Abstract
Long noncoding RNAs (lncRNAs) and microRNAs (miRNAs) are noncoding RNAs (ncRNAs) that occupy over 90% of the human genome, and their main function is to directly or indirectly regulate messenger RNA (mRNA) expression and participate in the tumorigenesis and progression of malignances. In particular, some lncRNAs can interact with miRNAs as competing endogenous RNAs (ceRNAs) to modulate mRNA expression. Accordingly, these RNA molecules are interrelated and coordinate to form a dynamic lncRNA-mediated ceRNA regulatory network. Mounting evidence has revealed that lncRNAs that act as ceRNAs are closely related to tumorigenesis. To date, numerous studies have established many different regulatory networks in hepatocellular carcinoma (HCC), and perturbations in these ceRNA interactions may result in the initiation and progression of HCC. Herein, we emphasize recent advances concerning the biological function of lncRNAs as ceRNAs in HCC, with the aim of elucidating the molecular mechanism underlying these HCC-related RNA molecules and providing novel insights into the diagnosis and treatment of HCC.
Keywords: Hepatocellular carcinoma, Long noncoding RNA, MicroRNA, Competing endogenous RNA, Function, Mechanism
Core tip: Mounting evidence has revealed that long noncoding RNA (lncRNA)-mediated competitive endogenous RNA (ceRNA) regulatory network plays a crucial role in tumorigenesis. To date, numerous studies have established many different regulatory networks in hepatocellular carcinoma (HCC), and perturbations in these ceRNA interactions may result in the initiation and progression of HCC. Herein, we emphasize recent advances concerning the biological function of lncRNAs as ceRNAs in HCC, with the aim of elucidating the molecular mechanism underlying these HCC-related RNA molecules and providing novel insights into the diagnosis and treatment of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a malignant tumor with high morbidity and mortality worldwide[1]. However, the pathogenesis of HCC remains elusive. Although great progress has been made in the diagnosis and treatment of HCC in recent years, the overall and long-term effects of treatment in patients with advanced HCC are poor. Therefore, in-depth studies are needed to explore the mechanisms underlying HCC occurrence and development, which will contribute to the development of effective diagnostic biomarkers and therapeutic targets for HCC.
Protein-coding genes account for less than 2% of the human genome, while most of the genome is composed of genes that are transcribed into noncoding RNAs (ncRNAs)[2]. NcRNAs are divided into long noncoding RNAs (lncRNAs), small ncRNAs, and intermediate-sized ncRNAs by length[3]. LncRNAs have been identified as key regulators of transcription and translation and are involved in a variety of biological processes by regulating gene expression[4]. MicroRNAs (miRNAs) are small ncRNAs that interact with the 3’-untranslated region (3’-UTR) of target mRNAs to facilitate their degradation or inhibit their translation. MiRNAs play a critical role in tumorigenesis and tumor cell proliferation, migration, and invasion[5]. LncRNAs and miRNAs are regulatory ncRNAs, and dysregulation of lncRNAs or miRNAs is involved in tumor initiation and progression either via the activation or inhibition of target genes[6].
Existing evidence indicates that there are interactions among RNA molecules, such as lncRNAs and miRNAs[7], miRNAs and mRNAs[8], and lncRNAs and mRNAs[9]; these RNA molecules are interrelated and collaborate to form a dynamic regulatory network of lncRNAs acting as competitive endogenous RNAs (ceRNAs)[10]. The ceRNA mechanism is one of the important ways by which an lncRNA exerts its posttranscriptional gene regulation in the cytoplasm, and perturbations in these ceRNA interactions contribute to tumor initiation and progression. Currently, the identified ceRNAs include protein-coding RNAs (mRNAs) and ncRNAs, such as lncRNAs, pseudogene transcripts, viral RNAs, and circular RNAs (circRNAs). LncRNAs are the main component of the ceRNA network, as they regulate mRNA expression by acting as miRNA sponges. To date, numerous different regulatory networks of lncRNAs acting as ceRNAs in HCC have been established. Accumulating evidence has revealed that lncRNAs acting as ceRNAs play pivotal roles in HCC initiation and progression[11,12]. Herein, we emphasize recent advances concerning the biological function of lncRNAs acting as ceRNAs in HCC, with the aim of elucidating the molecular mechanism underlying these HCC-related RNA molecules and providing novel insights into the diagnosis and treatment of HCC.
MECHANISM OF ACTION OF LNCRNAS INVOLVED IN THE CERNA REGULATORY NETWORK
Theoretically, any RNA molecule with a miRNA binding site can bind to a miRNA to form an intricate ceRNA regulatory network. RNA transcripts in the ceRNA network are in a state of equilibrium under physiological conditions; once perturbed, this will lead to the occurrence of disease[13,14]. In the ceRNA regulatory network, miRNA acts as a bridge between ncRNA and mRNA and negatively regulates the expression of its target mRNA[15]. There is growing evidence that each miRNA can regulate many transcripts. In turn, RNA transcripts with different miRNA response elements (MREs) may also be targets of multiple miRNAs[16]. The multiplicity of targets allows RNA and RNA to interact with each other by competitively binding to a common MRE, and the same MRE is the structural basis for the binding of different RNAs[16].
In addition to directly regulating mRNAs, lncRNAs can also indirectly affect the expression of target genes by sponging miRNAs[10]. Structurally, most lncRNAs are similar to mRNAs, which makes their patterns of gene regulation more diverse and extensive and unaffected by translation[17]. This may be the reason why many lncRNAs can act as ceRNAs to sponge miRNAs to inhibit miRNAs from degrading their target mRNAs. In general, the more miRNA binding sites there are on an lncRNA, the stronger the competition[18]. When lncRNAs are expressed at low levels, they can bind only a few miRNAs, and the remaining miRNAs interact with mRNAs to promote their degradation. In contrast, when lncRNAs are expressed at high levels, they can combine with more miRNAs, thus relieving the inhibitory effect of miRNAs on their target mRNAs. The new regulatory pattern of lncRNA-miRNA-mRNA is an extension of the traditional miRNA-mRNA regulatory model[10].
ROLE OF LNCRNAS AS CERNAS IN HCC
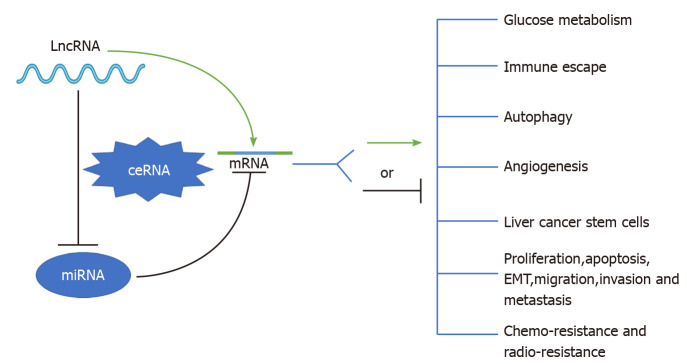
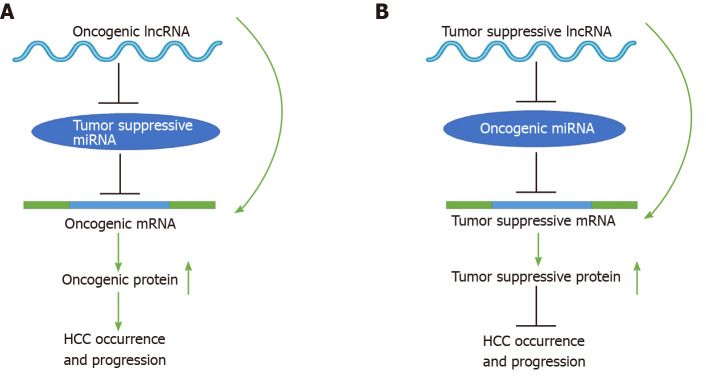
To date, mounting evidence indicates that oncogenic or tumor suppressive lncRNAs can regulate their target genes by acting as ceRNAs to sponge miRNAs[19,20], thereby affecting glucose metabolism, immune escape, autophagy, angiogenesis, liver cancer stem cells (LCSCs), proliferation, apoptosis, epithelial-mesenchymal transition (EMT), migration, invasion, metastasis, chemoresistance, and radioresistance in HCC (Figure 1). Specifically, the majority of the identified lncRNAs exhibit oncogenic properties that function as ceRNAs for tumor suppressive miRNAs, thereby activating the expression of oncogenic mRNAs to promote HCC occurrence and progression (Figure 2A). In addition, some lncRNAs exhibit tumor suppressive properties, acting as ceRNAs for oncogenic miRNAs (oncomiRs), thus upregulating the expression of tumor suppressive targets to inhibit HCC occurrence and progression (Figure 2B). Here, we elucidate the functions of some lncRNA-mediated ceRNA regulatory networks in HCC (Table 1). Note that there are many abbreviations in this paper, so they are listed and expanded in Table 1 below.
Figure 1.
Schematic diagram of the role of long noncoding RNA-mediated competitive endogenous RNA regulatory network in hepatocellular carcinoma. See the text for details. LncRNA: Long noncoding RNA; ceRNA: Competing endogenous RNA; miRNA: MicroRNA; mRNA: Messenger RNA; EMT: Epithelial-mesenchymal transition.
Figure 2.
Schematic diagrams of long noncoding RNA-mediated competitive endogenous RNA regulatory network that mediates the occurrence and progression of hepatocellular carcinoma. See the text for details. LncRNA: Long noncoding RNA; miRNA: MicroRNAs; mRNA: Messenger RNA.
Table 1.
