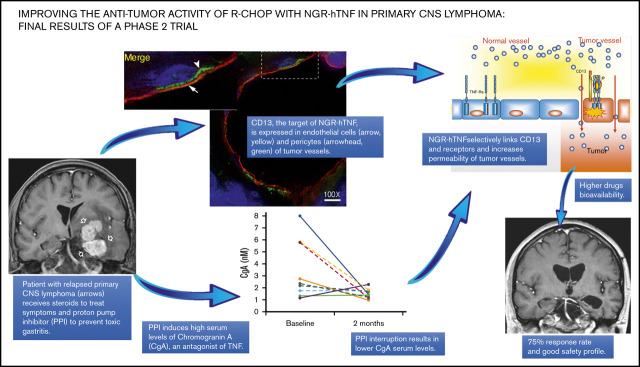
Key Points
R-CHOP preceded by NGR-hTNF was associated with a response rate of 75% and good safety profile in patients with relapsed or refractory PCNSL.
This activity is in line with CD13 expression in endothelial cells and pericytes of tumor vessels; chromogranin A is an antagonist of TNF.
Abstract
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the standard treatment of diffuse large B-cell lymphoma (DLBCL). Primary DLBCL of the central nervous system (CNS) (primary central nervous system lymphoma [PCNSL]) is an exception because of the low CNS bioavailability of related drugs. NGR–human tumor necrosis factor (NGR-hTNF) targets CD13+ vessels, enhances vascular permeability and CNS access of anticancer drugs, and provides the rationale for the treatment of PCNSL with R-CHOP. Herein, we report activity and safety of R-CHOP preceded by NGR-hTNF in patients with PCNSL relapsed/refractory to high-dose methotrexate-based chemotherapy enrolled in a phase 2 trial. Overall response rate (ORR) was the primary endpoint. A sample size of 28 patients was considered necessary to demonstrate improvement from 30% to 50% ORR. NGR-hTNF/R-CHOP would be declared active if ≥12 responses were recorded. Treatment was well tolerated; there were no cases of unexpected toxicities, dose reductions or interruptions. NGR-hTNF/R-CHOP was active, with confirmed tumor response in 21 patients (75%; 95% confidence interval, 59%-91%), which was complete in 11. Seventeen of the 21 patients with response to treatment received consolidation (ASCT, WBRT, and/or lenalidomide maintenance). At a median follow-up of 21 (range, 14-31) months, 5 patients remained relapse-free and 6 were alive. The activity of NGR-hTNF/R-CHOP is in line with the expression of CD13 in both pericytes and endothelial cells of tumor vessels. High plasma levels of chromogranin A, an NGR-hTNF inhibitor, were associated with proton pump inhibitor use and a lower remission rate, suggesting that these drugs should be avoided during TNF-based therapy. Further research on this innovative approach to CNS lymphomas is warranted. The trial was registered as EudraCT: 2014-001532-11.
Visual Abstract
Introduction
A combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the standard of care for most patients with diffuse large B-cell lymphoma (DLBCL). Patients with primary central nervous system (CNS) lymphoma (PCNSL) represent an important exception to this rule, because they are currently treated with high-dose methotrexate-based combinations, often in association with cytarabine, alkylating agents, and rituximab.1 These therapies are effective, but they require hospitalization and dedicated physicians with extensive clinical experience for treatment, and they have toxicity.2 In particular, infections are common, and iatrogenic complications that lead to treatment delays are responsible for nearly 50% of early treatment failures.3 Ideally, treating PCNSL with R-CHOP, a well-tolerated therapy that does not require hospitalization and that is widely used in onco-hematologic centers, could overcome these difficulties. However, R-CHOP is not used to treat PCNSL because these drugs and other related drugs are not capable of crossing the blood-brain barrier (BBB) and achieving efficient concentrations in the tumor.1 These pharmacokinetic limitations and the negative results of a randomized trial4 led to the CHOP regimen being excluded as treatment for patients with PCNSL. Thus, the induction of BBB permeabilization to enhance tumor penetration of R-CHOP could be an attractive investigational approach in PCNSL patients.
Tumor necrosis factor-α (TNF-α) is a good candidate for improving the bioavailability of anticancer drugs to tumors. This inflammatory cytokine alters endothelial cell-cell adhesion, thus inducing selective BBB permeabilization in animal models.5 However, the clinical use of TNF is limited by its unacceptable systemic toxicity.6 The therapeutic index of this cytokine can be enhanced by a vascular targeting approach, for example, by fusing its N terminus with CNGRCG, a tumor vasculature-homing peptide capable of recognizing an isoform of aminopeptidase N (CD13), which is upregulated in angiogenic tumor vessels and which is expressed only a little or not at all by normal blood vessels.6,7 The CNGRCG-human TNF (hTNF) fusion protein (NGR-hTNF; originally developed at the San Raffaele Scientific Institute, Milan, Italy) allows the delivery of extremely low yet pharmacologically active doses of TNF to the tumor vasculature, thereby avoiding systemic toxicity and counterregulatory mechanisms.8 The positive effect of NGR-hTNF on tumor vascular permeability and penetration of anticancer drugs has been demonstrated in several animal models.6,8 Safety and activity of NGR-hTNF in combination with different chemotherapeutic agents have been addressed in various clinical trials.6,9
On this background, we designed a phase 2 trial to assess whether NGR-hTNF can alter the BBB and enhance the tumor penetration and activity of R-CHOP in patients with relapsed or refractory (R/R) PCNSL (INGRID trial; ClinicalTrials.gov identifier: NCT03536039). Most patients with PCNSL have large B-cell morphology and non-germinal-center–like phenotype, a subtype less sensitive to R-CHOP, which led us to adopt NGR-hTNF/R-CHOP as exclusive therapy with caution. Accordingly, the use of consolidation with whole-brain radiotherapy (WBRT), autologous stem cell transplantation (ASCT), or lenalidomide maintenance was allowed. In the proof-of-principle part of the trial, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and single-photon emission computerized tomography (SPECT) demonstrated the positive effect of NGR-hTNF on vascular permeability in the lymphomatous lesions and peritumoral areas.10 These findings were in line with the activity of the NGR-hTNF/R-CHOP combination, which was associated with 9 tumor responses in 12 assessed patients, a figure that largely achieved the activity threshold required by the per-protocol first-step analysis and warranted completion of the planned accrual.10
Here, we report the results of the activity and safety of NGR-hTNF/R-CHOP on the whole population of the INGRID trial. This trial expands our knowledge of the potential interfering effects of known NGR-hTNF inhibitors such as chromogranin A (CgA) and soluble TNF receptors (sTNF-Rs). It also provides a deeper characterization of CD13 expression in PCNSL vasculature. CgA is a neurosecretory protein that can enhance the endothelial barrier and prevent the pro-permeabilizing activity of NGR-hTNF.11 Plasma levels of this protein increase during treatment with proton pump inhibitors (PPIs) and other acid-suppressive medications, a class of drugs often used to prevent gastric toxicity during steroid therapy and chemotherapy in PCNSL patients. sTNF-Rs can form complexes with NGR-hTNF and prevent its interaction with cell surface receptors on tumor vasculature.6 The results of this trial demonstrate that NGR-hTNF/R-CHOP is an undemanding, safe, and active treatment for patients with R/R PCNSL. Our findings also suggest an inhibitory effect of CgA, prove the expression of CD13 by pericytes and endothelial cells of PCNSL vasculature, and point toward the investigation of this innovative strategy as first-line therapy in future prospective trials.
