Abstract
Background
Through this prospective study, we aimed to explore the change of molecular modification after the transient scrotal hyperthermia on human sperm.
Methods
Ten healthy subjects selected with strict screening criteria underwent testicular warming in a 43 °C water bath for 30 min a day for 10 consecutive days. Semen samples were collected 2 weeks before the first heat treatment and 6 weeks after the first heat treatment. Proteins from the samples were labeled with isobaric tags for relative and absolute quantitation and analyzed by two-dimensional liquid chromatography–tandem mass spectrometry.
Results
In contrast to the control, of the 3446 proteins identified, 61 proteins were deregulated: 28 were up-regulated and 33 were down-regulated. Approximately 95% of the differentially expressed proteins were found to participate in spermatogenesis, fertilization, or other aspects of reproduction. In particular, the expression of sperm motility and energy metabolism-related proteins AKAP4, SPESP1, ODF1, ODF2, GAPDHS, and ACTRT2, validated by western blotting of the proteins obtained from human and mouse samples, tended to be reduced under scrotal hyperthermia.
Conclusions
The results indicated that the proteins AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2, play an important role in the heat-induced reversible reduction in sperm concentration and motility and have the potential to be the biomarkers and clinical targets for scrotal heat treatment induced male infertility.
Keywords: Sperm, Proteomics, ITRAQ, Hyperthermia
Introduction
In mammals, including humans, the scrotal temperature required for normal spermatogenesis within the testes is 2 °C to 8 °C lower than the core body temperature. Some patients with varicocele and people in certain occupations, such as boilermakers, cab drivers, and sedentary people, may develop scrotal hyperthermia, resulting in low sperm concentration and mobility and, consequently, infertility. However, once the influencing factor disappears and the scrotal temperature returns to 33 °C–35 °C, spermatogenesis, and sperm parameters revert to normal after one or two spermatogenic cycles [1–3].
Exposing the testes of mice [4], rats [5, 6], monkeys [7], bulls [8], sheep [9], and humans [10, 11] to high temperatures induced germ cell apoptosis in spermatogenesis [12]. Heat stress in the scrotum induced the apoptosis of germ cells [13–16], imbalances in antioxidant levels leading to oxidative stress [17–19], and deregulation in the gene expression pattern [20]. Comparative proteomics of testis biopsy specimens of men subjected to transient scrotal hyperthermia has revealed that these deregulated proteins were associated with germ cell proliferation and apoptosis [21]. Heat stress not only destroys spermatocytes but also affects sperm maturation in the caput epididymis [22]. Exposing sperm to hyperthermia in vitro has been shown to reduce total sperm motility and metabolic activity [23]. In a previous study, we found that oxidative stress, mitochondrial dysfunction, and tight packaging of sperm DNA could induce reversible impairment of spermatogenesis [24]. We also found that sperm concentration and motility in human subjects who experienced scrotal warming in a 43 °C water bath for 30 min a day for 10 consecutive days reduced to the lowest levels (30 and 65%, respectively) in the sixth week after treatment [24, 25]. Although many studies have evaluated the effect of scrotal hyperthermia, the molecular change underlying the oligospermia or asthenospermia induced by heat stress remains unclear.
To understand this molecular modification, we used isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomic analysis to explore the potential effects of scrotal temperatures on male reproductive function. Here we show that these deregulated proteins mainly participate in spermatogenesis, fertilization, and reproduction, of which these expressions of down-regulated proteins AKAP4, ODF1, ODF2, GAPDHS, SPESP1 and, ACTRT2 were validated by western blotting of proteins obtained from human and mouse sperm samples.
Material and methods
Subjects and human sperm specimens
This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Subjects were recruited using the same method and following the same exclusion criteria as in our previous study [25]. Briefly, men aged between 22 and 50 years, who had at least one child were included, and those with cryptorchidism or varicocele, without children, or with severe heart, brain, or renal disease or with sexually transmitted infections, epididymitis, epididymo-orchitis in anamnesis were excluded [26]. In addition, participants were instructed do not to keep in trousers pockets cell phones during the study because of their radiation negative influence on semen quality [27]. Ten subjects met our criteria, of which the subjects underwent testicular warming in a 43 °C water bath for 30 min a day for 10 consecutive days. The lower half body of each subject was immersed in the bathtub with the water regulated to 43 °C. To keep the water temperature constant, we continuously added the adjusted hot water (43 °C) into the bathtub. Meanwhile, we also drained the water from the bathtub by the same flow rate. Sperm specimens were collected 2 weeks before and 6 weeks after the first heat treatment and were prepared for proteomic analysis by iTRAQ and two-dimensional liquid chromatography–tandem mass spectrometry (2D-LC–MS/MS) to identify differentially expressed proteins.
Heat treatment in mice
Fifty adult male ICR mice (8–10 weeks old) were obtained from the Center for Disease Control and Prevention of Hubei Province in China (Grade SPF, Certificate No. SCXK 2015–0018). All mice were fed and maintained under a controlled environment of 20 °C–22 °C, 12/12-h light/dark cycle, and 60% humidity, with ad libitum access to food and water. All animal procedures were approved by the Institutional Review Board of Tongji Medical College and were performed by the National Institutes of Health Guiding Principles on the Care and Use of Animals. The mice were anesthetized with intraperitoneal injections of sodium pentobarbital at 30–50 mg/kg body weight. As described previously [28], the anesthetized mice were placed on a handmade raft in a posture that kept the scrotum immersed in water for 30 min at 43 °C (heat treatment group) or 33 °C (control). The mice were then transferred to a warm room until recovery from anesthesia.
Mature sperm samples were obtained from the epididymis of the mice as previously described [28]. Briefly, sperm samples were obtained by the oil drop method 21 days after the heat treatment, which is when the sperm concentration and motility after hyperthermia were considered to be lowest.
