Abstract
Background
Artificial meniscal scaffolds are being developed to prevent development of osteoarthritis after meniscectomy. Previously, it was reported that 3-dimensional (3D) anatomic scaffolds loaded with connective tissue growth factor (CTGF) and transforming growth factor β3 (TGF- β3) achieved meniscal regeneration in an ovine model. This was a relatively short-term study (3 months postoperative), and outcome analyses did not include magnetic resonance imaging (MRI).
Purpose
To evaluate long-term outcome of meniscal replacement with growth factor–laden poly-ε-caprolactone (PCL) scaffolds.
Study Design
Controlled laboratory study.
Methods
Anatomically shaped ovine meniscal scaffolds were fabricated from PCL with a 3D printer based on MRI data. Skeletally mature sheep (N = 34) were randomly allocated to 3 groups: scaffold without growth factor (0-μg group), scaffold with CTGF microspheres (μS) (5 μg) + TGF-β3 μS (5 μg) (5-μg group), and scaffold with CTGF μS (10 μg) + TGF-β3 μS (10 μg) (10-μg group). Unilateral medial meniscal replacement was performed. Animals were euthanized at 6 or 12 months. Regenerated meniscus, articular cartilage status, and synovial reaction were evaluated quantitatively with gross inspection, histology, and MRI. Kruskal-Wallis and Dunn tests were used to compare the 3 groups.
Results
Remnants of the PCL scaffold were evident in the 6-month specimens and were decreased but still present at 12 months in most animals. There were no significant differences among groups in gross inspection, histology, or MRI for either meniscal regeneration or articular cartilage protection. All experimental groups exhibited articular cartilage degeneration as compared with control (nonoperated). In terms of synovitis, there were no clear differences among groups, suggesting that growth factors did not increase inflammation and fibrosis. MRI revealed that meniscal extrusion was observed in most animals (82.7%).
Conclusion
Previously, the combination of CTGF and TGF-β3 was shown to stimulate mesenchymal stem cells into a fibrochondrocyte lineage. CTGF and TGF- β3 did not aggravate synovitis, suggesting no adverse response to the combination of 3D-printed PCL scaffold combined with CTGF and TGF- β3. Further work will be required to improve scaffold fixation to avoid meniscal extrusion.
Clinical Relevance
A significant advantage of this technique is the ability to print custom-fit scaffolds from MRI-generated templates. In addition, average-size menisci could be printed and available for off-the-shelf applications. Based on the 1-year duration of the study, the approach appears to be promising for meniscal regeneration in humans.
Keywords: meniscus, 3D printed scaffolds, growth factors, ovine knee
Since the meniscus plays a pivotal role in joint homeostasis, meniscectomy is a well-established risk factor for osteoarthritis.5,25 There is a critical unmet need for meniscal replacement implants. Allograft meniscal transplantation is suitable for certain patients after complete meniscectomy, but donor-recipient size match and tissue availability limit its application.28 Tissue-engineering approaches may allow novel solutions to meniscal replacement. Combinations of cells, biomaterials, and appropriate signaling molecules may allow formation of an organized structure with appropriate biomechanical properties that approximate the native meniscus. There are currently 2 artificial meniscal scaffolds available for clinical use. One is a biological scaffold, the Collagen Meniscus Implant (Stryker),40 while the other is a synthetic scaffold (Actifit; Orteq Limited)39 fabricated from polyurethane and polycaprolactone and approved for use in the European Union but not the United States.
Additive manufacturing techniques (3-dimensional [3D] printing) may allow creation of meniscal scaffolds based on a patient’s magnetic resonance imaging (MRI) or other imaging data, providing the advantage of patient-specific implants. 3D-printed meniscal scaffolds could also be used to produce average-size grafts that could be available for off-the-shelf applications and offer an appropriate microarchitectural environment for connective tissue progenitor cells and appropriate biomechanical properties to optimize the 3D orientation of extracellular matrix deposition while facilitating cellular infiltration. Previously, it was reported that 3D-printed anatomic meniscal scaffolds loaded with poly(lactic-co-glycolic acid) (PLGA) microspheres (μS) encapsulated with connective tissue growth factor (CTGF) and transforming growth factor β3 (TGF- β3) achieved meniscal regeneration in an ovine model, as shown by biomechanical and histological evaluation.19 However, this was a relatively short-term study, and outcome analyses did not include MRI or quantitative histological analyses. The purpose of the present study was to evaluate the longer-term outcome of meniscal replacement with growth factor–laden poly-ε-caprolactone (PCL) scaffold.
METHODS
Study Design
Anatomically shaped ovine meniscal scaffolds were fabricated from PCL (100-μm fibers; 100- to 200- μm channels). Skeletally mature sheep (n = 42) were used in this study (Figure 1). Eight sheep were eliminated from analysis owing to complications such as osteonecrosis, nonunion, and infection. Consequently, we analyzed 34 sheep with a mean age of 2.4 years (range, 1–4 years) and mean weight of 55.3 kg (range, 42.0–75.9 kg). They were randomly allocated to 3 groups: growth factor–free scaffold (0-μg group: n = 6 at 6 months, n = 5 at 12 months), growth factor– containing scaffold with CTGF μS (5 μg) + TGF-β3 μS (5 μg) (5-μg group: n = 8 at 6 months, n = 8 at 12 months), and growth factor–containing scaffold with CTGF μS (10 μg) + TGF-β3 μS (10-μg group: n = 6 at 6 months) (Table 1). These doses of growth factors encapsulated in μS were selected from our previous works19 to provide sufficient bioactivities while maintaining the structural integrity of scaffolds. The body weight and age were not significantly different among the 3 groups. Unoperated contralateral limbs served as controls. A single additional animal underwent sham surgery consisting of arthrotomy, followed by osteotomy, to evaluate the effect of surgical approach.
Figure 1.
