Abstract
Heart rate variability (HRV) is the inter-beat interval variation between consecutive heartbeats and an autonomic reflection of emotional regulation abilities to flexibly respond to challenges, such as psychosocial stress. Whereas there are known sex differences in stress-induced hormonal and emotional responses, we identified a gap in our understanding of sex-specific autonomic cardiac control during stress. Thus, we assessed HRV prior to, during and after administration of a public speech task in healthy participants (n=929) according to sex. Our meta-analysis found that during stress, women had lower HRV than men, with an overall Hedges’ g of 0.29 (p<0.0001) and 0.29 (p=0.0003) for fixed and random effects models, respectively. We did not find significant heterogeneity or evidence of publication bias. Analyses of additional timepoints showed no baseline difference and marginally lower HRV in women during anticipation and recovery. Findings of the present meta-analysis confirm sex differences in stress-induced hyperarousal and form a justification for implementation of mechanistic studies evaluating gonadal hormones, their potent metabolites and pro-inflammatory cytokines as mediators of this relationship.
Keywords: Heart rate variability, psychosocial stress, sex differences, Trier Social Stress Test
1. Introduction
Adaptive responses to environmental stressors are essential to maintaining health and well-being. Physiological and psychological responses to stress involve activation of the autonomic nervous system (ANS), the hypothalamic-pituitary-adrenal (HPA) axis, and immune systems that work in concert to restore homeostatic balance. Stress differentially affects men and women – whereas women are more vulnerable to stress-induced hyperarousal, men are more vulnerable to stress-induced attention deficits (Bangasser et al., 2018). In general, women exhibit lower cortisol reactivity (Kajantie and Phillips, 2006; Kudielka and Kirschbaum, 2005), greater subjective distress (Kelly et al., 2008) and increased heart rate (Kudielka et al., 2004) in response to acute psychosocial stress. However, there is much less agreement on the sex-specific effects of stress on autonomic cardiac control, as commonly indexed by heart rate variability (HRV) (Liu et al., 2017). For example, studies have reported blunted HRV responses (Espin et al., 2019), lower overall HRV (Brugnera et al., 2018), or higher overall HRV in women than in men (Adjei et al., 2018; Dimitrov et al., 2018). It is plausible that sex differences in acute stress responses may be related to health disparities between men and women with regard to stress-related diseases such as cardiovascular disease, depression and substance dependence(Beauchaine and Thayer, 2015; Thayer et al., 2010). Thus, in order to fully understand sex differences in stress-related disease and to develop sex-specific treatment approaches, a more comprehensive understanding of differences in all aspects of responses to acute stress, including HRV, is essential.
Heart rate variability refers to the natural variation in the inter-beat interval between consecutive heartbeats. Regular oscillations in inter-beat intervals can be observed in several frequency bands, and originate from many periodic processes, such as respiration, sympathetic outflow to the heart, and circadian rhythms, among others. Of primary interest in the literature on stress is high frequency heart rate variability (HF-HRV), also called respiratory sinus arrhythmia (RSA). HF-HRV consists of rhythmic oscillations in inter-beat intervals in the frequency range of respiration. Influential models of brain-autonomic nervous system interaction suggest that HF-HRV is an autonomic reflection of emotional regulation abilities. Of note, the same models also state that this reflection is one factor of HF-HRV, as the construct, in fact, remains multi-factorial (Porges, 2001; Thayer and Lane, 2000). As such, one aspect that HF-HRV indexes is parasympathetic nervous system (PNS) inhibition of autonomic arousal via vagal nerve influence (Porges, 2001).
Cardiac responses to stress are modulated by both branches of the ANS; the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS). The ANS is particularly important in adaptive responses to stressors because it can rapidly engage in anticipatory action and preparation to satisfy increased regulatory demands even before they occur, instead of relying on reflexive responses. Although also important aspects of the stress response, the HPA-axis and immune systems are not as functionally flexible as the ANS (Sterling, 2012). SNS activation triggers the “fight or flight” response to enable rapid reactions to a threat and accelerate heart rate. By contrast, the PNS promotes a “rest and digest” response that serves to limit stress reactions, decelerate heart rate, and restore equilibrium once the threat has passed. HRV, in particular HF-HRV, has been proposed to reflect the strength of this deceleratory PNS influence on the heart (Porges, 2001). Abnormal autonomic function during acute stress, i.e., imbalances between the SNS and PNS, may lead to hyperarousal, attenuated HRV, and an inability to effectively regulate emotions. It is important to note that HRV, including HF-HRV, can be affected by many factors such as age and fitness and should not be taken to be a “pure” measure of PNS influence; nevertheless, it has proven to be a useful and predictive measure of general emotional regulation ability and autonomic flexibility across many contexts (Balzarotti et al., 2017).
HRV can be assessed with a variety of different metrics, including time-domain and frequency-domain indices, or via non-linear metrics (for review see Shaffer & Ginsberg, 2017). One of the most commonly used time-domain measures of HRV is root mean square of successive differences (RMSSD), which is calculated using the time in milliseconds between normal heart beats over a given range (typically between one and five minutes) (Shaffer et al., 2014). PNS influences are thought to dominate in this measure, although some SNS influences may also be present (Berntson et al., 1997; Berntson et al., 2005). Other time domain measures, such as standard deviation of all R-R intervals (SDNN), or the proportion of number of pairs of successive beat-to-beat intervals that differ more than 50 ms (PNN50), are thought to have comparatively greater contributions of the SNS, circadian rhythms, and other periodic processes. However, with short recording periods such as those typically used in stress studies (e.g. 5 min.), respiratory and PNS influences are still thought to predominate in these measures (Shaffer and Ginsberg, 2017).
Frequency-domain measures of HRV separate heart rate oscillations into different frequency bands. In frequency metrics, HF-HRV is defined by the respiratory frequency band (0.15 to 0.4 Hz). HRV within this band is thought to primarily reflect PNS-based cardiac control. HRV at lower frequencies is influenced by a combination of PNS and SNS effects on the heart; however, the upper limit of SNS influence is approximately 0.1 Hz (Shaffer and Ginsberg, 2017). It is also important to note that over short recording periods, time and frequency domain metrics are typically correlated, consistent with the idea that they both measure primarily high-frequency, PNS-driven changes in inter-beat intervals (Kleiger et al., 2005). Thus, the focus of this review is on measures of HRV thought to index primarily PNS influences on the heart, although the degree of “purity” of these measures may vary somewhat. Although we will continue to use the general term “HRV” throughout this review, metrics thought to primarily index the low, very low or ultra-low components of HRV, which capture more SNS, circadian and other influences, are out of scope here.