Protein sample preparation and iTRAQ labeling
The human sperm samples were washed three times with phosphate-buffered saline (PBS) and ground to the powder with liquid nitrogen. The total proteins in the control group (sperm specimens collected 2 weeks before the first heat treatment) and heat group (sperm specimens collected 6 weeks after the first heat treatment) were dissolved in protein extraction solution [9 M urea, 4% CHAPS, 1% DTT, 1% IPG buffer (GE Healthcare)] at 30 °C for 1 h. The reaction liquid was then centrifuged at 15,000×g for 15 min at room temperature, and the supernatant was collected and analyzed by the Bradford method to determine the protein concentration. Equal amounts of each protein sample were dissolved and reacted with reductive alkylation and digested with trypsin according to the iTRAQ instructions. The digested peptides were collected and incubated with iTRAQ reagent (SCIEX, USA). Two biological replicates for before and after heat treatment samples were prepared for both groups. The replicates of one group were labeled with isobaric tags 113 and 114, and those of the other group were labeled with tags 114 and 115. The freeze-dried samples being treated by nitrogen were dissolved in Buffer A solution, and SCX chromatography was performed using an Agilent 1200 HPLC System (Agilent) using the following parameters: Poly-SEA, 5 μ, 300 Å, 2.0 × 150 mm, 0.5-ml/min flow, and UV detection at 215 nm and 280 nm. Mixed labeled proteins were separated into 12 segments by liquid chromatography. The data were acquired using a Triple TOF 5600 System (SCIEX, USA) together with a Nanospray III source (SCIEX, USA) and a pulled quartz tip as the emitter (New Objectives, USA), with the ion spray voltage at 2.5 kV, the curtain gas pressure at 30 psi, the nebulizer gas pressure at 5 psi, and the interface heater temperature at 150 °C.
Protein identification and quantification
Protein data were analyzed using ProteinPilot Software v.4.5 (SCIEX, USA), which uses the Paragon algorithm for database searching against the human database. The parameters were as follows: TripleTOF 5600, iTRAQ 4-plex quantification, cysteine modified with iodoacetamide, biological modification selected as ID focus, and trypsin digestion. Using an automated bait database search strategy, the false discovery rate (FDR; i.e., false-positive matches divided by total matches) was evaluated using Proteomics System Performance Evaluation Pipeline Software integrated with ProteinPilot Software. The iTRAQ 4-plex was then selected for protein quantification with unique peptides during the search. The peptides of the sperm samples before and after heat treatment were labeled with isobaric tags 113/115 and 114/116, respectively. One biological replicate of each before and after heat treatment samples was again labeled with isobaric tags 115 and 116. The isobaric tag-labeled samples were then pooled. All proteins with FDR < 1% and the number of peptides > 1 were further analyzed. The differentially expressed proteins were determined by p-values based on the ratios of different protein markers. The proteins that conformed to the requirements of a mean fold change of > 1.5 or < 0.67 and a p-value of < 0.05 were considered to be differentially expressed.
GO and KEGG pathway enrichment analysis
All proteins of interest were matched by a homology search against the human sperm protein database for gene ontology (GO) and KEGG pathway enrichment analysis. The linkages between the protein ID and GO information were established using the public databases NCBI, KEGG, and GO. GO analysis was performed using the Quick GO database, and statistical methods, including the Fisher’s exact test, were used to obtain p values. The annotations for pathways of enriched proteins were used as query data for KEGG pathway enrichment analysis. The protein-protein interactions (PPIs) were identified from the String database. All annotations were associated with their information code.
Western blotting
Sperm samples were homogenized in 80 μl of an ice-cold lysis buffer containing a cocktail of protease inhibitors and lysed by sonication several times. These homogenates were centrifuged at 12,000×g for 15 min at 4 °C, and the supernatant was collected for the quantification of total proteins. Samples with equal protein quantity (50 μg/lane) were electrophoresed on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride membranes (Beyotime, China). The membranes were blocked with 5% milk for 1 h at room temperature and then incubated with primary antibodies overnight at 4 °C. After washing with Tris-buffered saline with Tween 20, the membranes were incubated with secondary antibodies for 1 h at room temperature. Antibodies are listed in Supplementary Table 1. The band intensities were quantified by densitometry using ImageJ analysis software (Research Services Branch).
Statistical analysis
Data were analyzed using the Student’s t-test or the Mann–Whitney U test in case of differences in variance (Fisher’s exact test). All analyses were performed using SPSS 19.0 and GraphPad Prism 7.0. The results are presented as the mean ± SEM and were considered significant for p values of < 0.05. Asterisks indicate: *, p < 0.05.
Results
Experimental design and workflow for the identification of altered proteins in human sperm before and after heat treatment
To identify the heat stress-response proteins in human sperm and achieve a better understanding of the underlying metabolic process and the mechanism of spermatogenesis, we collected sperms from human sperm samples obtained 2 weeks before and 6 weeks after the first treatment and analyzed using an iTRAQ-based quantitative proteomic approach combined with 2D-LC–MS/MS (Fig. 1). The proteins in the sperm samples were quantitatively identified in two biological replicates. In total, 3446 proteins with an FDR of < 1% and a p-value of < 0.05 were identified (Supplementary Table 2).
Fig. 1.