Patients and methods
Study population and selection criteria
The INGRID study is a single-arm phase 2 trial focused on an experimental treatment consisting of 6 courses of standard R-CHOP21 (repeated every 21 days) preceded by NGR-hTNF infusion in HIV-negative adults with R/R PCNSL. The trial has 2 distinct phases: an exploratory phase focused on the feasibility of NGR-hTNF/R-CHOP and another proof-of-principle phase to assess the effects of NGR-hTNF on vascular permeability in the first 10 enrolled patients, which has been previously published.10 The second expansion phase focused on activity and tolerability of the experimental treatment in the whole trial population. There were 6 selection criteria: (1) histologically proven diagnosis of DLBCL according to the World Health Organization criteria,12 (2) disease exclusively localized in the CNS (ie, brain, cranial nerves, meninges, eyes) at first diagnosis and at trial registration, (3) lymphoma relapsed after or refractory to high-dose methotrexate-based chemotherapy, (4) measurable disease, (5) age 18 to 80 years, and (6) Eastern Cooperative Oncology Group (ECOG) performance status score ≤3. As usual in PCNSL trials, brain biopsy at relapse was not mandatory. Patients with previous organ transplant or other forms of immunosuppression; with hepatitis B virus, hepatitis C virus, and/or HIV infections; or with other malignancies were excluded. Patients with positive cerebrospinal fluid (CSF) cytologic examination were enrolled, whereas patients with positive results for flow cytometry examination of the CSF but negative results in conventional cytology and without any other evidence of CNS disease were excluded to avoid the risk of false-positive results. Before trial registration, histopathologic diagnostic specimens and neuroimaging examinations performed at diagnosis and relapse were centrally reviewed (M.P., G.M.C., and N.A., respectively), and all the enrolled patients were assessed by physical and neurologic examination, hemogram, biochemical serum profiles, echocardiography, enhanced total-body computed tomography scan, bone marrow biopsy, contrast-enhanced brain MRI scan, CSF examination, ophthalmologic evaluation, and [18F]fluorodeoxyglucose positron emission tomography scans. Risk was defined according to the International Extranodal Lymphoma Study Group (IELSG) score.13 There is no consensus on the definition of refractoriness in PCNSL patients; thus, we used an ad hoc definition of refractory disease: the progression of lymphoma within the 3 months since the last day of the last line of treatment before trial registration. Written informed consent was obtained from each patient. This trial conformed to the Declaration of Helsinki and was approved by the Institutional Review Board of the San Raffaele Scientific Institute (Milan, Italy).
Experimental treatment
Enrolled patients received 6 courses of R-CHOP21 preceded by NGR-hTNF. Treatment was delivered over 2 days: rituximab 375 mg/m2 was delivered on day 1, and NGR-hTNF 0.8 µg/m2 was delivered by a 1-hour infusion on day 2, at 2 hours before CHOP drugs were administered. Per protocol, the first course of R-CHOP was not preceded by NGR-hTNF in the first 10 patients.10 The rationale for the timing and administration schedule of NGR-hTNF has been previously reported.10 Oral or intravenous acetaminophen and paracetamol at 1.000 mg was delivered as prophylaxis for infusion-related reactions 30 to 60 minutes before starting each infusion of NGR-hTNF. At trial registration, steroids (if indicated earlier to treat symptoms) were interrupted, and ongoing PPIs were replaced with histamine 2 blockers (ie, ranitidine). As part of the CHOP regimen, prednisone was indicated for 5 days (from day 2 to day 6 of each course). Recombinant human granulocyte–colony stimulating factor for primary prophylaxis was recommended for heavily pretreated patients.
Patients who completed the 6 planned courses and achieved a complete response (CR) or partial response (PR) were evaluated for consolidative therapy. Per protocol and according to previous treatments, WBRT 30-36 Gy, carmustine-thiotepa–conditioned ASCT, or lenalidomide maintenance was allowed.
Toxicity and response assessments
Treatment adverse effects (AEs) were assessed separately for each chemotherapy course and graded according to the National Cancer Institute-National Cancer Information Center Common Toxicity Criteria (NCI-NCIC CTC) version 3.0.14 The worst toxicity per organ, per course, and per patient were considered. Routine clinical assessments by a cardiologist, electrocardiogram and echocardiography were performed, and troponin and pro-B-type natriuretic peptide levels were determined every 2 courses to exclude cardiac toxicity. Dose reduction was not considered for NGR-hTNF; per protocol, a maximum of a 2-week delay for re-treatment was allowed in case of grade ≥3 toxicity on the day of re-treatment. R-CHOP drug dose reductions, delay, and interruptions followed the well-known international guidelines used worldwide in routine practice; in particular, R-CHOP drug doses were reduced only in the case of severe complications after grade 4 toxicity.
All registered patients were considered for response evaluation. Response was assessed by gadolinium-enhanced MRI of the brain performed on a 1.5 Tesla scanner after the first (only in the first 10 enrolled patients10), second, fourth, and sixth courses of treatment. In cases with concomitant CSF and/or vitreous samples showing lymphomatous cells, examination was performed after the second, fourth, and sixth courses. Response was defined according to the International PCNSL Collaborative Group (IPCG) criteria15 (supplemental Table 1). As an important change within the IPCG criteria, a response was considered only when tumor regression was confirmed in 2 serial MRI scans. The maximum response was considered for analysis. After treatment, the disease was assessed every 3 months.
Biomarkers and target assessments
Plasma levels of CgA and sTNF-R1 and -R2 were tested by enzyme-linked immunosorbent assay on samples collected at the same time points as tumor response assessment (details regarding methods are provided in supplemental Data). The relationships between plasma levels of CgA or sTNF-Rs (continuous variables) and therapeutic response (CR vs no CR) and PPI therapy (yes vs no) were assessed by using the Mann-Whitney U test.
To characterize the expression of CD13 (the target of NGR) in PCNSL vasculature, we performed double immunofluorescence staining experiments on tumor tissue sections from 7 registered patients with anti-CD13 and anti-CD31 (a marker of endothelial cells) antibodies and with anti-CD13 and anti-platelet–derived growth factor receptor-β (PDGFR-β; a marker of pericytes) antibodies (details regarding methods are provided in supplemental Data).
Statistical considerations
Overall response rate (ORR: CR and PR) was the primary end point, and the 2-stage Simon Minimax design was used. The maximum ORR considered of low interest was 30% (rate reported in previous prospective trials focused on salvage treatment in PCNSL patients performed at our institution16,17), and the minimum ORR considered of interest was 50%; to demonstrate that difference, 28 patients were needed (1-sided test; type I error × 10; power × 9). At the first step, 12 patients would be registered and, if at least 4 responses were observed, the study would have continued to accrue up to 28 patients. The experimental treatment would be declared active if at least 12 responses were recorded among the 28 assessable patients. Tolerability and duration of response were the secondary end points. Tolerability was defined by the incidence of grade 3 to 4 AEs according to the NCI Common Terminology Criteria for Adverse Events (CTCAE).14 Duration of response was measured from the date of maximum response to the date of relapse, progression, death as a result of any cause, or last follow-up visit.