Study design. Skeletally mature sheep (n = 42) were randomly allocated to 3 groups: (1) 0-μg group: scaffold without growth factor; (2) 5-μg group: scaffold with CTGF μS (5μg) + TGF-β3 μS (5 μg); (3) 10-μg group: scaffold with CTGF μS (10μg) + TGF-β3 μS (10 μg). Eight animals were eliminated because of complications, and 34 animals were analyzed. The contralateral knee served as control. Animals were euthanized at either 6 or 12 months after surgery. CTGF, connective tissue growth factor; TGF-β3, transforming growth factor β3; μS, microspheres.
TABLE 1.
MRI Score for Regenerated Meniscusa
| Meniscal Position | Signal Intensity | Fibrocartilage | Meniscal Size | Capsular Healing | Meniscal Morphology | Total score |
|---|---|---|---|---|---|---|
| 6 mo | ||||||
| Control (n = 10) | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 μg (n = 5) | 1.8 ± 0.8 | 1.8 ± 0.8 | 0 | 0.6 ± 0.9 | 1.4 ± 0.5 | 5.6 ± 2.6b |
| 5 μg (n = 6) | 1.5 ± 1.1 | 1.7 ± 0.5 | 0 | 0 | 1 ± 0.6 | 4.2 ± 2.0c |
| 10 μg (n = 5) | 1 ± 0.7 | 2.0 ± 1.0 | 0 | 0 | 1.6 ± 0.6 | 4.6 ± 1.7b |
| 12 mo | ||||||
| Control (n = 9) | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 μg (n = 5) | 1.0 ± 1.4 | 2.0 ± 1.4 | 0 | 0 | 1.5 ± 0.7 | 4.5 ± 3.6b |
| 5 μg (n = 8) | 1.3 ± 1.3 | 1.4 ± 0.8 | 0 | 0 | 0.9 ± 1.1 | 3.6 ± 2.7b |
Values are presented as mean ± SD. P value was calculated with the Kruskal-Wallis test, followed by post hoc Dunn multiple-comparison tests. MRI, magnetic resonance imaging.
P < .01, vs the control group.
P < .05, vs the control group.
Design and Synthesis of 3D-Printed PCL Anatomic Meniscal Scaffolds Loaded With CTGF and TGF- β3
Anatomically correct meniscal scaffolds were fabricated with 3DBioplotter (Envision TEC) as previously described.18,19 Briefly, the anatomic contour of the medial meniscus of skeletally mature sheep was captured by multislice laser scanning and reconstructed by computer-aided design. PCL (molecular weight, ~65,000; Sigma) was molten at 120°C and dispensed, following the layer path dictated by 3D design of internal microstructures. Microstrands (300 μm) and microchannels (100 μm) were determined to be optimal to approximate the mechanical properties of the native meniscus.19 Recombinant human CTGF and TGF-β3 were encapsulated in 50:50 and 75:25 PLGA μS, respectively, with double emulsion.20 Two PLGA ratios were applied to yield controlled CTGF and TGF-β3 release as described previously.19 PLGA μS (5 or 10 μg/mL) containing CTGF or TGF-β3 were then incorporated onto scaffold microstrands via ethanol treatment as reported previously.38 To recapitulate the fibrocartilage distribution in native meniscus, CTGF-encapsulated μS were applied to the microchannels in the scaffold’s outer/middle zones, whereas TGF-β3– encapsulated μS were applied to the inner/middle zones. This technique enables the scaffolds to sustain release of CTGF and TGF-β3 up to 42 days in vitro.19
Surgical Procedure
All study procedures were approved by the Cornell University Institutional Animal Care and Use Committee. Sheep were operated unilaterally in the supine position under general anesthesia. A lateral arthrotomy was performed, and the patella was subluxated medially. To expose the medial meniscus, a sagittal-plane medial femoral condylar osteotomy was performed with an oscillating saw from the medial side of the intercondylar notch toward the distal medial femoral metaphysis.14 All the associated joint ligaments were preserved. A subtotal meniscectomy was performed by resecting all but 10% of the meniscal outer zone, and both anterior and posterior roots of the meniscus remained intact. The resulting meniscal defect was immediately grafted with an anatomically correct meniscal scaffold. The scaffold was sutured to the peripheral remaining meniscus and capsule with a braided permanent suture (2–0 Ethibond Excel). On the basis of our experience, we found that the anatomic shape and contour of meniscus were almost identical among animals regardless of age. However, there was a slight variance in the anterior-posterior length. Thus, we prepared multiple scaffolds with the same design but various lengths (small, medium, and large) and selected the implant that best fit the specific animal at the time of surgery. The medial femoral condyle was repaired with two 4.5 mm–diameter lag screws. The joint capsule and subcutaneous tissue were closed separately with continuous absorbable suture. The skin was stapled closed and covered with a sterile bandage (Figure 2; Video Supplement, available online). The operated sheep were confined to small pens (4 × 4 ft2) to minimize sudden or extended movements. Pain medication (narcotics and nonsteroidal anti-inflammatory drugs) was administered for a minimum of 2 weeks postoperatively. Upon complete condylectomy healing on radiographs (approximately 2 months), sheep were group housed in a small paddock (6 sheep per 12 3 12 ft2) for 1 week for acclimation and then transferred to a large paddock (96 3 96 ft2) until euthanasia. Animals were euthanized at 6 or 12 months postoperatively.
Figure 2.
Surgery and postoperative radiograph. (A) Surgical procedure. Medial femoral condyle osteotomy (a). Total medial meniscectomy (b). Transplantation of artificial meniscal scaffold (yellow arrow) (c). Fixation of medial femoral condyle with cannulated cancellous screw (white arrow) (d). (B) Radiograph at 2 months: anterior-posterior (a) and lateral (b). After confirmation of bone healing, sheep were allowed free paddock exercise. AP, anteroposterior; Lat, lateral.
