Abstract
Proper lung development depends on the precise temporal and spatial expression of several morphogenic factors, including Fgf10, Fgf9, Shh, Bmp4, and Tgf-β. Over- or under-expression of these molecules often leads to aberrant embryonic or postnatal lung development. Herein, we deleted the Tgf-β1 gene specifically within the lung embryonic mesenchymal compartment at specific gestational stages to determine the contribution of this cytokine to lung development. Mutant embryos developed severe lung hypoplasia and died at birth due to the inability to breathe. Despite the markedly reduced lung size, proliferation and differentiation of the lung epithelium was not affected by the lack of mesenchymal expression of the Tgf-β1 gene, while apoptosis was significantly increased in the mutant lung parenchyma. Lack of mesenchymal expression of the Tgf-β1 gene was also associated with reduced lung branching morphogenesis, with accompanying inhibition of the local FGF10 signaling pathway as well as abnormal development of the vascular system. To shed light on the mechanism of lung hypoplasia, we quantified the phosphorylation of 226 proteins in the mutant E12.5 lung compared with control. We identified five proteins, Hrs, Vav2, c-Kit, the regulatory subunit of Pi3k (P85), and Fgfr1, that were over- or under-phosphorylated in the mutant lung, suggesting that they could be indispensable effectors of the TGF-β signaling program during embryonic lung development. In conclusion, we have uncovered novel roles of the mesenchyme-specific Tgf-β1 ligand in embryonic mouse lung development and generated a mouse model that may prove helpful to identify some of the key pathogenic mechanisms underlying lung hypoplasia in humans.
Introduction
Over the past few decades, numerous research groups have highlighted the critical roles of several growth factors and signaling pathways in branching morphogenesis, cell proliferation, differentiation, and alveologenesis in the embryonic and postnatal lung [1, 2]. Despite the vast amount of data available, new findings and functions are discovered by adjusting either the time (embryonic versus postnatal versus adult) or the cellular location (epithelium versus mesenchyme versus specific lung cell type), in which these signaling pathways are manipulated.
The TGF-β signaling pathway is known to play critical roles in embryonic and postnatal lung development and hemostasis as well as in the pathogenesis of several adult lung diseases [3]. Activation of TGF-β signaling is initiated by the binding of the TGF-β ligand to the TGF-β receptors I and II. This leads to an intracellular protein phosphorylation cascade which drives the nuclear translocation of Smad2/3 proteins and transcription of TGF-β target genes [4]. In mammals three TGF-β ligands exist, Tgf-β1, -β2, and -β3. Genetic deletion of the Tgf-β ligands and receptors have uncovered several critical roles of the TGF-β signaling pathway during embryonic and postnatal lung development, adult lung regeneration after injury and onset and progression of lung diseases [5-8]. Conventional deletion of the Tgf-β1 gene in mice with C57BL/6 strain background resulted in intrauterine early embryonic lethality for most of the homozygous mutants, while Tgf-β1 conventional knockout in mice with strain background 129/Sv x CF-1 resulted in 50% mutants that died embryonically. The mutant mice that survived postnatally developed massive inflammation primarily in heart and lung by 3–4 weeks of age [9, 10]. Conventional deletion of the Tgf-β1 gene in mice was associated with multiple developmental defects of the heart, eye, inner ear, urogenital tract, facial bones, and palate. The lung of E18.5 mutant Tgfb2−/− fetuses appeared normal; however, the airways of the mutant pups collapsed at birth [11]. Tgf-β3−/− null embryos developed cleft palate [12, 13] and displayed delayed pulmonary development [14, 15].
All Tgf-β ligands act on cells through paracrine and autocrine manners. However, Tgf-β1 is able to stimulate cells through an additional endocrine mechanism [16]. Tgf-β1 can be produced by bone marrow, maternal and pathological sources and secreted into the plasma through which it can target distant cells and tissues. For example, Dickson et al. has shown that Tgf-β1 is delivered by the Tgf-β1+/− mother to the Tgf-β1−/− embryos through the placenta [17].
Conventional genetic deletion may mask key roles of a gene in specific organs by allowing the embryo to activate compensatory genes or signaling pathways. As mentioned above, for Tgf-β1, the compensatory mechanism may involve the maternal source of Tgf-β1 and other unknown factors in different genetic backgrounds. Furthermore, lung phenotypes in whole-body genetic deletion models may also be secondary to developmental defects in other organs [18] and potentially lead to cause-effect misinterpretation of the observed phenotype.
During lung development, Tgf-β1 is expressed by the mesenchyme adjacent to the epithelium [19]. Herein, to clarify and deepen our understanding of the role of Tgf-β1 ligand in lung development, we conditionally deleted the Tgf-β1 gene (Tgf-β1 CKO) specifically in the lung mesenchyme by utilizing the Tbx4-rTta/Tet-On-Cre mouse line [20].