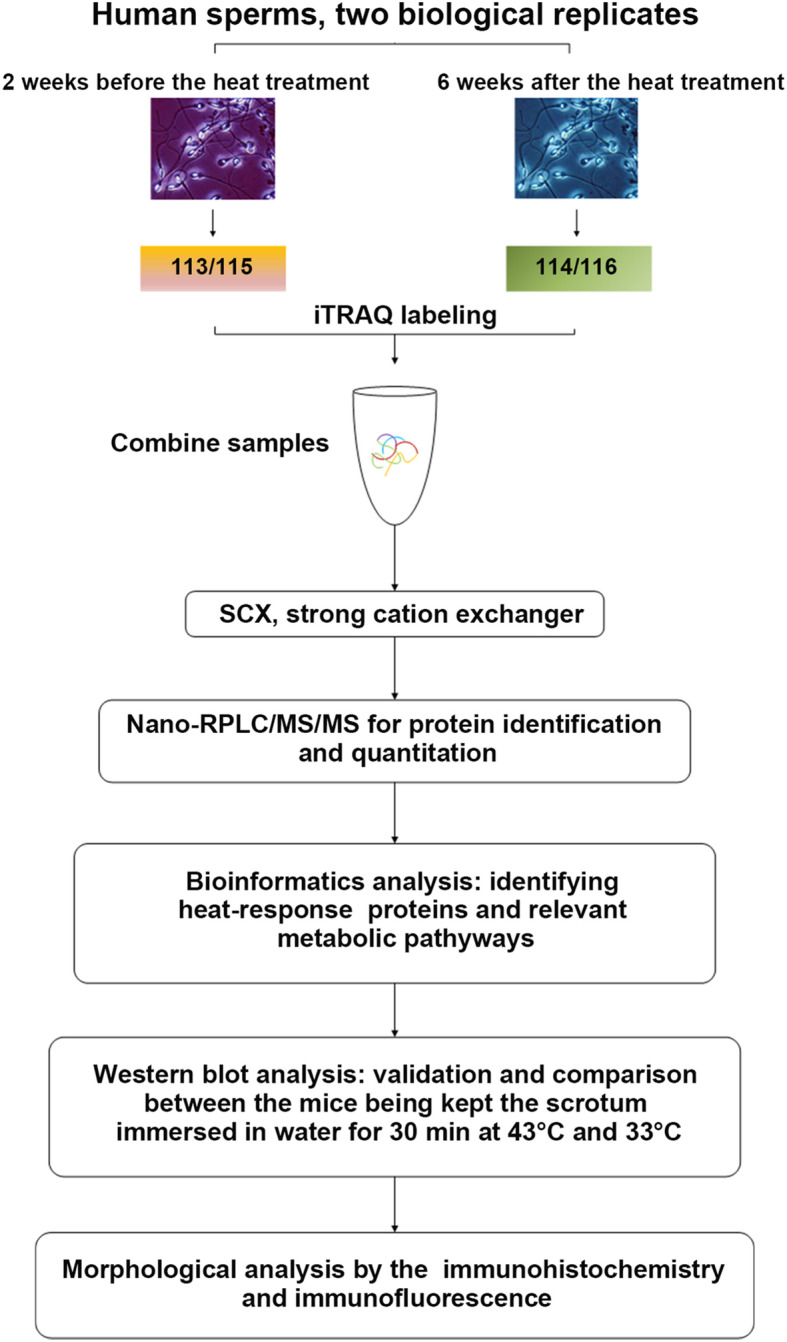
Experimental design and the workflow in this study. Two sets of biological replicate samples were analyzed by iTRAQ, using the hpRP-nanoLC-MS/MS workflow for examining proteome changes between human sperms of 2 weeks before the first treatment (control) and 6 weeks after the first local heat stress of the scrotum (Heat treatment 6w). Proteins were identified using Protein Pilot Software
Functional characterization and annotation of the differentially expressed proteins
To define fold change values that reflect the effect of scrotal hyperthermia on human sperm, the ratios of sperm proteins 2 weeks before and 6 weeks after scrotal hyperthermia in two independent biological duplicate groups were determined by protein labeling with isobaric tags 114/113 and 116/115. In total, 139 proteins in group 1 and 135 in group 2 were found to be differentially expressed based on fold change values of > 1.50 or < 0.67. Combining the results of these two groups, 61 proteins were found to be significantly upregulated (28 proteins) or downregulated (33 proteins) in the heat-treated group 6 weeks after treatment compared with the control group (Table 1).
Table 1.
Differentially expressed proteins in the sixth week after heat-treated group compared with those in the control group
| No. | Gene | Accession | Name | Fold change (avg) |
|---|---|---|---|---|
| 1 | AKAP4 | Q5JQC9 | A-kinase anchor protein 4 | 0.60675 |
| 2 | ODF2 | Q5BJF6 | Outer dense fiber protein 2 | 0.63015 |
| 3 | HK1 | P19367 | Hexokinase-1 | 0.62455 |
| 4 | CCIN | Q13939 | Calicin | 0.62445 |
| 5 | GAPDHS | O14556 | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 0.65205 |
| 6 | TEKT5 | Q96M29 | Tektin-5 | 0.61830 |
| 7 | FAM71B | Q8TC56 | Protein FAM71B | 0.55335 |
| 8 | LRRC37B | Q96QE4 | Leucine-rich repeat-containing protein 37B | 0.62535 |
| 9 | TPI1 | P60174 | Triosephosphate isomerase | 0.59305 |
| 10 | ODF1 | Q14990 | Outer dense fiber protein 1 | 0.60095 |
| 11 | GLUL | P15104 | Glutamine synthetase | 0.65995 |
| 12 | SPESP1 | Q6UW49 | Sperm equatorial segment protein 1 | 0.63020 |
| 13 | RAB2A | P61019 | Ras-related protein Rab-2A | 0.62420 |
| 14 | ZPBP | Q9BS86 | Zona pellucida-binding protein 1 | 0.57560 |
| 15 | ACTRT2 | Q8TDY3 | Actin-related protein T2 | 0.66370 |
| 16 | RSPH9 | Q9H1X1 | Radial spoke head protein 9 homolog | 0.65810 |
| 17 | FAM71A | Q8IYT1 | Protein FAM71A | 0.59755 |
| 18 | MENT | Q9BUN1 | Protein MENT | 0.55490 |
| 19 | ACTL9 | Q8TC94 | Actin-like protein 9 | 0.65075 |
| 20 | CYCL1 | P35663 | Cylicin-1 | 0.57220 |
| 21 | CYCL2 | Q14093 | Cylicin-2 | 0.50275 |
| 22 | VRK3 | Q8IV63 | Inactive serine/threonine-protein kinase VRK3 | 0.62290 |
| 23 | ATP1B3 | P54709 | Sodium/potassium-transporting ATPase subunit beta-3 | 0.63620 |
| 24 | FNDC8 | Q8TC99 | Fibronectin type III domain-containing protein 8 | 0.54235 |
| 25 | PPIL6 | Q8IXY8 | Peptidyl-prolyl cis-trans isomerase-like 6 | 0.64450 |
| 26 | SMCP | P49901 | Sperm mitochondrial-associated cysteine-rich protein | 0.51795 |
| 27 | SLC2A5 | P22732 | Solute carrier family 2, facilitated glucose transporter member 5 | 0.65610 |
| 28 | PFN3 | P60673 | Profilin-3 | 0.58565 |
| 29 | TMEM190 | Q8WZ59 | Transmembrane protein 190 | 0.60795 |
| 30 | SPATA31D1 | Q6ZQQ2 | Spermatogenesis-associated protein 31D1 | 0.66080 |
| 31 | DYDC1 | Q8WWB3 | DPY30 domain-containing protein 1 | 0.65775 |
| 32 | CD46 | P15529 | Membrane cofactor protein | 0.63320 |
