Abstract
Background
Evidence is emerging that surgery in the neonatal period is associated with increased risk of suboptimal neurodevelopmental outcomes (SNDO). The aim of this study was to describe neurodevelopmental outcomes (at 1 year) of neonatal surgery for congenital gastrointestinal surgical conditions (CGSC) and to explore risk factors.
Methods
Retrospective study (2005–2014) of infants born ≥34 weeks gestation with CGSC and admitted to the surgical neonatal intensive care unit of Perth Children’s Hospital, Western Australia. Clinical details and 1-year developmental outcomes based on Griffiths Mental Developmental Assessment Scales were collated from the database and by reviewing the medical records of study infants. SNDO was defined as one or more of the following: a general quotient less than 88 (ie, >1 SD below mean), cerebral palsy, blindness or sensorineural deafness. Univariable and multivariable logistic regression analyses were carried out to explore risk factors for SNDO. A total of 413 infants were included, of which 13 died. Median gestation was 37.6 weeks (IQR: 36.4–39.1). Information on developmental outcomes was available from 262 out of 400 survivors. A total of 43/262 (16.4%) had SNDO. On univariable analysis, lower z scores for birth weight, prolonged duration of antibiotics, increased episodes of general anaesthesia and prolonged duration of hospital stay were associated with SNDO. On multivariable analysis, lower z scores for birth weight and prolonged hospital stay were associated with increased risk of SNDO.
Conclusions
Late preterm and term infants undergoing neonatal surgery for CGSC may be at risk for SNDO. Studies with longer duration of follow-up are needed to further evaluate the role of potentially modifiable risk factors on their neurodevelopmental outcomes.
Keywords: gastroenterology, neonatology
What is known about the subject?
Surgery in the neonatal period may have an adverse effect on neurodevelopment outcomes.
Infection and excessive inflammation are harmful to the developing brain.
What this study adds?
Nearly 16% of late preterm and term infants who underwent neonatal surgery for congenital gastrointestinal conditions had suboptimal neurodevelopment at one year of age.
Lower z scores for birth weight and prolonged hospital stay were associated with increased risk of suboptimal neurodevelopmental outcomes.
C reactive protein levels and infections were not associated with suboptimal neurodevelopmental outcomes at 1 year of age.
Introduction
Survival following neonatal surgery has improved in the recent years, but short-term and long-term complications continue to have significant effects on these infants and their families.1 A recent population-based study that compared developmental outcomes of 124 neonates undergoing non-cardiac surgery versus 92 who underwent cardiac surgery and 162 healthy infants found that cardiac surgery carried the highest risk of developmental delay, but infants undergoing non-cardiac surgeries also had 7%–14% incidence of developmental delay.2
Factors associated with poor developmental outcomes in neonates undergoing surgery include low birth weight,3 chromosomal anomalies, growth restriction,4 prolonged hospital stay,5 need for Extracorporeal Membrane Oxygenation,6 7 chronic lung disease,8 increasing number of surgeries5 and low socioeconomic status.9 One factor that has not been adequately explored in neonates undergoing surgery is the influence of infection and inflammation. Exploring this area is important because infection and excessive inflammation are potentially harmful to the developing brain.10–14
We conducted this retrospective study to evaluate 1-year developmental outcomes of late preterm and term infants who underwent surgery for congenital gastrointestinal surgical conditions (CGSC) in our unit and to explore the potential risk factors. Another aim of the study was to analyse the impact of inflammation on neurodevelopmental outcomes of those infants.
Methods
This was a retrospective cohort study of all late preterm and term infants born at ≥340/7 weeks gestation between January 2005 and December 2014 with CGSC who underwent surgery in the neonatal period at the tertiary neonatal intensive care unit (NICU) of Perth Children’s Hospital, Western Australia.
The following conditions were included in the study—gastroschisis, exomphalos, duodenal atresia, malrotation, jejunoileal atresia, large bowel atresia, meconium ileus, Hirschsprung disease, multiple gut anomalies, gut perforations/stenoses, short bowel syndrome, biliary atresia, anorectal anomalies and benign abdominal cysts. We included oesophageal atresia and congenital diaphragmatic hernia because they also involve the gastrointestinal tract and have long-term gastrointestinal complications.
Infants were identified by interrogating the departmental database. Infants with chromosomal anomalies and syndromes known to adversely affect developmental outcomes were excluded. Infants born at <34 weeks gestation were excluded because they carry a higher risk of adverse developmental outcomes due to prematurity compared with late preterm and term infants.