Results
Study population
Twenty-eight patients (median age, 58 years; range, 26-78 years; 14 males) were registered between May 2016 and November 2018. The trial was ended after accrual was completed. The database lock for the primary analysis was 31 January 2020. All patients were assessable for activity and tolerability. Most patients had unfavorable prognosis features at trial registration, with an intermediate-high IELSG risk in 23 patients (82%) (Table 1). Previous lines of treatment per patient are reported in Table 2: 8 patients (29%) received 2 or more previous lines of treatment and 17 patients (61%) had received ASCT, WBRT, or both. The median time to progression after the previous line of treatment was 1 month (range, 0-84 months). Nineteen patients (68%) had refractory disease (progression within 3 months from the last day of the last line of treatment before trial registration).
Table 1.
Patient characteristics (n = 28)
| Characteristic | n/N | % |
|---|---|---|
| Median age (range), y | 58 (26-78) | |
| Ratio of males to females | 1 | |
| ECOG performance status >1 | 15 | 53 |
| High lactate dehydrogenase serum level | 11 | 40 |
| High CSF protein concentration* | 11/22 | 50 |
| Involvement of deep areas | 12 | 43 |
| IELSG risk score | ||
| Low | 5 | 18 |
| Intermediate | 19 | 68 |
| High | 4 | 14 |
| Sites of disease | ||
| Brain parenchyma | 28 | 100 |
| Intraocular disease | 3 | 10 |
| Meningeal dissemination† | 0 | 0 |
| Previous lines of therapy | ||
| ≥2 | 8 | 29 |
| ASCT | 7 | 25 |
| WBRT | 6 | 21 |
| Both ASCT and WBRT | 4 | 14 |
| Refractory disease‡ | 19 | 68 |
ECOG, Eastern Cooperative Oncology Group.
Lumbar puncture was contraindicated in 6 patients; per protocol, CSF protein concentration was considered an unfavorable prognostic feature in IELSG risk score in these patients.
Meningeal/CSF involvement was not an exclusion criteria.
Refractory disease was defined by the progression of lymphoma within the 3 months since the completion of the last line of treatment before trial registration.
Table 2.
Previous lines of treatment per patient
| First line | Second line | Third line | Response to the previous line† | TTP‡ | |||
|---|---|---|---|---|---|---|---|
| Treatment* | Response | Treatment | Response | Treatment | Response | ||
| MATRix (4) | PD | PD | 0 | ||||
| MATRix (4) > ASCT > WBRT | CR | CR | 9 | ||||
| MTX-ARAC (3) | PD | PD | 0 | ||||
| R-MPV (3) > WBRT + ITT | CR | MATRix (4) > ASCT | CR | CR | 8 | ||
| R-MTX-ARAC (4) > WBRT | PD | PD | 0 | ||||
| R-MTX-ARAC (4) > ASCT | CR | CR | 2 | ||||
| R-MTX-ARAC (4) | PD | Resection | PD | PD | 0 | ||
| MTX-ARAC (2) > WBRT | CR | CR | 1 | ||||
| R-MTX (3) > WBRT | PR | TMZ (2) | PD | R (4) | PD | PD | 0 |
| R-MTX-ARAC (4) > ASCT | CR | WBRT | CR | CR | 3 | ||
| MATRix (4) | PD | WBRT | CR | CR | 2 | ||
| R-MTX-ARAC (3) + ITT | PD | R-ITX-VP16 (2) | PD | WBRT | CR | CR | 3 |
| MTX-TMZ (5) + ITT | CR | CR | 8 | ||||
| MATRix (4) > De-VIC | PD | PD | 0 | ||||
| MTX (17) | CR | R-TMZ (4) | PD | PD | 0 | ||
| MATRix (4) > ASCT | CR | CR | 27 | ||||
| MATRix (4) > ASCT | CR | CR | 84 | ||||
| MATRix (4) > ASCT > WBRT | PD | PD | 0 | ||||
| R-MTX-ARAC (4) | PD | PD | 0 | ||||
| MATRix (2) | PD | PD | 0 | ||||
| MATRix (4) > ASCT | CR | CR | 12 | ||||
| MATRix (4) | PD | PD | 0 | ||||
| R-MTX-ARAC (2) | PD | R-ITX-VP16 (4) | PD | PD | 0 | ||
| MATRix (4) > WBRT | CR | CR | 1 | ||||
| MATRix (4) > ASCT + IVT | CR | CR | 4 | ||||
| MATRix (4) > ASCT | CR | CR | 11 | ||||
| R-MTX-ARAC (2) | PD | PD | 0 | ||||
| R-MTX-ARAC (4) > ASCT | CR | CR | 11 | ||||
ARAC, high-dose cytarabine; De-VIC, dexamethasone, etoposide, ifosfamide, and carboplatin; ITT, intrathecal chemotherapy; ITX, high-dose ifosfamide; IVT, intravitreal therapy; MATRix, methotrexate, cytarabine, thiotepa, and rituximab; MPV, methotrexate, procarbazine and vincristine; MTX, high-dose methotrexate; R, rituximab; TMZ, temozolomide; VP16, etoposide.
Number in parentheses indicates number of chemotherapy courses.
Response to the last line of treatment before trial registration.
TTP is the time to progression since the completion of the last line of treatment before trial registration.
Toxicity
NGR-hTNF/R-CHOP was well tolerated (Table 3; supplemental Table 2); 132 (79%) of the 168 planned courses of R-CHOP were delivered; NGR-hTNF was delivered in 158 of the 168 courses because, per protocol, the first 10 patients received only 5 doses of NGR-hTNF. Missed treatment courses were a result of progressive disease (PD). There were no cases of unexpected toxicity or interruptions because of toxicity, and no patient needed a reduction in dose of either NGR-hTNF or R-CHOP. Only 6 courses (4%) were delayed (cytopenia). Sixteen serious AEs were recorded in 12 patients: grade 1 to 2 seizures (3), grade 1 to 2 deep venous thrombosis (2), grade 3 infections (5), grade 3 syncope (2), grade 3 constipation, grade 4 febrile neutropenia, pulmonary aspergillosis, and grade 2 left ventricular function reduction. There were no cases of iatrogenic neurotoxicity. The 3 episodes of seizures occurred in 2 patients with active CNS lymphoma: 1 experienced seizures (2 episodes) after the first course of NGR-hTNF/R-CHOP and died of progressive lymphoma after the second course, and the other patient experienced seizures after the first course was administered. Anticonvulsant drug doses were optimized, and no other episodes occurred during the other 5 courses of NGR-hTNF/R-CHOP after consolidative ASCT and during 2 years of follow-up. Three patients achieved PR after the first 4 courses of NGR-hTNF/R-CHOP, with improvement of focal neurologic deficits but concomitant, progressive cognitive decline was followed early by PD (see “Activity (primary end point)”). Both syncope events occurred in the same 78-year-old woman after the first NGR-hTNF/R-CHOP course; they were attributed to vasovagal phenomena because no abnormal findings were detected during the electrocardiogram, echocardiography, brain MRI scan, and coronography; bisoprolol was indicated, and the patient did not experience syncopal events during the other 5 courses of NGR-hTNF/R-CHOP and 13 months of follow-up.
Table 3.