Magnetic Resonance Imaging
After euthanasia, the lower extremities were removed and stored on ice, and MRI was performed within 24 hours. All images were acquired on a clinical 3-T scanner (GE Healthcare) with an 8-channel phased array transmitreceive knee coil (Invivo). Two-dimensional fast spin echo proton density scans were acquired in the coronal and sagittal planes to assess morphologic appearance of the knee and meniscus. Acquisition parameters were as follows: echo time = 24 ms, repetition time = 4000 ms, echo train length = 12, field of view = 12 cm, acquisition matrix = 512 × 480, receiver bandwidth = ±62.5 kHz, number of excitations = 2, slice thickness = 1.3 mm, slice spacing = 0.0 mm. The images were evaluated to assess healing of the scaffold to the joint capsule, scaffold integrity and position, and newly formed tissue in the scaffold (Appendix Table A1, available in the online version of this article).35 The articular surfaces of the medial femoral condyle and tibial plateau were evaluated with a modified Noyes score (Appendix Table A2, available online).12,29 MRI scoring was done in a blinded fashion (H.G.P.).
Macroscopic and Histological Analysis
The joints were dissected, and macroscopic findings were scored in a blinded manner by 2 individuals (L.A.F. and S.A.R.) (Appendix Table A3, available online). Meniscal, articular cartilage, and synovial tissues were collected. The specimens were fixed in 10% neutral buffered formalin for 24 hours and decalcified with Immunocal (Decal). After embedding in paraffin, the tissues were sectioned to 5 mm and stained with safranin O and hematoxylin and eosin. Imaging of histological sections was performed with bright-field microscopy on a Nikon E800 microscope. For semiquantitative scoring, we developed a meniscal regeneration score based on a modified Pauli score with modifications for use in ovine meniscal tissue (Appendix Table A4, available online).34 This meniscal histological score was composed of 8 subcategories (size, meniscal morphology, integrity, integration to the capsule, cellularity, cell morphology, collagen organization, and matrix staining).30,34 The first 4 criteria analyzed the whole meniscus, and the final 4 analyzed 3 microscopic subsections. The final score was then summed. There was near-perfect interrater reliability for each histological score component and total histological score between 2 observers according to the Landis and Koch criteria (Appendix Table A5, available online).17
The Osteoarthritis Research Society International (OARSI) histological score was used for articular cartilage grading (Appendix Table A6, available online; modified Mankin score).21 We divided the articular surface into 3 zones and scored each. The most severe lesion was scored by 5 subcategories to determine stage. The percentage of affected surface area was scored as grade. The final score was calculated by multiplying stage and grade and adding up the 3 zones. For evaluation of synovitis, the OARSI histopathologic synovial scoring system was applied (Appendix Table A7, available online).21 Each sample was scored in 4 locations with 10× and 20× magnification, and the average total score was recorded. Histological scoring was done in a blinded fashion by 2 individuals (T.J.U., B.C.). Blinding was maintained throughout the scoring process to prevent bias.
Statistical Analysis
Data are shown as mean ± SD. Statistical analysis was performed with the Kruskal-Wallis test, followed by post hoc analysis with Dunn multiple-comparison tests and with a Spearman signed rank test for correlation analysis with GraphPad Prism (v 7.03; GraphPad Software Inc). The interobserver reliability of histological scoring was examined by calculating the intraclass correlation coefficient with SPSS Statistics (v 20; IBM). Statistical significance was defined as P < .05.
RESULTS
Gross Inspection
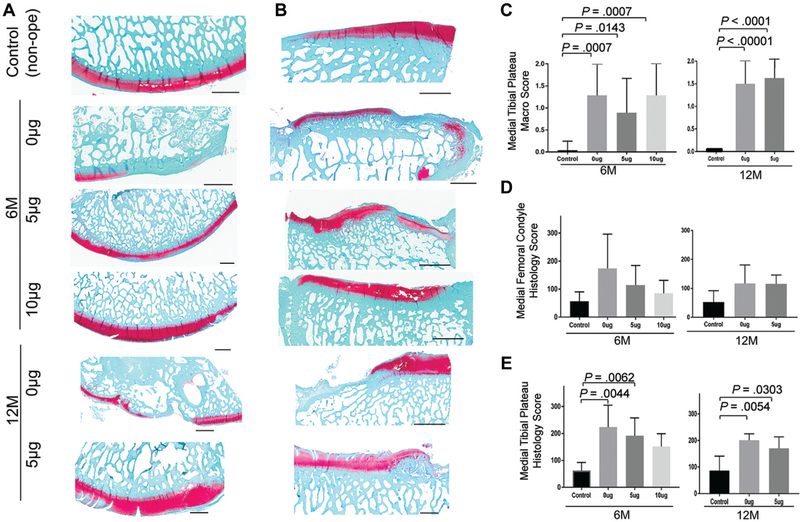
There were significant differences between the 3 experimental groups and the control group based on gross inspection and histology scores, but no significant difference was found among the 3 experimental groups (Figure 3). There was variability in the size and position of the meniscal scaffold, with some degree of extrusion of the scaffold in the majority of animals. Remnants of the PCL scaffold were evident in the 6-month specimens and were decreased but still present at 12 months in most animals. There was a variable degree of degenerative changes in the medial compartment. Macroscopic grading of the articular surfaces demonstrated significant differences between the 3 experimental groups and the control group. There was thinning and loss of articular cartilage on the medial femoral condyle and medial tibial plateau, with osteophytes along the joint margin. Sham operation (skin incision and medial femoral condylectomy) did not seem to have a significant effect on articular cartilage (Appendix Figure A1, available online). Articular cartilage on the tibial plateau generally showed more advanced degenerative changes as compared with the femoral surface (Figure 4). There was mild synovitis in some knees, generally localized around the intercondylar area.
Figure 3.
Macro- and microscopic evaluation of regenerated medial meniscus. (A) Representative pictures of gross specimens and histology stained with safranin O / fast green. (B) Macro score (0, best; 3, worst). (C) Histology score (0, best; 45, worst). The data are presented as mean ± SD. P value was calculated with the Kruskal-Wallis test, followed by post hoc Dunn multiple-comparison tests.
Figure 4.