Clinical characteristics of study infants were extracted from their medical records by one author (VB) and verified for accuracy by a second author (SR). Two neonatologists with expertise in developmental follow-up (DW and JKGT) collated the results of 1-year outcomes based on Griffiths Mental Development Scales (GMDS-II) from the departmental database. The GMDS-II assesses development in five areas: locomotor, personal and social, hearing and speech, eye and hand coordination, and performance. The five subscales are assessed and scored separately and then combined to provide an overall general quotient (GQ) reflecting the child’s developmental performance level relative to the general population. On these scales, a combined GQ of 100.2 (SD 12) is considered normal.15 The GMDS-II is a well-recognised tool for identifying neurosensory disability and is used widely.16 17
Outcome of interest for this study was suboptimal neurodevelopmental outcomes (SNDO) at 1 year of age. SNDO was defined as one or more of the following: (1) a GQ of <88 (ie, >1 SD below mean) on GMDS-II,15 (2) cerebral palsy (based on assessment by neurologist or developmental paediatrician) (3) blindness (visual acuity of <6/60 in the better eye) and (4) sensorineural deafness (based on audiometry assessment) requiring hearing aids.
Healthcare-associated infection (HAI)-included urinary tract infection (UTI) or healthcare-associated blood stream infection (HABSI), meningitis or surgical site infection or any type of viral infection. HABSI was defined as positive blood culture on a sample taken 48 hours after admission to the NICU. UTI was defined as positive culture based on a sample collected from suprapubic sample or in-and-out catheter. Meningitis was diagnosed based on positive culture on cerebrospinal fluid (CSF) samples collected with aseptic precautions. The diagnosis of wound infection was based on the presence of erythema/oedema/induration at the surgical site and positive culture on the wound swab. Respiratory viral infection was diagnosed based on PCR on postnasal aspirate samples taken in infants who presented clinical symptoms of respiratory illness.
C reactive protein (CRP) was used as the marker of inflammation. We stratified the CRP levels based on the timing in relation to the surgical procedure. Empirically, a CRP done in the preoperative period was considered to be a surrogate marker of early onset sepsis, whereas CRP performed within 72 hours of surgery was considered to be related to the degree of surgical injury and CRP performed after 72 hours of surgery to indicate hospital acquired infection.
Statistical analysis was done using the STATA V.16 software (StataCorp). The summary statistics for normally distributed continuous variables were expressed as mean and SD; those with skewed distribution were expressed as median and IQR. Categorical variables were expressed as frequency and percentage. Univariable and multivariable random effect logistic regression models were carried out to derive unadjusted and adjusted odds ratios and 95% CIs. Random effect was included in the fitted model to minimise bias due to the presence of correlated data (ie, multiple measurements of CRP values from individual patients). One-sample t-test was used to compare the mean GQ scores to the population mean (100.2).15 For all analyses, a two-tailed p<0.05 was considered statistically significant.
This retrospective study was approved by the institutional ethics committee as a quality assurance activity. All clinical variables and the results of developmental assessments (GMDS-II) collected for this study were retrospective in nature. Strengthening the Reporting of Observational Studies in Epidemiology guidelines were used to report this study.18
Patient and public involvement
The development of research question and outcome measures for this retrospective study were not informed by patients’ priorities, experience and preferences. Patients were not involved in the design, in the recruitment to and conduct of the study. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy. There are no plans to disseminate the results of this study to study participants.