Toxicity per course of treatment
| Grade 1-2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5 | |
|---|---|---|---|---|
| Neutropenia | 9 (7) | 17 (13) | 57 (43) | — |
| Thrombocytopenia | 34 (26) | 25 (19) | 26 (20) | — |
| Anemia | 86 (65) | 12 (9) | 2 (2) | — |
| Febrile neutropenia | — | 5 (4) | 1 (1) | — |
| Hepatotoxicity | 27 (20) | 4 (3) | 1 (1) | — |
| Oral mucositis | 1 (1) | 3 (2) | — | — |
| Infections | — | 5 (4) | — | — |
| Seizures | 3 (2) | — | — | — |
| Deep vein thrombosis | 2 (2) | — | — | — |
| Syncope | — | 2 (2) | — | — |
| LVEF reduction | 1 (1) | — | — | — |
| Constipation | 2 (2) | 1 (1) | — | — |
| Nausea and vomiting | 4 (3) | — | — | — |
| TNF infusion reaction* | 9 (7) | — | — | — |
All toxic events other than alopecia are reported. The denominator is the total number of delivered courses (n = 132).
LVEF, left ventricular ejection fraction.
Fever (4), chills (4), arterial hypertension (1).
Twelve patients required blood or platelet transfusions (7 of them had received previous ASCT). There were 9 cases of reaction to NGR-hTNF infusion; all of them, fever (4), chills (4), and arterial hypertension were of grade 1 to 2 and resolved after infusion was interrupted for 15 minutes, and symptomatic medication was administered; per protocol, infusion was completed 1 hour later. These patients received the following planned courses of NGR-hTNF/R-CHOP with per protocol prophylaxis and did not experience any further infusion reaction.
Activity (primary end point)
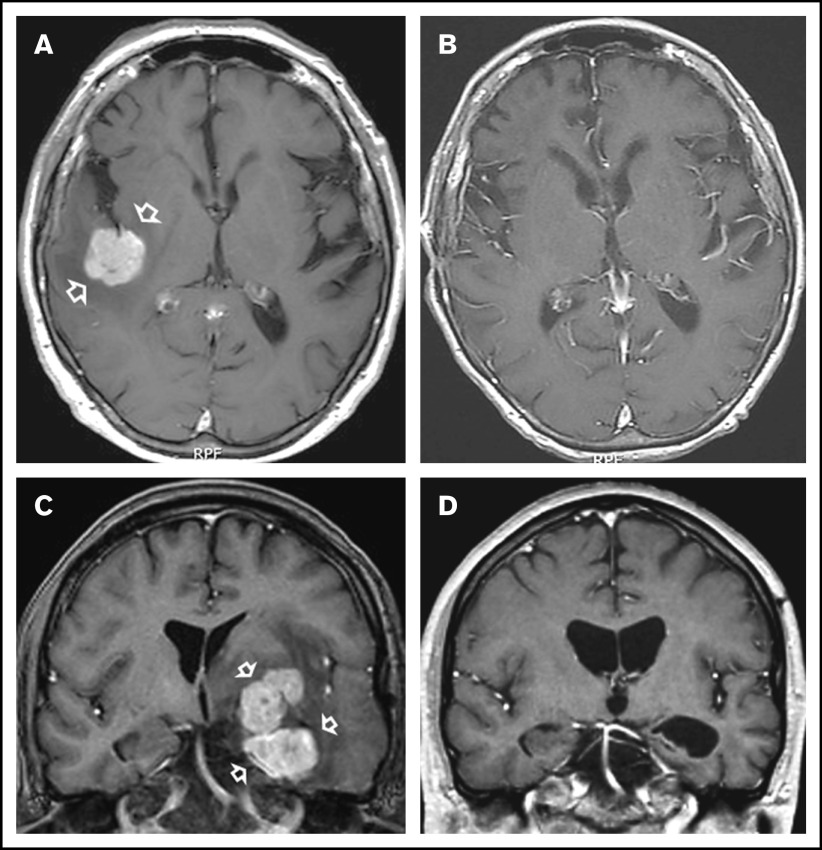
In all, 11 patients who received NGR-hTNF/R-CHOP had CR (examples in Figure 1) and 10 patients had PR, with an ORR of 75% (95% confidence interval, 59%-91%); 7 patients experienced PD. Thus, the predetermined activity threshold of ≥12 responses was largely achieved. The best response was achieved after the second course in 14 patients and after the fourth course in 7 patients. Responses were equally distributed in analyzed subgroups according to IELSG risk variables, site and number of lesions, previous therapies, and refractoriness (supplemental Table 3). Two of the 3 patients with intraocular disease achieved tumor regression at that site that lasted 3+ and 28+ months, respectively. Response lasted more than 6 months in all patients with CR (median, 11 months; range, 6-25+ months).
Figure 1.
Examples of responses to R-CHOP preceded by NGR-hTNF. (A) Gadolinium-enhanced T1-weighted scan shows a large homogeneous enhancing lesion in the right parietal lobe (arrows) in a 65-year-old man at second relapse after high-dose methotrexate and after salvage high-dose ifosfamide-based therapy plus WBRT. (B) Tumor regression after 4 courses of experimental treatment. (C) Gadolinium-enhanced T1-weighted scan shows a large polylobate, enhancing lesion infiltrating the basal ganglia, diencephalon, and left temporal lobe (arrows) in a 39-year-old man with PCNSL refractory to previous high-dose methotrexate-based chemoimmunotherapy. (D) Tumor regression after 4 courses of experimental treatment.
Seventeen of the 21 responding patients received consolidation (Table 4): WBRT in 7 patients, ASCT in 5, lenalidomide maintenance in 1, and combinations of these therapies in 4. Four responsive patients did not receive consolidation. A 78-year-old woman who achieved a CR refused consolidation WBRT and experienced relapse at 13 months of follow-up; the other 3 patients achieved PR after 4 courses of NGR-hTNF/R-CHOP, and focal neurologic deficits improved, but concomitant, progressive cognitive skills declined. Concomitant brain MRI scans excluded PD, and the patients refused further treatment and experienced relapse after 5, 5, and 6 months (Table 4).
Table 4.
Response to NGR-hTNF/RCHOP, consolidation, relapse, and survival
| Response to NGR-hTNF/R-CHOP | Consolidation | Response to consolidation | Treatment failure | TTTF, mo* | Status | Survival, mo* |
|---|---|---|---|---|---|---|
| PD | None | — | Yes | 4 | DoD | 5 |
| CR | Lenalidomide | CR | Yes | 11 | DoD | 11 |
| PD | None | — | Yes | 4 | DoD | 14 |
| CR | WBRT > lenalidomide | CR | Yes | 6 | DoD | 18 |
| PD | None | — | Yes | 4 | DoD | 6 |
| CR | WBRT > lenalidomide | CR | Yes | 9 | DoD | 22 |
| CR | ASCT > WBRT | CR | Yes | 6 | Alive | 31 |
| CR | ASCT > lenalidomide | CR | Yes | 17 | Alive | 30 |
| CR | ASCT | CR | Yes | 9 | DoD | 11 |
| PR | WBRT | CR | No | 10 | DUC | 10 |
| CR | ASCT | CR | Yes | 6 | DoD | 7 |
| CR | ASCT | CR | No | 25 | Alive | 25 |
| PD | None | — | Yes | 1 | DoD | 2 |
| PD | None | — | Yes | 1 | DoD | 2 |
| CR | None | — | Yes | 13 | DoD | 19 |
| PR | WBRT | CR | Yes | 10 | DoD | 11 |
| PR | WBRT | PR | Yes | 5 | DoD | 7 |
| PR | WBRT | PR | Yes | 5 | DoD | 7 |
| PR | None | — | Yes | 5 | DoD | 6 |
| PD | None | — | Yes | 0 | DoD | 1 |
| PR | None | — | Yes | 5 | DoD | 7 |
| PR | None | — | Yes | 6 | DoD | 9 |
| PR | WBRT | PR | Yes | 7 | DoD | 8 |
| CR | ASCT | CR | No | 17 | Alive | 17 |
| PD | None | — | Yes | 1 | DoD | 3 |
| PR | WBRT | PR | Yes | 3 | DoD | 6 |
| CR | ASCT | CR | No | 14 | Alive | 14 |
| PR | WBRT | CR | No | 14 | Alive | 14 |
Patients are reported in the same order as in Table 2.
