Abstract
Acute graft-versus-host disease (aGVHD) is a life-threatening complication after allogeneic hematopoietic cell transplantation (allo-HCT). Despite the use of prophylactic immunosuppression including calcineurin inhibitors, antimetabolites, antithymocyte globulin, or posttransplant cyclophosphamide, patients still develop severe aGVHD. In particular, patients with glucocorticoid-refractory GVHD (SR-GVHD) have a dismal prognosis with a low 1-year post–allo-HCT survival rate. Most classical drugs used to prevent or treat aGVHD target 1 specific pathway such as calcineurin inhibitors or mammalian target of rapamycin inhibitors, or they interfere with fast-dividing activated cells (eg, methotrexate, mycophenolate, and cyclophosphamide). In contrast to these drugs, inhibition-of-signaling molecules, used by multiple immune cells and critical for signal transduction of multiple proinflammatory cytokines, could be more efficacious at blocking GVHD. Ruxolitinib blocks Janus kinases 1 and 2, which are required to mediate the downstream signaling of multiple cytokine receptors. Recently, a multicenter phase 3 clinical trial showed that ruxolitinib led to significant improvements in efficacy outcomes compared to best available therapy, which will lead to a paradigm shift in the treatment of SR-GVHD.
Introduction
Systemic glucocorticoids are the standard of care for the initial treatment of grade II-IV acute graft-versus-host disease (aGVHD).1 However, many patients with aGVHD do not respond to glucocorticoids, and 6-month survival rates among glucocorticoid-refractory (SR) patients are ∼50% with long-term survival rates of only 5% to 30%.2 There were no US Food and Drug Administration (FDA)–approved medications for SR-aGVHD for several decades, and there was a paucity of well-controlled phase 2/3 clinical trial results to support the use of alternative therapies.3-5 Cytokines play a major role in aGVHD and a common denominator of many cytokines is the downstream activation of Janus kinases 1 and 2 (JAK1/2). Therefore, inhibition of JAK1/2 was tested as an approach to interfere with aGVHD in different mouse models and found to have activity.6-8 This led to further functional testing in preclinical models showing that JAK1/2 inhibition reduced major histocompatibility complex class II (MHC-II) expression on antigen-presenting cells9 and decreased migration of neutrophils toward mesenteric lymph nodes.10 In parallel, patients who had failed multiple previous therapies for aGVHD were treated with ruxolitinib in an individualized therapy approach showed encouraging response rates.11 Based on these findings, prospective trials were initiated including a single-arm phase 2 study (REACH-1) showing activity of ruxolitinib and leading to FDA approval.12 To clarify the question of whether ruxolitinib was superior to best available therapy (BAT), a multicenter randomized phase 3 trial was performed (REACH-2).13 Here, we review the development of ruxolitinib in SR-aGVHD, culminating with the results of the successful phase 3 randomized trial.