Results
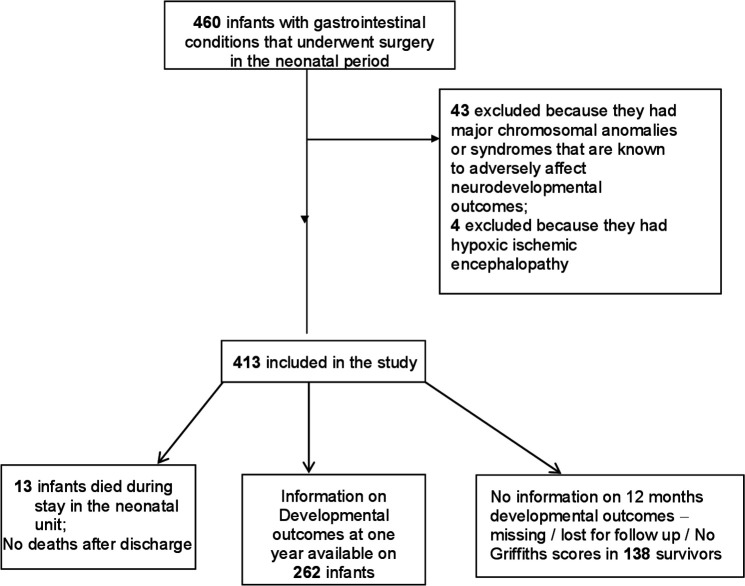
A total of 460 neonates underwent surgery for CGSC during the study period, of which 43 were excluded because of chromosomal anomalies or syndromes that are known to adversely affect neurodevelopmental outcomes. Four infants were excluded because they had moderate to severe hypoxic ischaemic encephalopathy due to perinatal asphyxia. The remaining 413 infants were included in the study. Of them 13 died, and of the 400 surviving infants, full information on developmental outcomes was available for 262/400 (65%) surviving infants. The flow diagram of patient selection process is given in figure 1.
Figure 1.
Study flow diagram.
The median gestation was 37.6 weeks (IQR: 36.4–39.1) and median birthweight 3000 grams (IQR: 2590–3405). The median duration of hospital stay was 18 days (IQR: 11–26 days, range: 1–153 days). There were 13 deaths, all of which were during initial hospital stay. There were no deaths after discharge from the hospital. Table 1 summarises the clinical characteristics in detail.
Table 1.
Characteristics of study infants
| Clinical characteristic | Median or no (percentage) | IQR | Range | N |
| Gestation (weeks) | 37.6 | 36.4 to 39.1 | 34.1 to 41.5 | 413 |
| Gender (male:female) | 57%:43% | NA | NA | 413 |
| Birth weight (grams) | 3000 | 2590 to 3405 | 1664 to 5060 | 413 |
| Birthweight z scores | −0.23 | −0.87 to 0.41 | −2.81 to 4.78 | 413 |
| Birth length (cm) | 49 | 47 to 51 | 40 to 58 | 403 |
| Birth length z scores | −0.03 | −0.75 to 0.57 | −3.38 to 4.23 | 403 |
| Birth head circumference (cm) | 34 | 32.5 to 35 | 28.5 to 47 | 409 |
| Birth head circumference z scores | 0.07 | −0.62 to 0.78 | −3.32 to 3.29 | 408 |
| APGAR 5 min | 9 | 9 to 9 | 4 to 10 | 409 |
| Presurgery C reactive protein (CRP) levels (mg/dL) | 7.5 | 5 to 20 | 1 to 188 | 933 |
| CRP levels within 72 hours of initial surgery (mg/dL) | 35.5 | 19 to 69 | 3 to 346 | 747 |
| CRP levels after 72 hours of surgery (mg/dL) | 15 | 7 to 29 | 1 to 325 | 2912 |
| Healthcare-associated blood stream infection | 27 (6.5%) | NA | NA | 413 |
| CSF culture positive | 1 (0.25%) | NA | NA | 413 |
| Lumbar puncture done | 14 (3.4%) | NA | NA | 413 |
| Viral infections (all respiratory) | 17 (4.1%) | NA | NA | 413 |
| Urinary tract infections (UTI) | 1 (0.24%) | NA | NA | 413 |
| Culture positive surgical site infections | 14 (3.4%) | NA | NA | 413 |
| Any healthcare-associated infection (blood stream or CSF or viral or UTI or wound infection) |
51 (12.4%) | NA | NA | 413 |
| No of antibiotic courses | 2 | 1 to 2 | 1 to 14 | 406 |
| Cumulative duration of antibiotics (days) | 6 | 4 to 8 | 1 to 56 | 406 |
| Surgery episodes under GA | 1 | 1 to 2 | 1 to 5 | 413 |
| No of episodes of hypoglycaemia (blood glucose <2.6 mmol/L) | 0 | 0 to 0 | 0 to 15 | 413 |
| Length of stay (days) | 18 | 11 to 26 | 1 to 153 | 413 |
| Post conception age at discharge (weeks) | 41 | 39.4 to 42.4 | 35.4 to 60.2 | 413 |
| Death before discharge | 13 (3.1%) | NA | NA | 413 |
| Death before 1 year | 13 (3.1%) | NA | NA | 413 |
| Corrected age at Griffiths assessment (months) | 12 | 12 to 12.5 | 10 to 15.5 | 270 |
| GQ scores at 12 months | 96.5 | 92 to 102 | 49 to 131 | 270 |
| SNDO | 43/262 (16.4%) | NA | NA | 262 |
GA, gestational age; GQ, general quotient; NA, not applicable; SGA, small for gestational age;SNDO, Suboptimal developmental outcomes.