There are a number of reasons to expect sex differences in both tonic (resting) and phasic (responses to a stimulus) HRV. There are sex differences in brain structures and circuitry related to cardiac autonomic control and emotion regulation. For example, studies have revealed associations between HRV and areas including the prefrontal cortex, amygdala and paraventricular nucleus(Coote, 2013; Dimitrov et al., 2018; Thayer and Lane, 2009). It is also known that there are sex differences in the anatomy of these regions and their relationship to stress-responses (Dimitrov et al., 2018; Nugent et al., 2011; Ruigrok et al., 2014). Sex differences in HRV between men and women may reflect differences in trait emotionality and self-regulation, and regulation of emotional and physiological responses to stressors that may influence individual susceptibility to stress-related disease and effectiveness of prevention and treatment efforts. The first step to an understanding of potential sex differences in HRV responses to acute stress is to review the existing literature to identify overall patterns of results and critical gaps for future research.
In this meta-analytic study, we specifically sought to examine sex differences in stress-induced changes in HRV, considered as a biomarker of ANS activity. The purpose of the meta-analysis was to gain a better understanding of psychosocial stress reactivity in women – whereas the HPA axis function and emotional regulation during social evaluative stress testing in women are now well-characterized, we identified a gap in the literature related to sex-specific autonomic cardiac control during stress. We focused on studies that used laboratory-based social evaluative stressors as, for example, the Trier Social Stress Test (Kirschbaum et al., 1993) is a reliable, social-evaluative method of inducing an acute stress response, including decreased HRV (Espin et al., 2019; Hermann et al., 2019; Reinelt et al., 2019). It is postulated that women tend to respond to stress in more socially oriented ways (Taylor, 2014) and are more vulnerable to stress-induced hyperarousal (Bangasser et al., 2018). Thus, we hypothesized that, in comparison to men, women would exhibit lower HRV during laboratory-based social evaluative stressor testing.
2. Methods
2.1. Search Strategy
We conducted a literature search in PubMed, Web of Knowledge and PsychInfo, and included eligible studies published through July 26th, 2019. Two authors (AH and SC) completed their search independently according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., 2009). Any discrepancies were reconciled by reviewing the literature jointly for specific points of difference. We used the following search string: ((“Heart Rate Variability” OR “HRV”) AND (“Trier Social Stress Test” OR “TSST”) AND (“Public Speech” OR “Social Evaluative” OR “Psychosocial Stress”) AND (DOCUMENT TYPE: (Article)). We compiled the results in EndNote X8.
2.2. Inclusion/Exclusion Criteria
Included studies had to measure HRV in healthy volunteer adults (Supplementary Table 1) using an electrocardiogram or a portable device during a psychosocial stress task involving public speaking. They had to report mean and variance by sex and use a frequency-domain measure of HRV between 0.15 and 0.4 Hz, a time domain measure such as square root of the mean-squared difference between successive R-R intervals (RMSSD), standard deviation of all R-R intervals (SDNN), the proportion of number of pairs of successive beat-to-beat intervals that differ more than 50 ms (PNN50) or a non-linear measure (e.g., approximate entropy; ApEn). We selected these measures based on a published meta-analysis evaluating parasympathetic function in psychiatric conditions (Alvares et al., 2016) in which only one measure was analyzed according to the above-specified hierarchy in the event that more than one measure was reported. We also performed a sub-analysis separately for time- vs. frequency-based HRV measures. When results were reported only in a figure, we used Web Plot Digitizer to extract data. If there was no report by sex, we contacted the corresponding authors.
2.3. Data Extraction
Data extraction form included the following fields: first author, year, sample size, age, task duration (baseline, anticipation, stress, recovery), analysis window (in minutes) for the respective parts of the stress task, recording method and HRV measure.
As results from different time points cannot be combined unless individual level data is available (Higgins and Green, 2011), in the event stress data was reported separately for speech and arithmetic, we recorded the speech timepoint for the analysis. Similarly, if recovery was reported at multiple timepoints, we recorded and analyzed the timepoint closest to the timepoint reported by the greatest number of studies (5 min).
2.4. Data Analysis
Analyses of HRV for baseline, anticipation, stress and recovery were carried out by calculating the Cohen’s d effect size (Cohen, 1988). In the event mean and variance values were provided for groups of men and women, those were combined using the following formulas:
where, N1= sample size group 1, N2= sample size group 2, M1= mean group 1, M2= mean group 2, SD1=standard deviation group 1, SD2=standard deviation group 2.
We divided the mean difference between the two groups by the pooled standard deviation. Next, we used the J-correction factor to obtain the Hedges’ g effect size, which corrects for small samples (Hedges and Olkin, 1985) and is considered small, medium, and large for values 0.2, 0.5, and 0.8, respectively. We used a random effects model to calculate the pooled effect size with an associated 95% CI and a p-value (Borenstein, 2009), though we present results of both fixed and random effects models. We assessed source-study heterogeneity using the χ2-based Q test with its associated p-value. A statistically significant Q statistic suggests different effect sizes across studies, implying that methodological or population sample differences may be introducing variance across individual studies. We quantified heterogeneity using I2 with values 25%, 50%, and 75% suggestive of small, medium and large heterogeneity and calculated potential publication bias using the Classical Tests (Egger et al., 1997). Finally, we performed a sub-analysis of frequency domain measures vs. RMSSD separately. All the analyses were performed using the “meta” package and the “metacont” function (Schwarzer et al., 2007) in R.
3. Results
3.1. Characteristics of Individual Studies
After removal of duplicate studies, literature search identified 177 individual abstracts as shown in the PRISMA figure (Figure 1). Those abstracts were screened for relevance, with 87 full-text studies reviewed, and 43 studies meeting the inclusion/exclusion criteria. Following the review of full texts, three studies reported HRV by sex (Brugnera et al., 2018; Buchanan et al., 2010; Espin et al., 2019) and 14 corresponding authors sent their summary statistics.
Figure 1.