We discovered that Tgf-β1 mutant embryos displayed agonal breathing at birth and died within a few hours after delivery. The lungs in these animals were severely hypoplastic [21], yet the differentiation of the lung epithelial cells was preserved. Cellular apoptosis was increased in the mutant lung parenchyma, while branching morphogenesis was significantly reduced. Real-time PCR and in situ hybridization revealed that mesenchyme-specific Tgf-β1 deletion strongly inhibited the FGF10 signaling pathway. Finally, using a protein microarray approach, we identified five proteins whose phosphorylation was changed in the mutant lungs and therefore could be involved in the genesis of the hypoplastic lung phenotype.
Materials and methods
Ethics statement
We adhered to the The National Institute of Health guidelines for animal care and safety. All animal experimental protocols were approved by the Animal Care and Use Committee of the Children’s Hospital of Los Angeles.
Animals
The Tbx4-rtTA mouse line was developed by Dr. Wei Shi who cloned a lung specific enhancer of the Tbx4 gene promoter upstream to the rtTA gene and, thus, allows genetic manipulation specifically in the lung mesenchyme [20]. Tet-O-Cre and Tgf-β1fl/fl mouse strains were bought from Jackson. Tgf-β2 mice were provided by Mohamed Azhar (University of South Carolina) [22]. To knockout the Tgf-β1 gene in the embryonic lung, Tbx4-rtTA/Tet-O-Cre/Tgf-β1fl/fl males were generated and crossed with Tgf-β1fl/fl females. Pregnant females were fed with doxycycline chow beginning at gestational stage E7.5 until lungs were harvested (unless otherwise indicated). All pregnant females in this study were fed with doxycycline containing chow. Lungs from embryos missing Tbx4-rtTA, TeT-O-Cre, or both were used as negative controls.
Histology and immunofluorescence staining
Embryonic lungs were fixed for up to 4 h at room temperature, while adult lungs were fixed overnight before being embedded in paraffin. Four-micrometer-thick tissue sections were used for immunohistochemistry and immunofluorescence. The following primary antibodies were used: α-SMAam, Ctgf, (GeneTex), Cc10 (Santa Cruz Biotechnology), Sftpc (Seven Hills Bioreagents), T1-α (Developmental Studies Hybridoma Bank), E-Cadherin, Phospho-H3 (Cell Signaling Technology), and Tubb4b (Biogenex). Secondary antibodies (Alexa-donkey-anti-mouse-546, Alexa-donkey-anti-rabbit-488 and Alexa-donkey-anti-goat-633) were bought from Thermo Fisher Scientific.
Whole mount PECAM1 staining
E12.5 lungs were harvested in cold PBS and fixed overnight at 4 °C in freshly prepared 4% paraformaldehyde. The next day, lungs were dehydrated in methanol, treated with H2O2/methanol (4:1) for 20 min, rehydrated to PBST (PBS, 0.5% Tween) and washed three times in PBST. Lungs were submerged in blocking buffer (with PBST/5% donkey serum) for 2 h and then incubated for 3 days at room temperature with the rat-anti-Pecam1 antibody (1/200, in PBS-T/5% donkey serum) (BD Pharmigen). On the third day, lungs were washed five times with PBST/5% donkey serum (1 h each time) and incubated with the secondary antibody (Alexa-donkey-anti-rat-555, 1/200 in PBST/5% donkey serum, Thermo Fisher Scientific) for 2 days. Unbound secondary antibody was washed away with PBS-T/5% donkey serum for 4–5 h. Embryonic lungs were then dehydrated in methanol and cleared with BABB for fluorescence confocal imaging.
In situ hybridization
E12.5 lungs were harvested and fixed with 4% formalin in phosphate buffered saline (PBS) at room temperature for 20 min. The samples were washed in PBS for 5 min, dehydrated and stored in 100% ethanol. Whole mount in situ hybridization was performed as previously described [23-25], with Fgf10, Bmp4, and Shh antisense riboprobes transcribed from murine cDNA templates cloned into bacterial plasmids.