The major surgical conditions were gastroschisis, malrotation, oesophageal atresia with or without tracheo-oesophageal fistula, Hirschsprung disease and congenital diaphragmatic hernia (table 2).
Table 2.
Developmental outcomes of neonates with CGISC*
| Major gastrointestinal anomaly | No | Mortality | SNDO among infants who were assessed | Median GQ |
| Gastroschisis | 92 (22.3%) | 3/92 (3.3%) | 8/55 (14.5%) | 98.5 (IQR:92.5–103) n=60 |
| Malrotation | 48 (11.6%) | 3/48 (6.2%) | 4/33 (12.1%) | 96 (IQR:93–103) n=34 |
| Oesophageal atresia | 44 (10.6%) | 1/44 (2.3%) | 10/27 (37%) | 93 (IQR:85–100) n=27 |
| Hirschsprung disease | 44 (10.6%) | 1/44 (2.3%) | 6/32 (18.7%) | 98 (IQR:93–102) n=33 |
| Congenital diaphragmatic hernia | 42 (10.2%) | 1/42 (2.4%) | 5/34 (14.7%) | 94.5 (IQR:92–105) n=34 |
| Ano-rectal anomalies | 39 (9.4%) | 0/39 (0%) | 3/21 (14.3%) | 96 (IQR:90–101) n=22 |
| Gut perforations and stenoses | 19 (4.6%) | 1/19 (5.3%) | 1/12 (8.3%) | 101.5 (IQR:94.5–106) n=12 |
| Duodenal atresia | 19 (4.6%) | 0/19 (0%) | 1/13 (7.7%) | 99 (IQR:94–110) n=15 |
| Jejuno-Ileal atresia | 16 (3.9%) | 0/16 (0%) | 1/9 (11.1%) | 97.5 (IQR:95–102) n=10 |
| Exomphalos | 13 (3.1%) | 0/13 (0%) | 1/6 (16.7%) | 99 (IQR:89–100) n=7 |
| Meconium Ileus | 12 (2.9%) | 0/12 (0%) | 0/5 (0%) | 98 (IQR:96–99) n=5 |
| Multiple gut anomalies | 10 (2.4%) | 1/10 (10%) | 3/7 (42.8%) | 88 (IQR:84–100) n=7 |
| Short bowel syndrome | 5 (1.2%) | 2/5 (40%) | 0/1 (0%) | 95 n=1 |
| Large bowel atresia | 5 (1.2%) | 0/5 (0%) | 0/1 (0%) | 104 n=1 |
| Benign abdominal cysts and tumours | 4 (0.97%) | 0/4 (0%) | 0/1 (0%) | 103 n=1 |
| Biliary atresia | 1 (0.24%) | 0/1 (0%) | 0/1 (0%) | 92 n=1 |
*For all outcomes, infants who underwent at least one episode of surgery were included; infants who died prior to undergoing any surgery were excluded. The information on neurodevelopmental outcomes was available for 65% of survivors.
CGISC, congenital gastrointestinal surgical conditions; GQ, general quotient; SNDO, suboptimal neurodevelopmental outcomes.
A total of 43/262 (16.4%) infants had SNDO, with nine infants having a GQ <76 (ie, more than 2 SD below the mean). One infant had deafness, one had cerebral palsy and none had blindness. The mean GQ was 96.3 (SD 10.3), which was significantly lower than the population mean of 100.2; p<0.001. Infants with multiple gut anomalies, oesophageal atresia, Hirschsprung disease, exomphalos and congenital diaphragmatic hernia had highest rates of SNDO among survivors (table 2).
HABSI occurred in 27 infants (6.5%). A total of 51 (12.4%) infants developed at least one episode of HAI (UTI or HABSI or viral infection or surgical site infection). None of the infants had early-onset sepsis. Coagulase negative Staphylococcus, Klebsiella spp and Eschericia Coli were the most common pathogens isolated (table 3).
Table 3.