Long noncoding RNA-mediated competitive endogenous RNA network in hepatocellular carcinoma
| LncRNA | Dysregulation | Sponged miRNA(s) | Affected mRNA(s)/ signaling pathway(s) | Biological functions | Ref. |
| RAET1K | Up-regulated | miR-100-5p | LDHA | Enhances HCC glycolysis and progression | Zhou et al[24] |
| TUG1 | Up-regulated | miR-455-3p | HK2 | Promotes HCC glycolysis and metastasis | Lin et al[26] |
| HOTAIR | Up-regulated | miR-130a-3p | HIF1 | Promotes glycolysis | Hu et al[28] |
| miR-218 | Bmi-1 | Promotes HCC cell proliferation | Fu et al[74] | ||
| miR-1 | FOXC1 | Promotes HCC cell proliferation, migration, and invasion | Su et al[75] | ||
| miR-214-3p | FLOT1 | Promotes HCC cell proliferation, migration, and invasion | Liu et al[77] | ||
| miR-23b-3p | ZEB1 | Promotes HCC invasion and metastasis | Yang et al[78] | ||
| MEG3 | Down-regulated | miR-483-3p | ERp29 | Inhibits glycolysis | Li et al[29] |
| miR-9-5p | SOX11 | Promotes HCC cell apoptosis and inhibits cell growth | Liu et al[93] | ||
| miRNA-10a-5p | PTEN | Inhibits HCC cell proliferation, migration, and invasion | Zhang et al[94] | ||
| NEAT1 | Up-regulated | miR-155 | Tim-3 | Facilitates CD8+T cells-mediated immune escape | Yan et al[35] |
| miR-335 | c-Met-Akt pathway | Enhances HCC resistance to sorafenib | Chen et al[130] | ||
| FENDRR | Down-regulated | miR-423-5p | GADD45β | Suppresses Treg-mediated immune escape | Yu et al[38] |
| PVT1 | Up-regulated | miR-365 | ATG3 | Promotes autophagy | Yang et al[44] |
| HNF1A-AS1 | Up-regulated | hsa-miR-30b-5p | ATG5 | Stimulates autophagy | Liu et al[45] |
| CCAT1 | Up-regulated | miR-181a-5p | ATG7 | Induces autophagy | Guo et al[46] |
| Let-7 | HMGA2 and c-Myc | Promotes HCC cell proliferation and migration | Deng et al[81] | ||
| miR-490-3p | CDK1 | Promotes HCC cell proliferation and invasion | Dou et al[82] | ||
| miR-30c-2-3p | CCNE1 | Promotes HCC cell proliferation | Zhang et al[83] | ||
| HCG11 | Up-regulated | miR-26a-5p | ATG12 | Promotes autophagy | Li et al[47] |
| LINC00665 | Up-regulated | miR-186-5p | MAP4K3 | Facilitates autophagy | Shan et al[49] |
| HULC | Up-regulated | miR-107 | SPHK1 | Promotes angiogenesis | Lu et al[52] |
| miR-200a-3p | ZEB1 | Enhances EMT and promotes HCC growth and metastasis | Li et al[65] | ||
| miR-2052 | MET | Promotes HCC cell proliferation, migration, and invasion | Zhang et al[66] | ||
| miR-186 | HMGA2 | Promotes HCC growth and metastasis | Wang et al[68] | ||
| miR-372-3p | Rab11a | Promotes HCC growth and metastasis | Cao et al[71] | ||
| miR-6825-5p, miR-6845-5p, and miR-6886-3p | USP22 | Increases HCC resistance to oxaliplatin | Xiong et al[142] | ||
| MALAT1 | Up-regulated | miR-3064-5p | FOXA1/CD24/Src pathway | Promotes angiogenesis | Zhang et al[53] |
| miR-140 | VEGF-A | Promotes angiogenesis | Hou et al[54] | ||
| miR-124 | PI3K/Akt pathway | Enhances HBx-induced CSC properties | He et al[59] | ||
| miR-216b | HIF-2α | Enhances HCC resistance to 5-FU | Yuan et al[155] | ||
| LINC00488 | Up-regulated | miR-330-5p | TLN1 | Facilitates angiogenesis | Gao et al[55] |
| DANCR | Up-regulated | miR-214, miR-320a and miR-199a | CTNNB1 | Enhances the stemness of HCC cells | Yuan et al[61] |
| ANRIL | Up-regulated | miR-384 | STAT3 | Promotes HCC cell proliferation, migration, and invasion | Ji et al[85] |
| miR-122-5p | N/A | Promotes HCC cell proliferation, metastasis, and invasion | Ma et al[86] | ||
| miR-144 | PBX3 | Promotes HCC cell growth, migration, and invasion | Ma et al[87] | ||
| GAS5 | Down-regulated | miR-21 | PDCD4 and PTEN | Suppresses HCC cell migration and invasion | Hu et al[89] |
| miR-135b | RECK | Inhibits HCC invasion | Yang et al[90] | ||
| miR-1323 | TP53INP1 | Inhibits HCC cell proliferation and invasion and promotes apoptosis | Zhang et al[91] | ||
| miR-222 | N/A | Increases HCC sensitivity to cisplatin | Zhao et al[151] | ||
| ASB16-AS1 | Up-regulated | miR-1827 | FZD4 Wnt/β-catenin pathway | Promotes HCC growth and invasion | Yao et al[97] |
| DSCR8 | Up-regulated | miR-485-5p | FZD7 Wnt/β-catenin pathway | Promotes HCC cell proliferation and cell cycle | Wang et al[99] |
| LINC00662 | Up-regulated | miR-15a, miR-16, and miR-107 | WNT3A Wnt/β-catenin pathway | Promotes HCC growth and metastasis | Tian et al[101] |
| SNHG5 | Up-regulated | miR-26a-5p | GSK3β Wnt/β-catenin pathway | Promotes HCC metastasis and EMT | Li et al[103] |
| SOX9-AS1 | Up-regulated | miR-5590-3p | SOX9 Wnt/β-catenin pathway | Facilitates HCC growth and metastasis | Zhang et al[105] |
| DLGAP1-AS1 | Up-regulated | miR-26a-5p and miR-26b-5p | IL-6 JAK2/STAT3 pathway and CDK8/LRP6 Wnt/β-catenin pathway | Facilitates HCC EMT and progression | Lin et al[109] |
| MIR22HG | Down-regulated | miR-10a-5p | NCoR2 Wnt/β-catenin pathway | Inhibits HCC growth, migration, and invasion | Wu et al[111] |
| PTTG3P | Up-regulated | miR-383 | CCND1/PARP2 and PI3K/Akt pathway | Promotes HCC cell proliferation, migration, and invasion and inhibits apoptosis | Zhou et al[115] |
| DLEU1 | Up-regulated | miR-133a | IGF-1R PI3K/AKT pathway | Promotes HCC cell proliferation, migration, and invasion | Zhang et al[116] |
| TCL6 | Down-regulated | miR-106a-5p | PTEN PI3K/AKT pathway | Inhibits HCC cell proliferation, migration, and invasion | Luo et al[117] |
| CDKN2B-AS1 | Up-regulated | let-7c-5p | NAP1L1 PI3K/AKT/ mTOR pathway | Promote HCC growth and metastasis | Huang et al[118] |
| GAS6-AS2 | Up-regulated | miR-493-5p | OTUB1 PI3K-AKT- FoxO3a pathway | Promotes HCC cell proliferation, migration, and invasion | Liang et al[119] |
| SNHG12 | Up-regulated | miR-199a/b-5p | MLK3 NF-κB pathway | Promotes HCC cell proliferation and tumorigenicity | Lan et al[125] |
| CASC2 | Down-regulated | miR-362-5p | CYLD NF-κB pathway | Inhibits HCC cell migration and invasion | Zhao et al[127]; Ni et al[128] |
| miR-222 | N/A | Enhances HCC sensitivity to cisplatin | Liu et al[150] | ||
| LINC-ROR | Up-regulated | miR-876-5p | FOXM1 | Increases HCC resistance to sorafenib | Zhi et al[134] |
| miR-145 | RAD18 | Enhances radiation resistance of HCC cells | Chen et al[163] | ||
| LINC00160 | Up-regulated | miR-132 | PIK3R3 | Promotes HCC resistance to sorafenib | Zhang et al[136] |
| FOXD2-AS1 | Up-regulated | miR-150-5p | TMEM9 | Facilitates HCC resistance to sorafenib | Sui et al[138] |
| SNHG3 | Up-regulated | miR-128 | CD151 | Promotes HCC resistance to sorafenib | Zhang et al[140] |
| SNHG16 | Up-regulated | miR-140-5p | N/A | Increases HCC resistance to sorafenib | Ye et al[141] |
| let-7b-5p | N/A | Enhances HCC resistance to oxaliplatin | Li et al[149] | ||
| NR2F1-AS1 | Up-regulated | miR-363 | ABCC1 | Enhances HCC resistance to oxaliplatin | Huang et al[144] |
| KCNQ1OT1 | Up-regulated | miR-7-5p | ABCC1 | Increases HCC resistance to oxaliplatin | Hu et al[145] |
| NRAL | Up-regulated | miR-340-5p | Nrf2 | Facilitates HCC resistance to cisplatin | Wu et al[147] |
| LINC01234 | Up-regulated | miR-31-5p | MAGEA3 | Promotes HCC resistance to cisplatin | Chen et al[148] |
| CRNDE | Up-regulated | miR-33a | HMGA2 | Promotes HCC resistance to 5-FU | Han et al[152] |
| KRAL | Down-regulated | miR-141 | Keap1 | Reverses HCC resistance to 5-FU | Wu et al[158] |
| NEAT1_2 | Up-regulated | miR-101-3p | WEE1 | Enhances radio-resistance of HCC cells | Chen et al[160] |
| LINC00473 | Up-regulated | miR-345-5p | FOXP1 | Promotes radio-resistance of HCC cells | Zhang et al[165] |
HCC: Hepatocellular carcinoma; LncRNAs: Long noncoding RNAs; ceRNA: Competitive endogenous RNA; RAET1K: Retinoic acid early transcript 1K; LDHA: Lactate dehydrogenase isoform A; TUG1: Taurine up-regulation gene 1; HK2: Hemikinase 2; HOTAIR: Homeobox transcript antisense RNA; HIF1: Hypoxia-inducible factor 1; Bmi-1:B lymphoma moloney murine leukemia virus insertion region 1; FOXC1: Forkhead box C1; FLOT1: Flotillin 1; ZEB1: Zinc finger E-box binding homeobox 1; MEG3: Maternally expressed gene 3; ERp29: ER protein 29; SOX11: SRY-related HMG-box transcription factor 11; PTEN: Phosphatase and tensin homolog; NEAT1: Nuclear enriched abundant transcript 1; Tim-3: T cell immunoglobulin mucin-3; FENDRR: Fetal-lethal noncoding developmental regulatory RNA; GADD45β: Growth arrest and DNA damage-inducible beta; PVT1: Plasmacytoma variant translocation 1; ATG3: Autophagy related genes 3; HNF1A-AS1: HNF1A antisense RNA 1; ATG5: Autophagy related genes 5; CCAT1: Colon cancer associated transcript 1; ATG7: Autophagy related genes 7; HMGA2: High mobility group AT-hook 2; CDK1: Cyclin-dependent kinase 1; CCNE1: Cyclin E1; HCG11: HLA complex group 11; ATG12: Autophagy related genes 12; LINC00665: Long intergenic non-protein coding RNA 665; MAP4K3: Mitogen-activated protein kinase kinase kinase kinase 3; HULC: Highly upregulated in liver cancer; SPHK1: Sphingosine kinase 1; MET: Hepatocyte growth factor receptor; Rab11a: Member RAS oncogene family; USP22: Ubiquitin-specific peptidase 22; MALAT1: Metastasis-associated lung adenocarcinoma transcript 1; FOXA1: Forkhead box A1; VEGF-A: Vascular endothelial growth factor A; PI3K/Akt pathway: Phosphoinositide-3-kinase/protein kinase B; CSC: Liver cancer stem cell; HIF-2α: Hypoxia-inducible factor 2α; TLN1: Talin 1; DANCR: Differentiation antagonizing non-protein coding RNA; CTNNB1: Catenin beta-1; ANRIL: Antisense noncoding RNA in the INK4 locus; STAT3: Signal transducer and activator of transcription 3; N/A: Not available.; PBX3: Pre-B-cell leukemia homeobox 3; GAS5: Growth arrest-specific 5; PDCD4: Programmed cell death 4; RECK: Cysteine-rich protein with Kazal motifs; TP53INP1:Tumor protein p53-induced nuclear protein 1;ASB16-AS1:ASB16 antisense RNA 1; FZD4: Frizzled 4; DSCR8: Down syndrome critical region 8; FZD7: Frizzled-7; WNT3A:Wingless-type MMTV integration site family 3A; SNHG5: Small nucleolar RNA host gene 5; GSK3β: Glycogen synthase kinase 3β; EMT: Epithelial-mesenchymal transition; SOX9-AS1: SOX9 antisense RNA 1; SOX9: Sex determining region Y-box 9; DLGAP1-AS1: Long noncoding RNA DLGAP1 antisense RNA 1; IL-6: Interleukin- 6; JAK2: Janus kinase 2; CDK8: Cyclin-dependent kinase 8; LRP6: Low-density lipoprotein receptor-related protein 6; MIR22HG: MIR22 host gene; NCoR2: Nuclear receptor corepressor 2; PTTG3P: Pituitary tumor-transforming 3; CCND1: Cyclin D1; PARP2: Poly ADP-ribose polymerase 2; DLEU1: Deleted in lymphocytic leukaemia 1; IGF-1R : Insulin-like growth factor 1 receptor; TCL6: T cell leukemia/lymphoma 6; CDKN2B-AS1: CDKN2B antisense RNA 1; NAP1L1: Nucleosome assembly protein 1 like 1; mTOR: Mammalian rapamycin; GAS6-AS2: Growth arrest specific 6 antisense RNA 2; OTUB1: OTU domain-containing ubiquitin aldehyde-binding protein 1; FOXO3a: Forkhead Box O3a; SNHG12: Small nucleolar RNA host gene 12; MLK3: Mixed-lineage kinase 3; NF-kB: Nuclear factor kappa-B; CASC2: Cancer susceptibility candidate 2; CYLD: Cylindromatosis; LINC-ROR: Intergenic non-protein coding RNA, regulator of reprogramming; FoxM1: Forkhead box M1; RAD18: A RING-type ubiquitin ligase E3; PIK3R3: Phosphoinositide-3-kinase regulatory subunit 3; FOXD2-AS1: FOXD2 adjacent opposite strand RNA 1; TMEM9: Transmembrane protein 9; SNHG3: Small nucleolar RNA host gene 3; SNHG16: Small nucleolar RNA host gene 16; NR2F1-AS1: NR2F1 antisense RNA 1; ABCC1: Multidrug resistance-associated protein 1; KCNQ1OT1: KCNQ1 overlapping transcript 1; NRAL: Nrf2 regulation-associated lncRNA; Nrf2: Nuclear factor erythroid-2-related factor 2; MAGEA3: Melanoma-associated antigen A3; CRNDE: Colorectal neoplasia differentially expressed; KRAL: Keap1 regulation-associated lncRNA; Keap1: Kelch-like ECH-associated protein 1; NEAT1_2: Nuclear enriched abundant transcript 1_2; WEE1: WEE1 G2 checkpoint kinase; FOXP1: Forkhead box protein P1.