Embryonic lung culture
Embryonic lungs were collected from E12.5 old embryos and cultured on top of a polycarbonate membrane (0.8 μm) (EMD Millipore) in a 4-well plate with 1 ml of DMEM/F12/HEPES and 15mM cell culture medium with antibiotics (penicillin and streptomycin). The cell medium was changed every other day. Lungs were cultured for up to 3 days. Wortmannin and PD173074 were bought from SelleckChem and resuspended in DMSO.
cDNA synthesis and real-time qRT-PCR
Total RNA was isolated from mouse E12.5 and E13.5 lungs using Trizol (Thermo Fisher Scientific). Three to four embryonic lungs were pooled together to get sufficient good-quality RNA for expression analysis. In total, 500–1000 ng of RNA was reverse-transcribed using the Iscript cDNA synthesis kit (Bio-Rad). Real-time quantitative PCR was performed in a Lightcycler II (Roche Applied Science) using gene-specific primers (Operon, Huntsville, AL, USA) and fluorescence probes (Roche Applied Science). The sequence of the primers used was Fgf10 (s-TCAGGACATGGTGTCACAGG, as-CATCTCCTTGGAGGTGATTGT); Bmp4 (s-GAGGAGTTTCCATCACGAAGA, as-GCTCTGCCGAGGAGATCA); Shh (s-TTTTCGTTATTGTCTTATATGGGTTG, as-TCTTGAAGGTCCGTTCATATTTTT); Aldh1a2 (s-CATGGTATCCTCCGCAATG, as-GCGCATTTAAGGCATTGTAAC); Fgf9 (s-TTGGATCATTTAAAGGGGATTCT, as-CCCACTGCTATACTGATAAATTCCA); and β-Catenin (s-TCCCCTGACAGAGTTACTCCA, as-TGTGGCTTGTCCTCAGACAT).
Tyrosine Phosphorylation ProArray Kit
Tyrosine Phosphorylation ProArray was bought from Full Moon Biosystems (Sunnyvale, CA, USA) (#PST228). To retrieve enough protein for the array, ten E12.5 control and twelve E12.5 mutant lungs were pooled together. Protein extract was quantified with using DC protein assay (Bio-Rad Laboratories): 40 μg of protein lysate was conjugated with biotin and incubated on the phospho-protein array. The fluorescence intensity of each spot was quantified using Image-J and spots were compared after subtracting background and negative control signals. The signal intensity for the six replicates were averaged and showed as average ± standard deviation.
Statistical analysis
The data are presented as the means ± standard deviation (SD). The differences between the groups were analyzed using a Student’s t-test.
Results
Mesenchymal knockout of the Tgf-β1 gene was associated with severe hypoplasia of the embryonic lung
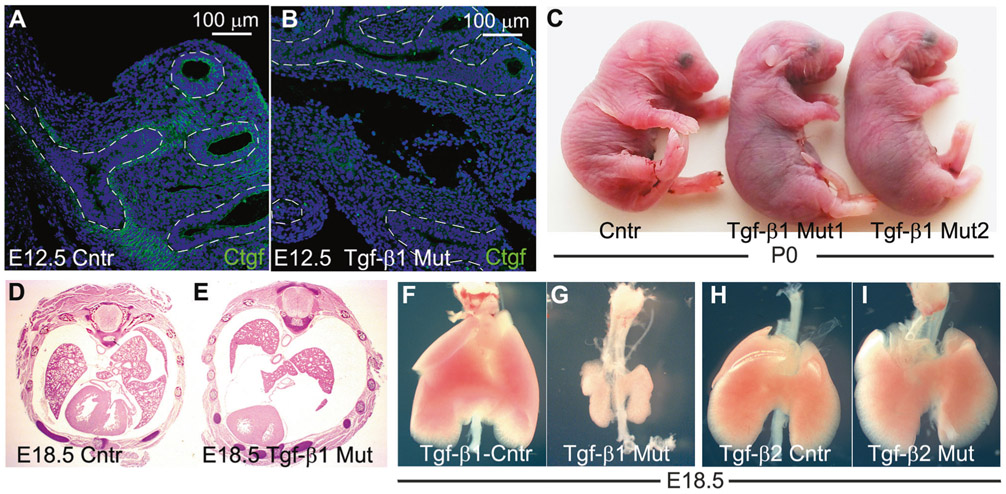
Tgf-β1fl/fl females were crossed with Tgf-β1fl/fl/Tbx4-rtTA/Tet-O-Cre males and fed with doxycycline chow from the gestational stage E7.5 until harvest of the embryos in which the Tbx4-rtTA induced Cre mouse drives the floxed-Tgf-β1 deletion specifically in the lung mesenchyme (Tgf-β1 CKO) [20]. Ctgf is one of the several target proteins of the TGF-β signaling pathway [26, 27]. Protein expression of the Ctgf gene was reduced in the E12.5 mutant lungs, which suggests effective genetic deletion of the Tgf-β1 in the mutant fetuses (Fig. 1a, b). At birth, control and mutant pups were similar in body size, yet the latter ones displayed agonal breathing, became cyanotic (Fig. 1c) and died within a few hours after delivery. Analysis of E18.5 fetuses, showed that almost all (more than 97%) mutant lungs exhibited severe bilateral hypoplasia in the parenchyma (Fig. 1d-g). In contrast, mesenchymal deletion of the Tgf-β2 gene did not affect embryonic (Fig. 1h, i) nor postnatal lung development (data not shown).