Micro-organisms isolated from infants with healthcare-associated infections
| Micro-organism | Blood | CSF | Urine | Viral infections | Wound/skin swab |
| CONS | 16 | 1 | – | – | 3 |
| Eschericia coli | 4 | – | – | – | 3 |
| Klebsiella | 3 | – | – | – | – |
| Pseudomonas | 1 | – | – | – | 2 |
| Streptococcus mitis | 1 | – | – | – | – |
| Moraxella | 1 | – | – | – | – |
| Enterococcus | 1 | – | – | – | 1 |
| Candida albicans | – | – | 1 | – | 2 |
| Staphylococcus aureus | – | – | – | – | 2 |
| Enterobacter cloacae | – | – | – | – | 1 |
| Rhino virus | – | – | – | 12 | – |
| RSV | – | – | – | 2 | – |
| Influenza A | – | – | – | 2 | – |
| Parainfluenza | – | – | – | 1 | – |
| Total | 27 | 1 | 1 | 17 | 14 |
CONS, coagulase negative Staphylococcus; RSV, respiratory syncytial virus.
Association between neonatal risk factors and SNDO among survivors
On univariable analysis, lower birthweight z scores, prolonged duration of antibiotic therapy increasing episodes of general anaesthesia and prolonged duration of hospital stay were associated with higher odds of SNDO among survivors (table 4). On multivariable analysis, lower birthweight z scores and longer duration of hospital stay were associated with increased odds of SNDO among survivors (table 4).
Table 4.
Risk factors for SNDO
| Variable | Unadjusted OR and 95% CI | P value | Adjusted OR and 95% CI | P value |
| Gestational age at birth (≥37 weeks) | 1.07 (0.53 to 2.19) | 0.840 | 1.54 (0.65 to 3.63) | 0.321 |
| Birthweight z scores | 0.64 (0.47 to 0.89) | 0.008* | 0.69 (0.49 to 0.98) | 0.038* |
| Female gender | 0.71 (0.36 to 1.39) | 0.313 | 0.53 (0.24 to 1.16) | 0.112 |
| No of episodes of hypoglycaemia (<2.6 mmol/L) | 1.06 (0.75 to 1.49) | 0.730 | 0.98 (0.60 to 1.58) | 0.923 |
| General anaesthesia (>3 episodes) | 3.31 (1.29 to 8.50) | 0.013* | 0.77 (0.17 to 3.59) | 0.745 |
| Preoperative CRP levels | 0.90 (0.59 to 1.37) | 0.627 | 0.92 (0.60 to 1.40) | 0.698 |
| CRP levels within 72 hours of surgery | 0.90 (0.63 to 1.28) | 0.555 | 1.06 (0.69 to 1.62) | 0.769 |
| CRP levels after 72 hours of surgery | 0.64 (0.41 to 1.01) | 0.053 | 0.99 (0.63 to 1.56) | 0.985 |
| Any infection | 1.20 (0.49 to 2.96) | 0.683 | 0.44 (0.11 to 1.77) | 0.247 |
| Cumulative duration of antibiotics | 1.05 (1.01 to 1.10) | 0.043* | 1.00 (0.91 to 1.10) | 0.990 |
| Degree of postnatal growth restriction | 2.00 (0.59 to 6.81) | 0.265 | 1.56 (0.62 to 3.97) | 0.348 |
| Length of stay | 1.02 (1.00 to 1.03) | 0.003* | 1.03 (1.00 to 1.06) | 0.034* |
*Statistically significant associations
CRP, C reactive protein.
Discussion
Our study found an overall mortality rate of 3.1% and SNDO in 16.4% of neonates undergoing surgery for CGSC. These findings are similar to a recent study that reported an incidence of 7%–14% in various domains of assessment at 3 years among 124 children who underwent surgery for non-cardiac conditions in the neonatal period.2 While the mean GQ of 96.3 in our cohort might not appear too low, it is important to note that the GMDS-II norms are based on population sample more than two decades ago. It is well known that developmental quotients and intelligence quotients in the general population increase by 2–3 points each decade (Flynn effect).19 If assessed using the GMDS-II tools, healthy 12-month-old infants during the study period of 2005–2014 would probably have scored a mean of 103 rather than 100.
Since the study spanned over 10 years (January 2005 to December 2014), advances in anaesthesia, surgical techniques, intensive care management, and changes to family and societal environment during that period could have influenced the in-hospital clinical outcomes and 1-year developmental outcomes of study infants. Contemporary multicentre studies with adequate sample size are needed enhance knowledge in this area.
