
| Pneumonitis/lung infectiona | 3 | (0.5) | 8 | (11) | 0 | (0.0) | 2 | (0.6) | |
| Pneumothorax | 4 | (0.7) | 14 | (2.0) | 1 | (0.3) | 6 | (18) | |
| Bronchopleural fistula /pleural infectiona | 5 | (0.8) | 4 | (0.6) | 3 | (0.9) | 1 | (0.3) | |
| Chylothorax | 2 | (0.3) | 4 | (0.6) | 1 | (0.3) | 2 | (0.6) | |
| Others | 0 | (0.0) | 2 | (0.3) | 0 | (0.0) | 1 | (0.3) | |
| Cardiac and vascular disorders | 5 | (0.8) | 14 | (2.0) | 3 | (0.9) | 8 | (2.4) | 0.2 |
| Acute coronary syndrome | 2 | (0.3) | 1 | (0.1) | 2 | (0.6) | 0 | (0.0) | |
| Atrial fibrillation | 0 | (0.0) | 6 | (0.9) | 0 | (0.0) | 2 | (0.6) | |
| Thromboembolic event | 3 | (0.5) | 5 | (0.7) | 1 | (0.3) | 5 | (1.5) | |
| Others | 0 | (0.0) | 2 | (0.3) | 0 | (0.0) | 1 | (0.3) | |
| Nervous system disorders | 2 | (0.3) | 2 | (0.3) | 2 | (0.6) | 1 | (0.3) | ¶ |
| Stroke | 1 | (0.2) | 2 | (0.3) | 1 | (0.3) | 1 | (0.3) | |
| Others | 1 | (0.2) | 0 | (0.0) | 1 | (0.3) | 0 | (0.0) | |
| Renal and urinary disorders | 2 | (0.3) | 6 | (0.9) | 1 | (0.3) | 1 | (0.3) | ¶ |
| Acute kidney injury | 1 | (0.2) | 5 | (0.7) | 0 | (0.0) | 1 | (0.3) | |
| Urinary tract infectiona | 1 | (0.2) | 1 | (0.1) | 1 | (0.3) | 0 | (0.0) | |
| Gastrointestinal and hepatobiliary disorders | 1 | (0.2) | 2 | (0.3) | 0 | (0.0) | 0 | (0.0) | ¶ |
| Colitis | 0 | (0.0) | 1 | (0.1) | 0 | (0.0) | 0 | (0.0) | |
| Ileus | 0 | (0.0) | 1 | (0.1) | 0 | (0.0) | 0 | (0.0) | |
| Others | 1 | (0.2) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |
| Postoperative bleeding | 2 | (0.3) | 1 | (0.1) | 1 | (0.3) | 0 | (0.0) | ¶ |
| Other category | 7 | (1.2) | 2 | (0.3) | 4 | (1.2) | 0 | (0.0) | ¶ |
| Anemia | 3 | (0.5) | 1 | (0.1) | 0 | (0.0) | 1 | (0.3) | |
| Delirium | 0 | (0.0) | 1 | (0.1) | 0 | (0.0) | 1 | (0.3) | |
| Wound infection | 2 | (0.3) | 0 | (0.0) | 2 | (0.6) | 0 | (0.0) | |
| Others | 2 | (0.3) | 0 | (0.0) | 2 | (0.6) | 0 | (0.0) | |
Data are number (%)
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events
Included in “infections and infestations” in the CTCAE category.
No statistical comparison due to small number of events.
Table 4 shows the cause of death at 30 days, 90 days, 1 year, and 5 years after surgery, before and after matching. Before matching, the proportions of patients with lung cancer-specific death and noncancer-specific death were greater among those who had undergone thoracotomy than in those had undergone MIS. However, after matching, the proportion of lung cancer-specific death was similar between MIS and thoracotomy whereas noncancer-specific death, especially death due to respiratory disorders, was still more frequent in the MIS group than in the thoracotomy group.