DoD, death [as a result] of disease; DUC, death [as a result of an] unknown cause.
TTTF, time to treatment failure and survival were estimated from trial registration.
Sixteen of the 21 responders experienced relapse; sites of recurrence included the primary area of disease (ie, area involved at trial registration) in all but 2 patients. In detail, relapse sites consisted of only primary sites in 10 patients, combined primary and secondary (areas uninvolved at trial registration) brain sites in 4, and only secondary brain sites in 2. Relapses involving the eyes, meninges, or extra-CNS organs were not recorded. At a median follow-up of 21 months (range, 14-31 months), 5 patients remain relapse free (supplemental Table 4), and 6 patients are alive (supplemental Figure 1). Three of the 5 relapse-free survivors had achieved CR after NGR-hTNF/R-CHOP, and they received consolidative carmustine thiotepa–conditioned ASCT; the other 2 relapse-free survivors had achieved PR after NGR-hTNF/R-CHOP and received complementary WBRT.
NGR-hTNF inhibitors
We observed an association between plasma levels of CgA at trial registration (baseline) and CR rate (CRR) (P = .066; Mann-Whitney U test) (Figure 2A). When patients were grouped into those with low or high CgA levels, using a receiver operating characteristic curve–driven cutoff of 1.4 nM, we observed 8 of 13 and 3 of 15 patients, respectively, achieving a CR (62% vs 20%; P = .05, Fisher’s exact test). Notably, plasma levels of CgA at trial registration were associated with the use of PPIs during previous steroid therapy. Median plasma levels of CgA were 1.05 nM (range, 0.29-3.27 nM) and 2.26 nM (range, 0.33-7.99 nM; P = .008, Mann-Whitney U test), respectively, in patients who did not receive PPIs (n = 14) and in patients who did receive PPIs (n = 14). Complete data (samples from at least 3 time points) on changes of plasma CgA levels during treatment were available for 14 patients: CgA concentrations were progressively reduced in 6 of the 9 patients after PPI interruption mandated by the protocol (Figure 2B), whereas the values remained stable in the 5 patients who had not received a PPI (Figure 2C).
Figure 2.
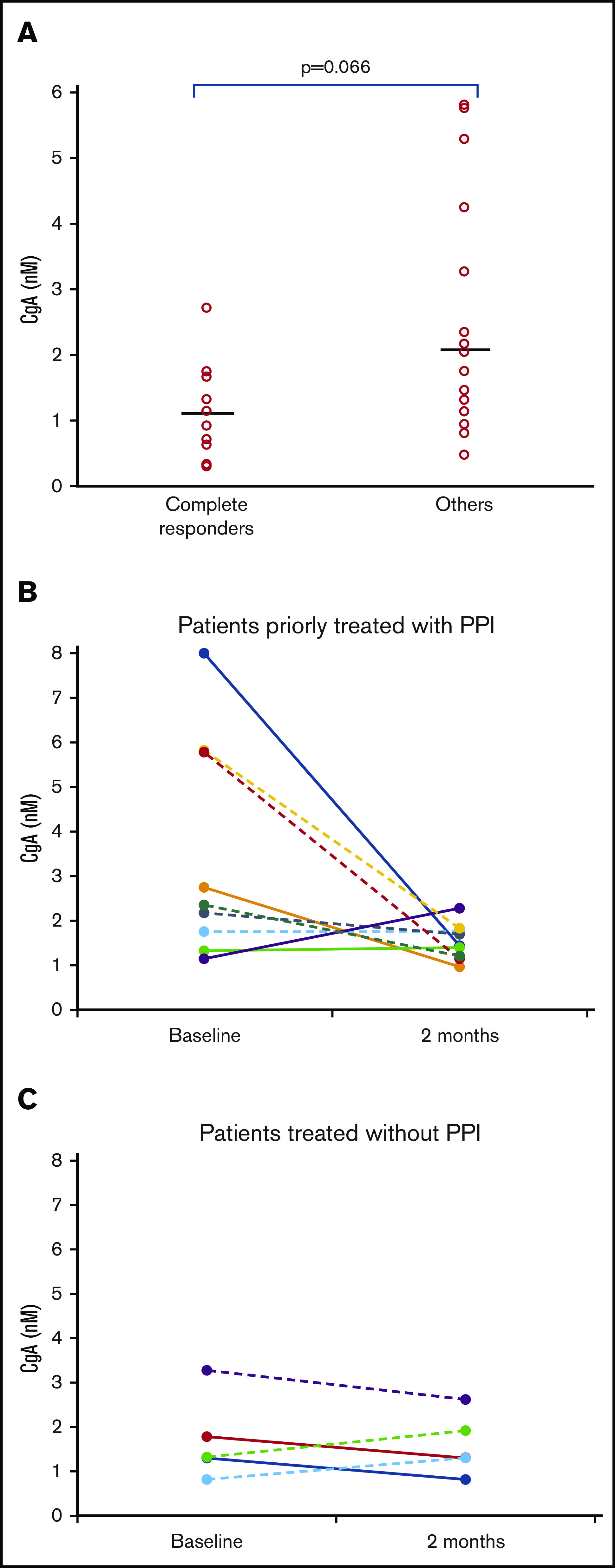
CgA plasma levels, PPI therapy, and responses. (A) Relationship between CgA plasma levels and response to NGR-hTNF/R-CHOP. Baseline plasma levels of CgA of patients who achieved a CR (n = 12) and patients who did not (others, n = 16). Median CgA levels were 1.14 nM (range, 0.29-2.72 nM) and 2.10 nM (range, 0.47-5.81 nM), respectively (P = .066). (B-C) Changes in CgA plasma levels after PPI interruption. The comparison of CgA concentrations in plasma samples collected at trial registration (baseline) and before the third course (2 months) showed level reduction in some patients after PPI interruption (B), whereas the values remained stable in patients who had not received this drug (C). No differences were detected between patients achieving a CR (continued lines) or a PR (dotted lines). These analyses were performed in the 14 patients who had samples taken at 3 time points at least.
Median plasma levels of sTNF-R1 and sTNF-R2 at trial registration were 0.66 nM (range, 0.32-4.88 nM) and 2.14 nM (range, 0.98-7.26 nM), respectively. sTNF-R concentrations were not associated with response to NGR-hTNF/R-CHOP, did not change after PPI interruption, and remained stable during treatment (data not shown).