| 33 | EFCAB3 | Q8N7B9 | EF-hand calcium-binding domain-containing protein 3 | 0.59870 |
| 34 | HNPNPM | P52272 | Heterogeneous nuclear ribonucleoprotein M | 1.89130 |
| 35 | CLGN | O14967 | Calmegin | 2.11610 |
| 36 | CANX | P27824 | Calnexin | 1.81915 |
| 37 | HNRNPA2B1 | P22626 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 1.84040 |
| 38 | XRCC6 | P12956 | X-ray repair cross-complementing protein 6 | 1.98985 |
| 39 | DDX4 | Q9NQI0 | Probable ATP-dependent RNA helicase DDX4 | 1.72575 |
| 40 | RCN2 | Q14257 | Reticulocalbin-2 | 1.67800 |
| 41 | HNRNPU | Q00839 | Heterogeneous nuclear ribonucleoprotein U | 1.91345 |
| 42 | DDX39A | O00148 | ATP-dependent RNA helicase DDX39A | 1.95795 |
| 43 | HNRNPK | P61978 | Heterogeneous nuclear ribonucleoprotein K | 1.94255 |
| 44 | NPM1 | P06748 | Nucleophosmin | 1.80460 |
| 45 | DDX17 | Q92841 | Probable ATP-dependent RNA helicase DDX17 | 1.76955 |
| 46 | UMOD | P07911 | Uromodulin | 2.17185 |
| 47 | HNRNPA3 | P51991 | Heterogeneous nuclear ribonucleoprotein A3 | 2.18345 |
| 48 | SMC3 | Q9UQE7 | Structural maintenance of chromosomes protein 3 | 1.63640 |
| 49 | RANBP1 | P43487 | Ran-specific GTPase-activating protein | 1.70640 |
| 50 | HNRNPL | P14866 | Heterogeneous nuclear ribonucleoprotein L | 1.74625 |
| 51 | RAN | P62826 | GTP-binding nuclear protein Ran | 1.85995 |
| 52 | DHX15 | O43143 | Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX15 | 1.76235 |
| 53 | ELAVL1 | Q15717 | ELAV-like protein 1 | 1.66060 |
| 54 | HNRNPF | P52597 | Heterogeneous nuclear ribonucleoprotein F | 2.04530 |
| 55 | KHDRBS1 | Q07666 | KH domain-containing, RNA-binding, signal transduction-associated protein 1 | 2.13890 |
| 56 | HNRNPD | Q14103 | Heterogeneous nuclear ribonucleoprotein D0 | 2.00500 |
| 57 | SF3B3 | Q15393 | Splicing factor 3B subunit 3 | 1.82035 |
| 58 | RBMXL2 | O75526 | RNA-binding motif protein, X-linked-like-2 | 1.60315 |
| 59 | RBMY1C | P0DJD4 | RNA-binding motif protein, Y chromosome, family 1 member C | 2.45005 |
| 60 | MYEF2 | Q9P2K5 | Myelin expression factor 2 | 1.66025 |
| 61 | REG3G | Q6UW15 | Regenerating islet-derived protein 3-gamma | 1.68985 |
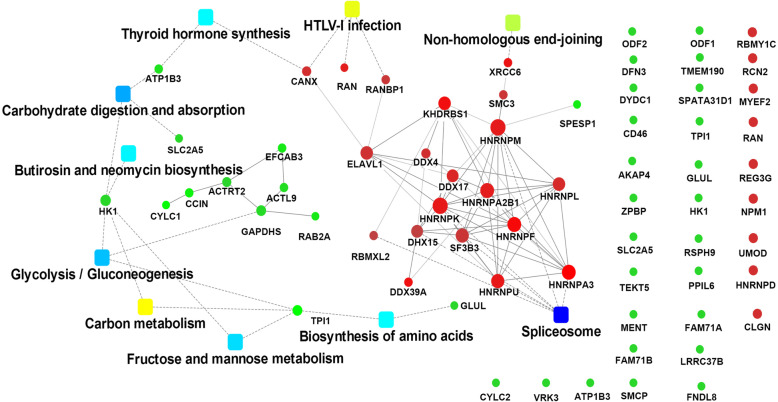
To understand the functions and pathways of the differentially expressed proteins, we used the network tools provided with the DAVID, String, and KEGG databases. The identified proteins were analyzed through GO enrichment analysis (FDR q < 0.05, Fisher’s exact test) in the biological process (BP), cell component (CC), and molecular function (MF) categories. Sexual reproduction, fertilization, gamete generation, and sperm motility were list with most significance in BP analysis (Fig. 2a). The top 10 CC classifications of these differentially expressed proteins included intracellular organelle, ribonucleoprotein complex, cytoplasm, and cilium are shown in Fig. 2b. The top 10 MF categories are shown in Fig. 2c. KEGG analysis revealed that the most active pathways (p ≤ 0.05) involving the differentially expressed proteins included the splicing process, carbohydrate digestion and absorption, glycolysis/gluconeogenesis, and fructose and mannose metabolism (Fig. 2d). Probable interaction analysis of the differentially expressed proteins against the String database revealed two independent PPI networks, one containing 19 proteins and the other containing 7 proteins. The PPI networks with the top nine KEGG enriched pathways are shown in Fig. 3.
Fig. 2.