Fig. 1.
Deletion of Tgf-β1 in the embryonic lung mesenchyme causes lung hypoplasia. a, b Ctgf immunostaining on E12.5 control and mutant lungs. The protein expression of the Ctgf gene, a downstream gene of the TGF-β signaling pathway, was reduced in the mutant lung, especially in the lung mesenchyme, compared with the control (dashed line marks the border between epithelium and mesenchyme) (n = 3). c Control P0 pups were healthy and had bright pink colored skin which indicates appropriate lung function and oxygenation of the blood. Conversely, mutant P0 pups were cyanotic, displayed agonal breathing and died soon after birth. d–i Cross section E18.5 embryos (d, e) and whole lung imaging (f, g) of E18.5 embryonic lungs showed that deletion of the Tgf-β1 gene led to severe lung hypoplasia (n > 40). On the other hand, deletion of Tgf-β2 (h, i) in the embryonic lung mesenchyme did not affect lung development (n = 3)
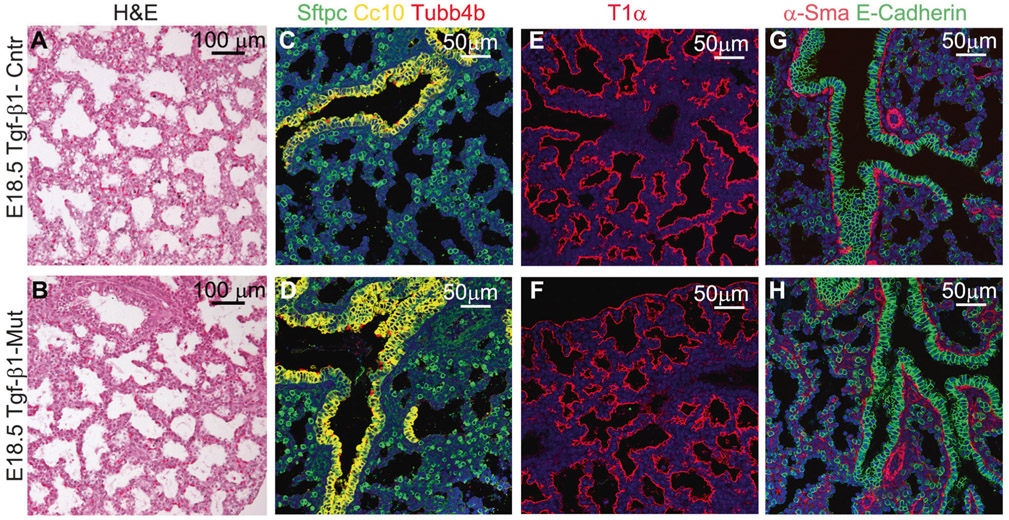
At the histological level, the gross saccular structure in E18.5 mutant lungs was similar to the control (Fig. 2a, b). Differentiation of proximal and distal airway epithelial cells, including club cells (Cc10 +), ciliated epithelial cells (Tubb4b+), type 1 alveolar epithelial cells (AEC1, T1-α +), and type 2 alveolar epithelial cells (AEC2, Sftpc+) was unchanged. In addition, differentiation of smooth muscle α-Sma+cells, which include vascular smooth muscle cells, bronchial smooth muscle cells and pericytes, was also normal in the mutant embryonic lung (Fig. 2c-h).
Fig. 2.