While many variables were found to be associated with increased risk of SNDO on univariable analysis, only lower birthweight z scores and longer duration of hospital stay were found to be having significant association on multivariable analysis. Lower birthweight z scores indicate fetal growth restriction and prolonged hospitalisation is usually related to the complex nature of the underlying surgical condition. Hence their association with adverse neurodevelopmental outcomes is not unexpected. The width of the CI for birthweight z-scores was very wide, ranging between a drop in the odds between 2% and 50%. The probable reason for this wide range could be related to the timing of intrauterine growth restriction (IUGR). For the same degree of IUGR, the one that starts early during pregnancy is known to have worse outcomes compared with late gestation IUGR.
Each additional day of stay in the hospital resulted in a change in the odds of SNDO by 3%. Many surgical infants stay for a protracted period of time in the hospital and hence these odds are likely to be clinically significant.
The burden of HAI and HABSI in neonates with CGSC has not been explored adequately. Donnell et al and van Saene et al conducted a prospective study of surgical infants<6 months to find infection rates.20 21 Thirty-two infants developed blood culture positive sepsis (15%); predominant micro-organisms (86%) were coagulase-negative staphylococci and enterococci. Other pathogens, including aerobic gram-negative bacilli, were responsible for the remainder. They suggested that gut translocation was the main factor behind sepsis in surgical infants rather than central lines and cautioned that prevention is unlikely to be successful if abnormal gut flora is ignored.21 Another study by Bishay et al reported that 31 out of 112 surgical infants (28%) had a total of 65 episodes of septicaemia.22
In very preterm infants, it is well established that neonatal sepsis is associated with higher risk of adverse neurodevelopmental outcomes. A recent systematic review by Cai et al23 found that preterm infants with neonatal sepsis were at a higher risk of neurodevelopmental impairments such as cerebral palsy and neurosensory deficits, compared with infants without sepsis (OR 3.18; 95% CI 2.29 to 4.41).23 Hence, we had expected similar findings in our cohort of surgical infants. However, in our study, HAI was not associated with increased risk of SNDO, either on univariable or multivariable analysis. Similarly, higher levels of CRPs were not associated with SNDO irrespective of the timing in relation to the surgeries. This could be related to the resilience of the brain of late preterm and term infants to the harmful effects of infection and inflammation, unlike the vulnerable extremely preterm infants. However, prolonged duration of antibiotic therapy, which could be a surrogate marker of clinically suspected infection, was associated with SNDO on univariable, but not multivariable analysis. Further studies with larger sample size and a longer duration of follow-up beyond 1 year of age are needed to explore the role of infection and inflammation in late preterm and term infants undergoing neonatal surgery.
The harmful effect of exposures to general anaesthesia on developing brain is an area of debate and active research.24 25 While animal studies have consistently shown general anaesthesia to be toxic to the developing brain,26 one recent large RCT27 and a large prospective cohort study28 found no significant association. Both these studies evaluated a single exposure to general anaesthesia, and hence do not address the issue of repeated exposures. A recent large data linkage study found that children exposed to general anaesthesia before 4 years have poorer development outcomes at school entry and school performance.29 In another cohort study,30 children who had multiple exposure to gestational age (GA) before 3 years of age scored 1.3 points (95% CI −3.8 to 1.2; p=0.32) less than unexposed children on intelligence tests; children who had one exposure to GA scored 0.5 points (95% CI −2.8 to 1.9; p=0.70) less than unexposed children. However, the parents of children who had multiple exposure to GA reported increased problems related to executive function, behaviour and reading.30 In our cohort, increasing episodes of general anaesthesia were associated with higher risk of SNDO on univariable analysis, but not on multivariable analysis. Further studies with long duration of follow-up are needed in this area.
While we found lower birthweight z scores and prolonged hospital stay to be associated with increased risk of SNDO, one should not ignore the possibility that the underlying surgical condition in itself could be an important risk factor that drives other morbidities leading to SNDO. In our cohort, multiple gut anomalies and oesophageal atresia had the highest incidence of SNDO (42.8% and 37%, respectively), which is not unexpected because these infants have significant in-hospital and postdischarge morbidities, which puts them at a higher risk of SNDO.