Table 4:
Cause of death at 30 days, 90 days, 1 year, and 5 years after surgery before/after propensity score matching
| At 30 days |
At 90 days |
At 1 year |
At 5 years |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIS | Thoracotomy | MIS | Thoracotomy | MIS | Thoracotomy | MIS | Thoracotomy | |||||||||
| Before matching | n = 607 | n = 696 | n = 607 | n = 696 | n = 607 | n = 696 | n = 607 | n = 696 | ||||||||
| Any cause of mortality | 1 | (0.2) | 10 | (1.4) | 3 | (0.5) | 18 | (2.6) | 19 | (3.1) | 69 | (9.9) | 106 | (17.5) | 245 | (35.2) |
| Cause-specific mortality | ||||||||||||||||
| Lung cancer specific | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 11 | (18) | 28 | (4.0) | 56 | (9.2) | 125 | (18.0) |
| Other cancer specific | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (0.3) | 3 | (0.4) | 14 | (2.3) | 15 | (2.2) |
| Noncancer specific | 1 | (0.2) | 10 | (1.4) | 3 | (0.5) | 18 | (2.6) | 3 | (0.5) | 31 | (4.5) | 12 | (2.0) | 51 | (7.3) |
| Respiratory | 0 | (0.0) | 6 | (0.9) | 0 | (0.0) | 11 | (16) | 0 | (0.0) | 16 | (2.3) | 2 | (0.3) | 24 | (3.4) |
| Cardiovascular | 0 | (0.0) | 2 | (0.3) | 1 | (0.2) | 2 | (0.3) | 1 | (0.2) | 3 | (0.4) | 2 | (0.3) | 3 | (0.4) |
| Nervous system | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Renal/Urinary tract | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Gastrointestinal | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (0.1) | 0 | (0.0) | 1 | (0.1) | 1 | (0.2) | 1 | (0.1) |
| Other* | 1 | (0.2) | 2 | (0.3) | 2 | (0.3) | 4 | (0.6) | 2 | (0.3) | 11 | (16) | 7 | (1.2) | 23 | (3.3) |
| Unknown | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 3 | (0.5) | 7 | (1.0) | 24 | (4.0) | 54 | (7.8) |
| After matching | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | ||||||||
| Any cause of mortality | 0 | (0.0) | 2 | (0.6) | 1 | (0.3) | 7 | (2.1) | 13 | (3.8) | 19 | (5.6) | 77 | (22.8) | 90 | (26.6) |
| Cause-specific mortality | ||||||||||||||||
| Lung cancer specific | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 8 | (2.4) | 6 | (18) | 42 | (12.4) | 46 | (13.6) |
| Other cancer specific | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (0.6) | 2 | (0.6) | 11 | (3.3) | 7 | (2.1) |
| Noncancer specific | 0 | (0.0) | 2 | (0.6) | 1 | (0.3) | 7 | (2.1) | 1 | (0.3) | 10 | (3.0) | 6 | (18) | 21 | (6.2) |
| Respiratory | 0 | (0.0) | 2 | (0.6) | 0 | (0.0) | 4 | (1.2) | 0 | (0.0) | 6 | (18) | 1 | (0.3) | 11 | (3.3) |
| Cardiovascular | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Nervous system | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Renal/Urinary tract | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Gastrointestinal | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (0.3) | 0 | (0.0) | 1 | (0.3) | 1 | (0.3) | 1 | (0.3) |
| Other* | 0 | (0.0) | 0 | (0.0) | 1 | (0.3) | 2 | (0.6) | 1 | (0.3) | 3 | (0.9) | 4 | (1.2) | 9 | (2.7) |
| Unknown | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (0.6) | 1 | (0.3) | 18 | (5.3) | 16 | (4.7) |
Data are number (%)
Abbreviations: MIS, minimally invasive surgery
Including unknown death within 6 months after the last follow-up, in the absence of recurrence or other malignant disease. Statistic comparison between MIS and thoracotomy after matching is shown in Table 1.
DISCUSSION
Our study demonstrated that, in elderly patients with early-stage NSCLC, MIS is associated with lower risk of noncancer-specific death compared with open thoracotomy. The novelty and strength of our study are as follows: 1) this is the first study to compare noncancer-specific death between MIS and thoracotomy using competing risks analysis; 2) propensity score matching and subsequent prognostic analysis included factors that are known to contribute to selection of surgical approaches and lung cancer- and noncancer-specific outcomes after surgery, including years of surgery, comorbidity, and ppo lung function, that are more strongly linked to postoperative morbidities28; 3) multivariable analysis demonstrated that thoracotomy is a significant risk factor for noncancer-specific death independent of older age, male sex, and lower ppoDLCO, all of which are risk factors for worse noncancer outcomes after surgery4; and 4) we focused on the elderly patient population where noncancer-specific death is a significant competing event against lung cancer-specific death.4
Previous studies have demonstrated equivalent OS or disease-free survival (DFS) between patients who had undergone MIS and thoracotomy.13, 14, 16, 18, 19, 22–25, 38 However, our study has shown that MIS is associated with lower noncancer-specific mortality in elderly patients. This discrepancy can be explained by two reasons—our study only focused on elderly patients and, in addition to noncancer-specific death, OS and DFS can be affected by death due to other malignant diseases that we regarded as competing events in this analysis.4, 39 When comparing causes of death between MIS and thoracotomy, death due to respiratory diseases was a main difference between the cohorts (Table 3 and e-Table 3). Postoperative morbidity can affect patient survival40 and our study demonstrates that differential noncancer-specific survival is impacted by postoperative morbidity status and surgical approach. Interestingly, higher incidence of noncancer-specific death after severe morbidity was observed only in the thoracotomy group (Figure 3). Further studies are warranted to investigate the potential relationship between noncancer-specific death, especially due to respiratory diseases, and patient symptomatic/functional status including pain, quality of life, respiratory physiology, and respiratory morbidity.