Expression of CD13 in PCNSL vasculature
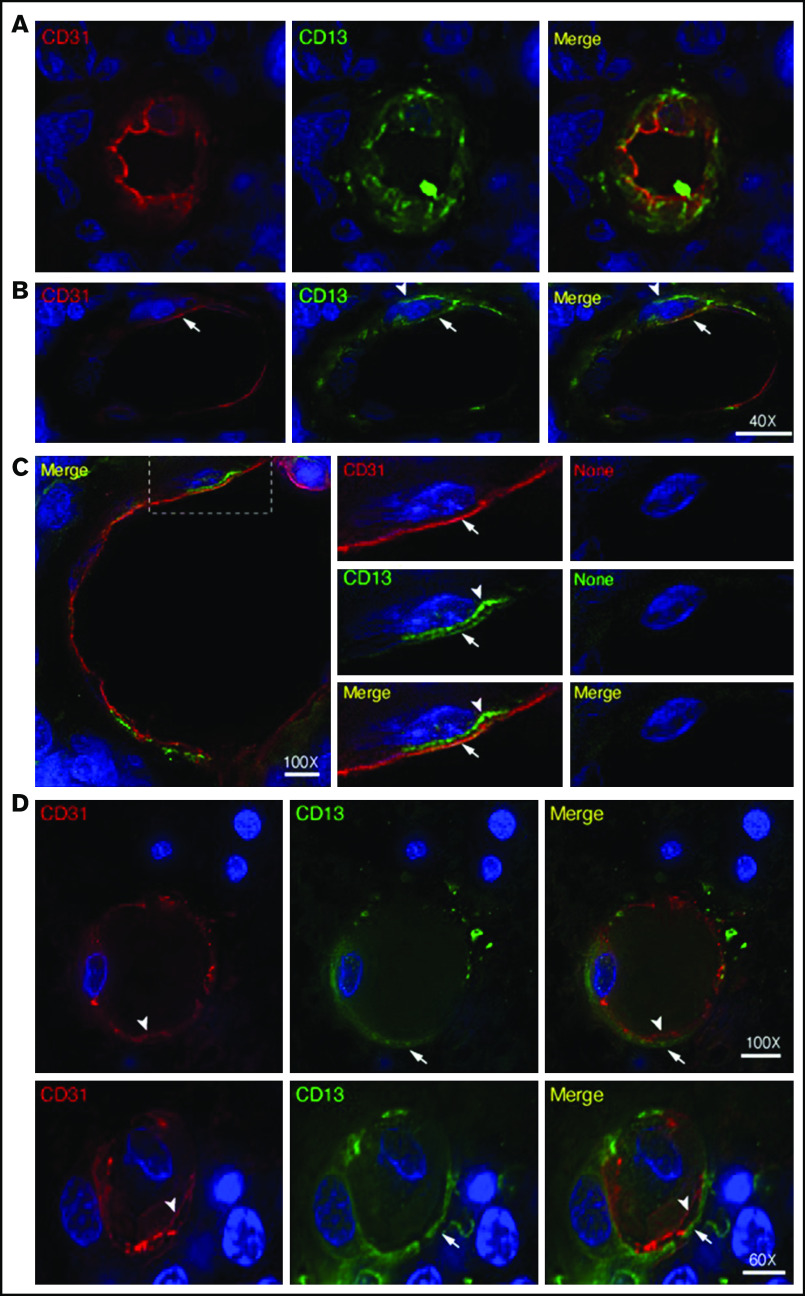
Double staining experiments with anti-CD13 (NGR target) and anti-PDGFR-β (pericyte marker) antibodies revealed the expression of CD13 in pericytes of most lymphoma-associated vessels (supplemental Figure 2). Double staining experiments with anti-CD13 and anti-CD31 (endothelial marker) antibodies also revealed CD13 expression in the endothelial lining of tumor vessels (Figure 3A-C; supplemental Movie 1). Tumor vessels with CD13+ pericytes and CD13– endothelial cells were also observed (Figure 3D).
Figure 3.
Expression of CD13 by endothelial cells and pericytes in primary CNS lymphoma vasculature. High-resolution wide-field co-immunofluorescence analysis of PCNSL tissue sections from 2 enrolled patients. The sections were stained with a polyclonal anti-CD13 antibody (green) and polyclonal anti-CD31, a marker of endothelial cells (red). Nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI; blue). (A-C) Examples of vessels with CD13+ endothelial cells (arrows, yellow in the merged figure) and CD13+ mural cells (arrowheads, green, likely pericytes). See also supplemental Movie 1 for z-stack images of the vessel reported in panel A. (C) Central panel: electronic enlargement of the highlighted area (dashed rectangle) of the vessel shown in the left panel. Right panel (control): a consecutive tissue section with secondary antibodies alone, showing lack of staining. (D) Examples of vessels with CD13+ mural cells (arrows, green) and CD13– endothelial cells (arrowheads, red) in the merged figure. Scale bar, 5 µm; magnification is shown in each panel.
Discussion
The INGRID trial demonstrates that the NGR-hTNF/R-CHOP combination is well tolerated and is highly active in patients with R/R PCNSL. This study also highlights the significant association among use of PPI, increased CgA plasma levels, and reduced response to NGR-hTNF/R-CHOP, and it demonstrates that CD13, the NGR target, is expressed by PCNSL endothelial cells and pericytes. The therapeutic activity of this innovative strategy follows selective enhancement of vascular permeability in the tumor and peritumoral areas, as previously demonstrated by using DCE-MRI and SPECT scans for this cohort,10 which likely favored R-CHOP penetration. All together, these findings support the hypothesis that NGR can be exploited for delivering TNF to the PCNSL vasculature and that increasing vascular permeability and drug penetration in the tumor by noninvasive procedures is an attractive approach that deserves to be further investigated in PCNSL patients.
This trial has some limitations. The single-arm design does not demonstrate that the clinical activity seen in enrolled patients derives from the NGR-hTNF/R-CHOP combination and not simply from the activity of R-CHOP. However, available evidence suggests that our encouraging results should be attributed to the biological effects of NGR-hTNF. In effect, several studies have demonstrated that CHOP with or without rituximab is ineffective in PCNSL patients4,18-20 and have confirmed the common belief that its lack of efficacy is mostly a result of the poor CNS bioavailability of related drugs. When used as first-line treatment, CHOP chemotherapy has been associated with short-lived responses in 28% to 55% of patients and did not improve disease control in combination with high-dose methotrexate-based chemotherapy or with WBRT.4,18-20 In the RTOG8806 trial,20 pre-irradiation with cyclophosphamide, doxorubicin, vincristine, and dexamethasone (CHOD) chemotherapy did not significantly improve survival over WBRT alone, with a 19% CRR after CHOD, and a 2-year overall survival of 40% after CHOD-WBRT. Similar results have been reported with the addition of the CHOP regimen after WBRT in a randomized trial.4 There are no studies focused on CHOP with or without rituximab in patients with R/R PCNSL; however, the disappointing results reported for first-line treatment4,18-20 suggest that this chemoimmunotherapy will also be inactive as salvage therapy. This view is also supported by the lack of objective responses after the first R-CHOP course delivered (without NGR-hTNF) in the first 10 patients enrolled in this trial,10 which contrasts sharply with the 75% ORR to NGR-hTNF/R-CHOP and with the fact that tumor regression was maintained until consolidation therapy and was started in 17 of the 21 responding patients. Somehow, these data can be considered a limitation of this study because consolidation could have had a relevant effect on survival, which does not allow us to demonstrate that duration of response was exclusively attributable to NGR-hTNF/R-CHOP. This choice was an attempt to offer our patients an additional possibility of cure, which is an uncommon event in patients with R/R PCNSL.