Glucose metabolism
The “reprogramming” of glucose metabolism is regarded as a prominent characteristic of cancer cells. A large amount of lactic acid produced by glycolysis forms an inflammatory microenvironment around the tumor, which contributes to tumor cell proliferation, EMT, invasion, metastasis, immune escape, and resistance to chemotherapy and radiotherapy. Existing evidence indicates that aberrant glucose metabolism plays a pivotal role in the invasion and metastasis of HCC[21,22]. The mechanism of abnormal activation of glycolysis in cancer cells is complex, and many studies have confirmed that lncRNAs play a significant role in modulating glycolysis by sponging miRNAs in HCC, among which oncogenic lncRNAs that act as ceRNAs can promote glycolysis. For instance, lactate dehydrogenase isoform A (LDHA), a glycolytic enzyme, can mediate aerobic glycolysis in cancer cells[23]. The lncRNA RAET1K, as a miR-100-5p sponge, can enhance LDHA expression and facilitate hypoxia-induced glycolysis, thereby promoting HCC progression[24]. In addition, hemikinase 2 (HK2) is another glycolytic enzyme related to glycolysis in cancer cells[25], and the lncRNA TUG1 induces glycolysis and promotes HCC metastasis by acting as a ceRNA to enhance HK2 expression by sponging miR-455-3p[26]. Additionally, hypoxia-inducible factor (HIF) 1 has been confirmed to promote aerobic glycolysis in cancer[27], and the lncRNA HOTAIR promotes glycolysis by acting as a ceRNA for miR-130a-3p to increase HIF1 expression in HCC cells[28]. By contrast, tumor suppressive lncRNAs that act as ceRNAs can inhibit glycolysis in HCC. For example, endoplasmic reticulum protein 29 (ERp29), an endoplasmic reticulum protein, and the lncRNA MEG3 are downregulated in high-glucose (HG) HCC cells, while miR-483-3p is upregulated in HG HCC cells. Mechanistically, the overexpression of MEG3 inhibits glycolysis by sponging miR-483-3p to increase ERp29 expression in HCC[29]. Currently, antitumor drugs that target glucose metabolism are being researched and developed; the above findings suggest that the lncRNA-mediated ceRNA network could provide new ideas for inhibiting glycolysis in HCC.
Immune escape
Tumor immune escape refers to the phenomenon that tumor cells can survive and proliferate by escaping immune system-mediated recognition and attack by changing themselves or their tumor microenvironment[30]. Currently, the effectiveness of immunotherapy is limited by tumor immune escape. Thus, an in-depth exploration of the mechanisms of tumor immune escape may provide novel insights into tumor immunotherapy. Current studies have shown that lncRNAs can modulate immune escape in HCC by acting as ceRNAs of miRNAs, among which oncogenic lncRNAs that function as ceRNAs can promote the immune escape of HCC cells. For example, NEAT1, a newly discovered oncogenic lncRNA, is specifically localized in nuclear paraspeckles and participates in paraspeckle formation and the transcriptional regulation of many genes[31,32]. T cell immunoglobulin mucin-3 (Tim-3), an immune checkpoint molecule, can suppress the immune response[33], and the increased expression of Tim-3 within the tumor can inactivate killer T cells, thus preventing the death of tumor cells[34]. Mechanistically, NEAT1 facilitates the CD8+ T cell-mediated immune escape of HCC cells by acting as a ceRNA for miR-155 to enhance Tim-3 expression[35]. Conversely, tumor suppressive lncRNAs that act as ceRNAs can inhibit the immune escape of HCC cells. For instance, GADD45β, a tumor suppressor, is associated with antitumor immune responses[36], and CD4+ T cells lacking GADD45β are less responsive to the stimulation of T cell receptors or inflammatory cytokines[37]. FENDRR, a tumor suppressor lncRNA, upregulates GADD45β by sponging miR-423-5p, thereby suppressing the immune escape of HCC cells[38]. These findings suggest that the lncRNA-mediated ceRNA network is involved in mediating immune evasion in HCC and thus may be a promising therapeutic target for HCC immunotherapy.
Autophagy
Autophagy is closely associated with the development of malignant tumors and can promote tumor survival and proliferation by regulating interactions between the tumor and tumor microenvironment[39]. In HCC, autophagy plays a vital role in tumor immunity, oxidative stress, and the maintenance of hepatic homeostasis and thus participates in HCC initiation and progression and resistance to chemotherapy drugs[40]. Identification of the mechanisms by which autophagy is activated in HCC will help clarify HCC pathogenesis and reveal novel treatments for HCC patients. Numerous investigations have indicated that oncogenic lncRNAs that function as ceRNAs are required for promoting autophagy in HCC. For example, autophagy-related genes 3, 5, 7, and 12 (ATG3, ATG5, ATG7, and ATG12, respectively) are major regulators of the induction of autophagosome formation[41-43]. The lncRNA PVT1 promotes autophagy in HCC by enhancing ATG3 expression by sponging miR-365[44]. The lncRNA HNF1A-AS1 upregulates the expression of ATG5 in HCC by acting as a sponge of hsa-miR-30b-5p, thus stimulating autophagy[45]. The lncRNA CCAT1 serves as a ceRNA for miR-181a-5p to induce autophagy in HCC by enhancing ATG7 expression[46]. The lncRNA HCG11 promotes autophagy in HCC by enhancing ATG12 expression by sponging miR-26a-5p[47]. In addition, mitogen-activated protein kinase kinase kinase kinase 3 (MAP4K3), an upstream kinase of the MAPK pathway, is a key node in the regulation of autophagy[48]. The lncRNA LINC00665 facilitates autophagy by sponging miR-186-5p to enhance MAP4K3 expression[49]. Collectively, these results suggest that the lncRNA-mediated ceRNA network could provide novel treatments for HCC patients.
Angiogenesis
Angiogenesis is responsible for HCC growth, proliferation, invasion, and metastasis[50]. The mechanisms underlying HCC angiogenesis are complex, and exploration of the factors involved in regulating HCC angiogenesis is of great significance for improving antiangiogenic treatments for HCC. Emerging evidence indicates that oncogenic lncRNAs that act as ceRNAs are tightly linked to HCC angiogenesis. For instance, sphingosine kinase 1 (SPHK1), a key metabolic enzyme, is correlated with tumor angiogenesis[51]. A study found that the lncRNA HULC promotes angiogenesis by upregulating SPHK1 in HCC; HULC acts as a ceRNA to increase the expression of transcription factor E2F1 by competitively binding to miR-107 and subsequently results in the activation of the SPHK1 promoter, thus promoting HCC angiogenesis in vivo[52]. In addition, the lncRNA MALAT1 can promote HCC angiogenesis by sponging miR-3064-5p to activate the forkhead box A1 (FOXA1)/CD24/Src pathway[53] or by functioning as a miR-140 sponge to enhance vascular endothelial growth factor A expression[54]. Similarly, LINC00488, another lncRNA, upregulates the expression of talin 1 to facilitate HCC angiogenesis by sponging miR-330-5p[55]. These findings suggest that the lncRNA-mediated ceRNA network may be a promising target for antiangiogenic therapies for HCC.
Liver cancer stem cells
LCSCs exhibit high proliferation, self-renewal, high tumorigenicity, chemoresistance, and radioresistance[56-58], and their abundance is positively associated with the degree of HCC malignancy. Elucidation of the regulatory mechanisms of LCSCs will contribute to our understanding of the pathogenesis of HCC and the identification of novel therapeutic strategies. Existing evidence has shown that oncogenic lncRNAs help sustain cancer stem cell (CSC) traits by acting as ceRNAs for miRNAs to initiate HCC development. For instance, the lncRNA MALAT1 activates the phosphoinositide-3-kinase/protein kinase B (PI3K/Akt) pathway by acting as a miR-124 sponge to enhance HBx-induced CSC properties[59]. In addition, catenin beta-1 (CTNNB1)/β-catenin sustains the stemness properties of LCSCs[60]. In another recent study, it was found that the lncRNA DANCR was highly expressed in HCC tissues and stem-like HCC cells; DANCR can act as a ceRNA to enhance CTNNB1 expression by sponging miR-214, miR-320a, and miR-199a, thereby enhancing the stemness of HCC cells[61]. Thus, the lncRNA-mediated ceRNA network may serve as a potential therapeutic target for LCSCs.
Proliferation, migration, EMT, invasion, and metastasis
The lncRNA-mediated ceRNA regulatory network functions by regulating miRNA target genes: LncRNAs acting as ceRNAs can participate in cell proliferation, apoptosis, migration, EMT, invasion, and metastasis by modulating mRNAs in HCC. Currently, there are many lncRNA-miRNA-miRNA target gene (mRNA) networks reported in HCC. In this review, we provide only a few examples of lncRNA-miRNA-mRNA networks, including those involving oncogenic lncRNAs such as HULC, HOTAIR, CCAT, and ANRIL and tumor suppressive lncRNAs such as GAS5 and MEG3.
LncRNA HULC-miRNA-mRNA: HULC has been identified as a specifically highly expressed lncRNA in HCC[62]. In a recent study, high HULC expression in HCC was significantly connected to increased lymph node metastasis and advanced TNM stage[63]. This finding indicates that HULC can facilitate the proliferation, migration, invasion, and metastasis of HCC cells, leading to the malignant development of HCC. Thus far, it has been reported that HULC may exert its oncogenic function in HCC through diverse molecular mechanisms, of which the HULC-mediated ceRNA network is important. For instance, zinc finger E-box binding homeobox 1 (ZEB1), a key regulator of EMT, contributes to HCC cell invasion and metastasis[64]. As a ceRNA of miR-200a-3p, HULC increases the expression of ZEB1, thereby enhancing EMT and promoting HCC growth and metastasis[65]. In addition, HULC can enhance the expression of hepatocyte growth factor receptor (MET) by sponging miR-2052, thus promoting HCC cell proliferation, migration, and invasion[66]. High mobility group AT-hook 2 (HMGA2), an oncogene, has been shown to be closely associated with cancer progression and metastasis[67]. HULC promotes HCC growth and metastasis by enhancing HMGA2 expression by acting as a ceRNA of miR-186[68]. Rablla, a central regulatory protein, promotes exosome secretion, and exosomes significantly promote HCC progression[69,70]. A mechanistic investigation revealed that HULC increases RAS oncogene family member expression to induce exosome secretion by sponging miR-372-3p, contributing to HCC growth and metastasis[71].
LncRNA HOTAIR-miRNA-mRNA: The lncRNA HOTAIR has been reported to exert an oncogenic role in a variety of malignances[72,73]. Emerging evidence suggests that HOTAIR functions as a ceRNA and facilitates HCC cell proliferation, migration, invasion, and metastasis. For instance, a study confirmed that HOTAIR promotes HCC cell proliferation and tumorigenicity by competitively binding to miR-218 to activate B lymphoma Moloney murine leukemia virus insertion region 1 expression and inactivate P16Ink4a and P14ARF[74]. Another study also demonstrated that Forkhead box C1-activated HOTAIR promotes HCC cell proliferation, migration, and invasion by acting as a sponge of miR-1[75]. In addition, flotillin 1 (FLOT1), a marker of lipid rafts, is highly expressed in HCC and contributes to aggressive tumor characteristics[76]. HOTAIR enhances FLOT1 expression by sponging miR-214-3p, thereby promoting HCC cell proliferation, migration, and invasion[77]. Additionally, HOTAIR promotes HCC cell invasion and metastasis by sponging miR-23b-3p to upregulate ZEB1 expression[78].
LncRNA CCAT-miRNA-mRNA: The lncRNA CCAT1 is located on chromosome 8q24.21 and plays vital roles in promoting HCC cell proliferation and metastasis[79]. CCAT1 has been shown to upregulate the expression of its downstream gene c-Myc, thereby promoting tumorigenesis[80]. Subsequently, many studies have explored the potential mechanism by which CCAT1 upregulates c-Myc to promote tumorigenesis. In HCC, CCAT1 functions as a ceRNA of miRNA let-7, thus counteracting the inhibitory effect of Let-7 on its target genes, HMGA2 and c-Myc, which upregulates the expression of HMGA2 and c-Myc and ultimately facilitates HCC proliferation and migration[81]. In addition, CCAT1 upregulates cyclin-dependent kinase 1 expression by acting as a miR-490-3p sponge, thereby promoting HCC cell proliferation and invasion[82]. Furthermore, CCAT1 acts as a sponge of miR-30c-2-3p to upregulate the expression of cyclin E1, leading to HCC cell proliferation[83].
LncRNA ANRIL-miRNA-mRNA: The lncRNA ANRIL is located on chromosome 9p21 and plays an oncogenic role in tumorigenesis[84]. ANRIL functions as a ceRNA to sponge miRNAs, thereby regulating gene expression in HCC. The high expression of ANRIL in HCC is related to HCC cell proliferation, migration, and invasion; mechanistically, ANRIL exerts its biological action in HCC by sponging miR-384 to upregulate signal transducer and activator of transcription 3 (STAT3) expression[85]. ANRIL can also promote HCC cell proliferation, metastasis, and invasion by acting as a ceRNA of miR-122-5p[86]. In addition, ANRIL can upregulate the expression of pre-B-cell leukemia homeobox 3 by sponging miR-144 to facilitate HCC cell growth, migration, and invasion[87].