Figure 3. Noncancer-specific cumulative incidence of death curves by morbidity status and surgical approach.
When dividing patients into three morbidity grade-based groups (black curve, no morbidity or CTCAE grade 1 morbidity; green curve, grade 2 morbidity; and blue curve, grade ≥3 morbidity) for those who have undergone MIS (left), there was no difference in NC-CID curves between the three groups; however, in patients who had undergone thoracotomy (right), there were significant difference between the groups (p <0.001) where the grade ≥3 morbidity group had higher risk of NC-CID.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; Grade, CTCAE grade; MIS, minimally invasive surgery; NC-CID, noncancer-specific cumulative incidence of death
In this study, we focused on elderly patients (≥65 years of age) based on our recent observation that noncancer-specific mortality was found to be a significant competing event against lung cancer-specific mortality in resected NSCLC patients ≥65 years of age but not in patients <65 years of age.4 However, patient characteristics and outcomes between MIS vs. thoracotomy in 654 patients <65 years of age (used the same inclusion/exclusion criteria as the elderly cohort) are available in Online Only Supplemental Material (e-Table 4 and e-Figure 2). Similar to the elderly cohort before matching, MIS was associated with recent time period, female sex, non-smoker, better pulmonary function, adenocarcinoma history, and lower p-stage. Despite “favorable background” in MIS compared with thoracotomy, there is no difference between the 2 cohorts in terms of postoperative morbidity, short-term mortality, and long-term, noncancer-specific mortality in patients <65 years of age. Although we used 65 years of age as a cut-off,4, 41 with increasing age of cancer patients future analysis may consider an increased age cut-off (70 or 75 years).42 Analysis within our cohort with cut-offs 70 or 75 years did not change the observations.
The limitations of our study include inherent biases in patient selection in a retrospective series. Although we attempted to offset potential bias between MIS and thoracotomy by PS matching, remaining bias might still have affected the results. For example, we used pathologic stage instead of clinical stage for matching. After matching, pathologic stage was well balanced between MIS and thoracotomy; however, clinical stage was not balanced (e-Table 5). Although this might have caused potential remaining bias between the cohorts, LC-CID analysis between MIS and thoracotomy (Figure 2) has no demonstrable difference between both cohorts after matching and, therefore, we believe that our conclusions were not significantly changed. The change in patient population between early and late time periods might have affected our analyses due to MIS learning curve, a potential change in patient selection for MIS, and a potential improvement of perioperative patient care during the study period. Another limitation was that we excluded 113 patients with conversion before PS matching in order to evaluate differences between patients who had successfully undergone MIS and thoracotomy. Patient characteristics and outcomes of the conversion group are shown in e-Table 6 (MIS vs. conversion vs. thoracotomy) and e-Table 7 (by reason for conversion). Compared with the thoracotomy group, the conversion group has similar or higher preoperative noncancer-related risk (higher comorbidity and lower pulmonary function) and similar outcomes. The causes of severe morbidity and death are shown in e-Table 8 and 9, respectively. Similar noncancer-related backgrounds and outcomes between the conversion and thoracotomy groups may suggest that the noncancer-specific prognostic impact from thoracotomy that were converted from MIS would be similar to the impact from intentional thoracotomy. Another limitation of our study is the number of patients with an unknown cause of death. Of the 351 deaths within 5 years after surgery, 78 were due to unknown causes (34 after PS matching). The patient characteristics by cause of death among patients who died within 5 years after surgery, before and after PS matching, are shown in e-Table 10 and e-Table 11. Preoperative and postoperative variables in patients with unknown causes of death are more similar to the total cohort of patients. However, the characteristics of patients in unknown death are not similar to any one subcohort (lung cancer-, other cancer-, and noncancer-specific death). This suggests that it is unlikely that “unknown death” are coming from any one subcohort.43
In conclusion, we demonstrate that, in a PS-matching cohort, multivariable analysis indicates that lobectomy performed successfully by MIS is associated with lower incidence of noncancer-specific death compared with lobectomy performed by open thoracotomy in elderly patients with NSCLC.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alex Torres of the MSK Thoracic Surgery Service for their editorial assistance.