Preclinical findings and rationale for the clinical development of ruxolitinib
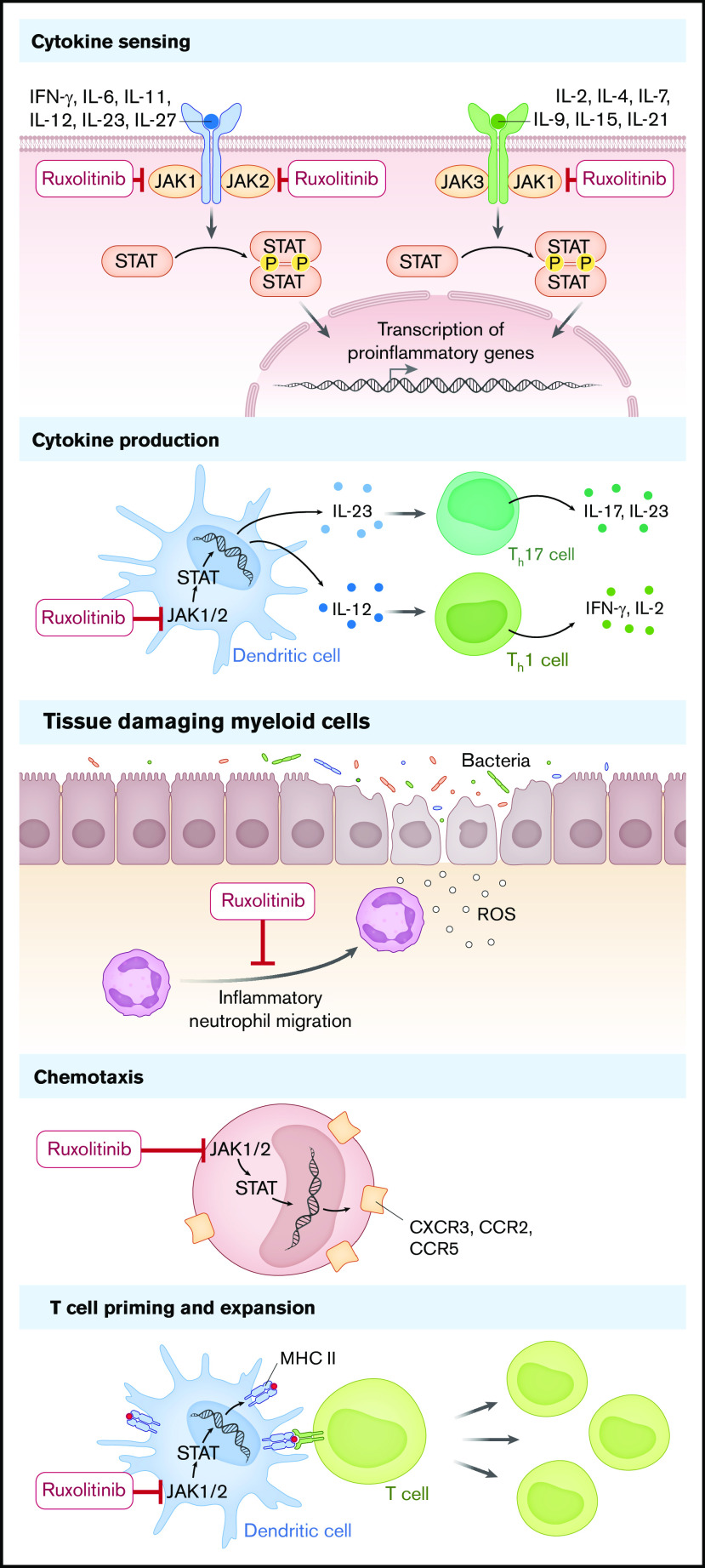
JAK1/2 inhibition may interfere with different levels of immune activation and different phases of aGVHD. In the early phase of aGVHD, the release of tissue damage–related molecules acting as danger-associated molecular patterns promotes GVHD including, besides others, hyaluronic acid, HMGB-1, adenosine triphosphate,14 S100 protein,15 and uric acid.16 Damage of barrier tissues leads to drainage of pathogen-associated molecular patterns into the microenvironment, which also triggers GVHD. Recently, strategies using antibacterial antibodies to eliminate invading bacteria have been tested in preclinical models.17 The invading bacteria cause recruitment of neutrophil granulocytes that promote local tissue damage by release of reactive oxygen species18 and migration to mesenteric lymph nodes where they present antigen to incoming T cells.10 This initial phase of GVHD requires inflammatory neutrophil migration, which is inhibited when JAK1/2 is blocked.10 The different levels of immune cell activation contributing to aGVHD, which are targeted by ruxolitinib, are illustrated in Figure 1.
Figure 1.
The proposed mechanism of action of ruxolitinib in aGVHD. Ruxolitinib interferes with cytokine sensing, cytokine production, recruitment of tissue-damaging myeloid cells, chemotaxis, MHC expression, and T-cell priming. IFN, interferon; IL, interleukin; P, phosphate; ROS, reactive oxygen species; Th17, T helper 17 cell.
In the second phase, T-cell priming, costimulation, and cytokines are crucial for the activation and survival of donor T cells that later cause GVHD. Donor T cells are primed more effectively when MHC-II is upregulated, which is blocked by JAK1/2 inhibition.9 In agreement, ruxolitinib blocked maturation of human dendritic cells in vitro.19 Interleukin-2 (IL-2) receptor common γ-chain (CD132) cytokines IL-2, IL-7, and IL-15 provide T-cell survival signals. Antibody-based inhibition of the common γ-chain reduced GVHD in the mouse model by reducing downstream signals in T cells including JAK3.20 Additionally, interferon-γ signaling is reduced when JAK1/2 is blocked, which reduced GVHD in the mouse model.6
The third phase of GVHD is characterized by the expansion of donor T cells and organ damage. Ruxolitinib was shown by independent groups to reduce T-cell expansion in vivo while allowing for expansion of regulatory T cells.6-8 Besides its effects on immune cells, topical ruxolitinib was shown to protect skin follicular stem cells in mice.21 Conversely, topical corticosteroids applied for treatment of skin inhibited skin stem cells and niche preadipocytes.21 Different JAK inhibitors interfere with immune function as reviewed in Schroeder et al22 and Chao.23
These findings were partly reported in parallel to the clinical development of ruxolitinib and further supported its use for SR-GVHD.