Mesenchymal deletion of Tgf-β1 did not affect lung epithelial cell differentiation. a, b Lung parenchyma of mutant and normal E18.5 embryonic lung appeared very similar to each other. c–f Staining for AEC2 (Sftpc +), club (Cc10 +), ciliated (Tubb4b +), and AEC1 (T1-α +) cell markers revealed normal epithelial cell differentiation in the mutant Tgf-β1 CKO mouse lungs. g, h Staining for α-Sma and E-Cadherin to highlight lung smooth muscle and epithelial cells, respectively. Differentiation of smooth muscle cells (vascular and bronchial smooth muscle cells and pericytes), and spatial organization of the epithelia cells were also comparable between control and mutant embryonic lungs (n = 4)
Apoptosis was increased in Tgf-β1 CKO embryonic lungs
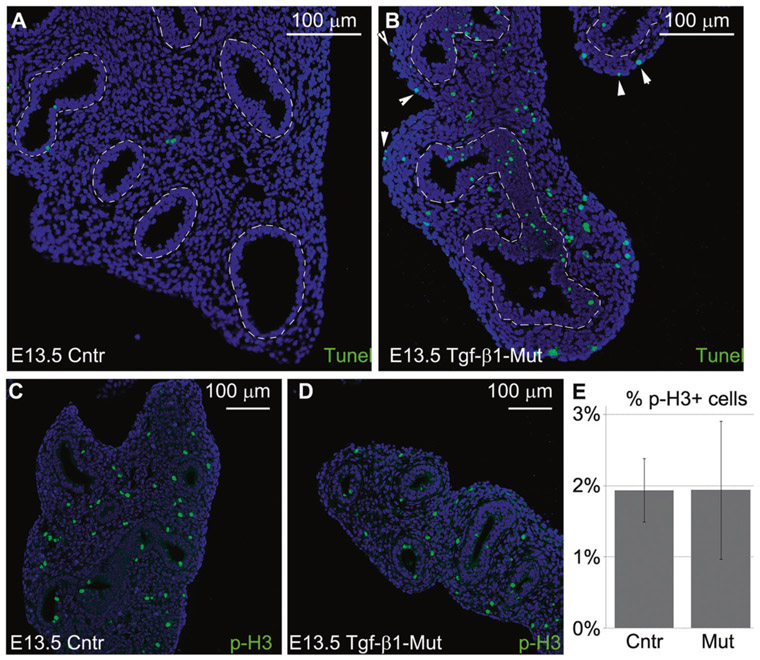
To determine the mechanism of lung hypoplasia in the mutant fetuses, we first investigated whether the lack of the mesenchymal expression of Tgf-β1 affected cell apoptosis and proliferation. Tunel assay on E13.5 lungs revealed an increase of apoptotic cells in all compartments, including the epithelium, mesenchyme, and mesothelium of the mutant lungs compared with the control (Fig. 3a, b). Meanwhile, cell proliferation, measured as the number of Phopho-H3 positive cells, was similar between mutant and control E13.5 lungs (Fig. 3c, d). Therefore, we concluded that the increase in cell death must have contributed to the extent of lung hypoplasia in the mutant embryos.
Fig. 3.
Lack of mesenchymal expression of Tgf-β1 increased cellular apoptosis. a, b Control and mutant E13.5 lungs were collected and analyzed for the presence of apoptotic cells. Lack of mesenchymal expression of Tgf-β1 was associated with markedly increased apoptosis of the lung epithelium, mesenchyme, and mesothelium (white arrowhead) (n = 3) (dashed line marks the border between epithelium and mesenchyme). c–e Meanwhile, the number of proliferating cells was similar between mutant and control E13.5 lungs (n = 3)
Lung mesenchymal knockout of the Tgf-β1 gene inhibited lung branching as well as the FGF10 signaling pathway
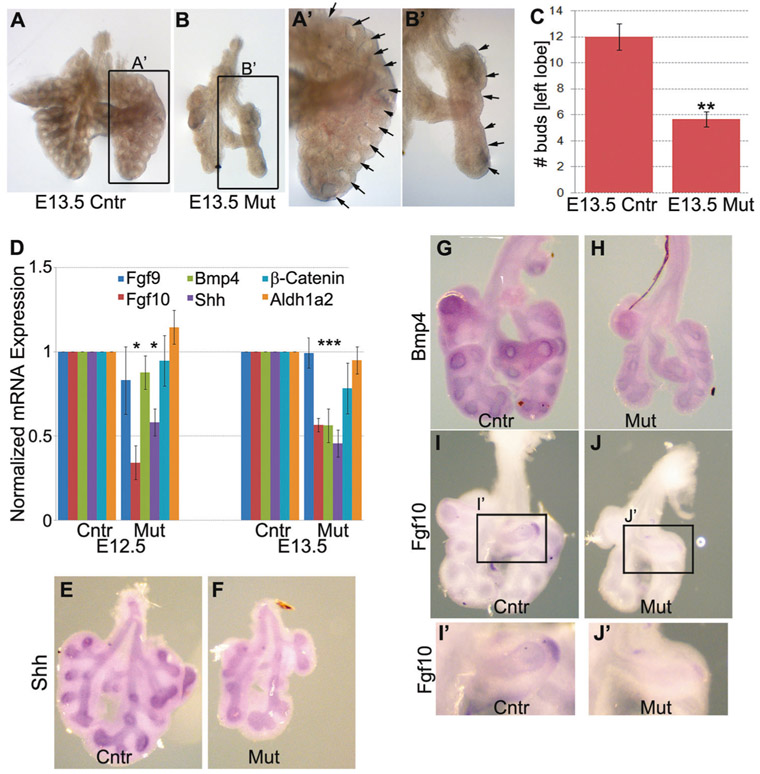
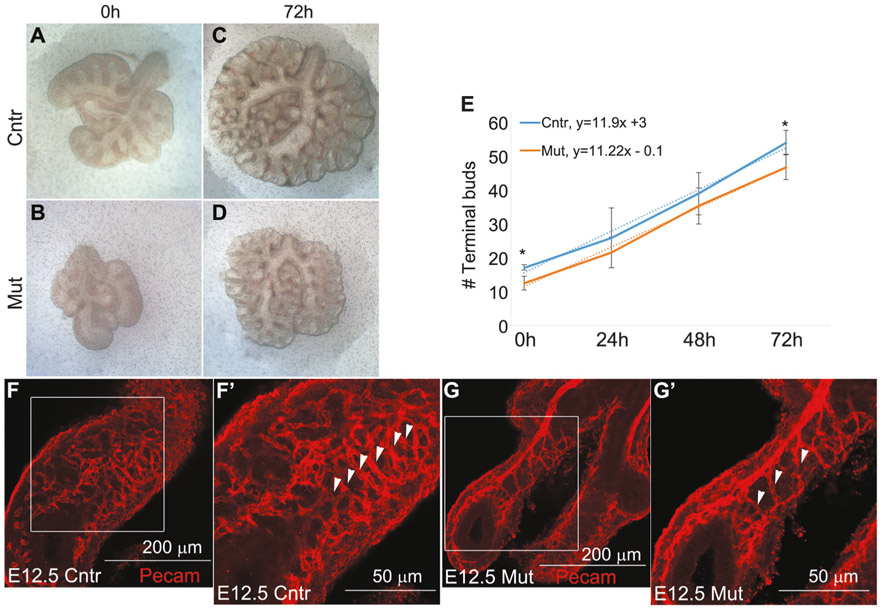
In the mouse, lung branching morphogenesis is initiated at the E10.5 and continues until the E16.5 [28]. During branching morphogenesis, the tree-like lung airways (bronchi and bronchioles) are generated; reduced lung branching morphogenesis is associated with delayed lung development, airway malformation and smaller lung size. To determine whether lung branching was reduced in the mutant lungs, we collected E13.5 embryonic lungs from both control and mutant fetuses and counted the number of terminal buds. We found that the number of terminal buds was significantly decreased in the mutant E13.5 embryonic lungs compared with the control (Fig. 4a-c), which suggests that the reduction of lung branching is responsible for the lung hypoplasia in the mutant fetuses.
Fig. 4.