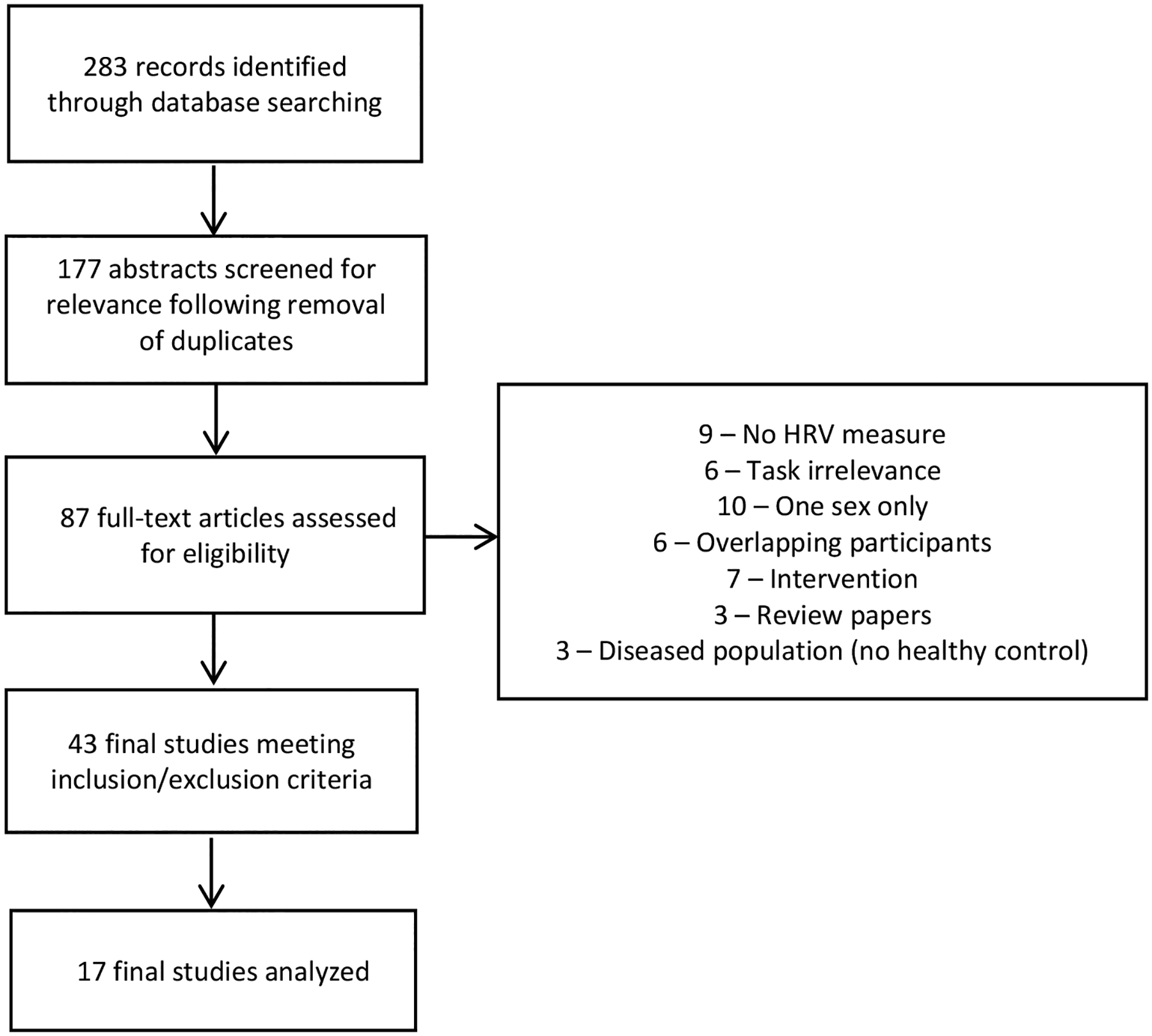
PRISMA flow diagram
As shown in Table 1, study participants included in this meta-analysis averaged 29 years of age. The ratio of men to women was 0.9:1, with 440 men and 489 women included in the meta-analysis. The duration of stress ranged from 2 minutes to 10 minutes, with analysis window ranging between 1 to 10 minutes of recording. The most frequent HRV measure was RMSSD (64%). A total of 9 studies used ECG and 8 studies used a portable device to measure HRV. Four studies (Espin et al., 2019; Gamaiunova et al., 2019; Klumbies et al., 2014; Strahler et al., 2010) controlled for menstrual cycle phase. Of the 17 studies which were included in the meta-analysis, 14 studies completed a manipulation check, verifying a successful induction of stress (Supplementary Table 2).
Table 1.
Individual Study Characteristics
| Author | Year | Sample Size | Participants’ Age | Stress Test Protocol | Analysis Window (min average) | Recording Method | HRV MEASURE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Mean | Standard Deviation | Preparation (min) | Test (min) | Recovery | Baseline | Preparation | Test | Recovery | ||||
| Baert | 2012 | 3 | 11 | 26.7 | 6.6 | 3 | NR | 30 | 20 | NR | NR | 20 | Polar S810i cardiac monitor | RMSSD |
| Brugnera | 2018 | 29 | 31 | 25.6 | 3.8 | 2 | 5 | NR | 5 | 2 | 5 | NR | Portable Device (Pulse) | RMSSD |
| Buchanan | 2010 | 27 | 27 | 50.2 | 10.7 | 10 | 10 | NR | 5 | 10 | 10 | NR | ECG | HF |
| Clamor | 2018 | 27 | 32 | 29.9 | 11.8 | 3 | 10 | 10 | 5 | 5 | 5 | 5 | ECG | RMSSD |
| Dijkhuis | 2019 | 19 | 7 | 20.6 | 1.5 | 7 | 6 | NR | 5 | 5 | 5 | 5 | ECG | RMSSD |
| Espin | 2019 | 25 | 51 | 19.1 | 1.6 | 10 | 10 | 20 | 5 | 5 | 5 | 5 | Portable Device (Suunto, model6) | RMSSD |
| Gamaiunova | 2019 | 12 | 10 | 47.6 | 10.5 | 10 | 10 | 30 | 2 | 2 | 2 | 2 | Portable Device (Polar RS800CX cardiac monitor) | RMSSD |
| Grimley | 2018 | 10 | 20 | 19.8 | 1.4 | NR | 10 | NR | 4 | 5 | 5 | 5 | Wrist-worn pulse oximeter (WristOx2) | HF |
| Jonsson | 2015 | 9 | 7 | 49.2 | 6.4 | 5 | 10 | 40 | 5 | 5 | 5 | 5 | ECG | RSA |
| Klumbies | 2014 | 38 | 30 | 30.2 | 10.0 | 5 | 10 | 60 | 2 | 2 | 2 | 2 | Polar S810i cardiac monitor | RMSSD |
| Maattanen | 2018 | 4 | 42 | 28.1 | 10.0 | 3 | 3 | 8 | NR | NR | NR | NR | ECG | HF |
| Moretta | 2018 | 4 | 17 | 23.3 | 2.9 | 3 | 5 | 3 | 3 | 3 | 3 | 3 | ECG | RMSSD |
| Pulopulos | 2018 | 85 | 74 | 30.0 | 11.1 | 5 | 10 | 15 | 5 | 5 | 5 | 5 | ECG | RMSSD |
| Schwarz | 2018 | 25 | 27 | 42.1 | 40.5 | 5 | 10 | 60 | 2 | 2 | 2 | 2 | Polar 800CX | RMSSD |
| Strahler | 2010 | 72 | 55 | 41.5 | 18.0 | 3 | 10 | 20 | 2 | 2 | 2 | 2 | Portable Device (Polar S810i cardiac monitor) | RMSSD |
| Van Hedger | 2017 | 25 | 16 | 20.0 | 1.3 | 2 | 3 | 5 | 5 | 2 | 3 | 5 | ECG | RSA |
| Wearne | 2019 | 26 | 32 | 22.2 | 6.4 | 10 | 10 | 20 | 5 | 5 | 5 | 5 | ECG | HF |
3.2. Evaluation of Sex Differences in HRV During Stress
Women had lower HRV than men, with an overall Hedges’ g of 0.29 (p<0.0001) for the fixed effects model and 0.29 (p=0.0003) for the random effects model (Figure 2). No significant between-study difference was detected (tau2=0.01; H =1.06 [1.00; 1.37]), with the level of heterogeneity in the small range (I2 = 15.1%, Q=18.85; p=0.27). A visual inspection of the funnel plot (Figure 3) suggested no evidence of publication bias, which was confirmed statistically (p=0.35). An analysis including only the 14 studies which reported successful induction of stress did not change the main finding (Supplementary Figure 1).