Macro- and microscopic evaluation of articular cartilage. Representative pictures of (A) medial femoral condyle and (B) medial tibial plateau. Scale bar = 2 mm. (C) Macroscopic score of medial tibial plateau (0, best; 2, worst). Histology score of (D) medial femoral condyle and (E) medial tibial plateau (0, best; 360, worst). The data are presented as mean ± SD. P value was calculated with the Kruskal-Wallis test, followed by post hoc Dunn multiple-comparison tests.
Histological Evaluation
Meniscal Scaffold
Microscopic analysis demonstrated that both growth factor–containing groups showed more regenerated tissue than the 0-μg group (Figure 3). Histological evaluation demonstrated formation of fibrous, fibrovascular, and fibrochondrocytic matrix in the meniscal scaffold, with variable amounts of proteoglycan-rich matrix. There was variability in the overall organization of the newly formed matrix. The 5-μg group had the best meniscal matrix organization, with proteoglycan-rich fibrocartilage-like tissue. To a lesser degree, the 10-μg group also yielded proteoglycan-rich regenerated meniscus at 6 months, and the 0-μg group contained only disorganized fibrous tissue, even at 12 months (matrix staining subcategory: 0 μg, 7.0 ± 2.1; 5 μg, 3.9 ± 1.6; 10 μg, 4.9 ± 2.0; 0-μg group vs 5-μg group, P < .05). Small remnants of the PCL material were still evident at 6 months.
Articular Cartilage Degeneration
Histological evaluation demonstrated degenerative cartilage changes, with cartilage fibrillation and thinning, loss of matrix staining, and subchondral bone thickening (Figure 4). Although all operated groups showed worse scores than the control in the femur, there were no statistically significant differences among all groups. In contrast, there were significant differences in the OARSI histological score between the controls and the 0- and 5-μg groups on the tibial side at 6 and 12 months.
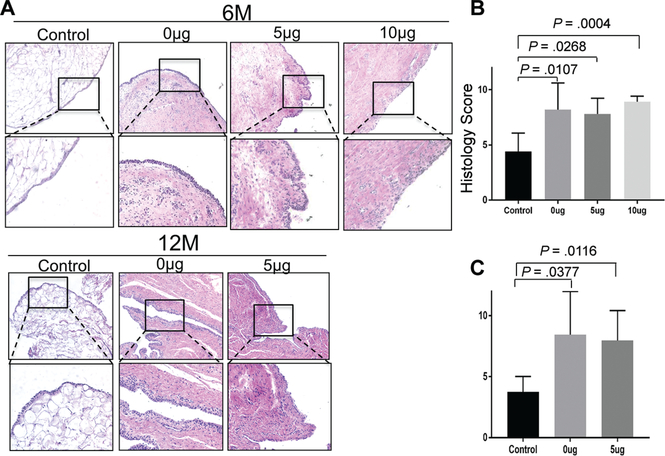
Synovitis
Synovial membrane samples from each treatment group demonstrated occasional lymphocytes in the absence of plasma cells or neutrophils (Figure 5). Numerous peripheral vascular structures were present in the experimental groups, with increased collagen matrix in the subsynovial tissue as compared with the control group. These findings were still observed at 12 months. There was a higher (more abnormal) histological score for each of the 3 treatment groups as compared with the control group. There was no significant difference in mean histological synovitis score among the 3 scaffold groups.
Figure 5.
Microscopic evaluation of synovitis. (A) Representative photomicrographs of synovium stained with hematoxylin and eosin at 6 and 12 months. Histology score at (B) 6 months and (C) 12 months (0, best; 12, worst). The data are presented as mean ± SD. P value was calculated with the Kruskal-Wallis test, followed by post hoc Dunn multiple-comparison tests.
MRI Analysis
Meniscal Scaffold
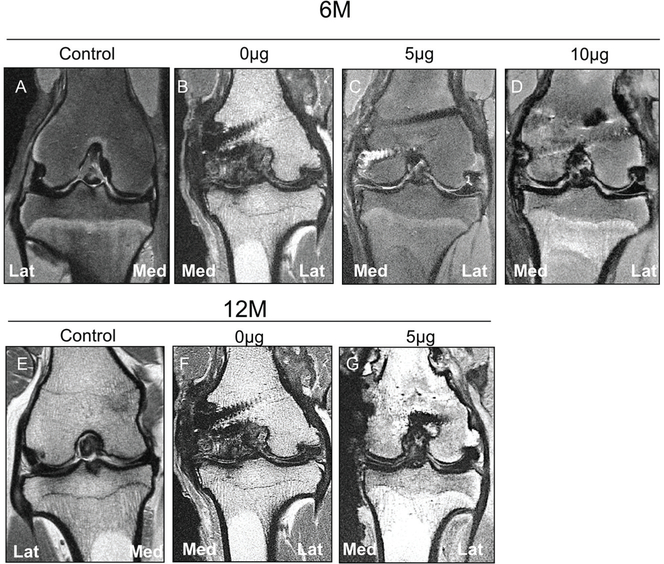
The MRI evaluation found that the 5-μg group tended to display lower scores than the 0and 10-μg groups for signal intensity and morphology. Meniscal extrusion (defined as the outer edge of the meniscus extending beyond the tibial margin) was observed in most animals (82.7%). The score followed a similar trend of macroscopic and histological scores (Figure 6, Table 1). All experimental groups displayed differences in the MRI meniscal score as compared with the control specimens, but differences were not detected among the experimental groups at 6 or 12 months.
Figure 6.