LncRNA GAS5-miRNA-mRNA: The lncRNA GAS5 is downregulated in diverse malignancies, including HCC[88]. Increasing evidence indicates that the GAS5-mediated ceRNA network may be one of the important mechanisms by which GAS5 exerts its biological functions in HCC. For example, GAS5 restrains HCC cell migration and invasion by sponging miR-21 to upregulate its targets, programmed cell death 4 and phosphatase and tensin homolog (PTEN)[89]. In addition, GAS5 suppresses HCC invasion by sponging miR-135b to enhance cysteine-rich protein with Kazal motifs expression[90]. GAS5 also functions as a miR-1323 sponge to upregulate tumor protein p53-induced nuclear protein 1 expression, thus inhibiting HCC cell proliferation and invasion and promoting apoptosis[91].
LncRNA MEG3-miRNA-mRNA: MEG3 is an imprinted gene and a tumor suppressive lncRNA. MEG3 is inversely related to tumorigenesis and plays an inhibitory role in many malignancies[92]. Acting as a ceRNA against miRNA is an important mechanism of action of MEG3 in HCC. For example, the overexpression of MEG3 can promote cell apoptosis and inhibit HCC growth by upregulating SRY-related HMG-box transcription factor 11 expression by acting as an miR-9-5p sponge[93]. In addition, MEG3 enhances the expression of PTEN to restrain HCC cell proliferation, migration, and invasion by sponging miRNA-10a-5p[94].
The lncRNA-mediated ceRNA regulatory network functions by modulating signaling pathways: LncRNAs acting as ceRNAs can also participate in HCC cell proliferation, migration, EMT, invasion, and metastasis by modulating various signaling pathways in HCC, including the Wnt/β-catenin pathway, PI3K/AKT pathway, and nuclear factor kappa-B (NF-kB) pathway.
Wnt/β-catenin pathway: Abnormal activation of the Wnt/β-catenin pathway, a key event implicated in HCC carcinogenesis, is believed to be a key target for the clinical diagnosis and treatment of HCC[95]. Thus, elucidation of the regulatory mechanisms of the Wnt/β-catenin pathway will provide new insights into a new anticancer therapy for HCC. Extensive evidence to date has indicated that lncRNAs can mediate the Wnt/β-catenin pathway by acting as ceRNAs of miRNAs, thereby modulating HCC cell proliferation and invasion; oncogenic lncRNAs that act as ceRNAs can perform their biological actions by activating the Wnt/β-catenin pathway in HCC. For example, frizzled (FZD) 4, a Wnt receptor, can activate the Wnt/β-catenin pathway in HCC[96], and the lncRNA ASB16-AS1 enhances FZD4 expression to activate the Wnt/β-catenin pathway by acting as a miR-1827 sponge and subsequently facilitates HCC growth and invasion[97]. Likewise, another Wnt receptor, FZD7, can also activate the Wnt/β-catenin pathway in HCC[98]. The lncRNA DSCR8 activates the Wnt/β-catenin pathway by enhancing FZD7 expression by acting as a miR-485-5-p sponge to facilitate HCC cell proliferation and the cell cycle[99]. Wingless-type MMTV integration site family 3A (WNT3A) is one of the crucial components of the Wnt/β-catenin pathway related to HCC progression[100], and the lncRNA LINC00662 activates the Wnt/β-catenin pathway by enhancing WNT3A expression via the competitive sponging of miR-15a, miR-16, and miR-107, thereby promoting HCC growth and metastasis[101]. Glycogen synthase kinase 3β (GSK3β) is a pivotal regulator of β-catenin signaling[102], and the lncRNA SNHG5 acts as a miR-26a-5p sponge to enhance GSK3β expression, thereby activating the Wnt/β-catenin pathway to facilitate HCC metastasis and EMT[103]. In addition, sex determining region Y-box (SOX) 9 can activate the Wnt/β-catenin pathway in HCC[104], and the lncRNA SOX9-AS1 facilitates HCC growth and metastasis by increasing SOX9 expression to activate the Wnt/β-catenin pathway by acting as a miR-5590-3p sponge[105]. In HCC, interleukin (IL)-6 is associated with the activation of Janus kinase 2 (JAK2)/STAT3 signaling[106]; cyclin-dependent kinase (CDK) 8 and low-density lipoprotein receptor-related protein 6 (LRP6) are associated with the activation of Wnt/β-catenin signaling[107,108], and the lncRNA DLGAP1-AS1 increases the expression of IL-6 and CDK8/LRP6 by functioning as a sponge of miR-26a-5p and miR-26b-5p, thereby activating JAK2/STAT3 and Wnt/β-catenin signaling to facilitate EMT and the progression of HCC, respectively[109]. Instead, tumor suppressive lncRNAs that act as ceRNAs function by inactivating Wnt/β-catenin signaling in HCC. For example, in HCC, nuclear receptor corepressor 2 is associated with inhibition of the activation of Wnt/β-catenin signaling[110]. MIR22HG, a tumor suppressive lncRNA, increases NCOR2 expression by sponging miR-10a-5p, thereby inactivating Wnt/β-catenin signaling to inhibit HCC cell growth, migration, and invasion[111]. The abovementioned findings suggest that different lncRNA-mediated ceRNA networks can exert their biological functions in HCC by mediating the Wnt/β-catenin pathway; these networks may become effective therapeutic targets for treating HCC patients.
PI3K/AKT pathway: PI3K/AKT, a highly activated pathway in HCC, is implicated in HCC carcinogenesis and chemoresistance[112-114]. At present, emerging evidence indicates that multiple lncRNA-mediated ceRNA networks can exert their biological functions by modulating the PI3K/AKT pathway, among which oncogenic lncRNAs that act as ceRNAs exert their biological function by activating the PI3K/AKT pathway in HCC. For instance, the lncRNA PTTG3P facilitates the proliferation, migration, and invasion and inhibits the apoptosis of HCC cells by increasing the expression of cyclin D1/poly ADP-ribose polymerase 2 and activating the PI3K/Akt pathway by acting as a ceRNA of miR-383[115]. Similarly, the lncRNA DLEU1 activates the PI3K/Akt pathway by increasing insulin-like growth factor 1 receptor-1R expression by sponging miR-133a, thereby facilitating HCC cell proliferation, migration and invasion[116]. By contrast, tumor suppressive lncRNAs that act as ceRNAs can exert their biological function by inactivating the PI3K/Akt pathway in HCC. For instance, TCL6, a tumor suppressive lncRNA, upregulates PTEN expression by sponging miR-106a-5p to suppress the PI3K/AKT pathway, thereby inhibiting HCC cell proliferation, migration, and invasion[117]. Intriguingly, several oncogenic lncRNAs that act as ceRNAs have been reported to exert their biological functions by activating the PI3K/AKT/mammalian rapamycin (mTOR) or PI3K/AKT/FoxO3a pathway in HCC. For example, the lncRNA CDKN2B-AS1, an oncogenic lncRNA, enhances nucleosome assembly protein 1 like 1 expression by acting as a ceRNA of let-7c-5p, thus activating the PI3K/AKT/mTOR pathway to promote HCC cell growth and metastasis[118]. In addition, the lncRNA GAS6-AS2 activates the PI3K/AKT /FoxO3a pathway by upregulating OTU domain-containing ubiquitin aldehyde-binding protein 1 expression by sponging miR-493-5p, which promotes HCC cell proliferation, migration, and invasion[119]. In short, the lncRNA-miRNA-PI3K/AKT, PI3K/AKT/mTOR or PI3K-AKT-FoxO3a regulatory network is expected to be a potential therapeutic target for the treatment of HCC patients.
NF-κB pathway: Numerous studies have shown that abnormal activation of the NF-kB pathway is related to HCC growth, EMT, and invasion[120-122]. Existing evidence suggests that lncRNAs can act as miRNA sponges and exert their biological function by mediating the NF-κB pathway in HCC, among which oncogenic lncRNAs that act as ceRNAs can exert their biological function by activating the NF-κB pathway in HCC. For example, in the NF-κB pathway, mixed-lineage kinase (MLK) 3 contributes to cancer migration, invasion, and metastasis[123,124], and the lncRNA SNHG12 enhances MLK3 expression by competitively sponging miR-199a/b-5p, thereby activating the NF-κB pathway to promote HCC proliferation and tumorigenicity[125]. By contrast, a tumor suppressive lncRNA that acts as a ceRNA can exert its biological function by inactivating the NF-κB pathway in HCC. For example, CYLD, a tumor suppressor, can negatively regulate the NF-κB pathway in HCC[126], and the lncRNA CASC2, a tumor suppressive lncRNA, suppresses the NF-κB pathway by enhancing CYLD expression by sponging miR-362-5p, thereby inhibiting HCC cell migration and invasion[127,128]. These findings indicate that the lncRNA-miRNA-NF-κB pathway network may serve as a therapeutic target for patients with HCC.
Chemoresistance and radioresistance
Although current chemotherapy and radiotherapy regimens can prolong the survival of HCC patients, tumor recurrence and metastasis due to chemoresistance and radioresistance lead to unsatisfactory long-term efficacy. The underlying mechanisms of therapeutic resistance in HCC are still unclear, and the exploration of such mechanisms will help improve the current treatment of HCC. Emerging evidence suggests that lncRNAs play a critical role in mediating chemoresistance and radioresistance by acting as ceRNAs of miRNAs in HCC.
Currently, the lncRNA-mediated ceRNA network has been proven to mediate HCC resistance to chemotherapy drugs, including sorafenib, oxaliplatin, cisplatin, and 5-fluorouracil (5-FU). Exploration of the resistance mechanisms to chemotherapy drugs in the treatment of HCC will provide new insights into overcoming chemoresistance.
Sorafenib has been approved for treating advanced HCC; however, the emergence of sorafenib resistance has affected the efficacy of HCC treatment. Existing studies have shown that the lncRNA-mediated ceRNA network is responsible for HCC resistance to sorafenib. Specifically, oncogenic lncRNAs that act as ceRNAs can enhance HCC resistance to sorafenib. For example, activation of the c-Met-Akt pathway can promote sorafenib resistance in HCC cells[129], and the lncRNA NEAT1 activates the c-Met-Akt pathway by sponging miR-335 to enhance sorafenib resistance in HCC cells[130]. In addition, recent studies have suggested that the abnormal activation or expression of forkhead box M1 (FoxM1) contributes to chemotherapy resistance in various cancer cells[131,132]. In HCC cells, FoxM1 knockout sensitizes drug-resistant HCC cells to sorafenib[133], and the lncRNA LINC-ROR increases sorafenib resistance in HCC cells by elevating FOXM1 expression by sponging miR-876-5p[134]. Phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3), a regulatory subunit of PI3K, activates the PI3K/AKT pathway to enhance the resistance of HCC cells to sorafenib-induced apoptosis[135], and the lncRNA LINC00160 acts as a miR-132 sponge to promote sorafenib resistance by increasing PIK3R3 expression in HCC cells[136]. Transmembrane protein 9 (TMEM9) plays a vital role in HCC cell growth[137], and the lncRNA FOXD2-AS1 upregulates TMEM9 expression by sponging miR-150-5p to facilitate the resistance of HCC cells to sorafenib[138]. The tetraspanin protein CD151 has been shown to attenuate drug-induced apoptosis in cancer cell lines[139], and the lncRNA SNHG3 enhances CD151 expression by acting as a ceRNA for miR-128 to promote HCC resistance to sorafenib[140]. Additionally, the lncRNA SNHG16 is upregulated in sorafenib-resistant HCC cells, and SNHG16 increases sorafenib resistance partly by competitively sponging miR-140-5p[141].
Oxaliplatin has been approved for the treatment of patients with locally advanced and metastatic HCC who are not eligible for surgical resection or local treatment; however, oxaliplatin resistance affects the efficacy of HCC treatment. The lncRNA-mediated ceRNA network has been confirmed to modulate oxaliplatin resistance in HCC. In particular, oncogenic lncRNAs that act as ceRNAs can enhance HCC resistance to oxaliplatin. For instance, HULC can upregulate the expression of the ubiquitin-specific peptidase 22 (USP22) protein by suppressing miR-6825-5p, miR-6845-5p, and miR-6886-3p at the epigenetic or transcriptional level in HCC cells; USP22 enhances the HULC-induced deubiquitination of Sirt1 and stabilizes it, and Sirt1 stability induces the autophagy of HCC cells, thus increasing the resistance of HCC cells to oxaliplatin[142]. Multidrug resistance-associated protein 1 (ABCC1) is indicative of chemotherapy resistance[143], and the lncRNA NR2F1-AS1 elevates ABCC1 expression by sponging miR-363 to enhance oxaliplatin resistance in HCC cells[144]. Similarly, the lncRNA KCNQ1OT1 increases oxaliplatin resistance in HCC cells by sponging miR-7-5p to elevate ABCC1 expression[145].