Figure 2.
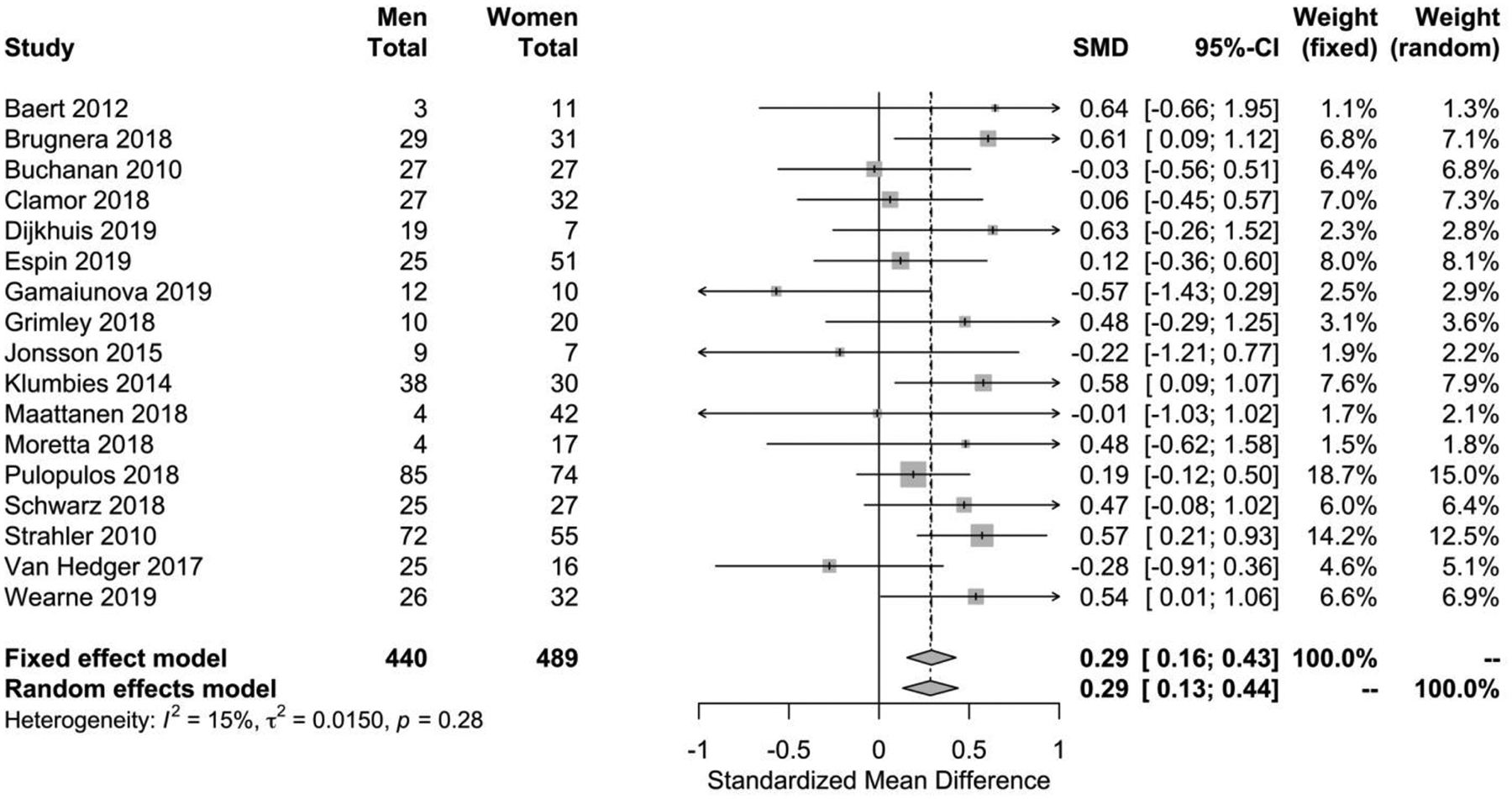
Forest plot of sex differences in HRV during stress. Positive standardized mean difference means that women have lower HRV compared to men (fixed effects model: p<0.0001; random effects model: p=0.0016)
Figure 3.