Magnetic resonance imaging evaluation of regenerated medial meniscus. Representative coronal 2-dimensional fast spin echo images of the sheep knee with a meniscal construct implanted in the medial compartment evaluated at 6 and 12 months postoperatively. (A) Control specimen at 6 months displays normal appearance of articular cartilage and meniscal position, with no extrusion present. (B) Specimen with 0-μg construct at 6 months demonstrates marked loss of the articular cartilage with bone-on-bone apposition in the medial compartment. There is extrusion of the meniscal remnant into the gutter, and hypertrophic scarring of the adjacent capsule is present. Osteophytes are located laterally, but the position of the lateral meniscus is preserved. (C) Specimen with 5-μg meniscal construct at 6 months demonstrates mild extrusion of the construct but maintains relatively low signal intensity similar to the native lateral meniscus. Superficial chondral wear is present. (D) Specimen with 10-μg meniscal construct at 6 months demonstrates slight extrusion of the construct with moderate signal hyperintensity within the implant, truncation of the free edge of the construct, and superficial chondral wear. (E) Control specimen at 12 months displays normal articular cartilage and meniscal position. (F) Specimen with 0-μg meniscal construct at 12 months demonstrates severe medial compartment osteoarthritis with marked subchondral sclerosis in the condyle, extrusion of the meniscal remnant into the gutter, and hypertrophic scarring of the medial capsule. Lateral compartment osteophytes are present without extrusion of the native meniscus. (G) Specimen with 5-μg construct at 12 months demonstrates no meniscal extrusion with preservation of the hypointense signal in the construct and minimal cartilage loss.
Articular Cartilage Degeneration
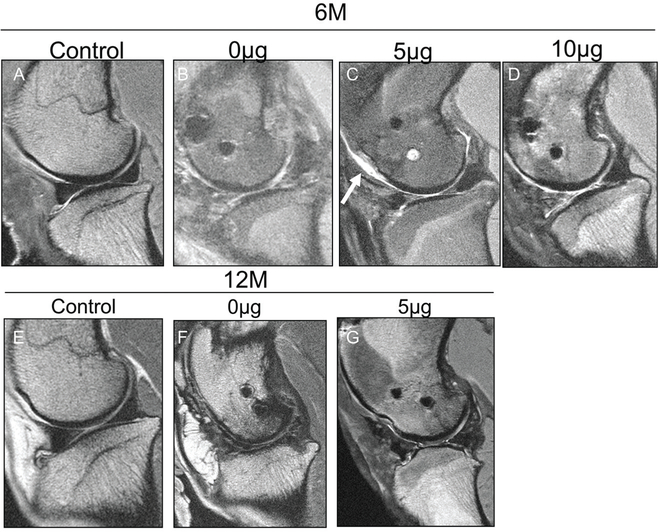
MRI detected significant degeneration of the articular cartilage for each experimental group at each time point as compared with the control group, but differences were not detected among the experimental groups at 6 or 12 months (Figure 7, Table 2).
Figure 7.
Magnetic resonance imaging evaluation of articular cartilage of medial compartment. Representative sagittal 2-dimensional fast spin echo images of the sheep knee with a meniscal construct implanted in the medial compartment evaluated at 6 and 12 months postoperatively. (A) Control specimen at 6 months displays preservation of the articular cartilage with a hypointense meniscus and no extrusion present. (B) Specimen with 0-μg meniscal scaffold at 6 months displays marked hyperintensity of the meniscal scaffold with extrusion of the anterior horn relative to the tibia, encased in thickened synovium. (C) Specimen with 5-μg meniscal scaffold at 6 months displays truncation of the free edge of the anterior and posterior horns, but the meniscus is not extruded and there is relative preservation of the hypointense signal. Chondral wear appears more severe over the trochlea (arrow). (D) Specimen with 10-μg scaffold at 6 months demonstrates marked hyperintensity in the anterior horn but no extrusion and with superficial chondral wear. (E) Control specimen at 12 months displays preservation of the articular cartilage with no meniscal extrusion present. (F) Specimen with 0-μg scaffold at 12 months demonstrates areas of full-thickness loss of articular cartilage and hyperintensity of the meniscal construct but only minimal anterior extrusion. (G) Specimen with 5-μg scaffold at 12 months demonstrates moderate extrusion of the anterior horn with truncation of the free edge and preservation of articular cartilage over the condyle, but there is focal wear over the tibia.
TABLE 2.
MRI Score for Cartilage Degenerationa
| Modified Noyes Score |
|||||
|---|---|---|---|---|---|
| Medial Femoral Condyle | Medial Tibial Plateau | Osteophytes | Subchondral Sclerosis | Total Score | |
| 6 mo | |||||
| Control (n = 10) | 0.5 ± 1.1 | 0 | 0.4 ± 0.5 | 0 | 0.9 ± 1.5 |
| 0 μg (n = 5) | 8 ± 2.7 | 9 ± 2.2 | 1 ± 0.7 | 0.4 ± 0.6 | 18.4 ± 3.0b |
| 5 μg (n = 6) | 6.7 ± 2.6 | 5.3 ± 3.8 | 1.8 ± 0.4 | 1.2 ± 1.0 | 15.0 ± 7.2b |
| 10 μg (n = 5) | 4.8 ± 3.3 | 5.6 ± 2.6 | 1.6 ± 0.5 | 0.6 ± 0.5 | 12.6 ± 6.1b |
| 12 mo | |||||
| Control (n = 9) | 0 | 0 | 0.3 ± 0.5 | 0 | 0.3 ± 0.5 |
| 0 μg (n = 5) | 5.6 ± 2.3 | 5.6 ± 2.3 | 2 ± 0 | 1.6 ± 0.8 | 14.8 ± 4.8c |
| 5 μg (n = 8) | 4.6 ± 2.4 | 4.3 ± 2.4 | 1.3 ± 0.7 | 0.6 ± 0.9 | 10.8 ± 5.5b |
Values are presented as mean ± SD. P value was calculated with the Kruskal-Wallis test, followed by post hoc Dunn multiple-comparison tests. MRI, magnetic resonance imaging.
P < .01, vs the control group.
P < .001, vs the control group.