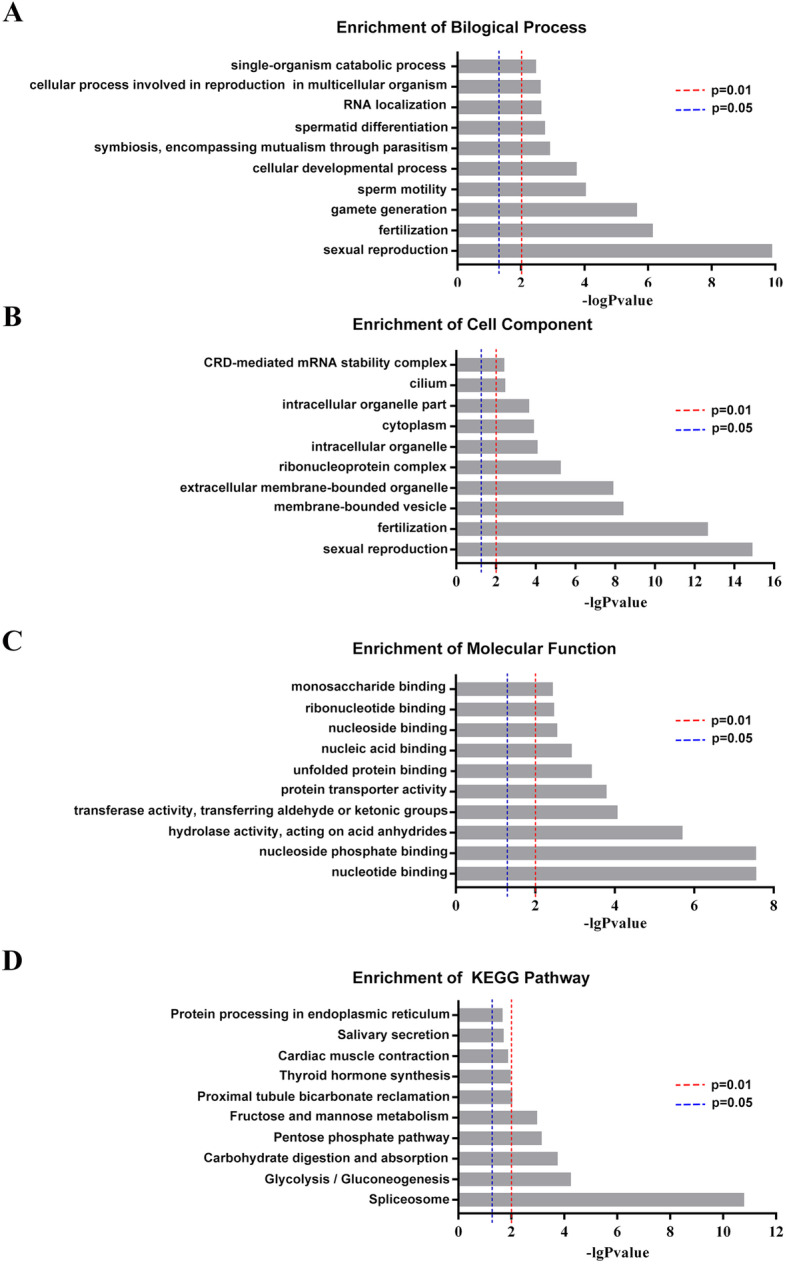
GO annotation indicated the 61 differentially expressed proteins involved the sperm motility and fertility. GO annotation in three categories (a: biological process (BP), b: cellular component (CC), c: molecular function (MF)), and distribution of enriched KEGG pathway (d). The columns are colored with a gradient from blue (p < 0.05) to red (p < 0.01)
Fig. 3.
Molecular pathways of deregulated proteins involved in asthenospermia and oligospermia after heat treatment. A network of protein-protein interactions (PPI) combined with fold changes of proteins, protein-protein interactions, and KEGG pathway enrichment. Red balls represent an up-regulated protein (p < 0.05), green balls represent a down-regulated protein (p < 0.05), and green squares represent the pathway (p < 0.05)
Confirmation of heat stress-induced proteins in mouse sperm by western blotting
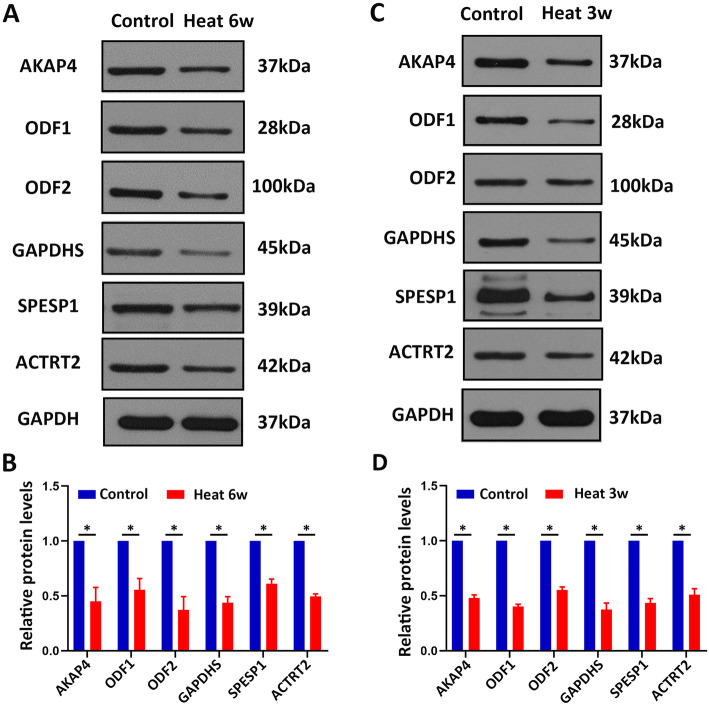
To validate the proteomics finding, the proteins related to sperm motility and energy metabolism AKAP4, SPESP1, ODF1, ODF2, GAPDHS, and ACTRT2 were evaluated in human sperms by western blotting. In line with our proteomics results, the proteins levels of sperm motility and energy metabolism-related proteins AKAP4, SPESP1, ODF1, ODF2, GAPDHS, and ACTRT2 were all significantly decreased in human sperms after heat treatment in comparison with control sperms (Fig. 4a-b), indicating that the proteomic reported here was reliable. Meanwhile, we operated mice subjected to testicular warming in a 43 °C or 33 °C water bath for 30 min once time. After 21 days, epididymis sperm samples were collected and used for western blotting. Meaningfully, these proteins AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 were down-regulated in mouse sperms of heat treatment compared with control (Fig. 4c-d). Collectively, these results indicated that these proteins AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 were significantly down-regulated after heat treatment of scrotum, suggesting that these proteins may serve as the biomarkers for scrotal heat treatment-induced male infertility.
Fig. 4.