Prognosis of patients with R/R PCNSL is poor, with a 1-year progression-free survival (PFS) of ∼20% and a median overall survival of 2.1 months in a series of 256 patients treated in routine practice, which included 12% of patients managed with palliative care.21 As expected, outcome was better in the 15% of patients who received salvage chemotherapy followed by consolidation, with a median PFS from the date of first relapse or progression of 13.5 months. Most patients with R/R PCNSL are treated with combinations of drugs active against systemic lymphomas and with a moderate ability to cross the BBB. High-dose ifosfamide-based combinations are often used, with CRRs between 27% and 48%17,22,23; one-third of these patients received consolidative ASCT.23 PCNSL cells often display activated survival signaling pathways,24 which are well-known therapeutic targets in other lymphomas. Recent clinical trials in patients with R/R PCNSL addressed drugs that target these molecules, such as temsirolimus,25 ibrutinib,26-29 lenalidomide,30,31 and pomalidomide,32 reporting mostly encouraging response rates but short PFS and varied toxicity profiles. The results of the INGRID trial are in line with these experiences; however, duration of response in this trial cannot be compared with those reported in most of the previous trials because a potential effect of consolidation should be considered. In any case, with mild and manageable toxicity and an ORR of 75%, NGR-hTNF/R-CHOP is an active and innovative salvage strategy that deserves to be further investigated in PCNSL patients.
Reversible BBB disruption by intra-arterial infusion of mannitol followed by intra-arterial chemotherapy aimed to increase drug concentrations in the lymphoma-infiltrated brain have been tested as salvage and first-line treatment in patients with PCNSL.33 In institutions with adequate expertise, this strategy has been associated with a 58% CR rate, a 5-year PFS of 31%, and acceptable morbidity and neurotoxicity.33 However, it is a procedurally intensive treatment that requires monthly intravascular interventions under general anesthesia over the course of 1 year, which is an important obstacle for wide use of this strategy. Conversely, BBB permeabilization by NGR-hTNF is a noninvasive, simple, and well-tolerated approach that could be used in most centers that routinely treat PCNSL patients. The encouraging activity of NGR-hTNF/R-CHOP was associated with an excellent safety profile, with no unexpected toxicities and with a maintained dose intensity in all cases. In line with previous trials,34,35 the addition of low-dose NGR-hTNF to chemotherapeutic agents was associated with good tolerability and, in particular, the combination with doxorubicin was not associated with severe cardiovascular events.
The selectivity of NGR-hTNF for tumor vessels requires interaction with specific receptors.8,36 When delivered at low doses, NGR-hTNF engages in high-avidity multivalent interactions with endothelial cells that express CD13, TNF-R1, and TNF-R2, as they occur in the angiogenic tumor vasculature,6 but not with endothelial cells lacking CD13, as they occur in normal tissues.8 In the exploratory phase of the INGRID trial, we showed that the pro-permeabilizing effect of NGR-hTNF is more evident in tumor and peritumoral areas, where CD13 expression by tumor vasculature has been demonstrated.10 This study was not designed to demonstrate whether NGR-hTNF increases the BBB permeability in non-enhancing regions that can be diffusely infiltrated by lymphoma cells and represent a sanctuary for lymphoma cells, which results in relapse. A deeper characterization of CD13 in PCNSL vessels demonstrates that this protein is expressed by endothelial cells and pericytes, which contrasts with the fact that CD13 is expressed only in pericytes in the normal brain vessels.37,38 This pattern of expression of CD13 provides the molecular basis for the antitumor activity of NGR-hTNF/R-CHOP, considering that both endothelial cells and pericytes of the PCNSL vessels are likely accessible to intravenously delivered NGR-hTNF, which is favored by the fact that, as shown by DCE-MRI and SPECT,10 PCNSL vasculature is more permeable than normal brain vessels.
Some issues regarding CgA and sTNF-Rs, 2 important inhibitors of NGR-hTNF activity,39 need to be discussed. Although plasma levels of sTNF-Rs were not related to therapeutic outcome, significant associations among CgA plasma levels, use of PPIs, and response to NGR-hTNF/R-CHOP were observed. Plasma levels of CgA are known to increase after treatment with PPIs, because these drugs induce hypergastrinemia and stimulate enterochromaffin cells to secrete CgA.40 In line with these notions, we observed that PCNSL patients taking PPIs (to prevent gastric toxicity from steroids and chemotherapy) had increased CgA levels that were associated with lower activity of NGR-hTNF/R-CHOP. These findings suggest that discontinuation of PPIs before administering NGR-hTNF/R-CHOP is advisable. When possible, NGR-hTNF/R-CHOP should be started once CgA reaches normal levels after PPI discontinuation, which may require anywhere from a few days to a few weeks.40
Because of the high activity, excellent safety profile, and the fact that R-CHOP is routinely used in the outpatient setting, NGR-hTNF/R-CHOP should be assessed as first-line treatment for PCNSL. The evidence suggests that this strategy would be better tested in combination with consolidative ASCT or WBRT. Combinations with other drugs previously tested in patients with relapsed PCNSL such as lenalidomide or ibrutinib should be also investigated. Likewise, overall activity of BBB permeabilization in patients with relapsed PCNSL may be improved by using other chemotherapy combinations. Use of BBB permeabilization in secondary CNS lymphomas and other CNS tumors (ie, gliomas and metastases) should be investigated.
In summary, the results of the INGRID trial suggest that the pro-permeabilizing effects of NGR-hTNF on the BBB can be exploited to enhance the activity of R-CHOP in PCNSL. The NGR-hTNF/R-CHOP combination is active and safe in patients with R/R PCNSL, and its antitumor activity is in line with the expression of CD13 in tumor vessels. PPIs should not be used during this therapy because they can mitigate the effects of NGR-hTNF by enhancing the plasma levels of CgA. Accordingly, NGR-hTNF/R-CHOP strategy needs to be addressed as first-line treatment in PCNSL patients, and new drug combinations with NGR-hTNF should be explored in the relapse setting.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors appreciate the excellent technical assistance and sustained scientific collaboration of the multidisciplinary team studying primary CNS lymphoma at the IRCCS San Raffaele Scientific Institute, Milan, Italy: Angelo Diffidenti and Maria Colia (research nurses in the Lymphoma Unit), Stefano Orezzi (Neuroradiology Unit), Anna Chiara (Radiotherapy and Tomotherapy Unit), Maria Rosa Terreni (Pathology Unit), Filippo Gagliardi (Neurosurgery Unit), Elisabetta Miserocchi and Giulio Modorati (Ophthalmology Unit), and Barbara Colombo (Tumor Biology and Vascular Targeting Unit). The authors also thank colleagues from other institutions in Italy who referred patients for trial enrollment and acknowledge hematologists and oncologists in the Department of Onco-Hematology, IRCCS San Raffaele Scientific Institute, Milan, Italy, for their excellent clinical assistance. The authors are indebted to enrolled patients and their families for their generous commitment.
This study was supported by a grant from the Leukemia and Lymphoma Society (6510-17) (A.J.M.F.) and in part by a grant from the Associazione Italiana Ricerca Contro il Cancro (IG-23470) (A.C.). NGR-hTNF was kindly provided by MolMed SpA (Milan, Italy).