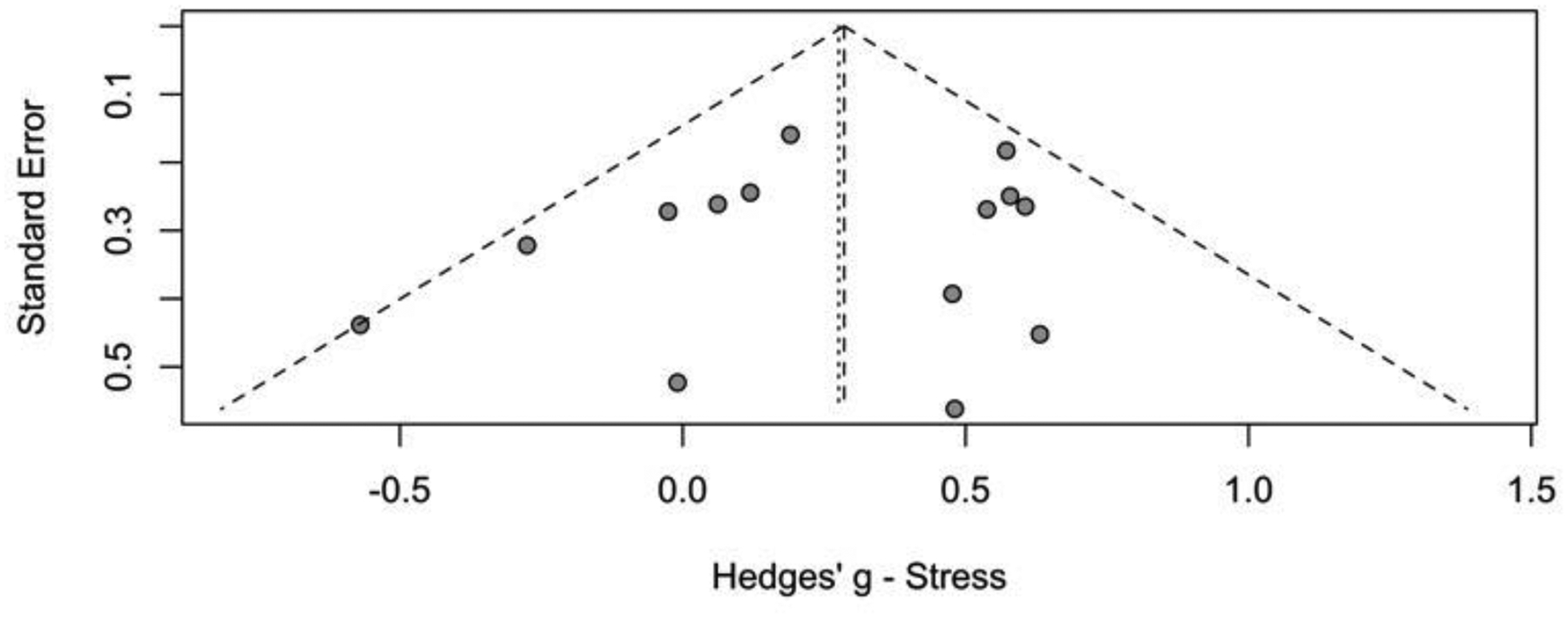
Funnel plot of included studies. Results of statistical analysis indicate absence of asymmetry and publication bias (p=0.50)
3.3. Evaluation of Sex Differences in HRV Before and After Stress
No baseline difference was detected between men and women, with identical fixed and random effect results (Hedges’ g=0.02, z=0.24, p=0.81), lack of heterogeneity (p=0.96) and marginal publication bias (p=0.07). Marginally significant difference between men and women was found during anticipation, with Hedges’ g of 0.14 (z=1.84, p=0.06) for both fixed and random effects models respectively, lack of heterogeneity (p=0.60) and publication bias (p=0.18). Marginally significant difference in the recovery between men and women was found, with identical fixed and random effects (Hedges’ g=0.11, z=1.54, p=0.12), absence of heterogeneity (p=0.80) and marginal publication bias (p=0.13). Figure 4 depicts effect size estimates with 95% confidence intervals for sex differences in HRV across four public speech test phases.
Figure 4.
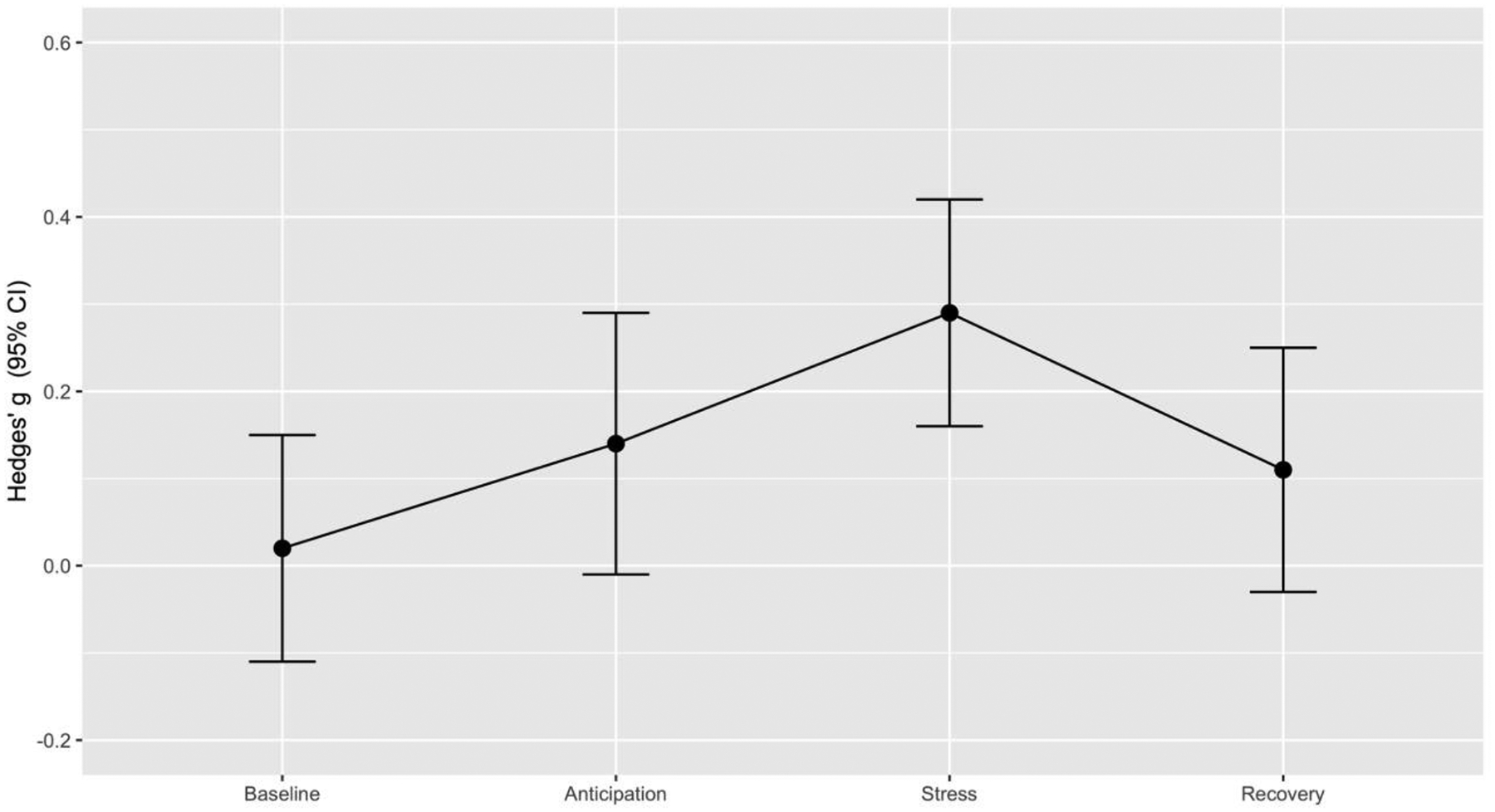
Effect size estimates for sex differences in HRV across four psychosocial stress test phases.
3.4. Sub-Analysis of HRV Measures During Stress
Evaluation of RMSSD showed that women had a statistically lower HRV with the Hedges’ g of 0.34 (p<0.0001) and 0.34 (p=0.0001) for fixed and random effects, respectively with no evidence of heterogeneity (p=0.3) or publication bias (p=0.98). The HF/RSA sub-analyses were not statistically significant for fixed or random effects models. Supplementary Figure 2 displays results of the sub-analysis.