Clinical trials and retrospective case studies
Following the description of the activity of therapeutic JAK1/2 blockade in experimental rodent models,6-8 the first 6 patients who were treated with ruxolitinib for acute (n = 4) or chronic (n = 2) SR-GVHD were reported.7 All 6 patients with advanced disease responded and 1 of these 6 patients achieved a complete remission (CR). Since then, numerous retrospective studies reported the activity of ruxolitinib in SR-aGVHD, but only a few included a meaningful number of patients. Table 1 summarizes these retrospective studies. Patients with either chronic or acute SR-GVHD are often mixed in the same reports, and some reports also mix adult and pediatric patients. The largest retrospective study, by Zeiser et al,11 included 54 patients with aGVHD. In this series, 42% of patients (n = 18) treated with ruxolitinib had a documented infectious event. Infectious events were significantly more common among patients treated for acute SR-GVHD than in patients treated for chronic SR-GVHD (P < .005). Among patients treated for acute SR-GVHD, both viral (n = 11) and bacterial (n = 10) events were frequently encountered. Overall, in these retrospective analyses summarized in Table 1, the CR rates were 22% to 69% among the patients but caution should be stressed given the heterogeneity of treated patients (some having received >3 lines before ruxolitinib).
Table 1.
Retrospective analyses
| No. overall | No. with SR-aGVHD | CR, n (%) | ORR, % | Comment | Reference |
|---|---|---|---|---|---|
| 95 | 54 | 25 (46.3) | 81.5 | Multicenter United States and European Union; previous lines 3 (1-7) | 11 |
| 43 | 19 | 12 (63) | 84 | 25 | |
| 23 | 23 | 5 (22) | 69.5 | Multicenter Spain Grupo Español de Trasplante Hematopoyético; previous lines 3 | 26 |
| 75 | 32 | 20 (63) | 75 | Single center: adults and children; median follow-up, 28 mo | 27 |
| 22 | 13 | 4 (31) | 77 | Pediatrics | 28 |
| 13 | 11 | 4 (36) | 45 | Pediatrics | 29 |
| 29 | 13 | 9 (69) | 84 | Pediatrics | 30 |
CR, complete remission; ORR, overall response rate.
The REACH-1 trial was the first formal open-label, phase 2 study (ClinicalTrials.gov identifier NCT02953678) that led to FDA approval in the United States.12 Patients aged ≥12 years with grades II to IV SR-aGVHD were eligible. The primary end point was overall response rate (ORR) at day 28; the key secondary end point was duration of response (DOR) at 6 months.
In this first prospective multicenter, open-label, single-cohort, phase 2 trial, patients were recruited at 26 medical centers across 17 US states. Patients underwent their first hematopoietic cell transplantation from any donor source for hematologic malignancies, had evidence of myeloid engraftment,16 and received no more than 1 systemic treatment in addition to corticosteroids for treatment of aGVHD. Patients received a starting oral dose of ruxolitinib at 5 mg twice daily, with an option to increase to 10 mg twice daily after 3 days in the absence of cytopenias.
Seventy-one patients were treated and most had grade III/IV GVHD (N = 48 patients [67.6%]) at enrollment. At day 28, 39 patients (54.9% [95% confidence interval (CI), 42.7% to 66.8%]) had an overall response, including 19 (26.8%) with CR responses. Best ORR at any time was 73.2% (complete response, 56.3%). Responses were observed across all target organs (skin [61.1%], upper [45.5%] and lower [46.0%] gastrointestinal tract, and liver [26.7%]). Median DOR was 345 days. Overall survival (OS) estimate at 6 months was 51.0%. The most common treatment-emergent adverse events were anemia (64.8%), thrombocytopenia (62.0%), hypokalemia (49.3%), neutropenia (47.9%), and peripheral edema (45.1%).
The median (range) follow-up interval was 156 days. Subgroup analysis of baseline characteristics demonstrated that the day 28 response was associated with aGVHD grade at enrollment; no other significant associations were observed for other factors evaluated (day 28 response grade II vs grade III/IV aGVHD; odds ratio, 0.15; P = .0042). The 12-month cumulative incidence rate for nonrelapse mortality (NRM) was 52.9%.