DISCUSSION
In this study, we found that a PCL meniscal replacement scaffold supported variable regeneration of fibrous and fibrochondrocytic tissue. There were no significant differences in the regenerated meniscal tissue between growth factor–containing and growth factor–free meniscal scaffolds in histological, macroscopic, or MRI evaluations. However, qualitative histological analysis demonstrated superior meniscal matrix organization in the 5-μg group, with proteoglycan-rich fibrocartilage-like tissue. To a lesser degree, the 10-μg group also showed proteoglycan-rich regenerated meniscus at 6 months, whereas the 0-μg group contained only disorganized fibrous tissue, even at 12 months. Among treatment groups, no difference was found in cartilage degeneration, synovitis, or meniscal extrusion at both time points. These findings suggest that the growth factor–containing meniscal scaffold may lead to better meniscal regeneration in terms of both the quantity and the quality of regenerated tissue.
The goal of meniscal transplantation or replacement is to restore the role of the meniscus in load transmission across the tibiofemoral compartment, thereby decreasing contact stress on the articular surfaces and possibly restoring the role of the meniscus as a secondary stabilizer for the anterior cruciate ligament. Despite varying degrees of new tissue formation in the meniscal scaffold, we still found progressive degenerative changes in all study groups in the medial compartment, especially on the tibial plateau. Although not statistically significant, there was a trend toward less degenerative change in the growth factor–containing groups as compared with the 0-μg group. This finding is consistent with the effect in the regenerating meniscus and suggests that CTGF and TGF-β3 release could have an anabolic effect on articular cartilage. We cannot directly comment on any chondroprotective effect of our scaffold, as we did not include an untreated, meniscectomy group. Rather, our purpose was to evaluate the ability of this novel cytokine-containing scaffold to support neomeniscal formation. Although there was variable formation of fibrovascular and fibrochondrocytic tissue, this regenerated tissue did not protect the articular surfaces.
The lack of a “chondroprotective” effect may have been due to several factors. First, we found varying degrees of meniscal extrusion. Extrusion of the meniscus has been reported as a complication after meniscal transplantation in clinical studies, and biomechanical studies have shown that meniscal extrusion leads to a decreased tibiofemoral contact area and consequently to increased tibiofemoral contact pressure.23 It has also been reported that extrusion of the meniscus is associated with the development of osteoarthritis.8
Reconstitution of the microstructure, composition, and material properties of the meniscus is a challenging tissue-engineering problem. An appropriate scaffold, cells, and signals for the cells are required. The nanostructure, chemistry, and degradation profile of the scaffold material will affect tissue formation. Furthermore, a source of pluripotent cells that can differentiate into the appropriate meniscal fibrochondrocyte phenotype is required. The proposed mechanism of action with our scaffold is that the CTGF and TGF-β3 would attract synovial-derived cells into the scaffold and induce their differentiation into a meniscal cell phenotype. In this study, we did not look at the release kinetics of the 2 growth factors in vivo. Despite the inevitable variance in the release rate between in vitro and in vivo environments owing to local enzymatic and physiochemical activities, the release pattern—fast release of CTGF and slower release of TGF-β3—is expected to be the same in vivo, as the release rate is primarily regulated by hydrolysis of PLGA. We also did not perform any biomechanical analysis, but histology demonstrated that the microstructure of the regenerative menisci was inferior to that of native meniscal tissue.
CTGF, also known as CCN2 (cysteine-rich angiogenic protein 61, CTGF, nephroblastoma overexpressed), is a multicellular protein of the CCN family of extracellular matrix–associated heparin-binding proteins.4 CTGF has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair.9 Regarding the meniscus, CTGF may have a pivotal role in regulating type I collagen synthesis.6 TGF-β also has several regulatory activities, such as stimulating collagen and proteoglycan production, improving meniscal cell attachment in repaired meniscal tissue, and encouraging matrix contraction.31,32 There are 3 isoforms, TGF-β1, TGF-β2, and TGF-β3, which have been well characterized in mammalian tissues. In particular, TGF-β3 appears to be important for soft tissue healing, as it has been associated with promoting dermal wound and tendon healing without scar formation in a fetal and neonatal environment.10 It was reported that the delivery of TGF-β3 with an injectable calciumphosphate matrix at the supraspinatus tendon footprint improved enthesis healing with new bone formation and improved collagen organization in addition to increased fibrocartilage tissue.16 The combination of CTGF and TGF-β3 could induce fibrochondrogenic differentiation of human mesenchymal stem cells (MSCs).19 Our results suggest that these 2 cytokines may be able to induce local stem cells to differentiate into meniscus-like cells, with resulting new tissue formation within the PCL scaffold.
Various materials have been used for fabrication of meniscal scaffolds, including bioabsorbable synthetic polymers such as polyurethane, polyglycolic acid, polylactic acid, and PCL. A significant advantage of synthetic scaffolds would be the relative ease of manipulation of their biomechanical properties. Specifically, PCL scaffolds have shown promise as a tissue-engineering solution for the meniscus and may provide an appropriate microarchitectural environment for meniscal cells.7,22 These nanofibers may have the advantage of providing adequate initial biomechanical properties to protect the regenerating meniscal tissue and to organize and optimize the 3D orientation of extracellular matrix deposition in the circumferential direction while facilitating cellular infiltration through the scaffold’s fibers.19
The sheep model for meniscal replacement is a challenging model. Exposure of the tibiofemoral compartment to allow precise scaffold placement and suturing is difficult. We used a medial femoral condylar osteotomy approach. Cutting of the bone and overlying hyaline cartilage may have changed the “biologic milieu” in the joint. However, there were generally only mild degenerative changes at the site of the condylectomy. An alternative approach is to release the medial collateral ligament with a bone block from the medial femoral epicondyle.2 However, in our experience, securing the meniscus to the most axial aspect of the joint is more difficult in this approach as compared with the condylar osteotomy approach used here.
Another limitation of the sheep model is the inability to control postoperative weightbearing. In human patients, minimal weightbearing loads are permitted in the first 4 to 6 weeks. The immediate loading in the sheep model may have contributed to extrusion of the scaffold. A further limitation of our model is the lack of secure fixation of the anterior and posterior horns to bone. Secure horn fixation of a meniscal transplant is critical for function, and the combination of immediate weightbearing and lack of direct horn fixation may have contributed to meniscal extrusion and compromised the biomechanical function of our scaffold. Other investigators using the sheep model have reported meniscal extrusion.15 We have redesigned the scaffold to contain extensions attached to the anterior and posterior horns to allow fixation into bone tunnels to anchor the horns.