4. Discussion
In the first meta-analysis evaluating sex differences in HRV during stress, we found that women consistently show lower HRV during social stress compared to men. The effect size was small, but highly significant. Our additional aim was to characterize the trajectory of HRV across different timepoints of a laboratory-based social stressor. At baseline, there were no statistically significant sex differences, presenting similar mean. Women displayed marginally lower HRV during stress anticipation and recovery periods.
Influential models of brain-autonomic nervous system interaction suggest that HRV is an autonomic reflection of emotional regulation (Porges, 2001; Thayer and Lane, 2000) abilities. In particular, the high-frequency component of HRV has been proposed to index parasympathetic inhibition of autonomic arousal via vagal nerve influence. Primarily this literature has examined HRV at rest as an indicator of a trait-like ability to flexibly respond to challenges (Beauchaine and Thayer, 2015). However, our findings suggest that examining HRV in response to stress contributes independent information. For example, in our meta-analysis, HRV did not differ between men and women at baseline, and a previous large meta-analysis by Koenig and Thayer (2016) found that women actually have increased HF-HRV power at rest compared to men. This latter finding might suggest the likelihood of more adaptive parasympathetic responses to stress in women. However, our results show lower HRV in women during an actual stress challenge, potentially reflecting poorer autonomic coping during stress. It is important to note that most of the studies included in this meta-analysis reported RMSSD as their measure of HRV. While PNS cardiac control is thought to dominate in this time-domain measure, it includes other influences such as SNS activity, and may also differ somewhat in its influences from frequency-domain measures of HF-HRV.
Our main finding contrasts the findings related to sex differences in other aspects of the stress response, including cortisol, which is the primary hormonal output of the HPA axis. A prior meta-analysis involving 640 women and 710 men (Liu et al., 2017), found that cortisol response to the TSST was lessened in women compared to men, suggesting a reduced stress response in women. Although both the HPA axis and the parasympathetic nervous system fall under the control of a group of forebrain, limbic and brainstem regions commonly termed the “central autonomic network” (Benarroch, 1993; Thayer and Lane, 2000), exact relationships between these aspects of stress responding are unclear. One previous study which jointly examined cortisol and HRV found that lower HRV during the anticipatory period was related to greater cortisol release after stress, but that HRV during the task was unrelated to cortisol release (Pulopulos et al., 2018). This suggests the intriguing possibility that men and women have different autonomic “strategies” for dealing with stress, such that men rely more on mobilization for challenge via HPA activation, and women on parasympathetic withdrawal. Although speculative, this could relate to the different behavioral strategies taken towards stress by men and women, i.e. that women tend to respond with more passive coping strategies (Bale and Epperson, 2015). This possibility should be explored more in future research in humans.
The reasons for these differences in stress response are unknown, but likely relate to both developmental and current hormonal influences (Bale and Epperson, 2015) such as menstrual cycle and use of hormonal contraceptives. Among the differences that may be developmental in origin are differences in the functionality of the paraventricular nucleus. Oxytocin neurons in the paraventricular nucleus activate cardiovagal neurons to influence HRV (Coote, 2013), and the paraventricular nucleus is also a site of sex differences in other aspects of the stress response, including contributions to differences in glucocorticoid release (Oyola and Handa, 2017). Other areas in the central autonomic network also show sex-related differentiation across development that may be related to these differences in HRV. For example, the amygdala and ventromedial prefrontal cortex are thought to be upstream contributors to parasympathetic activity and HRV. Studies in animals have identified sex-specific patterns of maturation in these areas during puberty (Bale and Epperson, 2015), and in humans, these areas display sex-related differences in structure (Ruigrok et al., 2014). Functionally, one study has found that amygdala activity is positively associated with HRV in women but negatively associated with HRV in men (Nugent et al., 2011). Given that these areas are also highly related to stress response, it will be important to further investigate whether they contribute to the differences found here.
Regarding the potential role of current hormonal status, results of both animal and human research are in an agreement that estradiol increases HRV. Intravenous injection of estrogen to ovariectomized female rats produces a dose-response increase of the vagal nerve activity (Saleh et al., 2000) with even low dose estradiol administration to rats increasing HFabs, an important index of cardiac parasympathetic modulation (Campos et al., 2014). In women, HRV is higher during the follicular phase when estrogen levels are increasing and unopposed than during the luteal phase (Bai et al., 2009; McKinley et al., 2009). Collectively, these findings support a potential protective role of estradiol on parasympathetic cardiac tone. The effect of progesterone and its potent metabolite allopregnanolone on ANS during stress is uncertain. Only four studies included in our meta-analysis controlled for menstrual cycle phase; therefore, we were not able to identify the role of current hormonal status on HRV in response to stress. Nonetheless, this analysis is critical in women’s health research as it may uncover important modulation of stress metrics, such as HRV, by gonadal hormones. If this is the case, it will be important to evaluate the potency of testosterone vs. estradiol in enhancing HRV.
Finally, sex differences in stress-induced changes in ANS may be critical factors that contribute to the epidemiology of stress-related psychiatric conditions. The inability to adapt is a central component in vulnerability-stress model of psychiatric disorders, and many psychiatric disorders are sex-specific in prevalence. Several meta-analyses of HRV at rest in psychiatric disorders have been published, with reduced resting HRV found across conditions (Alvares et al., 2016), including anxiety (Chalmers et al., 2014), bipolar (Faurholt-Jepsen et al., 2017) and somatic (Tak et al., 2009) disorders, relative to healthy controls. The effect size of HRV in psychotic disorders is exceptionally large (Hedges’ g = 0.94) (Alvares et al., 2016). Further, through its effects on the HPA and ANS, stress ultimately influences the immune system (Hendrickson and Raskind, 2016) by increasing proinflammatory cytokine levels (Nance and Sanders, 2007) which may be neurotoxic (Buntinx et al., 2004) and related to psychiatric conditions (Ongur et al., 1998; Rajkowska et al., 2007). The parasympathetic branch of the ANS stimulates cholinergic, anti-inflammatory pathways (Rosas-Ballina et al., 2008; Tracey, 2002), which inhibit cytokine production. Therefore, reduced activity in the parasympathetic branch of the ANS in response to stress may also contribute to inflammatory mechanisms in stress-related psychiatric conditions. In summary, this demonstration of insufficient autonomic nervous system responses to stress in women in the present study suggests several possible mechanistic paths that may underlie the epidemiologic phenomenon of greater prevalence of stress-related psychiatric disorders in women.