On 24 May 2019, the FDA approved ruxolitinib for SR-aGVHD.24 For the purposes of establishing efficacy, the FDA analysis only included patients who (a) progressed after 3 days of treatment with 2 mg/kg methylprednisolone (MP) per day (equivalent), (b) did not improve after 7 days of treatment with 2 mg/kg MP per day (equivalent), (c) progressed to a new organ after treatment with 1 mg/kg MP per day (equivalent) for skin and upper gastrointestinal GVHD, or (d) recurred during or after a steroid taper. Additionally, patients were excluded if they had received a systemic treatment other than corticosteroids for aGVHD. Using these parameters, the final population for the FDA efficacy analysis included 49 patients. Additional follow-up through at least day 180 was requested by the FDA to establish durability of the responses with additional evaluations performed weekly for 4 weeks and every 28 days thereafter, including days 100, 180, and 365. The safety population included all 71 patients treated. The FDA adjudicated the root cause of death. Within 30 days of the last dose of ruxolitinib, 21 patients (30%) died of GVHD, 2 (3%) died of infection, none died of relapse, and none died of an adverse reaction to ruxolitinib. An adverse reaction resulting in treatment discontinuation occurred in 31% of patients. The most common adverse reaction leading to treatment discontinuation was infection (10%). The most common adverse reactions (≥10%) leading to dose interruption or dose reduction were infection, thrombocytopenia, and neutropenia. Adverse events included infections, bleeding, thrombosis, relapse, and graft failure. Infection of any type was reported in 78% of patients (grades III-V in 62%). The most common infections were sepsis (25%) and cytomegalovirus infections (20%). Hemorrhage was reported in 49% of patients (grades III-V in 20%). The benefit/risk assessment of the FDA is summarized in Table 2.
Table 2.
FDA benefit/risk assessment
| Dimension | Evidence | Conclusions |
|---|---|---|
| Condition | SR-aGVHD potentially lethal | Poor prognosis of SR-aGVHD |
| Current option | No approved therapies; drugs often used off-label with low evidence of efficacy | Clinical medical need |
| Benefit | In NCT02953678, day 28 ORR was 57% and DOR 0.5 mo based on the analysis of 49 patients | The magnitude of ORR and DOR supports the use of ruxolitinib |
| Risks | Safety analysis included 71 patients; fatal infection occurred in 14% and fatal hemorrhage in 4%; the most common AEs were expected | Potential risks of cytopenias, infections, and hemorrhage can be mitigated |
Adapted from Table 7 in Przepiorka et al24 with permission.
AE, adverse event; DOR, duration of response.
The REACH-2 trial13 was a phase 3, multicenter, open-label, randomized trial comparing efficacy and safety of oral ruxolitinib (10 mg twice daily) with BAT, in patients with steroid-refractory aGVHD after allogeneic stem cell transplantation (ClinicalTrials.gov number NCT02913261). The primary end point was ORR at day 28, defined as the proportion of patients who achieved a complete response or partial response when compared with baseline organ staging without the use of additional systemic therapy for aGVHD. The key secondary end point was durable ORR at day 56, defined as the proportion of patients in each treatment group with response at day 28 that was maintained at day 56. Other secondary end points included duration of response, best overall response (complete or partial response up to day 28 before start of additional systemic therapy for aGVHD), failure-free survival, and NRM.
Eligible patients were aged ≥12 years and recipients of allogeneic stem cell transplantation (any donor source, any stem cell source) who developed grade II-IV steroid-refractory aGVHD requiring systemic immunosuppressive therapy. Steroid-refractory was defined as progressing based on organ assessment after at least 3 days of systemic steroid therapy, with or without calcineurin inhibitors; lack of response after 7 days; or failure during steroid taper.
Patients were randomized (1:1) to ruxolitinib or BAT for up to 24 weeks, stratified by baseline aGVHD grade (II vs III vs IV). Ruxolitinib was given orally at a dose of 10 mg twice daily. Tapering of ruxolitinib was permitted after day 56 for responding patients. The type of BAT was chosen by the investigator at time of randomization from the following options: antithymocyte globulin, extracorporeal photopheresis, mesenchymal stromal cells, low-dose methotrexate, mycophenolate mofetil, mammalian target of rapamycin inhibitors (everolimus or sirolimus), etanercept, or infliximab. Crossover from BAT to ruxolitinib was permitted if patients did not respond at day 28 or if they lost their response thereafter and required additional systemic therapy and did not have signs of chronic GVHD.