The antitumor efficacy of cisplatin in the treatment of advanced HCC patients is unsatisfactory due to drug resistance. The lncRNA-mediated ceRNA network has been demonstrated to modulate cisplatin resistance in HCC, among which oncogenic lncRNAs that act as ceRNAs can enhance HCC resistance to cisplatin. For example, nuclear factor erythroid-2-related factor 2 (Nrf2) is upregulated in HepG2/cisplatin cells and mediates the chemoresistance of HCC cells to cisplatin[146]. The lncRNA NRAL increases Nrf2 expression by sponging miR-340-5p, thereby facilitating cisplatin resistance in HCC cells[147]. Melanoma-associated antigen A3 (MAGEA3) enhances chemoresistance to cisplatin in HepG2 cells, and the lncRNA LINC01234 enhances MAGEA3 expression by sponging miR-31-5p to promote cisplatin resistance in HCC[148]. The lncRNA SNHG16 enhances cisplatin resistance in HCC cells by sponging let-7b-5p[149]. Conversely, tumor suppressive lncRNAs that act as ceRNAs can reduce HCC resistance to cisplatin. For example, the overexpression of CASC2, a tumor suppressor lncRNA, strengthens cisplatin sensitivity in HCC cells by sponging miR-222[150]. In addition, the overexpression of GAS5, another tumor suppressor lncRNA, enhances the sensitivity of HCC cells to cisplatin by sponging miR-222[151].
The inhibitory efficacy of 5-FU on HCC cells is limited by chemical resistance. Emerging evidence indicates that the lncRNA-mediated ceRNA network is correlated with 5-FU resistance in HCC cells, among which oncogenic lncRNAs that act as ceRNAs can enhance HCC resistance to 5-FU. For example, the lncRNA CRNDE acts as a ceRNA of miR-33a in HCC to enhance HMGA2 expression, thereby promoting chemoresistance to 5-FU in HCC cells[152]. HIHIF-2α is related to the resistance of HCC cells to doxorubicin and sorafenib[153,154], and the lncRNA MALAT1 acts as a ceRNA to increase HIF-2α expression by competitively sponging miR-216b, leading to the enhanced chemoresistance of HCC cells to 5-FU[155]. In contrast, tumor suppressive lncRNAs that act as ceRNAs can reduce HCC resistance to 5-FU. For example, Kelch-like ECH-associated protein 1 (Keap1) inactivation enhances the resistance of HCC cells to chemotherapy drugs such as sorafenib[156,157], and the overexpression of KRAL, a tumor suppressive lncRNA, enhances Keap1 expression by functioning as a ceRNA for miR-141 to reverse the resistance to 5-FU in HCC cell lines[158].
Currently, an oncogenic lncRNA-mediated ceRNA network has been demonstrated to enhance HCC resistance to radiation therapy. For instance, AZD1775, an inhibitor of WEE1, has been reported to sensitize HCC cells to radiation[159], suggesting that WEE1 can enhance radioresistance in HCC. The lncRNA NEAT1_2 upregulates WEE1 expression by acting as a ceRNA for miR-101-3p to reduce the radiosensitivity of HCC[160]. A RING-type ubiquitin ligase E3 (RAD18), an E3 ubiquitin-linked enzyme, can induce radiation resistance in glioma cells[161,162]. The lncRNA LINC-ROR competes with sponge miR-145 to increase RAD18 expression, thereby enhancing the radiation resistance of HCC cells[163]. Forkhead box protein P1 (FOXP1), a transcription factor, attenuates radioresistance in cervical cancer[164]. The lncRNA LINC00473 promotes radioresistance in HCC by increasing FOXP1 expression by sponging miR-345-5p[165]. These findings suggest that the lncRNA-mediated ceRNA network may provide new clues for overcoming radioresistance in HCC.
PROBLEMS AND PERSPECTIVES
The lncRNA-mediated ceRNA regulatory network provides a new mode of posttranscriptional regulation and plays a critical role in the initiation and progression of HCC. Nevertheless, investigations into the detailed mechanism of the ceRNA network and its relationship with HCC are still in the preliminary stage. Although there are increasing reports about lncRNAs as ceRNAs in HCC, several fundamental problems facing these studies need to be addressed. First, information on the roles of lncRNAs that act as ceRNAs in current studies is derived from overexpression and/or knockout experiments, and only when the abundance of lncRNAs is remarkably high can lncRNAs act as ceRNAs. As a result, the abundance of artificially controlled lncRNAs often far exceeds the abundance range of any endogenous lncRNA. Therefore, it is urgent to verify whether the lncRNA-mediated ceRNA network has the same effects under normal cellular conditions. Second, most of the current research on ceRNAs is still in the prediction stage of bioinformatics, and most studies lack biological validation; the regulatory relationships in the ceRNA network need to be effectively verified. Third, methodologically, there are few predictive tools available; most miRNA-mRNA predictions focus only on the binding of a miRNA with its target in the 3’-UTR. However, this is not always the case; miRNAs can also target the 5'-untranslated region and coding sequences of mRNAs[166-170]. Thus, provided that the prediction of a ceRNA is not limited to the 3’-UTR of its mRNA, the range of predicted ceRNAs should be improved. Fourth, one miRNA generally interacts with one target mRNA. However, some miRNAs may modulate many target mRNAs, and vice versa[167,171]. Thus, it is necessary to model the effect of ceRNAs in real scenarios. Fifth, the ceRNA hypothesis maintains that a miRNA is stably expressed; in fact, intracellular miRNA expression is dynamic, which inevitably influences the effectiveness of ceRNAs. Sixth, although ceRNA prediction methods are constantly updated, the current prediction algorithms cannot fully encompass several factors affecting ceRNA susceptibility (quantity and characteristics of MREs, miRNA/mRNA abundance, and subcellular location of RNAs)[172,173]. Therefore, the prediction methods and experimental methods still need to be further updated and improved. Seventh, the initiation and progression of HCC are a complex event, and it is unclear whether other mechanisms interact with lncRNAs acting as ceRNAs in HCC cells. Addressing the above problems will enable a better understanding of the lncRNA-mediated ceRNA network that can be used to more effectively diagnose and treat HCC.
Given the critical roles and the complex interactions among lncRNA-mediated ceRNA regulatory networks in HCC, future investigations with validation in large sample sizes and the exploration of in-depth molecular mechanisms are needed to probe the HCC-specific lncRNA-mediated ceRNA axis, which should identify new diagnostic and prognostic markers of HCC and provide promising targets for the treatment of patients with HCC.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests.
Manuscript source: Invited manuscript
Peer-review started: April 13, 2020
First decision: June 18, 2020
Article in press: July 15, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta S S-Editor: Liu M L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Zhao-Shan Niu, Laboratory of Micromorphology, School of Basic Medicine, Medical Department of Qingdao University, Qingdao 266071, Shandong Province, China. z.s.niu@qdu.edu.cn.
Wen-Hong Wang, Department of Pathology, School of Basic Medicine, Medical Department of Qingdao University, Qingdao 266071, Shandong Province, China.
Xian-Ning Dong, Department of Pathology, the Affiliated Hospital of Qingdao University, Qingdao 266061, Shandong Province, China.
Li-Mei-Li Tian, BGI Gene Innovation Class, School of Basic Medicine, Medical Department of Qingdao University, Qingdao 266071, Shandong Province, China.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wright MW, Bruford EA. Naming 'junk': human non-protein coding RNA (ncRNA) gene nomenclature. Hum Genomics. 2011;5:90–98. doi: 10.1186/1479-7364-5-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramón Y Cajal S, Segura MF, Hümmer S. Interplay Between ncRNAs and Cellular Communication: A Proposal for Understanding Cell-Specific Signaling Pathways. Front Genet. 2019;10:281. doi: 10.3389/fgene.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YA, Park KK, Lee SJ. LncRNAs Act as a Link between Chronic Liver Disease and Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:2883. doi: 10.3390/ijms21082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jámbor I, Szabó K, Zeher M, Papp G. [The importance of microRNAs in the development of systemic autoimmune disorders] Orv Hetil. 2019;160:563–572. doi: 10.1556/650.2019.31349. [DOI] [PubMed] [Google Scholar]
- 6.Dai X, Kaushik AC, Zhang J. The Emerging Role of Major Regulatory RNAs in Cancer Control. Front Oncol. 2019;9:920. doi: 10.3389/fonc.2019.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolenda T, Guglas K, Kopczyńska M, Teresiak A, Bliźniak R, Mackiewicz A, Lamperska K, Mackiewicz J. Oncogenic Role of ZFAS1 lncRNA in Head and Neck Squamous Cell Carcinomas. Cells. 2019;8 doi: 10.3390/cells8040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subat S, Inamura K, Ninomiya H, Nagano H, Okumura S, Ishikawa Y. Unique MicroRNA and mRNA Interactions in EGFR-Mutated Lung Adenocarcinoma. J Clin Med. 2018;7:419. doi: 10.3390/jcm7110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Le TD, Liu L, Li J. Inferring and analyzing module-specific lncRNA-mRNA causal regulatory networks in human cancer. Brief Bioinform. 2019;20:1403–1419. doi: 10.1093/bib/bby008. [DOI] [PubMed] [Google Scholar]
- 10.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Wang Z, Zong Y, Deng D, Chen P, Lu J. LncRNA MFI2-AS1 promotes HCC progression and metastasis by acting as a competing endogenous RNA of miR-134 to upregulate FOXM1 expression. Biomed Pharmacother. 2020;125:109890. doi: 10.1016/j.biopha.2020.109890. [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Deng J, Zhou J, Wang Z, Yuan XF, Pan S, Zhang HB. Long non-coding RNA TUG1 promotes cell progression in hepatocellular carcinoma via regulating miR-216b-5p/DLX2 axis. Cancer Cell Int. 2020;20:8. doi: 10.1186/s12935-019-1093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Z, Huang H, Huang L, Sun M, Yan Q, Song Y, Wei F, Bo H, Gong Z, Zeng Y, Li Q, Zhang W, Li X, Xiang B, Li X, Li Y, Xiong W, Li G. Regulation network and expression profiles of Epstein-Barr virus-encoded microRNAs and their potential target host genes in nasopharyngeal carcinomas. Sci China Life Sci. 2014;57:315–326. doi: 10.1007/s11427-013-4577-y. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 19.Du Z, Sun T, Hacisuleyman E, Fei T, Wang X, Brown M, Rinn JL, Lee MG, Chen Y, Kantoff PW, Liu XS. Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nat Commun. 2016;7:10982. doi: 10.1038/ncomms10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Hu W, Wang Y, An Y, Song L, Shang P, Yue Z. Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA. Mol Cancer. 2020;19:18. doi: 10.1186/s12943-020-1132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua S, Lei L, Deng L, Weng X, Liu C, Qi X, Wang S, Zhang D, Zou X, Cao C, Liu L, Wu D. miR-139-5p inhibits aerobic glycolysis, cell proliferation, migration, and invasion in hepatocellular carcinoma via a reciprocal regulatory interaction with ETS1. Oncogene. 2018;37:1624–1636. doi: 10.1038/s41388-017-0057-3. [DOI] [PubMed] [Google Scholar]