Authorship
Contribution: A.J.M.F. and A.C. conceived, designed, and supervised the study; A.J.M.F., T.C., G.C., L.S.P., F. Ciceri., C.B., N.A., and A.C. developed the methodology; E.S. and D.D.L. provided administrative support and managed the data; A.N. performed the statistical analyses; T.C., M.F., M.S., P.A., E.G., S.S., and V.T. treated patients; G.M.C., F.F., and N.A. assessed and analyzed the images; D.C. performed pharmacology assessments and analysis; M.P., F. Curnis, E.R., and A.C. performed the histopathology, immunohistochemistry, and immunofluorescence assays; T.C. and P.L. acquired the clinical data; A.J.M.F., T.C., M.P., F. Curnis, and A.C. analyzed and interpreted the data; A.J.M.F., M.P., and A.C. wrote the manuscript; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: C.B. was employed and had equity ownership in MolMed SpA. A.C. served as a consultant to MolMed SpA during the conduct of the study. A.C. and F. Curnis hold a patent on NGR-hTNF. The remaining authors declare no competing financial interests.
Correspondence: Andrés J. M. Ferreri, Lymphoma Unit, Department of Onco-Hematology, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, 20132 Milan, Italy; e-mail: ferreri.andres@hsr.it.
References
- 1.Batchelor TT. Primary central nervous system lymphoma. Hematology Am Soc Hematol Educ Program. 2016;2016:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJM, Holdhoff M, Nayak L, Rubenstein JL. Evolving treatments for primary central nervous system lymphoma. Am Soc Clin Oncol Educ Book. 2019;39(39):454-466. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG) . Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217-e227. [DOI] [PubMed] [Google Scholar]
- 4.Mead GM, Bleehen NM, Gregor A, et al. . A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89(6):1359-1370. [PubMed] [Google Scholar]
- 5.Connell JJ, Chatain G, Cornelissen B, et al. . Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst. 2013;105(21):1634-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti A, Curnis F, Rossoni G, Marcucci F, Gregorc V. Peptide-mediated targeting of cytokines to tumor vasculature: the NGR-hTNF example. BioDrugs. 2013;27(6):591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Matteo P, Arrigoni GL, Alberici L, et al. . Enhanced expression of CD13 in vessels of inflammatory and neoplastic tissues. J Histochem Cytochem. 2011;59(1):47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110(4):475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregorc V, Gaafar RM, Favaretto A, et al. . NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2018;19(6):799-811. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJM, Calimeri T, Conte GM, et al. . R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-α in primary CNS lymphoma. Blood. 2019;134(3):252-262. [DOI] [PubMed] [Google Scholar]
- 11.Dondossola E, Gasparri AM, Colombo B, Sacchi A, Curnis F, Corti A. Chromogranin A restricts drug penetration and limits the ability of NGR-TNF to enhance chemotherapeutic efficacy. Cancer Res. 2011;71(17):5881-5890. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Harris NL, et al. . WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 13.Ferreri AJ, Blay JY, Reni M, et al. . Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266-272. [DOI] [PubMed] [Google Scholar]
- 14.Trotti A, Colevas AD, Setser A, et al. . CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176-181. [DOI] [PubMed] [Google Scholar]
- 15.Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group . Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034-5043. [DOI] [PubMed] [Google Scholar]
- 16.Reni M, Zaja F, Mason W, et al. . Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mappa S, Marturano E, Licata G, et al. . Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol. 2013;31(3):143-150. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill BP, Wang CH, O’Fallon JR, et al. . Primary central nervous system non-Hodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. Int J Radiat Oncol Biol Phys. 1999;43(3):559-563. [DOI] [PubMed] [Google Scholar]
- 19.Laack NN, O’Neill BP, Ballman KV, et al. ; North Central Cancer Treatment Group and Mayo Clinic . CHOD/BVAM chemotherapy and whole-brain radiotherapy for newly diagnosed primary central nervous system lymphoma. Int J Radiat Oncol Biol Phys. 2011;81(2):476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz C, Scott C, Sherman W, et al. . Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996;14(2):556-564. [DOI] [PubMed] [Google Scholar]
- 21.Langner-Lemercier S, Houillier C, Soussain C, et al. . Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arellano-Rodrigo E, López-Guillermo A, Bessell EM, Nomdedeu B, Montserrat E, Graus F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70(4):219-224. [DOI] [PubMed] [Google Scholar]
- 23.Choi MK, Kang ES, Kim DW, et al. . Treatment outcome of relapsed/refractory primary central nervous system diffuse large B-cell lymphoma: a single-center experience of autologous stem cell transplantation. Int J Hematol. 2013;98(3):346-354. [DOI] [PubMed] [Google Scholar]
- 24.Ponzoni M, Issa S, Batchelor TT, Rubenstein JL. Beyond high-dose methotrexate and brain radiotherapy: novel targets and agents for primary CNS lymphoma. Ann Oncol. 2014;25(2):316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korfel A, Schlegel U, Herrlinger U, et al. . Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757-1763. [DOI] [PubMed] [Google Scholar]
- 26.Soussain C, Choquet S, Blonski M, et al. . Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II “proof-of-concept” iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121-130. [DOI] [PubMed] [Google Scholar]
- 27.Grommes C, Pastore A, Palaskas N, et al. . Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grommes C, Tang SS, Wolfe J, et al. . Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lionakis MS, Dunleavy K, Roschewski M, et al. . Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833-843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghesquieres H, Chevrier M, Laadhari M, et al. . Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective “proof of concept” phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol. 2019;30(4):621-628. [DOI] [PubMed] [Google Scholar]
- 31.Vu K, Mannis G, Hwang J, Geng H, Rubenstein JL. Low-dose lenalidomide maintenance after induction therapy in older patients with primary central nervous system lymphoma. Br J Haematol. 2019;186(1):180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tun HW, Johnston PB, DeAngelis LM, et al. . Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelov L, Doolittle ND, Kraemer DF, et al. . Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol. 2009;27(21):3503-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Laarhoven HW, Fiedler W, Desar IM, et al. . Phase I clinical and magnetic resonance imaging study of the vascular agent NGR-hTNF in patients with advanced cancers (European Organization for Research and Treatment of Cancer Study 16041). Clin Cancer Res. 2010;16(4):1315-1323. [DOI] [PubMed] [Google Scholar]
- 35.Gregorc V, Santoro A, Bennicelli E, et al. . Phase Ib study of NGR-hTNF, a selective vascular targeting agent, administered at low doses in combination with doxorubicin to patients with advanced solid tumours. Br J Cancer. 2009;101(2):219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. 2006;12(1):175-182. [DOI] [PubMed] [Google Scholar]
- 37.Kunz J, Krause D, Kremer M, Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J Neurochem. 1994;62(6):2375-2386. [DOI] [PubMed] [Google Scholar]
- 38.Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58(3):367-378. [PubMed] [Google Scholar]
- 39.Desar IM, van Herpen CM, van Asten JJ, et al. . Factors affecting the unexpected failure of DCE-MRI to determine the optimal biological dose of the vascular targeting agent NGR-hTNF in solid cancer patients. Eur J Radiol. 2011;80(3):655-661. [DOI] [PubMed] [Google Scholar]
- 40.Mosli HH, Dennis A, Kocha W, Asher LJ, Van Uum SH. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin A in healthy subjects. J Clin Endocrinol Metab. 2012;97(9):E1731-E1735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.