The primary study objective was met: the ORR at day 28 was statistically significantly higher with ruxolitinib than with BAT (62.3% vs. 39.4%; odds ratio, 2.64; 95% CI, 1.65-4.22; P < .001). The complete response rate was 34.4% and 19.4%, respectively. ORR was highest in patients with grade II (75.5% vs. 50.9%) and III (56.3% vs. 37.5%) aGVHD at baseline in the ruxolitinib and BAT groups, respectively; however, the odds ratio for ORR with ruxolitinib compared with BAT was highest in patients with grade IV aGVHD at baseline (53.3% vs. 23.3%; odds ratio, 3.76). The key secondary objective was also met: the rate of durable overall response at day 56 was statistically significantly higher with ruxolitinib (39.6% vs. 21.9%; odds ratio, 2.38; 95% CI, 1.43-3.94; P < .001). Best overall response was 81.8% with ruxolitinib and 60.6% with BAT (odds ratio, 3.07; 95% CI, 1.80-5.25; P < .001). Median failure-free survival with ruxolitinib was statistically significantly longer than with BAT (4.99 months vs 1.02 months; hazard ratio, 0.46; 95% CI, 0.35-0.60; P < .001), and the cumulative incidence of failure events at 1 month was lower with ruxolitinib than with BAT (18.5% vs 49.1%) and remained lower at all time points up to 18 months (61.0% vs 81.8%). For NRM, the cumulative incidence of events at 18 months was 49.4% with ruxolitinib and 50.8% with BAT.
No difference in OS between the ruxolitinib and BAT arms of the REACH-2 trial was found. However, OS was not the primary objective of the study, and the study was not powered to detect a difference in OS. In the REACH-2 study, ORR was used as surrogate end point. The median OS was 11.1 months vs 6.5 months in the ruxolitinib and control groups, respectively, which indicates a trend towards better survival in the ruxolitinib group.
The most common adverse events (any grade and grade III or higher) up to day 28 were thrombocytopenia and anemia. The incidence of relapse of the underlying disease was not different in the ruxolitinib compared to the BAT group.13 Infections of severity grade III up to day 28 occurred in 22.4% and 18.7% of patients, respectively. The safety profile of ruxolitinib was consistent with the safety profile of ruxolitinib found in the REACH-1 trial. Although 38% of patients required ruxolitinib dose modifications, the number of patients who discontinued ruxolitinib due to adverse events was low (11%). Infection rate, which is particularly relevant in SR-aGVHD, was generally similar with ruxolitinib and BAT.3-5 The incidence of cytomegalovirus infection was 25.7% in the ruxolitinib group, which might be seen higher than the 12.7% reported in the REACH-1 trial, at a first glance but not statistically different from BAT (25.7% vs 20.7%). A total of 72 patients (47.4%) and 77 patients (51.3%) died by the cutoff date in the ruxolitinib and BAT arms, respectively, including 43 (28.3%) and 36 (24.0%) during the randomized treatment period (median, 63 days vs 29 days for ruxolitinib vs BAT). Most deaths were attributed to aGVHD (34 [22.4%] and 37 [24.7%], respectively).
Lessons from the REACH trials and conclusions
Despite numerous phase 2 trials and 2 previous randomized phase 3 trials for acute SR-GVHD, no treatment has demonstrated superiority over other treatments, and, with the exception of ruxolitinib, no new drugs have been approved either as first- or second-line treatment for aGVHD in the last 30 years.3-5 The REACH-2 trial thus represents the first successful randomized trial in this setting, a strong argument for a practice change with ruxolitinib now being considered the gold standard in acute SR-GVHD.13 The median OS was not different between the ruxolitinib and the control group, possibly because of the relatively short follow-up.13 Whether crossover from BAT to ruxolitinib, which occurred in over 50%,13 can fully explain this lack of survival advantage will need longer follow-up. The long-term follow-up of the REACH-2 trial will also enable chronic GVHD estimates and long-term infectious-risk evaluation. Another important conclusion from the study is that treatment of a disease with such complex and multifaceted pathophysiology as GVHD might be most successful with agents that have broad biological “reach” into multiple pathways that contribute to the disease.
Finally, this trial included mainly adult patients: only 9 patients (2.9%) were 12 to 18 years old. Whether ruxolitinib response rates will be similar in a pediatric population warrants further studies, but data from retrospective studies summarized in Table 1 are encouraging.