Western blot analysis showing AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 proteins from control and heat treatment sperm of human and mouse. a Western blot analysis showing AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 proteins from human sperms of control and heat treatment. GAPDH was used as a loading control. b Quantification of the expression of the proteins shows significantly decreased levels in heat treatment groups. Data are presented as mean ± SEM, n = 3. *, P < 0.05. c-d Western blot analysis showing AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 proteins expression from mouse sperms of control and heat treatment (c) and the quantification of these proteins levels in (d). Data are presented as mean ± SEM, n = 3. *, P < 0.05
Discussion
In the study, we identified 61 proteins significantly deregulated in human sperms of 6 weeks after first heat treatment of the scrotum compared with control using iTRAQ and 2D-LC–MS/MS and selected some proteins related to sperm motility and energy metabolism for verification by western blotting in human and mouse sperms.
These deregulated proteins were found to be involved in several biological processes, including sexual reproduction, fertilization, gametogenesis, sperm motility, cell development, and sperm differentiation. The MF enrichment analysis suggests that the identified differentially expressed proteins are mainly involved in nucleotide binding and nucleoside phosphate binding, consistent with the main functions of these significantly change proteins reported in the previous proteomics study of local heat shock to the scrotum [21]. Our results revealed some new proteins associated with sperm motility, that might be transcribed completely before the metaphase of spermatogenesis and translated in spermatozoa, for example, AKAP4 and mitochondrial-encoded proteins [29].
It takes approximately 64 days in humans and 34.5 days in mice from the first spermatogenesis wave to sperm, and both require four spermatogenic cycles, of which latter 3/4 interval of the four spermatogenic cycles prepares the stage VII spermatids to form mature spermatozoa, which move to the epididymis [28, 30]. In humans, transient scrotal hyperthermia induced the lowest sperm concentration and motility in the latter 3/4 cycle of the four spermatogenic cycles, i.e., at 6 to 8 weeks after heat shock [25]. The sperm concentration and motility of mice after brief scrotal heat treatment have been reported to be lowest at approximately 21 days after transient scrotal heat [28]. The time of lowest sperm concentration and motility after transient scrotal hyperthermia in humans can be considered equivalent to that in mice.
Of these deregulated proteins, most of the upregulated proteins bind with poly-tail RNA, nucleotides, and proteins and participate in biological processes such as mRNA processing and gene expression. Whereas, the down-regulated proteins were mainly involved in fructose transmembrane transport, fructose metabolism to pyruvate, and cell growth and development. We found some down-regulated proteins, such as AKAP4, GAPDHS, SPESP1, ACTRT2, ODF2, and ODF1, play an important role in spermatogenesis. AKAP4 was mainly expressed in the tail of spermatozoa [31] and is involved in sperm motility. It acted as a signaling molecule to regulate spermatozoa and mature sperm acrosome activation by stimulating a signaling cascade [32–34], which was essential for sperm motility and fertility [35]. Heat treatment induces sperm DNA fragmentation significantly increasing and spermatozoa apoptosis [25, 36, 37]. Furthermore, knockdown of AKAP4 led to increased DNA fragmentation and apoptosis [38]. That indicated AKAP4 downregulation is associated with human sperm damage after the transient scrotal hyperthermia. GAPDHS was a testis-specific enzyme expressed at the tubular fibrous sheath of the main segment of the sperm flagellum. It participates in glycolysis and glycogen metabolism pathways and the synthesis of ATP, which is important for sperm motility. Gapdhs deficient mice exhibited non-motile sperm and male sterility [39, 40]. Abnormal GAPDHS expression led to non-obstructive azoospermia [41]. SPESP1 is involved in the fusion of sperm and egg cells and its abnormal expression leads to abnormal distribution of other proteins in spermatozoa. Meanwhile, Spesp1 mutant mice have been shown to low sperm viability and male infertility [42, 43]. The cytoskeletal protein ACTRT2 is expressed at the post-acrosomal region and middle part of human sperm and plays a role in sperm motility. ACTRT2 expression in the mature spermatozoa of patients with varicocele was previously shown to be up-regulated [44], in contrast to the results of the ACTRT2 expression obtained in our study. This difference suggests that the short-term heat shock of scrotum causes its upregulation for compensation. ACTRT2 expression has not been evaluated in mice or other species. Our PPI analysis revealed an interaction between ACTRT2 and GAPDHS, but the specific functions of these proteins remain to be clarified. Abnormal ODF1 expression has been shown to cause abnormal sperm morphology and low sperm motility [45]. ODF1, also known as HSP10, plays an important role in sperm head and neck connections and Odf1 defect mice have been shown to exhibit impairment in the mitochondrial sheath movement of spermatozoa [46, 47]. Previous studies have demonstrated that ODF2 transcription was mainly initiated in primary spermatocyte and, in addition to ODF2 post-processing, combination, and storage is regulated by the hnRNP (A, M, U, K) family proteins [48, 49]. It is then expressed in round spermatids, which interacts with Tssk4 to regulate sperm movement [50]. Thus, these deregulated proteins are closely associated with low sperm viability and concentration after the heat treatment of scrotum and reversible male infertility.
In summary, we used iTRAQ and 2D-LC–MS/MS to identify differential protein expression profiles in human sperms from control and heat treatment of scrotum and found the expression of 61 sperm proteins deregulated after transient scrotal hyperthermia, which consequently decrease sperm concentration and motility. These proteins were mainly involved in sexual reproduction, fertilization, sperm motility, and cellular development. Besides, these proteins AKAP4, ODF1, ODF2, GAPDHS, SPESP1, and ACTRT2 were examined to better verify these results in mice after heat treatment. Further studies of the relationship between these proteins and heat treatment of scrotum are needed to confirm and to further dissect the mechanisms underlying, which are promising to be the biomarkers and clinical targets for scrotal heat treatment-induced male infertility.
Supplementary information
Acknowledgments
Not applicable.