Most tissue-engineering approaches use cell-seeded scaffolds. Various cell types have been investigated in preclinical animal models, including meniscal cells, articular chondrocytes, and MSCs. MSCs are the most frequent cell type studied in basic and clinical studies, owing to their multipotency and proliferation potential, as well as the fact that they are easily accessible from peripheral connective tissues, such as bone marrow,3,27 periosteum, adipose, and the synovium.26,36 Additionally, they have been shown to have a good safety profile.37 Zhang et al41 recently demonstrated that 3D-printed PCL scaffolds containing bone marrow–derived MSCs attained successful meniscal regeneration as compared with cell-free scaffolds in a rabbit total meniscectomy model. MSCs could differentiate into meniscus-like cells in the appropriate microenvironment and also produce a variety of cytokines to promote tissue regeneration. However, a significant limitation of cell-seeded scaffolds is the added cost and complexity associated with maintaining cell viability. Furthermore, the current regulatory environment in the United States does not permit cell culture to isolate and expand the small number of stem cells in adipose tissue and bone marrow. Thus, a strategy that exploits endogenous resident cells in the joint will be more readily translated to clinical application. Endogenous cells that repopulate the scaffolds could come from synovium, synovial fluid, peripheral blood, and/or bone marrow. It has been shown that MSCs increase in number in synovial fluid after meniscal injury.24 To expose the medial compartment, an osteotomy was performed, which would likely induce a large number of bone marrow MSCs into the joint cavity that may have contributed to healing to some extent.
The synovial membrane may play a key role in meniscal regeneration, as the host cells that populate the meniscal allograft likely originate in the synovium.13 Additionally, synovial blood vessels provide necessary nourishment for the regenerating meniscus. We did not see any evidence of an excessive synovial inflammatory or immunologic response to the PCL scaffold material. We also did not observe excessive subsynovial fibrosis, which was a concern given the profibrotic effect of CTGF.1 The lack of difference in histological score among the synovial membranes of the 3 treatment groups suggested that the addition of CTGF and TGF-β3 to the scaffold did not induce an inflammatory or fibrous response as compared with the no–growth factor meniscal scaffold. Rather, the presence of occasional lymphocytes was consistent with a response to surgical intervention. These data indicate that meniscal transplantation with PCL, CTGF, and TGF-β3 is safe and would not incite an excessive inflammatory or fibrotic response in the synovium.
Accurate histological scores allow careful and rigorous tissue evaluation, which will contribute to ultimate translation to human clinical trials. Analysis of larger tissue sections in large animal models presents challenges. We have developed a new scoring system to quantitatively evaluate regenerated meniscus in an ovine model. The results of this study suggest that the histological scoring system is a valid method that correlates with an established MRI scoring system. This scoring system will be useful for assessing meniscal regeneration in other large animals, such as pigs and goats.
There were several limitations in this study. First, our sample size was relatively small. However, in general, large animal studies with sheep or goat models are often underpowered because the number of animals per group (per time point) is often controlled by budgetary and institutional limitations. According to previous studies with ovine knee models, this number ranged from 3 to 8 per experimental group.11,15,33 Thus, we attempted to prepare at least 6 or 7 animals per group and per time point, to be able to conduct statistical analysis. Second, we did not perform histological analysis on the 1 sham surgery animal, as a larger number of animals would need to be studied to draw any conclusions. Additional sham surgery animals are required to address the effect of the surgical approach in this mode. Third, given the insufficient number of animals, we could not prepare a 10-μg group at 12 months. On the basis of our preliminary analysis, we found that 5 μg was more promising than 10 μg at 6 months in terms of meniscal regeneration, so we did not include a 10-μg group at 12 months. Last, biomechanical analysis was not performed to assess the regenerative meniscal tissue. Information about the biomechanical properties of the regenerated meniscus could provide insight into the lack of differences in terms of articular cartilage degeneration and synovitis. Analysis of biomarkers and growth factors in synovial fluids could be avenues for future study. Biomarkers would be useful to assess cartilage degeneration and synovitis. Measuring CTGF and TGF-β3 would provide insight into whether the kinetics of cytokine release from the scaffold is similar to our in vitro data described previously. Despite these limitations, our data suggest that this 3D-printed PCL scaffold supports neotissue formation at 1 year in this challenging intra-articular environment. Further development of this implant will require improved methods for scaffold fixation in the joint and further study to establish whether the addition of CTGF and TGF-β3 to the scaffold is beneficial and, if so, what is the optimal dose.
In conclusion, we evaluated 3D-printed PCL meniscal scaffolds loaded with CTGF and TGF-β3 using a subtotal meniscectomy model in the ovine knee up to 1 year. CTGF and TGF-β3 did not result in excessive synovitis, suggesting that the combination of PCL scaffold and growth factors was safe. MRI examination exhibited extrusion in most of the scaffolds, which may contribute to cartilage degeneration in the treatment groups.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge with thanks the important technical assistance of Ying Liang (Orthopaedic Soft Tissue Research Program, Hospital for Special Surgery) for preparing histological specimens; Sarah Pownder, DVM, PhD (Department of Radiology, Hospital for Special Surgery) for MRI; Michelle Delco, DVM, PhD (Cornell University) for surgery; and Solaiman Tarafder (Columbia University) for preparing scaffolds. The authors also recognize the valuable support of Amir Lebaschi, MD, Colin Danaher, Daniel Nemirov, Samuel Green, and Joo Young Sunwoo (Orthopaedic Soft Tissue Research Program, Hospital for Special Surgery) for histological analysis.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by National Institutes of Health grant R01AR065023. L.A.F. is a consultant for Arthrex, Inc, and a shareholder in Mitogen, Inc. J.J.M. is the founder of Mitogen, Inc. H.G.P. receives National Institutes of Health funding and institutional research support from GE Healthcare, has received consulting fees from Smith & Nephew and GE Healthcare, and has received hospitality payments from DePuy Synthes. S.A.R. is a shareholder in Mitogen, Inc, and Ortho RTI; is a consultant for Flexion Therapeutic; and has received royalties from Zimmer Biomet and fees for services other than consulting from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Footnotes
A Video Supplement for this article is available online.