- 22.Huang M, Xiong H, Luo D, Xu B, Liu H. CSN5 upregulates glycolysis to promote hepatocellular carcinoma metastasis via stabilizing the HK2 protein. Exp Cell Res. 2020;388:111876. doi: 10.1016/j.yexcr.2020.111876. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Wang X, Wu T, Yang S. miR-489 suppresses multiple myeloma cells growth through inhibition of LDHA-mediated aerobic glycolysis. Genes Genomics. 2020;42:291–297. doi: 10.1007/s13258-019-00900-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11:176. doi: 10.1038/s41419-020-2366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Chen J, Sun F, Wang Z, Xu W, Yu Y, Ding F, Shen H. miR-202 functions as a tumor suppressor in hepatocellular carcinoma by targeting HK2. Oncol Lett. 2020;19:2265–2271. doi: 10.3892/ol.2020.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY, Lin KH. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67:188–203. doi: 10.1002/hep.29462. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Q, Wei Z, Li Y, Zhou X, Chen J, Wang T, Shao G, Zhang M, Zhang Z. miR186 functions as a tumor suppressor in osteosarcoma cells by suppressing the malignant phenotype and aerobic glycolysis. Oncol Rep. 2018;39:2703–2710. doi: 10.3892/or.2018.6394. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, Fu Q, Jing C, Zhang X, Qin T, Pan Y. LncRNA HOTAIR knockdown inhibits glycolysis by regulating miR-130a-3p/HIF1A in hepatocellular carcinoma under hypoxia. Biomed Pharmacother. 2020;125:109703. doi: 10.1016/j.biopha.2019.109703. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Cheng T, He Y, Zhou S, Wang Y, Zhang K, Yu P. High glucose regulates ERp29 in hepatocellular carcinoma by LncRNA MEG3-miRNA 483-3p pathway. Life Sci. 2019;232:116602. doi: 10.1016/j.lfs.2019.116602. [DOI] [PubMed] [Google Scholar]
- 30.Mascaux C, Angelova M, Vasaturo A, Beane J, Hijazi K, Anthoine G, Buttard B, Rothe F, Willard-Gallo K, Haller A, Ninane V, Burny A, Sculier JP, Spira A, Galon J. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571:570–575. doi: 10.1038/s41586-019-1330-0. [DOI] [PubMed] [Google Scholar]
- 31.Lo PK, Wolfson B, Zhou Q. Cellular, physiological and pathological aspects of the long non-coding RNA NEAT1. Front Biol (Beijing) 2016;11:413–426. doi: 10.1007/s11515-016-1433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mello SS, Sinow C, Raj N, Mazur PK, Bieging-Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J, Hirose T, Nakagawa S, Rinn J, Attardi LD. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes Dev. 2017;31:1095–1108. doi: 10.1101/gad.284661.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahran AM, Hetta HF, Rayan A, Eldin AS, Hassan EA, Fakhry H, Soliman A, El-Badawy O. Differential expression of Tim-3, PD-1, and CCR5 on peripheral T and B lymphocytes in hepatitis C virus-related hepatocellular carcinoma and their impact on treatment outcomes. Cancer Immunol Immunother. 2020;69:1253–1263. doi: 10.1007/s00262-019-02465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol. 2020;13:100738. doi: 10.1016/j.tranon.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan K, Fu Y, Zhu N, Wang Z, Hong JL, Li Y, Li WJ, Zhang HB, Song JH. Repression of lncRNA NEAT1 enhances the antitumor activity of CD8+T cells against hepatocellular carcinoma via regulating miR-155/Tim-3. Int J Biochem Cell Biol. 2019;110:1–8. doi: 10.1016/j.biocel.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Ju S, Zhu Y, Liu L, Dai S, Li C, Chen E, He Y, Zhang X, Lu B. Gadd45b and Gadd45g are important for anti-tumor immune responses. Eur J Immunol. 2009;39:3010–3018. doi: 10.1002/eji.200839154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu B, Ferrandino AF, Flavell RA. Gadd45beta is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol. 2004;5:38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z, Zhao H, Feng X, Li H, Qiu C, Yi X, Tang H, Zhang J. Long Non-coding RNA FENDRR Acts as a miR-423-5p Sponge to Suppress the Treg-Mediated Immune Escape of Hepatocellular Carcinoma Cells. Mol Ther Nucleic Acids. 2019;17:516–529. doi: 10.1016/j.omtn.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazari Y, Bravo-San Pedro JM, Hetz C, Galluzzi L, Kroemer G. Autophagy in hepatic adaptation to stress. J Hepatol. 2020;72:183–196. doi: 10.1016/j.jhep.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 41.Samaka RM, Basha MA, Mansour E. Does the Autophagy Related Gene 7 (ATG7) Have a Role in Non-Melanoma Skin Cancer? Clin Cosmet Investig Dermatol. 2020;13:49–58. doi: 10.2147/CCID.S222051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji E, Kim C, Kang H, Ahn S, Jung M, Hong Y, Tak H, Lee S, Kim W, Lee EK. RNA Binding Protein HuR Promotes Autophagosome Formation by Regulating Expression of Autophagy-Related Proteins 5, 12, and 16 in Human Hepatocellular Carcinoma Cells. Mol Cell Biol. 2019;39:e00508–18. doi: 10.1128/MCB.00508-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX, Yao M, Zhang DD, Yi XF. Nrf2 induces cisplatin resistance through activation of autophagy in ovarian carcinoma. Int J Clin Exp Pathol. 2014;7:1502–1513. [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Peng X, Jin H, Liu J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma. Gene. 2019;697:94–102. doi: 10.1016/j.gene.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Wei X, Zhang A, Li C, Bai J, Dong J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem Biophys Res Commun. 2016;473:1268–1275. doi: 10.1016/j.bbrc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 46.Guo J, Ma Y, Peng X, Jin H, Liu J. LncRNA CCAT1 promotes autophagy via regulating ATG7 by sponging miR-181 in hepatocellular carcinoma. J Cell Biochem. 2019;120:17975–17983. doi: 10.1002/jcb.29064. [DOI] [PubMed] [Google Scholar]
- 47.Li ML, Zhang Y, Ma LT. LncRNA HCG11 accelerates the progression of hepatocellular carcinoma via miR-26a-5p/ATG12 axis. Eur Rev Med Pharmacol Sci. 2019;23:10708–10720. doi: 10.26355/eurrev_201912_19771. [DOI] [PubMed] [Google Scholar]
- 48.Hsu CL, Lee EX, Gordon KL, Paz EA, Shen WC, Ohnishi K, Meisenhelder J, Hunter T, La Spada AR. MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB. Nat Commun. 2018;9:942. doi: 10.1038/s41467-018-03340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shan Y, Li P. Long Intergenic Non-Protein Coding RNA 665 Regulates Viability, Apoptosis, and Autophagy via the MiR-186-5p/MAP4K3 Axis in Hepatocellular Carcinoma. Yonsei Med J. 2019;60:842–853. doi: 10.3349/ymj.2019.60.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gachechiladze M, Tichý T, Kolek V, Grygárková I, Klein J, Mgebrishvili G, Kharaishvili G, Janíková M, Smičková P, Cierna L, Pitson S, Maddelein ML, Cuvillier O, Škarda J. Sphingosine kinase-1 predicts overall survival outcomes in non-small cell lung cancer patients treated with carboplatin and navelbine. Oncol Lett. 2019;18:1259–1266. doi: 10.3892/ol.2019.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Z, Xiao Z, Liu F, Cui M, Li W, Yang Z, Li J, Ye L, Zhang X. Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1) Oncotarget. 2016;7:241–254. doi: 10.18632/oncotarget.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Ha M, Li L, Huang X, Liu C. MicroRNA-3064-5p sponged by MALAT1 suppresses angiogenesis in human hepatocellular carcinoma by targeting the FOXA1/CD24/Src pathway. FASEB J. 2020;34:66–81. doi: 10.1096/fj.201901834R. [DOI] [PubMed] [Google Scholar]
- 54.Hou ZH, Xu XW, Fu XY, Zhou LD, Liu SP, Tan DM. Long non-coding RNA MALAT1 promotes angiogenesis and immunosuppressive properties of HCC cells by sponging miR-140. Am J Physiol Cell Physiol. 2020;318:C649–C663. doi: 10.1152/ajpcell.00510.2018. [DOI] [PubMed] [Google Scholar]
- 55.Gao J, Yin X, Yu X, Dai C, Zhou F. Long noncoding RNA LINC00488 functions as a ceRNA to regulate hepatocellular carcinoma cell growth and angiogenesis through miR-330-5. Dig Liver Dis. 2019;51:1050–1059. doi: 10.1016/j.dld.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Khosla R, Hemati H, Rastogi A, Ramakrishna G, Sarin SK, Trehanpati N. miR-26b-5p helps in EpCAM+cancer stem cells maintenance via HSC71/HSPA8 and augments malignant features in HCC. Liver Int. 2019;39:1692–1703. doi: 10.1111/liv.14188. [DOI] [PubMed] [Google Scholar]
- 57.Gordeeva O. Cancer-testis antigens: Unique cancer stem cell biomarkers and targets for cancer therapy. Semin Cancer Biol. 2018;53:75–89. doi: 10.1016/j.semcancer.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16:4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He B, Peng F, Li W, Jiang Y. Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced cancer stem cell properties in HepG2 through PI3K/Akt signaling. J Cell Biochem. 2019;120:2908–2918. doi: 10.1002/jcb.26823. [DOI] [PubMed] [Google Scholar]
- 60.Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ, Liu F, Cao JG, Deng XY. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells via downregulation of β-catenin. World J Gastroenterol. 2013;19:7680–7695. doi: 10.3748/wjg.v19.i43.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan SX, Wang J, Yang F, Tao QF, Zhang J, Wang LL, Yang Y, Liu H, Wang ZG, Xu QG, Fan J, Liu L, Sun SH, Zhou WP. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63:499–511. doi: 10.1002/hep.27893. [DOI] [PubMed] [Google Scholar]
- 62.Sayad A, Taheri M, Arsang-Jang S, Glassy MC, Ghafouri-Fard S. Hepatocellular carcinoma up-regulated long non-coding RNA: a putative marker in multiple sclerosis. Metab Brain Dis. 2019;34:1201–1205. doi: 10.1007/s11011-019-00418-z. [DOI] [PubMed] [Google Scholar]
- 63.Zhou C, Wu H, Liu Y, Yin C, Yang B. [Long non-coding RNA HULC affects downstream-related targets to regulate migration and invasion of hepatoma cells] Zhonghua Gan Zang Bing Za Zhi. 2018;26:513–518. doi: 10.3760/cma.j.issn.1007-3418.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Yuan K, Xie K, Lan T, Xu L, Chen X, Li X, Liao M, Li J, Huang J, Zeng Y, Wu H. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ. 2020;27:1355–1368. doi: 10.1038/s41418-019-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ, Wang CY, Zhang HM, Zhang RX, Zhang JJ, Yao Z, Shen ZY. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7:42431–42446. doi: 10.18632/oncotarget.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Liao Z, Liu F, Su C, Zhu H, Li Y, Tao R, Liang H, Zhang B, Zhang X. Long noncoding RNA HULC promotes hepatocellular carcinoma progression. Aging (Albany NY) 2019;11:9111–9127. doi: 10.18632/aging.102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawsawi O, Henderson V, Burton LJ, Dougan J, Nagappan P, Odero-Marah V. High mobility group A2 (HMGA2) promotes EMT via MAPK pathway in prostate cancer. Biochem Biophys Res Commun. 2018;504:196–202. doi: 10.1016/j.bbrc.2018.08.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Chen F, Zhao M, Yang Z, Li J, Zhang S, Zhang W, Ye L, Zhang X. The long noncoding RNA HULC promotes liver cancer by increasing the expression of the HMGA2 oncogene via sequestration of the microRNA-186. J Biol Chem. 2017;292:15395–15407. doi: 10.1074/jbc.M117.783738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao SQ, Zheng H, Sun BC, Wang ZL, Liu T, Guo DH, Shen ZY. Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion. World J Gastroenterol. 2019;25:5283–5299. doi: 10.3748/wjg.v25.i35.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arshi A, Raeisi F, Mahmoudi E, Mohajerani F, Kabiri H, Fazel R, Zabihian-Langeroudi M, Jusic A. A Comparative Study of HOTAIR Expression in Breast Cancer Patient Tissues and Cell Lines. Cell J. 2020;22:178–184. doi: 10.22074/cellj.2020.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajagopal T, Talluri S, Akshaya RL, Dunna NR. HOTAIR LncRNA: A novel oncogenic propellant in human cancer. Clin Chim Acta. 2020;503:1–18. doi: 10.1016/j.cca.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 74.Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang H, Liang WC, Wang SS, Ko CH, Waye MM, Kung HF, Li G, Zhang JF. Hotair mediates hepatocarcinogenesis through suppressing miRNA-218 expression and activating P14 and P16 signaling. J Hepatol. 2015;63:886–895. doi: 10.1016/j.jhep.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Su DN, Wu SP, Chen HT, He JH. HOTAIR, a long non-coding RNA driver of malignancy whose expression is activated by FOXC1, negatively regulates miRNA-1 in hepatocellular carcinoma. Oncol Lett. 2016;12:4061–4067. doi: 10.3892/ol.2016.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang SH, Wang CJ, Shi L, Li XH, Zhou J, Song LB, Liao WT. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One. 2013;8:e64709. doi: 10.1371/journal.pone.0064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Shang Z, Ma Y, Ma J, Song J. HOTAIR/miR-214-3p/FLOT1 axis plays an essential role in the proliferation, migration, and invasion of hepatocellular carcinoma. Int J Clin Exp Pathol. 2019;12:50–63. [PMC free article] [PubMed] [Google Scholar]