Abbreviations
- iTRAQ
isobaric Tags for Relative and Absolute Quantitation
- 2D-LC-MS/MS
Two-dimensional liquid chromatography–tandem mass spectrometry
- AKAP4
A-Kinase anchor protein 4
- ODF2
Outer dense fiber protein 2
- ODF1
Outer dense fiber protein 1
- SPESP1
Sperm equatorial segment protein 1
- ACTRT2
Actin-related protein t2
- GO
Gene ontology
- hnRNP
Heterogeneous Nuclear Ribonucleoprotein GAPDHS, Glyceraldehyde-3-Phosphate dehydrogenase, testis-specific
- FDR
False discovery rate
Authors’ contributions
WX conceived and direct the study. QW and WX wrote the manuscript. QW, MR, SH, and DK performed all experiments. WX and CZ supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81671507), Fundamental Research Funds for the Central Universities (HUST; grant no. 2015ZDTD050), and National Key Research and Development Program (grant no. 2016YFC1000903).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All the human procedures were approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. All the animal operations were permitted by the Institutional Animal Care and Use Committee (IACUC) of Tongji Medical College, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan-Qing Wu and Meng Rao contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12958-020-00640-w.
References
- 1.Thonneau P, Ducot B, Bujan L, Mieusset R, Spira A. Effect of male occupational heat exposure on time to pregnancy. Int J Androl. 1997;20(5):274–278. doi: 10.1046/j.1365-2605.1997.d01-303.x. [DOI] [PubMed] [Google Scholar]
- 2.Hjollund NH, Bonde JP, Jensen TK, Olsen J. Diurnal scrotal skin temperature and semen quality. The Danish first pregnancy planner study team. Int J Androl. 2000;23(5):309–318. doi: 10.1046/j.1365-2605.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Garolla A, Torino M, Sartini B, Cosci I, Patassini C, Carraro U, Foresta C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum Reprod. 2013;28(4):877–885. doi: 10.1093/humrep/det020. [DOI] [PubMed] [Google Scholar]
- 4.Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65(1):229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- 5.CHOWDHURY AK, STEINBERGER E. A quantitative study of the effect of heat on germinal epithelium of rat testes. Am J Anat. 1964;115:509–524. doi: 10.1002/aja.1001150307. [DOI] [PubMed] [Google Scholar]
- 6.Lue YH, Sinha HA, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of Intratesticular testosterone on stage specificity. Endocrinology. 1999;140(4):1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 7.Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, Ng CM, Hu ZY, Li YC, Leung A, Swerdloff RS. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91(2):539–545. doi: 10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- 8.Barth AD, Bowman PA. The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can Vet J. 1994;35(2):93–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Mieusset R, Quintana CP, Sanchez PL, Sowerbutts SF, Zupp JL, Setchell BP. Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J Reprod Fertil. 1992;94(2):337–343. doi: 10.1530/jrf.0.0940337. [DOI] [PubMed] [Google Scholar]
- 10.Mieusset R, Bujan L. The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int J Androl. 1994;17(4):186–191. doi: 10.1111/j.1365-2605.1994.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 11.Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988;49(1):1–23. doi: 10.1016/s0015-0282(16)59640-x. [DOI] [PubMed] [Google Scholar]
- 12.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18(4):169–184. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldman A, Rodriguez-Casuriaga R, Gonzalez-Lopez E, Capoano CA, Santinaque FF, Geisinger A. MTCH2 is differentially expressed in rat testis and mainly related to apoptosis of spermatocytes. Cell Tissue Res. 2015;361(3):869–883. doi: 10.1007/s00441-015-2163-2. [DOI] [PubMed] [Google Scholar]
- 14.Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod BioMed Online. 2015;30(1):14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Park SJ, Kim TS, Park HJ, Park J, Kim BK, Kim GR, Kim JM, Huang SM, Chae JI, et al. Testicular hyperthermia induces unfolded protein response signaling activation in spermatocyte. Biochem Biophys Res Commun. 2013;434(4):861–866. doi: 10.1016/j.bbrc.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Kanter M, Aktas C, Erboga M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: an immunohistochemical and ultrastructural study. Toxicol Ind Health. 2013;29(2):99–113. doi: 10.1177/0748233711425082. [DOI] [PubMed] [Google Scholar]
- 17.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80(5):913–919. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin C, Shin DG, Park SG, Chu SB, Gwon LW, Lee JG, Yon JM, Baek IJ, Nam SY. Curcumin dose-dependently improves spermatogenic disorders induced by scrotal heat stress in mice. Food Funct. 2015;6(12):3770–3777. doi: 10.1039/c5fo00726g. [DOI] [PubMed] [Google Scholar]
- 19.Kaur S, Bansal MP. Protective role of dietary-supplemented selenium and vitamin E in heat-induced apoptosis and oxidative stress in mice testes. ANDROLOGIA. 2015;47(10):1109–1119. doi: 10.1111/and.12390. [DOI] [PubMed] [Google Scholar]
- 20.Li YC, Hu XQ, Xiao LJ, Hu ZY, Guo J, Zhang KY, Song XX, Liu YX. An oligonucleotide microarray study on gene expression profile in mouse testis of experimental cryptorchidism. Front Biosci. 2006;11:2465–2482. doi: 10.2741/1983. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Cui Y, Xie J, Chen L, Chen X, Guo X, Zhu Y, Wang X, Tong J, Zhou Z, et al. Proteomic analysis of testis biopsies in men treated with transient scrotal hyperthermia reveals the potential targets for contraceptive development. PROTEOMICS. 2010;10(19):3480–3493. doi: 10.1002/pmic.201000281. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Liu F, Gao X, Liu X, Kong X, Wang H, Li J. Comparative proteomic analysis of heat stress proteins associated with rat sperm maturation. Mol Med Rep. 2016;13(4):3547–3552. doi: 10.3892/mmr.2016.4958. [DOI] [PubMed] [Google Scholar]
- 23.Sabes-Alsina M, Tallo-Parra O, Mogas MT, Morrell JM, Lopez-Bejar M. Heat stress has an effect on motility and metabolic activity of rabbit spermatozoa. Anim Reprod Sci. 2016;173:18–23. doi: 10.1016/j.anireprosci.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Rao M, Xia W, Yang J, Hu LX, Hu SF, Lei H, Wu YQ, Zhu CH. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology-US. 2016;4(6):1054–1063. doi: 10.1111/andr.12228. [DOI] [PubMed] [Google Scholar]