One of the limitations of our study was the shorter duration of follow-up of 1 year and the findings may not track subsequently. In a recent study, Fairbairn et al reported that Bayley-III results for all domains at 1 year of age were a weak predictor of outcomes at 3 years of age in infants who had early major cardiac and non-cardiac surgery and healthy infants.31 Hence all infants, irrespective of the results of developmental assessments at 1 year should be followed with formal developmental assessments at least until 5 years of age. At the same time, infants identified as high risk based on the 1-year assessments could be provided early developmental interventions to optimise their outcomes. Only recently, we have commenced routine developmental follow-up until 2 years of age with Bayley Scales of Infant Development to all infants undergoing surgery in the neonatal period.
Surgical infants who need prolonged duration of mechanical ventilation are at higher risk of hypoxic episodes and hence worse developmental outcomes. At the same time, prolonged ventilation could be a maker of severity of the underlying anomaly. A limitation of our study was the lack of reliable information on the duration of mechanical ventilation among the study infants.
The other limitations of our study were: (1) retrospective design without healthy controls, (2) the indication for doing CRP levels was at the discretion of clinicians rather than based on a standardised protocol, (3) full information on developmental outcomes was missing from nearly 35% of survivors, (4) lack of information on sociodemographic status of family and (5) missing information about duration of general anaesthesia which can have significant influence on developmental outcomes. The data were from a single centre from a high-income country and hence the findings may not be generalisable. The main strength of the study is the large sample size of surgical infants and the use of regression analyses to adjust for confounders.
Conclusions
Late preterm and term infants undergoing surgery for CGSC may be at risk for SNDO at 1 year of age. Studies with long-term follow-up are needed to further evaluate the influence of potentially modifiable risk factors on neurodevelopmental outcomes in such infants.
Supplementary Material
Acknowledgments
We sincerely thank Damber Shrestha for providing data from the departmental database. Patient advisers were not involved in this retrospective study.
Footnotes
Contributors: SR conceptualised and designed the study, coordinated and supervised data collection, verified the data for accuracy, drafted the initial manuscript, and reviewed and revised the manuscript. VB collected the data, assisted with drafting of the initial manuscript and reviewed the manuscript SP and KS critically reviewed and revised the manuscript. They also provided feedback at the design and analysis stage of the study DW and JKGT provided information regarding the developmental outcomes of all study infants. They critically reviewed and revised the manuscript. IG critically reviewed and revised the manuscript. MKB conducted the statistical analysis of the data, reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: Project Grant from Centre for Neonatal Research and Education, Faculty of Health and Medical Sciences, University of Western Australia.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This retrospective study was approved by the institutional ethics committee as a quality assurance activity.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The deindentified patient data are available from the correspondence author and will be provided on reasonable request.
References
- 1.Escobar MA, Caty MG. Complications in neonatal surgery. Semin Pediatr Surg 2016;25:347–70. 10.1053/j.sempedsurg.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Walker K, Loughran-Fowlds A, Halliday R, et al. Developmental outcomes at 3 years of age following major non-cardiac and cardiac surgery in term infants: a population-based study. J Paediatr Child Health 2015;51:1221–5. 10.1111/jpc.12943 [DOI] [PubMed] [Google Scholar]
- 3.Newton LE, Abdessalam SF, Raynor SC, et al. Neurodevelopmental outcomes of tracheoesophageal fistulas. J Pediatr Surg 2016;51:743–7. 10.1016/j.jpedsurg.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 4.Aite L, Bevilacqua F, Zaccara A, et al. Short-Term neurodevelopmental outcome of babies operated on for low-risk esophageal atresia: a pilot study. Dis Esophagus 2014;27:330–4. 10.1111/dote.12114 [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua F, Ravà L, Valfrè L, et al. Factors affecting short-term neurodevelopmental outcome in children operated on for major congenital anomalies. J Pediatr Surg 2015;50:1125–9. 10.1016/j.jpedsurg.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 6.Danzer E, Gerdes M, D'Agostino JA, et al. Preschool neurological assessment in congenital diaphragmatic hernia survivors: outcome and perinatal factors associated with neurodevelopmental impairment. Early Hum Dev 2013;89:393–400. 10.1016/j.earlhumdev.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 7.Danzer E, Gerdes M, Bernbaum J, et al. Neurodevelopmental outcome of infants with congenital diaphragmatic hernia prospectively enrolled in an interdisciplinary follow-up program. J Pediatr Surg 2010;45:1759–66. 10.1016/j.jpedsurg.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 8.Cortes RA, Keller RL, Townsend T, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg 2005;40:36–46. Discussion 45-6. 10.1016/j.jpedsurg.2004.09.037 [DOI] [PubMed] [Google Scholar]