- 78.Yang T, He X, Chen A, Tan K, Du X. LncRNA HOTAIR contributes to the malignancy of hepatocellular carcinoma by enhancing epithelial-mesenchymal transition via sponging miR-23b-3p from ZEB1. Gene. 2018;670:114–122. doi: 10.1016/j.gene.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 79.Wang F, Xie C, Zhao W, Deng Z, Yang H, Fang Q. Long non-coding RNA CARLo-5 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Clin Exp Med. 2017;17:33–43. doi: 10.1007/s10238-015-0395-9. [DOI] [PubMed] [Google Scholar]
- 80.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, Yang L, Chen LL. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18. doi: 10.1186/s13046-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dou C, Sun L, Jin X, Han M, Zhang B, Li T. Long non-coding RNA colon cancer-associated transcript 1 functions as a competing endogenous RNA to regulate cyclin-dependent kinase 1 expression by sponging miR-490-3p in hepatocellular carcinoma progression. Tumour Biol. 2017;39:1010428317697572. doi: 10.1177/1010428317697572. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Cai M, Jiang D, Xu L. Upregulated LncRNA-CCAT1 promotes hepatocellular carcinoma progression by functioning as miR-30c-2-3p sponge. Cell Biochem Funct. 2019;37:84–92. doi: 10.1002/cbf.3375. [DOI] [PubMed] [Google Scholar]
- 84.Wang CH, Li QY, Nie L, Ma J, Yao CJ, Chen FP. LncRNA ANRIL promotes cell proliferation, migration and invasion during acute myeloid leukemia pathogenesis via negatively regulating miR-34a. Int J Biochem Cell Biol. 2020;119:105666. doi: 10.1016/j.biocel.2019.105666. [DOI] [PubMed] [Google Scholar]
- 85.Ji Y, Sun H, Liang H, Wang Y, Lu M, Guo Z, Lv Z, Ren W. Evaluation of LncRNA ANRIL Potential in Hepatic Cancer Progression. J Environ Pathol Toxicol Oncol. 2019;38:119–131. doi: 10.1615/JEnvironPatholToxicolOncol.2019028282. [DOI] [PubMed] [Google Scholar]
- 86.Ma J, Li T, Han X, Yuan H. Knockdown of LncRNA ANRIL suppresses cell proliferation, metastasis, and invasion via regulating miR-122-5p expression in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018;144:205–214. doi: 10.1007/s00432-017-2543-y. [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Zhang H, Li G, Hu J, Liu X, Lin L. LncRNA ANRIL promotes cell growth, migration and invasion of hepatocellular carcinoma cells via sponging miR-144. Anticancer Drugs. 2019;30:1013–1021. [Google Scholar]
- 88.Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q, Liu Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol Med Rep. 2016;13:1541–1550. doi: 10.3892/mmr.2015.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y, Yang X, Shen J, Liu Q, Zhang J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol. 2016;37:2691–2702. doi: 10.1007/s13277-015-4111-x. [DOI] [PubMed] [Google Scholar]
- 90.Yang L, Jiang J. GAS5 Regulates RECK Expression and Inhibits Invasion Potential of HCC Cells by Sponging miR-135b. Biomed Res Int. 2019;2019:2973289. doi: 10.1155/2019/2973289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F, Yang C, Xing Z, Liu P, Zhang B, Ma X, Huang L, Zhuang L. LncRNA GAS5-mediated miR-1323 promotes tumor progression by targeting TP53INP1 in hepatocellular carcinoma. Onco Targets Ther. 2019;12:4013–4023. doi: 10.2147/OTT.S209439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghafouri-Fard S, Taheri M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed Pharmacother. 2019;118:109129. doi: 10.1016/j.biopha.2019.109129. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z, Chen JY, Zhong Y, Xie L, Li JS. lncRNA MEG3 inhibits the growth of hepatocellular carcinoma cells by sponging miR-9-5p to upregulate SOX11. Braz J Med Biol Res. 2019;52:e8631. doi: 10.1590/1414-431X20198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Liu J, Lv Y, Zhang C, Guo S. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am J Transl Res. 2019;11:4089–4099. [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Y, Wei A, Zhang H, Chen X, Wang L, Zhang H, Yu X, Yuan Q, Zhang J, Wang S. Retraction Note: α2,6-Sialylation mediates hepatocellular carcinoma growth in vitro and in vivo by targeting the Wnt/β-catenin pathway. Oncogenesis. 2020;9:28. doi: 10.1038/s41389-020-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai H, Chen Y, Yang X, Ma S, Wang Q, Zhang Y, Niu X, Ding G, Yuan Y. Let7b modulates the Wnt/β-catenin pathway in liver cancer cells via downregulated Frizzled4. Tumour Biol. 2017;39:1010428317716076. doi: 10.1177/1010428317716076. [DOI] [PubMed] [Google Scholar]
- 97.Yao X, You G, Zhou C, Zhang D. LncRNA ASB16-AS1 Promotes Growth And Invasion Of Hepatocellular Carcinoma Through Regulating miR-1827/FZD4 Axis And Activating Wnt/β-Catenin Pathway. Cancer Manag Res. 2019;11:9371–9378. doi: 10.2147/CMAR.S220434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Quan H, Li B, Yang J. MicroRNA-504 functions as a tumor suppressor in hepatocellular carcinoma through inhibiting Frizzled-7-mediated-Wnt/β-catenin signaling. Biomed Pharmacother. 2018;107:754–762. doi: 10.1016/j.biopha.2018.07.150. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Sun L, Wang L, Liu Z, Li Q, Yao B, Wang C, Chen T, Tu K, Liu Q. Long non-coding RNA DSCR8 acts as a molecular sponge for miR-485-5p to activate Wnt/β-catenin signal pathway in hepatocellular carcinoma. Cell Death Dis. 2018;9:851. doi: 10.1038/s41419-018-0937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan LH, Yao M, Cai Y, Gu JJ, Yang XL, Wang L, Yao DF. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J Gastroenterol. 2016;22:3829–3836. doi: 10.3748/wjg.v22.i14.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian X, Wu Y, Yang Y, Wang J, Niu M, Gao S, Qin T, Bao D. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol Oncol. 2020;14:462–483. doi: 10.1002/1878-0261.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Z, Chang L, Zhou H, Liu X, Li Y, Mi T, Tong D. Arctigenin Attenuates Tumor Metastasis Through Inhibiting Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma via Suppressing GSK3β-Dependent Wnt/β-Catenin Signaling Pathway In Vivo and In Vitro. Front Pharmacol. 2019;10:937. doi: 10.3389/fphar.2019.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Guo D, Zhao Y, Ren M, Lu G, Wang Y, Zhang J, Mi C, He S, Lu X. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3β signal pathway. Cell Death Dis. 2018;9:888. doi: 10.1038/s41419-018-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawai T, Yasuchika K, Ishii T, Miyauchi Y, Kojima H, Yamaoka R, Katayama H, Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Komori J, Hatano E, Kawaguchi Y, Uemoto S. SOX9 is a novel cancer stem cell marker surrogated by osteopontin in human hepatocellular carcinoma. Sci Rep. 2016;6:30489. doi: 10.1038/srep30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang W, Wu Y, Hou B, Wang Y, Deng D, Fu Z, Xu Z. A SOX9-AS1/miR-5590-3p/SOX9 positive feedback loop drives tumor growth and metastasis in hepatocellular carcinoma through the Wnt/β-catenin pathway. Mol Oncol. 2019;13:2194–2210. doi: 10.1002/1878-0261.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, Chen Y, Jiang R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer. 2020;8:e000285. doi: 10.1136/jitc-2019-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, Zhang X, Wang T, Wang L, Tan Z, Wei W, Yan B, Zhao J, Wu K, Yang A, Zhang R, Jia L. MicroRNA-26a is a key regulon that inhibits progression and metastasis of c-Myc/EZH2 double high advanced hepatocellular carcinoma. Cancer Lett. 2018;426:98–108. doi: 10.1016/j.canlet.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Zeng XC, Liu FQ, Yan R, Yi HM, Zhang T, Wang GY, Li Y, Jiang N. Downregulation of miR-610 promotes proliferation and tumorigenicity and activates Wnt/β-catenin signaling in human hepatocellular carcinoma. Mol Cancer. 2014;13:261. doi: 10.1186/1476-4598-13-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin Y, Jian Z, Jin H, Wei X, Zou X, Guan R, Huang J. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/β-catenin pathway. Cell Death Dis. 2020;11:34. doi: 10.1038/s41419-019-2188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qu C, He, Lu X, Dong L, Zhu Y, Zhao Q, Jiang X, Chang P, Jiang X, Wang L, Zhang Y, Bi L, He J, Peng Y, Su J, Zhang H, Huang H, Li Y, Zhou S, Qu Y, Zhao Y, Zhang Z. Salt-inducible Kinase (SIK1) regulates HCC progression and WNT/β-catenin activation. J Hepatol. 2016;64:1076–1089. doi: 10.1016/j.jhep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Wu Y, Zhou Y, Huan L, Xu L, Shen M, Huang S, Liang L. LncRNA MIR22HG inhibits growth, migration and invasion through regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells. Cancer Sci. 2019;110:973–984. doi: 10.1111/cas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang YF, Zhang MF, Tian QH, Fu J, Yang X, Zhang CZ, Yang H. SPAG5 interacts with CEP55 and exerts oncogenic activities via PI3K/AKT pathway in hepatocellular carcinoma. Mol Cancer. 2018;17:117. doi: 10.1186/s12943-018-0872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang H, Wang Q, Liu J, Cao H. Inhibition of the PI3K/Akt signaling pathway reverses sorafenib-derived chemo-resistance in hepatocellular carcinoma. Oncol Lett. 2018;15:9377–9384. doi: 10.3892/ol.2018.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou Q, Zhang W, Wang Z, Liu S. Long non-coding RNA PTTG3P functions as an oncogene by sponging miR-383 and up-regulating CCND1 and PARP2 in hepatocellular carcinoma. BMC Cancer. 2019;19:731. doi: 10.1186/s12885-019-5936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang W, Liu S, Liu K, Liu Y. Long non-coding RNA deleted in lymphocytic leukaemia 1 promotes hepatocellular carcinoma progression by sponging miR-133a to regulate IGF-1R expression. J Cell Mol Med. 2019;23:5154–5164. doi: 10.1111/jcmm.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo LH, Jin M, Wang LQ, Xu GJ, Lin ZY, Yu DD, Yang SL, Ran RZ, Wu G, Zhang T. Long noncoding RNA TCL6 binds to miR-106a-5p to regulate hepatocellular carcinoma cells through PI3K/AKT signaling pathway. J Cell Physiol. 2020;235:6154–6166. doi: 10.1002/jcp.29544. [DOI] [PubMed] [Google Scholar]
- 118.Huang Y, Xiang B, Liu Y, Wang Y, Kan H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018;437:56–66. doi: 10.1016/j.canlet.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 119.Liang C, Pang L, Ke Y, Ji W, Xiong J, Ding R, Ding Y. LncRNA GAS6-AS2 facilitates tumor growth and metastasis of hepatocellular carcinoma by activating the PI3K/AKT/FoxO3a signaling pathway. Int J Clin Exp Pathol. 2019;12:4011–4023. [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson CL, Jurk D, Fullard N, Banks P, Page A, Luli S, Elsharkawy AM, Gieling RG, Chakraborty JB, Fox C, Richardson C, Callaghan K, Blair GE, Fox N, Lagnado A, Passos JF, Moore AJ, Smith GR, Tiniakos DG, Mann J, Oakley F, Mann DA. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat Commun. 2015;6:6818. doi: 10.1038/ncomms7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mo SJ, Hou X, Hao XY, Cai JP, Liu X, Chen W, Chen D, Yin XY. EYA4 inhibits hepatocellular carcinoma growth and invasion by suppressing NF-κB-dependent RAP1 transactivation. Cancer Commun (Lond) 2018;38:9. doi: 10.1186/s40880-018-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He Z, Dong W, Hu J, Ren X. AQP5 promotes hepatocellular carcinoma metastasis via NF-κB-regulated epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2017;490:343–348. doi: 10.1016/j.bbrc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 123.Rattanasinchai C, Gallo KA. MLK3 Signaling in Cancer Invasion. Cancers (Basel) 2016;8 doi: 10.3390/cancers8050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chadee DN. Involvement of mixed lineage kinase 3 in cancer. Can J Physiol Pharmacol. 2013;91:268–274. doi: 10.1139/cjpp-2012-0258. [DOI] [PubMed] [Google Scholar]