- 25.Rao M, Zhao XL, Yang J, Hu SF, Lei H, Xia W, Zhu CH: Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. In., vol. 17; 2015: 668–675. [DOI] [PMC free article] [PubMed]
- 26.Banyra O, Nikitin O, Ventskivska I. Acute epididymo-orchitis: relevance of local classification and partner's follow-up. Cent European J Urol. 2019;72(3):324–329. doi: 10.5173/ceju.2019.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorpinchenko I, Nikitin O, Banyra O, Shulyak A. The influence of direct mobile phone radiation on sperm quality. Cent European J Urol. 2014;67(1):65–71. doi: 10.5173/ceju.2014.01.art14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Crespo M, Pintado B, Gutierrez-Adan A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75(1):40–47. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- 29.Amaral A, Castillo J, Ramalho-Santos J, Oliva R. The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Hum Reprod Update. 2014;20(1):40–62. doi: 10.1093/humupd/dmt046. [DOI] [PubMed] [Google Scholar]
- 30.OAKBERG EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99(3):507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 31.Moretti E, Baccetti B, Scapigliati G, Collodel G. Transmission electron microscopy, immunocytochemical and fluorescence in situ hybridisation studies in a case of 100% necrozoospermia: case report. ANDROLOGIA. 2006;38(6):233–238. doi: 10.1111/j.1439-0272.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang SM, Li HB, Wang JX, Shi YC, Cheng HB, Wang W, Li H, Hou JQ, Wen DG. Morphological characteristics and initial genetic study of multiple morphological anomalies of the flagella in China. Asian J Androl. 2015;17(3):513–515. doi: 10.4103/1008-682X.146100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teijeiro JM, Marini PE. The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro-AKAP4 phosphorylation. Reproduction. 2012;143(6):773–785. doi: 10.1530/REP-11-0314. [DOI] [PubMed] [Google Scholar]
- 34.Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75(2):270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 35.Moretti E, Scapigliati G, Pascarelli NA, Baccetti B, Collodel G. Localization of AKAP4 and tubulin proteins in sperm with reduced motility. Asian J Androl. 2007;9(5):641–649. doi: 10.1111/j.1745-7262.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton T, Siqueira A, de Castro LS, Mendes CM, Delgado JC, de Assis PM, Mesquita LP, Maiorka PC, Nichi M, Goissis MD, et al. Effect of heat stress on sperm DNA: protamine assessment in ram spermatozoa and testicle. Oxidative Med Cell Longev. 2018;2018:5413056. doi: 10.1155/2018/5413056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baskaran S, Agarwal A, Panner SM, Finelli R, Robert KA, Iovine C, Pushparaj PN, Samanta L, Harlev A, Henkel R. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: a scientometric analysis. Reprod Biol Endocrinol. 2019;17(1):110. doi: 10.1186/s12958-019-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jagadish N, Devi S, Gupta N, Suri V, Suri A. Knockdown of A-kinase anchor protein 4 inhibits proliferation of triple-negative breast cancer cells in vitro and in vivo. Tumour Biol. 2020;42(4):1390175187. doi: 10.1177/1010428320914477. [DOI] [PubMed] [Google Scholar]
- 39.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101(47):16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margaryan H, Dorosh A, Capkova J, Manaskova-Postlerova P, Philimonenko A, Hozak P, Peknicova J. Characterization and possible function of glyceraldehyde-3-phosphate dehydrogenase-spermatogenic protein GAPDHS in mammalian sperm. Reprod Biol Endocrinol. 2015;13:15. doi: 10.1186/s12958-015-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorosh A, Tepla O, Zatecka E, Ded L, Koci K, Peknicova J. Expression analysis of MND1/GAJ, SPATA22, GAPDHS and ACR genes in testicular biopsies from non-obstructive azoospermia (NOA) patients. Reprod Biol Endocrinol. 2013;11:42. doi: 10.1186/1477-7827-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujihara Y, Murakami M, Inoue N, Satouh Y, Kaseda K, Ikawa M, Okabe M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J Cell Sci. 2010;123(Pt 9):1531–1536. doi: 10.1242/jcs.067363. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy NS, Melton PE, Cadby G, Yazar S, Franchina M, Moses EK, Mackey DA, Hewitt AW. Meta-analysis of human methylation data for evidence of sex-specific autosomal patterns. BMC Genomics. 2014;15:981. doi: 10.1186/1471-2164-15-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Guo Y, Song N, Fan Y, Li K, Teng X, Guo Q, Ding Z. Proteomic pattern changes associated with obesity-induced asthenozoospermia. Andrology-US. 2015;3(2):247–259. doi: 10.1111/andr.289. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Wang Y, Xu X, Yu Z, Gui YT, Cai ZM. Differential expression of ODF1 in human ejaculated spermatozoa and its clinical significance. Zhonghua Nan Ke Xue. 2009;15(10):891–894. [PubMed] [Google Scholar]
- 46.Yang K, Grzmil P, Meinhardt A, Hoyer-Fender S. Haplo-deficiency of ODF1/HSPB10 in mouse sperm causes relaxation of head-to-tail linkage. Reproduction. 2014;148(5):499–506. doi: 10.1530/REP-14-0370. [DOI] [PubMed] [Google Scholar]
- 47.Yang K, Meinhardt A, Zhang B, Grzmil P, Adham IM, Hoyer-Fender S. The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol Cell Biol. 2012;32(1):216–225. doi: 10.1128/MCB.06158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pletz N, Medack A, Riess EM, Yang K, Kazerouni ZB, Huber D, Hoyer-Fender S. Transcriptional activation of Odf2/Cenexin by cell cycle arrest and the stress activated signaling pathway (JNK pathway) Biochim Biophys Acta. 2013;1833(6):1338–1346. doi: 10.1016/j.bbamcr.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Wei Y, Fu G, Li H, Saiyin H, Lin G, Wang Z, Chen S, Yu L. Tssk4 is essential for maintaining the structural integrity of sperm flagellum. Mol Hum Reprod. 2015;21(2):136–145. doi: 10.1093/molehr/gau097. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Li H, Fu G, Wang Y, Du S, Yu L, Wei Y, Chen S. Testis-specific serine/threonine protein kinase 4 (Tssk4) phosphorylates Odf2 at Ser-76. Sci Rep. 2016;6:22861. doi: 10.1038/srep22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.