Branching morphogenesis and FGF10 signaling pathway were inhibited in the Tgf-β1 CKO lungs. a–c To detect changes in the rate of lung branching morphogenesis, we collected E13.5 control and mutant lungs and counted the number of terminal buds in the left lung lobe. Lung branching was significantly delayed in the freshly isolated mutant lungs (n = 3). d–j Real-time qRT-PCR (d) and in situ hybridization (e–j) revealed that the FGF10 signaling pathway was strongly inhibited in the Tgf-β1 CKO embryonic lungs as suggested by the significantly reduced mRNA expression of the Bmp4, Shh and Fgf10 genes (n = 4) (**p < 0.01, *p < 0.05)
FGF10 signaling pathway is crucial for proper lung airway epithelial branching morphogenesis in mice. Fgf10 is secreted by the distal lung mesenchyme and acts as an attractant and morphogen for the adjacent lung epithelium during bud growth. Deletion of the Fgf10 gene in the embryonic lung mesenchyme results in complete lung agenesis [29-32]. Retinoic acid signals upstream of the FGf10 signaling pathway by inducing the expression of Fgf10 mRNA in the lung mesenchyme, while Bmp4 and Shh gene expression are induced in the lung epithelium by Fgf10 to drive proper embryonic lung morphogenesis [33, 34].
To test if reduced branching morphogenesis in our mutant lung was caused by the inhibition of the FGF10 signaling pathway, expression of Fgf10 in E12.5 and E13.5 control and mutant lungs were compared by real-time PCR and in situ hybridization. Indeed, Fgf10 expression at the mRNA level was halted in the mutant lung, and consistently, Bmp4 and Shh mRNAs were also significantly reduced in the mutant lung compared with the control (Fig. 4d-j).
Taken together, these findings suggest that mesenchymal expression of Tgf-β1 is necessary for proper lung branching morphogenesis, possibly by regulating FGF10 expression and signaling in the embryonic lung.
This possible mechanism was tested in E12.5 embryonic lung explant culture. Interestingly, mutant lungs grew at the same pace as control lungs and did not require exogenous FGF10 (Fig. 5). After 3 days in culture, the number of terminal buds in the mutant lung was lower than the control, but that was most likely caused by the fact that the mutant lungs had a lower number of terminal buds from the beginning (E12.5), when they were harvested (Fig. 5a-d). However, in terms of increment of the number of terminal buds per day, control and mutant lungs did not differ (Fig. 5e, see slope of trend curves), suggesting that Tgf-β1 may be involved in the early rather than late-branching process. In addition to airway branching, we also examined the pulmonary vasculature by whole mount staining for the endothelial marker Pecam1 (Cd31) in E12.5 embryonic lungs. Although the vascular vessels were well ramified and organized and several capillaries were visible in the control lung (Fig. 5f), the vascular system was significantly immature in the mutant lungs (Fig. 5g). The difference was particularly evident for the capillaries oriented on the right-left axis (white arrowhead of Fig. 5f′, g′).