- 9.Wynn J, Aspelund G, Zygmunt A, et al. Developmental outcomes of children with congenital diaphragmatic hernia: a multicenter prospective study. J Pediatr Surg 2013;48:1995–2004. 10.1016/j.jpedsurg.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bright HR, Babata K, Allred EN, et al. Neurocognitive outcomes at 10 years of age in extremely preterm newborns with late-onset bacteremia. J Pediatr 2017;187:43–9. 10.1016/j.jpeds.2017.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 2015;11:192–208. 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2012;71:444–57. 10.1002/ana.22620 [DOI] [PubMed] [Google Scholar]
- 13.Jiang NM, Cowan M, Moonah SN, et al. The impact of systemic inflammation on neurodevelopment. Trends Mol Med 2018;24:794–804. 10.1016/j.molmed.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi D, Qiao L, Bergelson I, et al. Staphylococcus epidermidis bacteremia induces brain injury in neonatal mice via Toll-like receptor 2-dependent and -independent pathways. J Infect Dis 2015;212:1480–90. 10.1093/infdis/jiv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntley M. The griffiths mental development scales from birth to two years: manual. Amersham: Association for Research in Infant and Child Development (ARICD), 1996. [Google Scholar]
- 16.Abdel-Latif ME, Bajuk B, Oei J, et al. Population study of neurodevelopmental outcomes of extremely premature infants admitted after office hours. J Paediatr Child Health 2014;50:E45–54. 10.1111/jpc.12028 [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Latif ME, Bajuk B, Ward M, et al. Neurodevelopmental outcomes of extremely premature infants conceived after assisted conception: a population based cohort study. Arch Dis Child Fetal Neonatal Ed 2013;98:F205–11. 10.1136/archdischild-2012-302040 [DOI] [PubMed] [Google Scholar]
- 18.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- 19.Trahan LH, Stuebing KK, Fletcher JM, et al. The Flynn effect: a meta-analysis. Psychol Bull 2014;140:1332–60. 10.1037/a0037173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnell SC, Taylor N, van Saene HKF, et al. Infection rates in surgical neonates and infants receiving parenteral nutrition: a five-year prospective study. J Hosp Infect 2002;52:273–80. 10.1053/jhin.2002.1318 [DOI] [PubMed] [Google Scholar]
- 21.van Saene HKF, Taylor N, Donnell SC, et al. Gut overgrowth with abnormal flora: the missing link in parenteral nutrition-related sepsis in surgical neonates. Eur J Clin Nutr 2003;57:548–53. 10.1038/sj.ejcn.1601578 [DOI] [PubMed] [Google Scholar]
- 22.Bishay M, Retrosi G, Horn V, et al. Septicaemia due to enteric organisms is a later event in surgical infants requiring parenteral nutrition. Eur J Pediatr Surg 2012;22:050–3. 10.1055/s-0031-1287853 [DOI] [PubMed] [Google Scholar]
- 23.Cai S, Thompson DK, Anderson PJ, et al. Short- and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis. Children 2019;6:1. 10.3390/children6120131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andropoulos DB. Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn Ther 2018;43:1–11. 10.1159/000475928 [DOI] [PubMed] [Google Scholar]
- 25.Hansen TG. Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatr Anaesth 2015;25:65–72. 10.1111/pan.12548 [DOI] [PubMed] [Google Scholar]
- 26.Rappaport BA, Suresh S, Hertz S, et al. Anesthetic neurotoxicity-clinical implications of animal models. N Engl J Med 2015;372:796–7. 10.1056/NEJMp1414786 [DOI] [PubMed] [Google Scholar]
- 27.Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (gas): an international multicentre, randomised controlled trial. Lancet 2016;387:239–50. 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun LS, Li G, Miller TLK, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 2016;315:2312–20. 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneuer FJ, Bentley JP, Davidson AJ, et al. The impact of general anesthesia on child development and school performance: a population-based study. Paediatr Anaesth 2018;28:528–36. 10.1111/pan.13390 [DOI] [PubMed] [Google Scholar]
- 30.Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo anesthesia safety in kids (mask) study. Anesthesiology 2018;129:89–105. 10.1097/ALN.0000000000002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairbairn N, Galea C, Loughran-Fowlds A, et al. Prediction of three year outcomes using the Bayley-III for surgical, cardiac and healthy Australian infants at one year of age. Early Hum Dev 2018;117:57–61. 10.1016/j.earlhumdev.2017.12.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.