- 125.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Urbanik T, Köhler BC, Boger RJ, Wörns MA, Heeger S, Otto G, Hövelmeyer N, Galle PR, Schuchmann M, Waisman A, Schulze-Bergkamen H. Down-regulation of CYLD as a trigger for NF-κB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int J Oncol. 2011;38:121–131. [PubMed] [Google Scholar]
- 127.Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR-362-5p/Nf-κB axis. J Cell Physiol. 2018;233:6661–6670. doi: 10.1002/jcp.26446. [DOI] [PubMed] [Google Scholar]
- 128.Ni F, Zhao H, Cui H, Wu Z, Chen L, Hu Z, Guo C, Liu Y, Chen Z, Wang X, Chen D, Wei H, Wang S. MicroRNA-362-5p promotes tumor growth and metastasis by targeting CYLD in hepatocellular carcinoma. Cancer Lett. 2015;356:809–818. doi: 10.1016/j.canlet.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 129.Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:159. doi: 10.1186/s13046-016-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen S, Xia X. Long noncoding RNA NEAT1 suppresses sorafenib sensitivity of hepatocellular carcinoma cells via regulating miR-335-c-Met. J Cell Physiol. 2019:epub ahead of print. doi: 10.1002/jcp.27567. [DOI] [PubMed] [Google Scholar]
- 131.Westhoff GL, Chen Y, Teng NNH. Targeting FOXM1 Improves Cytotoxicity of Paclitaxel and Cisplatinum in Platinum-Resistant Ovarian Cancer. Int J Gynecol Cancer. 2017;27:1602–1609. doi: 10.1097/IGC.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 132.Kongsema M, Wongkhieo S, Khongkow M, Lam EW, Boonnoy P, Vongsangnak W, Wong-Ekkabut J. Molecular mechanism of Forkhead box M1 inhibition by thiostrepton in breast cancer cells. Oncol Rep. 2019;42:953–962. doi: 10.3892/or.2019.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan D, Yan X, Dai X, Chen L, Sun L, Li T, He F, Lian J, Cai W. Activation of AKT/AP1/FoxM1 signaling confers sorafenib resistance to liver cancer cells. Oncol Rep. 2019;42:785–796. doi: 10.3892/or.2019.7192. [DOI] [PubMed] [Google Scholar]
- 134.Zhi Y, Abudoureyimu M, Zhou H, Wang T, Feng B, Wang R, Chu X. FOXM1-Mediated LINC-ROR Regulates the Proliferation and Sensitivity to Sorafenib in Hepatocellular Carcinoma. Mol Ther Nucleic Acids. 2019;16:576–588. doi: 10.1016/j.omtn.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samarin J, Laketa V, Malz M, Roessler S, Stein I, Horwitz E, Singer S, Dimou E, Cigliano A, Bissinger M, Falk CS, Chen X, Dooley S, Pikarsky E, Calvisi DF, Schultz C, Schirmacher P, Breuhahn K. PI3K/AKT/mTOR-dependent stabilization of oncogenic far-upstream element binding proteins in hepatocellular carcinoma cells. Hepatology. 2016;63:813–826. doi: 10.1002/hep.28357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang W, Liu Y, Fu Y, Han W, Xu H, Wen L, Deng Y, Liu K. Long non-coding RNA LINC00160 functions as a decoy of microRNA-132 to mediate autophagy and drug resistance in hepatocellular carcinoma via inhibition of PIK3R3. Cancer Lett. 2020;478:22–33. doi: 10.1016/j.canlet.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y, Ran Y, Xiong Y, Zhong ZB, Wang ZH, Fan XL, Ye QF. Effects of TMEM9 gene on cell progression in hepatocellular carcinoma by RNA interference. Oncol Rep. 2016;36:299–305. doi: 10.3892/or.2016.4821. [DOI] [PubMed] [Google Scholar]
- 138.Sui C, Dong Z, Yang C, Zhang M, Dai B, Geng L, Lu J, Yang J, Xu M. LncRNA FOXD2-AS1 as a competitive endogenous RNA against miR-150-5p reverses resistance to sorafenib in hepatocellular carcinoma. J Cell Mol Med. 2019;23:6024–6033. doi: 10.1111/jcmm.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hwang S, Takimoto T, Hemler ME. Integrin-independent support of cancer drug resistance by tetraspanin CD151. Cell Mol Life Sci. 2019;76:1595–1604. doi: 10.1007/s00018-019-03014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang PF, Wang F, Wu J, Wu Y, Huang W, Liu D, Huang XY, Zhang XM, Ke AW. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2019;234:2788–2794. doi: 10.1002/jcp.27095. [DOI] [PubMed] [Google Scholar]
- 141.Ye J, Zhang R, Du X, Chai W, Zhou Q. Long noncoding RNA SNHG16 induces sorafenib resistance in hepatocellular carcinoma cells through sponging miR-140-5p. Onco Targets Ther. 2019;12:415–422. doi: 10.2147/OTT.S175176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiong H, Ni Z, He J, Jiang S, Li X, He J, Gong W, Zheng L, Chen S, Li B, Zhang N, Lyu X, Huang G, Chen B, Zhang Y, He F. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36:3528–3540. doi: 10.1038/onc.2016.521. [DOI] [PubMed] [Google Scholar]
- 143.Piatkov I, Caetano D, Assur Y, Lau SL, Jones T, Boyages SC, McLean M. ABCB1 and ABCC1 single-nucleotide polymorphisms in patients treated with clozapine. Pharmgenomics Pers Med. 2017;10:235–242. doi: 10.2147/PGPM.S142314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang H, Chen J, Ding CM, Jin X, Jia ZM, Peng J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J Cell Mol Med. 2018;22:3238–3245. doi: 10.1111/jcmm.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu H, Yang L, Li L, Zeng C. Long non-coding RNA KCNQ1OT1 modulates oxaliplatin resistance in hepatocellular carcinoma through miR-7-5p/ ABCC1 axis. Biochem Biophys Res Commun. 2018;503:2400–2406. doi: 10.1016/j.bbrc.2018.06.168. [DOI] [PubMed] [Google Scholar]
- 146.Shi L, Chen ZG, Wu LL, Zheng JJ, Yang JR, Chen XF, Chen ZQ, Liu CL, Chi SY, Zheng JY, Huang HX, Lin XY, Zheng F. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac J Cancer Prev. 2014;15:10439–10444. doi: 10.7314/apjcp.2014.15.23.10439. [DOI] [PubMed] [Google Scholar]
- 147.Wu LL, Cai WP, Lei X, Shi KQ, Lin XY, Shi L. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J Cell Commun Signal. 2019;13:99–112. doi: 10.1007/s12079-018-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen Y, Zhao H, Li H, Feng X, Tang H, Qiu C, Zhang J, Fu B. LINC01234/MicroRNA-31-5p/MAGEA3 Axis Mediates the Proliferation and Chemoresistance of Hepatocellular Carcinoma Cells. Mol Ther Nucleic Acids. 2020;19:168–178. doi: 10.1016/j.omtn.2019.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Li S, Peng F, Ning Y, Jiang P, Peng J, Ding X, Zhang J, Jiang T, Xiang S. SNHG16 as the miRNA let-7b-5p sponge facilitates the G2/M and epithelial-mesenchymal transition by regulating CDC25B and HMGA2 expression in hepatocellular carcinoma. J Cell Biochem. 2020;121:2543–2558. doi: 10.1002/jcb.29477. [DOI] [PubMed] [Google Scholar]
- 150.Liu Z, Dang C, Xing E, Zhao M, Shi L, Sun J. Overexpression of CASC2 Improves Cisplatin Sensitivity in Hepatocellular Carcinoma Through Sponging miR-222. DNA Cell Biol. 2019;38:1366–1373. doi: 10.1089/dna.2019.4882. [DOI] [PubMed] [Google Scholar]
- 151.Zhao P, Cui X, Zhao L, Liu L, Wang D. Overexpression of Growth-Arrest-Specific Transcript 5 Improved Cisplatin Sensitivity in Hepatocellular Carcinoma Through Sponging miR-222. DNA Cell Biol. 2020;39:724–732. doi: 10.1089/dna.2019.5282. [DOI] [PubMed] [Google Scholar]
- 152.Han S, Han B, Li Z, Sun D. Downregulation of long noncoding RNA CRNDE suppresses drug resistance of liver cancer cells by increasing microRNA-33a expression and decreasing HMGA2 expression. Cell Cycle. 2019;18:2524–2537. doi: 10.1080/15384101.2019.1652035. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.He C, Sun XP, Qiao H, Jiang X, Wang D, Jin X, Dong X, Wang J, Jiang H, Sun X. Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci. 2012;103:528–534. doi: 10.1111/j.1349-7006.2011.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan P, Cao W, Zang Q, Li G, Guo X, Fan J. The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance of hepatocellular carcinoma cells via modulating autophagy. Biochem Biophys Res Commun. 2016;478:1067–1073. doi: 10.1016/j.bbrc.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 156.Yasuda D, Ohe T, Takahashi K, Imamura R, Kojima H, Okabe T, Ichimura Y, Komatsu M, Yamamoto M, Nagano T, Mashino T. Inhibitors of the protein-protein interaction between phosphorylated p62 and Keap1 attenuate chemoresistance in a human hepatocellular carcinoma cell line. Free Radic Res. 2020:1–13. doi: 10.1080/10715762.2020.1732955. [DOI] [PubMed] [Google Scholar]
- 157.Zheng A, Chevalier N, Calderoni M, Dubuis G, Dormond O, Ziros PG, Sykiotis GP, Widmann C. CRISPR/Cas9 genome-wide screening identifies KEAP1 as a sorafenib, lenvatinib, and regorafenib sensitivity gene in hepatocellular carcinoma. Oncotarget. 2019;10:7058–7070. doi: 10.18632/oncotarget.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu L, Pan C, Wei X, Shi Y, Zheng J, Lin X, Shi L. lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun Signal. 2018;16:47. doi: 10.1186/s12964-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cuneo KC, Morgan MA, Davis MA, Parcels LA, Parcels J, Karnak D, Ryan C, Liu N, Maybaum J, Lawrence TS. Wee1 Kinase Inhibitor AZD1775 Radiosensitizes Hepatocellular Carcinoma Regardless of TP53 Mutational Status Through Induction of Replication Stress. Int J Radiat Oncol Biol Phys. 2016;95:782–790. doi: 10.1016/j.ijrobp.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen X, Zhang N. Downregulation of lncRNA NEAT1_2 radiosensitizes hepatocellular carcinoma cells through regulation of miR-101-3p/WEE1 axis. Cell Biol Int. 2019;43:44–55. doi: 10.1002/cbin.11077. [DOI] [PubMed] [Google Scholar]
- 161.Wu B, Wang H, Zhang L, Sun C, Li H, Jiang C, Liu X. High expression of RAD18 in glioma induces radiotherapy resistance via down-regulating P53 expression. Biomed Pharmacother. 2019;112:108555. doi: 10.1016/j.biopha.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 162.Xie C, Wang H, Cheng H, Li J, Wang Z, Yue W. RAD18 mediates resistance to ionizing radiation in human glioma cells. Biochem Biophys Res Commun. 2014;445:263–268. doi: 10.1016/j.bbrc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 163.Chen Y, Shen Z, Zhi Y, Zhou H, Zhang K, Wang T, Feng B, Chen Y, Song H, Wang R, Chu X. Long non-coding RNA ROR promotes radioresistance in hepatocelluar carcinoma cells by acting as a ceRNA for microRNA-145 to regulate RAD18 expression. Arch Biochem Biophys. 2018;645:117–125. doi: 10.1016/j.abb.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 164.Cheng L, Shi X, Huo D, Zhao Y, Zhang H. MiR-449b-5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur J Pharmacol. 2019;856:172399. doi: 10.1016/j.ejphar.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Y, Huang B, Chen Z, Yang S. Knockdown of LINC00473 Enhances Radiosensitivity in Hepatocellular Carcinoma via Regulating the miR-345-5p/FOXP1 Axis. Onco Targets Ther. 2020;13:173–183. doi: 10.2147/OTT.S240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J, Zhou W, Liu Y, Liu T, Li C, Wang L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5'UTR of RUNX3. Oncol Lett. 2018;15:7215–7220. doi: 10.3892/ol.2018.8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Atambayeva S, Niyazova R, Ivashchenko A, Pyrkova A, Pinsky I, Akimniyazova A, Labeit S. The Binding Sites of miR-619-5p in the mRNAs of Human and Orthologous Genes. BMC Genomics. 2017;18:428. doi: 10.1186/s12864-017-3811-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ivashchenko A, Berillo O, Pyrkova A, Niyazova R, Atambayeva S. MiR-3960 binding sites with mRNA of human genes. Bioinformation. 2014;10:423–427. doi: 10.6026/97320630010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yurikova OY, Aisina DE, Niyazova RE, Atambayeva SA, Labeit S, Ivashchenko AT. [The Interaction of miRNA-5p and miRNA-3p with the mRNAs of Orthologous Genes] Mol Biol (Mosk) 2019;53:692–704. doi: 10.1134/S0026898419040189. [DOI] [PubMed] [Google Scholar]
- 170.Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol Cell. 2016;64:320–333. doi: 10.1016/j.molcel.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sen R, Ghosal S, Das S, Balti S, Chakrabarti J. Competing endogenous RNA: the key to posttranscriptional regulation. Scientific World Journal. 2014;2014:896206. doi: 10.1155/2014/896206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bosson AD, Zamudio JR, Sharp PA. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol Cell. 2014;56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]