Footnotes
Data-sharing requests may be e-mailed to the corresponding authors, Robert Zeiser (robert.zeiser@uniklinik-freiburg.de) and Gérard Socié (gerard.socie@aphp.fr).
Authorship
Contribution: R.Z. and G.S. wrote the manuscript together.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Zeiser, Department of Hematology, Oncology and Stem Cell Transplantation, University Medical Center Freiburg, D-79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de; and Gérard Socié, Hematology-Transplantation, Hospital St. Louis, AP-HP, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: gerard.socie@aphp.fr.
References
- 1.Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socié G, Milpied N, Yakoub-Agha I, et al. Long-term follow-up of a phase 3 clinical trial of inolimomab for the treatment of primary steroid refractory aGVHD. Blood Adv. 2019;3(2):184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socié G, Vigouroux S, Yakoub-Agha I, et al. A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD. Blood. 2017;129(5):643-649. [DOI] [PubMed] [Google Scholar]
- 5.Macmillan ML, Couriel D, Weisdorf DJ, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109(6):2657-2662. [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Ziga ED, Ritchey J, et al. IFNγR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120(19):4093-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832-3842. [DOI] [PubMed] [Google Scholar]
- 8.Carniti C, Gimondi S, Vendramin A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740-3749. [DOI] [PubMed] [Google Scholar]
- 9.Stickel N, Hanke K, Marschner D, et al. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT signaling in dendritic cells after stem cell transplantation. Leukemia. 2017;31(12):2732-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hülsdünker J, Ottmüller KJ, Neeff HP, et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood. 2018;131(16):1858-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiser R, von Bubnoff N, Butler J, et al. ; REACH2 Trial Group . Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm K, Ganesan J, Müller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;12:1434-1438. [DOI] [PubMed] [Google Scholar]
- 15.Reinhardt K, Foell D, Vogl T, et al. Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J Immunol. 2014;193(7):3355-3365. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic D, Ganesan J, Bscheider M, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210(10):1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hülsdünker J, Thomas OS, Haring E, et al. Immunization against poly-N-acetylglucosamine reduces neutrophil activation and GVHD while sparing microbial diversity. Proc Natl Acad Sci USA. 2019;116(41):20700-20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab L, Goroncy L, Palaniyandi S, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648-654. [DOI] [PubMed] [Google Scholar]
- 19.Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192-1202. [DOI] [PubMed] [Google Scholar]
- 20.Hechinger AK, Smith BA, Flynn R, et al. Therapeutic activity of multiple common γ-chain cytokine inhibition in acute and chronic GVHD. Blood. 2015;125(3):570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi S, Hashimoto D, Hayase E, Teshima T. Topical ruxolitinib protects LGR5+ stem cells in the hair follicle and ameliorates skin graft-versus-host disease [abstract]. Biol Blood Marrow Transplant. 2016;22(suppl 3):S21-S22. [Google Scholar]
- 22.Schroeder MA, Choi J, Staser K, DiPersio JF. The role of Janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transplant. 2018;24(6):1125-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao N. Finally, a successful randomized trial for GVHD. N Eng J Med. 2020;382(19):1853-1854. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25(2):e328-e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abedin S, McKenna E, Chhabra S, et al. Efficacy, toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1689-1694. [DOI] [PubMed] [Google Scholar]
- 26.Escamilla Gómez V, García-Gutiérrez V, López Corral L, et al. ; Grupo Español de Trasplante Hematopoyético (GETH) . Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55(3):641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moiseev IS, Morozova EV, Bykova TA, et al. Long-term outcomes of ruxolitinib therapy in steroid-refractory graft-versus-host disease in children and adults. Bone Marrow Transplant. 2020;55(7):1379-1387. [DOI] [PubMed] [Google Scholar]
- 28.González Vicent M, Molina B, González de Pablo J, Castillo A, Díaz MÁ. Ruxolitinib treatment for steroid refractory acute and chronic graft vs host disease in children: clinical and immunological results. Am J Hematol. 2019;94(3):319-326. [DOI] [PubMed] [Google Scholar]
- 29.Khandelwal P, Teusink-Cross A, Davies SM, et al. Ruxolitinib as salvage therapy in steroid-refractory acute graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2017;23(7):1122-1127. [DOI] [PubMed] [Google Scholar]
- 30.Uygun V, Karasu G, Daloğlu H, et al. Ruxolitinib salvage therapy is effective for steroid-refractory graft-versus-host disease in children: a single-center experience. Pediatr Blood Cancer. 2020;67(4):e28190. [DOI] [PubMed] [Google Scholar]