Funding information: The authors’ laboratory research is supported by grants from the National Institutes of Health (R01 CA217169 to D.R.J., P30 CA008748 to P.S.A.); the U.S. Department of Defense (BC132124 and LC160212 to P.S.A.); the Derfner Foundation; the Joanne and John DallePezze Foundation; Mr. William H. Goodwin and Mrs. Alice Goodwin; the Commonwealth Foundation for Cancer Research; and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Abbreviations
- ASMD
absolute standardized mean difference
- BMI
body mass index
- CID
cumulative incidence of death
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- CVD
cardiovascular disease
- DFS
disease-free survival
- DM
diabetes mellitus
- LC-CID
lung cancer—specific cumulative incidence of death
- MIS
minimally invasive surgery
- MSK
Memorial Sloan Kettering Cancer Center
- NC-CID
noncancer-specific cumulative incidence of death
- NSCLC
non-small cell lung cancer
- OS
overall survival
- ppoDLCO
predicted postoperative diffusion capacity of the lung for carbon monoxide
- ppoFEV1
predicted postoperative forced expiratory volume in one second
- p-Stage
pathologic stage
- VATS
video-assisted thoracic surgery
Footnotes
Summary conflict of interest statement: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stat Bite Percentage of New Caces by Age Group For Lung and Bronchus Cancer (2008–2012). J Natl Cancer Inst 2016; 108(3). [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan KS, Eguchi T, Adusumilli PS. Competing risks and cancer-specific mortality: why it matters. Oncotarget 2018; 9(7):7272–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017; 35(3):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Centers: NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer v4 2016. Available at:http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 6.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012; 84(5):1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012; 142(6):1620–35. [DOI] [PubMed] [Google Scholar]
- 8.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010; 139(2):366–78. [DOI] [PubMed] [Google Scholar]
- 9.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012; 256(3):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016; 17(6):836–44. [DOI] [PubMed] [Google Scholar]
- 11.Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995; 109(5):997–1001; discussion 1001–2. [DOI] [PubMed] [Google Scholar]
- 12.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000; 24(1):27–30; discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 13.Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009; 138(1):11–8. [DOI] [PubMed] [Google Scholar]
- 15.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009; 138(2):419–25. [DOI] [PubMed] [Google Scholar]
- 16.Pages PB, Delpy JP, Orsini B, et al. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy With Thoracotomy: A French Nationwide Study. Ann Thorac Surg 2016; 101(4):1370–8. [DOI] [PubMed] [Google Scholar]
- 17.Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016; 49(2):602–9. [DOI] [PubMed] [Google Scholar]
- 18.Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015; 99(2):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. Bmj 2014; 349:g5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014; 148(2):637–43. [DOI] [PubMed] [Google Scholar]
- 21.Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013; 43(4):813–7. [DOI] [PubMed] [Google Scholar]
- 22.Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014; 46(4):607–13. [DOI] [PubMed] [Google Scholar]
- 23.Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014; 98(1):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna WC, de Valence M, Atenafu EG, et al. Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy? Eur J Cardiothorac Surg 2013; 43(6):1121–5. [DOI] [PubMed] [Google Scholar]
- 25.Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013; 44(5):849–54. [DOI] [PubMed] [Google Scholar]
- 26.Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016; 101(3):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge SB, American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed New York: Springer, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl):e166S–90S. [DOI] [PubMed] [Google Scholar]
- 29.Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007; 84(4):1085–91; discussion 1091. [DOI] [PubMed] [Google Scholar]
- 30.Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007; 25(31):4993–7. [DOI] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 2010. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 32.Rosenbaum PR, Rubin DB. Constructing a Control Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score. The American Statistician 1985; 39(1):33–38. [Google Scholar]
- 33.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011; 46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10(2):150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine JP. Regression modeling of competing crude failure probabilities. Biostatistics 2001; 2(1):85–97. [DOI] [PubMed] [Google Scholar]
- 37.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999; 94(446):496–509. [Google Scholar]
- 38.Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2017. [DOI] [PubMed] [Google Scholar]
- 39.Lu S, Tan KS, Kadota K, et al. Spread Through Air Spaces (STAS) is an Independent Predictor of Recurrence and Lung Cancer Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez FG, Kosinski AS, Furnary AP, et al. Differential impact of operative complications on survival following surgery for primary lung cancer [abstract]. American Association for Thoracic Surgery Centennial 2017. [Google Scholar]
- 41.Organization WH. Proposed working definition of an older person in Africa for the MDS project 2002. Available at: http://www.who.int/healthinfo/survey/ageingdefnolder/en/.
- 42.Stinchcombe TE, Zhang Y, Vokes EE, et al. Pooled Analysis of Individual Patient Data on Concurrent Chemoradiotherapy for Stage III Non-Small-Cell Lung Cancer in Elderly Patients Compared With Younger Patients Who Participated in US National Cancer Institute Cooperative Group Studies. J Clin Oncol 2017; 35(25):2885–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamel JW, Vogel RL. Non-parametric comparison of relative versus cause-specific survival in Surveillance, Epidemiology and End Results (SEER) programme breast cancer patients. Stat Methods Med Res 2001; 10(5):339–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.