Of the 17 studies included in the present meta-analysis, 12 report positive direction of standardized mean difference (i.e. greater HRV in men than in women) and 5 (Gamaiunova et al., 2019; Jonsson et al., 2015; Maattanen et al., 2018; Van Hedger et al., 2017, Buchanan et al., 2010) report negative direction for standardized mean difference. Several methodological differences may have contributed to this finding. For example, sample size for the male group in Gamaiunova et al (2019), Jonsson et al (2015) and Maattanen et al (2018) was smaller than majority of the studies (Table 1), raising the possibility that the studies were underpowered to show greater HRV in men. The duration of preparation and speech for Van Hedger et al (2017) was the shortest (5 minutes) of all studies and no report of manipulation check is provided in Buchanan et al (2010), presenting the possibility of inadequate stress induction, though, as shown in Supplementary Table 2, the modified version of the TSST used in the Van Hedger study did significantly alter HRV. Supplementary Table 2 indicates that 14 out of the 17 studies reported successful manipulation checks, showing significant stress-related changes in HPA, HRV or subjective mood states. In fact, our re-analysis of these 14 studies does not change the main findings. In our attempt to try to understand the source of variation between studies in reported effect size estimates, we obtained correlations between HRV during stress and cortisol output, however, generally those correlations were non-significant across studies (data not shown), confirming the previously-reported non-significant relationship between HPA and HRV (Pulopulos et al., 2020; Pulopulos et al., 2018). Nonetheless, the above-specified, or other factors we are unable to decipher from the current data, contributed to the negative direction of standardized means difference in Gamaiunova et al., 2019; Jonsson et al., 2015; Maattanen et al., 2018; Buchanan et a., 2010 and Van Hedger et al., 2017.
This study does present several limitations. A general limitation of the meta-analytic approach is lack of complete data availability. The availability of data from publications in our analysis was 40%. Despite this challenge, in our sample of 929 individuals, we found no evidence of heterogeneity or publication bias across all four of the evaluated timepoints. A conceptual limitation of the present study is that effects in the context of an acute stressor, like a laboratory-based stress-induction paradigm, do not necessarily generalize to the effects of chronic stress in everyday life. Although many theories relate HRV to parasympathetic activation and to vagal nerve activity specifically (Porges, 2001; Thayer and Lane, 2000), others have noted that when respiratory rate is not controlled for, this measure is less physiologically specific (Ritz, 2009). Furthermore, the articles included in this study contained several methodological differences in the collection and assessment of HRV. Durations of the baseline, preparation, test, and recovery phases for HRV measurement varied between studies from as short as two minutes to as long 20 minutes, and both time and frequency-domain measures were included. Further, in some cases measurement durations fell short of the ideal minimum durations for assessing these metrics. These differences could account for some of the variability observed in the present results. The studies included here also differed in whether or not menstrual cycle phase was recorded for women, and this is an important consideration for future studies. Finally, the articles presented here generally did not control for respiration rate.
In the present meta-analysis, we found that across studies comparing HRV in men and women, women show reduced HRV during acute social stress challenge. This finding addresses a gap in the literature related to sex-specific autonomic cardiac control during stress and contributes to a greater understanding of sex-specific physiologic reactivity to social evaluative stressors.
Supplementary Material
Highlights.
During psychosocial stress (i.e. speech delivery), women showed lower heart rate variability (HRV) than men.
Women displayed marginally lower HRV values during stress anticipation and recovery, while the baseline values between men and women were indistinguishable.
These findings contribute to our understanding of sex differences in stress-induced hyperarousal, measured by HRV.
Acknowledgements
The authors would like to thank the following individuals for contributing data for this meta-analysis:
David Braeuer (Technische Universitaet Dresden)
Annika Clamor (Universität Hamburg)
Renee Dijkhuis (Universiteit Leiden)
Rudi De Raedt (Universiteit Gent)
Liudmila Gamaiunova (Université de Lausanne)
Peter Jönsson (Kristianstad University)
Ilmari Määttänen (University of Helsinki)
Tania Moretta (Universita Degli Studi di Padova)
Lisa Olson (University of Redlands)
Matias Pulopulos (Universiteit Gent)
Johanna Schwarz (Stockholm University)
Jana Strahler (Technische Universitaet Dresden)
Travis Wearne (University of New South Wales, Sydney)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjei T, Xue J, Mandic DP, 2018. The Female Heart: Sex Differences in the Dynamics of ECG in Response to Stress. Frontiers in physiology 9, 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares G, Quintana D, Hickie I, Guastella A, 2016. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. JOURNAL OF PSYCHIATRY & NEUROSCIENCE 41, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Li J, Zhou L, Li X, 2009. Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. AMERICAN JOURNAL OF PHYSIOLOGY-HEART AND CIRCULATORY PHYSIOLOGY 297, H765–H774. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN, 2015. Sex differences and stress across the lifespan. Nat Neurosci 18, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarotti S, Biassoni F, Colombo B, Ciceri MR, 2017. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological psychology 130, 54–66. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Telenson AM, Salvatore M, 2018. Sex differences in stress regulation of arousal and cognition. Physiology & behavior 187, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF, 2015. Heart rate variability as a transdiagnostic biomarker of psychopathology. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 98, 338–350. [DOI] [PubMed] [Google Scholar]
- Benarroch E, 1993. The Central Autonomic Network - Functional - Organization, dysfunction, and perspective. Mayo Clinic Proceedings 68, 988–1001. [DOI] [PubMed] [Google Scholar]
- Berntson GG Jr., B.J.T. DL, E. P, G. PG, K. M, M. HN, N. SW, P. JP, S. PH, S. MW, V.d.M., 1997. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, 2005. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology 42, 246–252. [DOI] [PubMed] [Google Scholar]
- Borenstein M, 2009. Effect sizes for continuous data, in: Cooper H, Hedges L, Valentine J (Eds.), The handbook of research synthesis and meta analysis Russell Sage Foundation, New York. [Google Scholar]
- Brugnera A, Zarbo C, Tarvainen M, Marchettini P, Adorni R, Compare A, 2018. Heart rate variability during acute psychosocial stress: A randomized cross-over trial of verbal and non-verbal laboratory stressors. INTERNATIONAL JOURNAL OF PSYCHOPHYSIOLOGY 127, 17–25. [DOI] [PubMed] [Google Scholar]
- Buchanan T, Driscoll D, Mowrer S, Sollers J, Thayer J, Kirschbaum C, Tranel D, 2010. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. PSYCHONEUROENDOCRINOLOGY 35, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M, 2004. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. JOURNAL OF NEUROSCIENCE RESEARCH 76, 834–845. [DOI] [PubMed] [Google Scholar]
- Campos C, Casali K, Baraldi D, Conzatti A, Araujo A, Khaper N, Llesuy S, Rigatto K, Bello-Klein A, 2014. Efficacy of a Low Dose of Estrogen on Antioxidant Defenses and Heart Rate Variability. OXIDATIVE MEDICINE AND CELLULAR LONGEVITY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J, Quintana D, Abbott M, Kemp A, 2014. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. FRONTIERS IN PSYCHIATRY 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York, NY. [Google Scholar]
- Coote JH, 2013. Myths and realities of the cardiac vagus. J Physiol 591, 4073–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov A, Demin K, Fehlner P, Walter H, Erk S, Veer IM, 2018. Differences in Neural Recovery From Acute Stress Between Cortisol Responders and Non-responders. Front Psychiatry 9, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ-BRITISH MEDICAL JOURNAL 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin L, Villada C, Hidalgo V, Salvador A, 2019a. Effects of sex and menstrual cycle phase on cardiac response and alpha-amylase levels in psychosocial stress. Biological psychology 140, 141–148. [DOI] [PubMed] [Google Scholar]
- Faurholt-Jepsen M, Kessing L, Munkholm K, 2017. Heart rate variability in bipolar disorder: A systematic review and meta-analysis. NEUROSCIENCE AND BIOBEHAVIORAL REVIEWS 73, 68–80. [DOI] [PubMed] [Google Scholar]
- Gamaiunova L, Brand P, Bondolfi G, Kliegel M, 2019a. Exploration of psychological mechanisms of the reduced stress response in long-term meditation practitioners. PSYCHONEUROENDOCRINOLOGY 104, 143–151. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I, 1985. Statistical methods for meta-analysis. Academic Press, San Diego, CA. [Google Scholar]
- Hendrickson R, Raskind M, 2016. Noradrenergic dysregulation in the pathophysiology of PTSD. EXPERIMENTAL NEUROLOGY 284, 181–195. [DOI] [PubMed] [Google Scholar]
- Hermann R, Biallas B, Predel H, Petrowski K, 2019. Physical versus psychosocial stress: effects on hormonal, autonomic, and psychological parameters in healthy young men. STRESS-THE INTERNATIONAL JOURNAL ON THE BIOLOGY OF STRESS 22, 103–112. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Collaboration.