Fig. 5.
Branching morphogenesis in the mutant lung is rescued in vitro. a–e Control and mutant E12.5 lungs were harvested and cultured on air–liquid interface on semipermeable polycarbonate porous membranes for 72 h. Even though at 72 h the number of terminal buds was still reduced in the mutant lung compared with the control lung, the rate (slope of the interpolated line) at which control, and mutant embryonic lungs branched in culture over time did not differ (n = 4) (*p < 0.05). f, g Whole mount Pecam1 staining on E12.5 control and mutant lungs showed that the vascular system was immature and less organized in the Tgf-β1 CKO lungs (n = 5)
Tyrosine phosphorylation protein array identifies novel effectors of the lung mesenchymal TGF-β signaling pathway
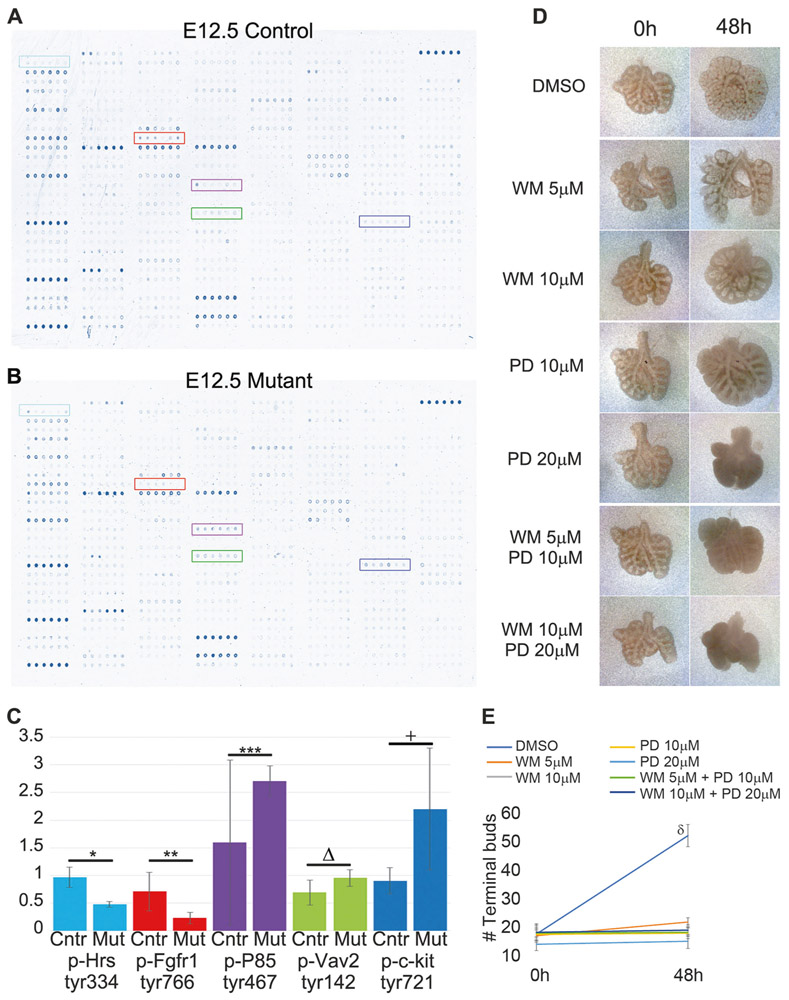
We hypothesized that the Tgf-β1 deletion may affect several signaling pathways in the embryonic lung. We utilized our genetic mouse model as a platform to identify novel genes and signaling pathways potentially regulated by Tgf-β1 cytokine signaling in the embryonic lung. To achieve that, we utilized an antibody microarray approach which assesed the change of tyrosine phosphorylation on 228 proteins. Since the phenotype was clearly evident at the E12.5 stage, we decided to utilize lungs from this embryonic stage for the phospho-protein profiling. Among the 228 proteins analyzed, five proteins displayed a clear difference in phosphorylation (Fig. 6): phospho-Hrs (tyr334), phospho-Fgfr1(tyr766), phospho-P85 (tyr467), phospho-Vav2 (tyr142), and phospho-c-Kit (tyr721).
Fig. 6.