REFERENCES
- 1.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2010;4(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brzezinski A, Ghodbane SA, Patel JM, Perry BA, Gatt CJ, Dunn MG.The ovine model for meniscus tissue engineering: considerations of anatomy, function, implantation, and evaluation. Tissue Eng Part C Methods. 2017;23(12):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators.J Cell Biochem. 2006;98(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- 4.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41(4):771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–419. [DOI] [PubMed] [Google Scholar]
- 6.Furumatsu T, Kanazawa T, Miyake Y, Kubota S, Takigawa M, Ozaki T. Mechanical stretch increases Smad3-dependent CCN2 expression in inner meniscus cells. J Orthop Res. 2012;30(11):1738–1745. [DOI] [PubMed] [Google Scholar]
- 7.Gao S, Chen M, Wang P, et al. An electrospun fiber reinforced scaffold promotes total meniscus regeneration in rabbit meniscectomy model. Acta Biomater. 2018;73:127–140. [DOI] [PubMed] [Google Scholar]
- 8.Guermazi A, Eckstein F, Hayashi D, et al. Baseline radiographic osteoarthritis and semi-quantitatively assessed meniscal damage and extrusion and cartilage damage on MRI is related to quantitatively defined cartilage thickness loss in knee osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2015;23(12):2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci. 2011;68(19):3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosokawa R, Nonaka K, Morifuji M, Shum L, Ohishi M. TGF-beta 3 decreases type I collagen and scarring after labioplasty. J Dent Res. 2003;82(7):558–564. [DOI] [PubMed] [Google Scholar]
- 11.Kelly BT, Robertson W, Potter HG, et al. Hydrogel meniscal replacement in the sheep knee: preliminary evaluation of chondroprotective effects. Am J Sports Med. 2007;35(1):43–52. [DOI] [PubMed] [Google Scholar]
- 12.Kijowski R, Blankenbaker DG, Davis KW, Shinki K, Kaplan LD, De Smet AA. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250(3):839–848. [DOI] [PubMed] [Google Scholar]
- 13.King D The healing of semilunar cartilages. 1936. Clin Orthop Relat Res. 1990;252:4–7. [PubMed] [Google Scholar]
- 14.Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthritis Cartilage. 2013;21(8):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kon E, Filardo G, Tschon M, et al. Tissue engineering for total meniscal substitution: animal study in sheep model—results at 12 months. Tissue Eng Part A. 2012;18(15–16):1573–1582. [DOI] [PubMed] [Google Scholar]
- 16.Kovacevic D, Fox AJ, Bedi A, et al. Calcium-phosphate matrix with or without TGF-beta3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39(4):811–819. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 18.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014;6(266): 266RA171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120(9):3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage. 2010;18(suppl 3):S80–S92. [DOI] [PubMed] [Google Scholar]
- 22.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structurefunction, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37(1):124–129. [DOI] [PubMed] [Google Scholar]
- 24.Matsukura Y, Muneta T, Tsuji K, Koga H, Sekiya I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin Orthop Relat Res. 2014;472(5):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills PM, Wang Y, Cicuttini FM, et al. Tibio-femoral cartilage defects3–5 years following arthroscopic partial medial meniscectomy. Osteoarthritis Cartilage. 2008;16(12):1526–1531. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa Y, Muneta T, Kondo S, et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage. 2015;23(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa Y, Muneta T, Otabe K, et al. Cartilage derived from bone marrow mesenchymal stem cells expresses lubricin in vitro and in vivo. PLoS One. 2016;11(2):e0148777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noyes FR, Barber-Westin SD. Repair of complex and avascular meniscal tears and meniscal transplantation. J Bone Joint Surg Am. 2010;92(4):1012–1029. [PubMed] [Google Scholar]
- 29.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–513. [DOI] [PubMed] [Google Scholar]
- 30.Ozeki N, Muneta T, Matsuta S, et al. Synovial mesenchymal stem cells promote meniscus regeneration augmented by an autologous Achilles tendon graft in a rat partial meniscus defect model. Stem Cells. 2015;33(6):1927–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11(7–8):1141–1148. [DOI] [PubMed] [Google Scholar]
- 32.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytesin scaffolds. J Orthop Res. 2005;23(5):1184–1190. [DOI] [PubMed] [Google Scholar]
- 33.Patel JM, Merriam AR, Kohn J, Gatt CJ Jr, Dunn MG. Negative outcomes of poly(l-lactic acid) fiber-reinforced scaffolds in an ovine total meniscus replacement model. Tissue Eng Part A. 2016;22(17–18):1116–1125. [DOI] [PubMed] [Google Scholar]
- 34.Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potter HG, Rodeo SA, Wickiewicz TL, Warren RF. MR imaging of meniscal allografts: correlation with clinical and arthroscopic outcomes. Radiology. 1996;198(2):509–514. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8): 2521–2529. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473(7):2316–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng Part C Methods. 2008;14(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer SJ, Saithna A, Carmont MR, Dhillon MS, Thompson P, Spalding T. Meniscal scaffolds: early experience and review of the literature. Knee. 2012;19(6):760–765. [DOI] [PubMed] [Google Scholar]
- 40.Steadman JR, Rodkey WG. Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy. 2005;21(5):515–525. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZZ, Wang SJ, Zhang JY, et al. 3D-printed poly(epsilon-caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution: a 12- and 24-week animal study in a rabbit model. Am J Sports Med. 2017;45(7):1497–1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.