- Jonsson P, Osterberg K, Wallergard M, Hansen AM, Garde AH, Johansson G, Karlson B, 2015. Exhaustion-related changes in cardiovascular and cortisol reactivity to acute psychosocial stress. Physiology & behavior 151, 327–337. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL, 2008. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry 39, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The Trier Social Stress Test - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT, 2005. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 10, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumbies E, Braeuer D, Hoyer J, Kirschbaum C, 2014. The Reaction to Social Stress in Social Phobia: Discordance between Physiological and Subjective Parameters. PLOS ONE 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C, 2004. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. International Journal of Behavioral Medicine 11, 116–121. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biological Psychology 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6, e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ein N, Peck K, Huang V, Pruessner J, Vickers K, 2017. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. PSYCHONEUROENDOCRINOLOGY 82, 26–37. [DOI] [PubMed] [Google Scholar]
- Maattanen I, Ravaja N, Henttonen P, Puttonen S, Paavonen K, Swan H, Hintsa T, 2018. Type 1 long QT syndrome and psychological stress in a laboratory setting. J Health Psychol, 1359105317751617. [DOI] [PubMed] [Google Scholar]
- McKinley P, King A, Shapiro P, Slavov I, Fang Y, Chen I, Jamner L, Sloan R, 2009. The impact of menstrual cycle phase on cardiac autonomic regulation. PSYCHOPHYSIOLOGY 46, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance D, Sanders V, 2007. Autonomic innervation and regulation of the immune system (1987–2007). BRAIN BEHAVIOR AND IMMUNITY 21, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC, 2011. Sex differences in the neural correlates of autonomic arousal: a pilot PET study. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 80, 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets W, Price J, 1998. Glial reduction in the subgenual prefrontal cortex in mood disorders. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA 95, 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ, 2017. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress (Amsterdam, Netherlands) 20, 476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, 2001. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Baeken C, De Raedt R, 2020. Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Hormones and Behavior 117. [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Vanderhasselt M-A, De Raedt R, 2018. Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology 94, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier C, Miguel-Hidalgo J, 2007. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. NEUROPSYCHOPHARMACOLOGY 32, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinelt J, Uhlig M, Muller K, Lauckner M, Kumral D, Schaare H, Baczkowski B, Babayan A, Erbey M, Roebbig J, Reiter A, Bae Y, Kratzsch J, Thiery J, Hendler T, Villringer A, Gaebler M, 2019. Acute psychosocial stress alters thalamic network centrality. NEUROIMAGE 199, 680–690. [DOI] [PubMed] [Google Scholar]
- Ritz T, 2009. Studying noninvasive indices of vagal control: the need for respiratory control and the problem of target specificity. Biological psychology 80, 158–168. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish W, Ochani K, Harris Y, Huston J, Chavan S, Tracey K, 2008. Splenic nerve is required for cholinergic anti inflammatory pathway control of TNF in endotoxemia. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J, 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39, 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh T, Connell B, Saleh M, 2000. Acute injection of 17 beta-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. AUTONOMIC NEUROSCIENCE-BASIC & CLINICAL 84, 78–88. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, Antes G, Schumacher M, 2007. A test for publication bias in meta-analysis with sparse binary data. STATISTICS IN MEDICINE 26, 721–733. [DOI] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP, 2017. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 5, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL, 2014. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology 5, 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, 2012. Allostasis: a model of predictive regulation. Physiology & behavior 106, 5–15. [DOI] [PubMed] [Google Scholar]
- Strahler J, Mueller A, Rosenloecher F, Kirschbaum C, Rohleder N, 2010. Salivary alpha-amylase stress reactivity across different age groups. PSYCHOPHYSIOLOGY 47, 587–595. [DOI] [PubMed] [Google Scholar]
- Tak L, Riese H, de Bock G, Manoharan A, Kok I, Rosmalen J, 2009. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. BIOLOGICAL PSYCHOLOGY 82, 101–110. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, 2014. Physiological stress response to loss of social influence and threats to masculinity. Soc Sci Med 103, 51–59. [DOI] [PubMed] [Google Scholar]
- Thayer J, Yamamoto S, Brosschot J, 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology 141, 122–131. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders 61, 201–216. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2009. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33, 81–88. [DOI] [PubMed] [Google Scholar]
- Tracey K, 2002. The inflammatory reflex. NATURE 420, 853–859. [DOI] [PubMed] [Google Scholar]
- Van Hedger K, Necka EA, Barakzai AK, Norman GJ, 2017. The influence of social stress on time perception and psychophysiological reactivity. Psychophysiology 54, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne TA, Lucien A, Trimmer EM, Logan JA, Rushby J, Wilson E, Filipcikova M, McDonald S, 2019. Anxiety sensitivity moderates the subjective experience but not the physiological response to psychosocial stress. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 141, 76–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