Tgf-β1 deletion in the lung mesenchyme interferes with the phosphorylation of several proteins in the embryonic lung. a, b Black and white photo of the scanned arrays. Colored rectangles identified five proteins whose change of phosphorylation level was clearly changed. c Average and standard deviation of the phosphorylation levels of Hrs, Fgfr1, P85, Vav2, and c-Kit (*p = 2E–06, **p = 0.009, ***p = 0.1, Δp = 0.036, +p = 0.034). d, e Wild-type E12.5 lungs were cultured with DMSO, wortmannin (WM), PD173074 (PD) or WM+PD for 2 days to determine the effect of the inhibiton of Pi3k and Fgfr1 on lung branching morphogenesis. Both drugs inhibited lung growth and branching (n = 3) (δp < 0.05)
Next, we investigated whether inhibition of P85 and Fgfr1 proteins in the embryonic lung phenocopied the embryonic deletion of the Tgf-β1. We decided to follow-up with these two targets since they both feed into the FGF10 signaling pathway [35-37] and could easily be inhibited with well characterized and commercially available small molecules. P85 constitutes the regulatory subunit of the phosphoinositide-3-kinase (Pi3k), which can be specifically inhibited with wortmannin [38], meanwhile Fgfr1 can be blocked with PD173074 [39]. Wild-type E12.5 lungs were harvested and cultured on top of polycarbonate support for up to 48 h. Wortmannin and PD173074 were added to the cell culture medium at different concentrations, alone or in combination (Fig. 6d). Both drugs severely inhibited lung branching morphogenesis and lung growth (Fig. 6e).
The severity of the lung phenotype was dependent on the time of the Tgf-β-1 gene deletion
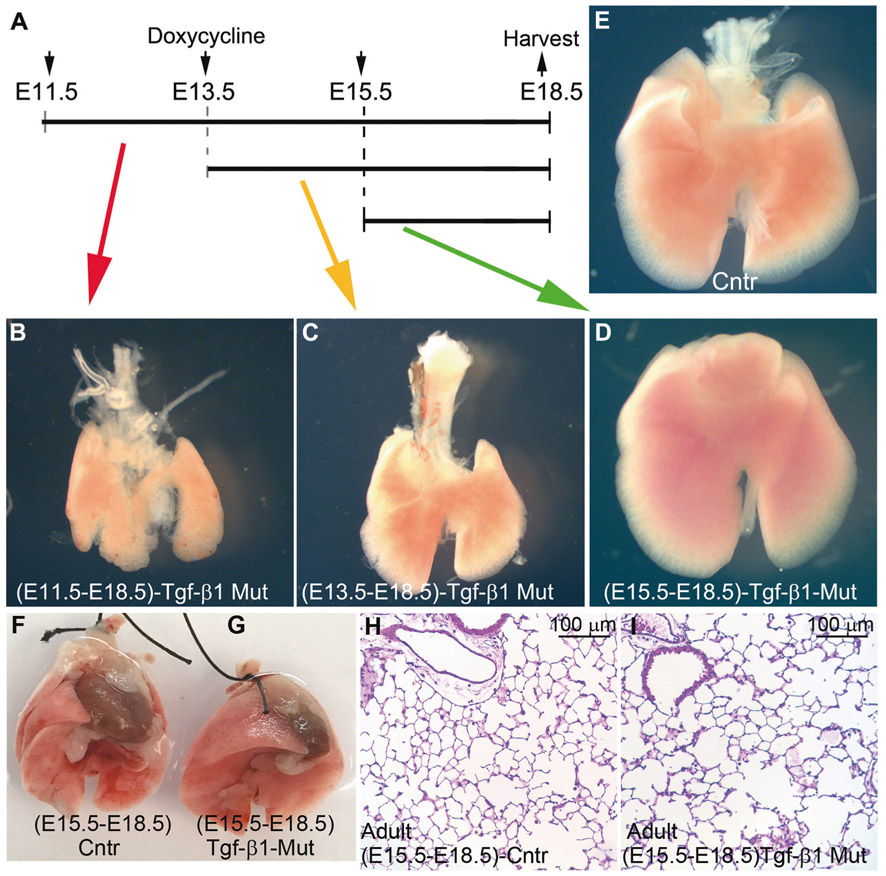
Depending on the gestational stage, the Tbx4-rtTA transgene is active in different lung cell subpopulations [20]. We investigated how the gestational stage at which the Tgf-β1 gene was deleted, affected the severity of the lung phenotype in the mutant embryos. We found that deletion of Tgf-β1 at E11.5 and E13.5 was still associated with significant lung hypoplasia, even though the severity of the phenotype was not as strong as observed for the E7.5 induction (Fig. 7a-e). Surprisingly, Tgf-β1 deletion at E15.5, did not affect embryonic lung development in the mutant fetuses: the size of the mutant and control lungs appeared very similar, and no gross abnormalities were visible in the mutant lungs (Fig. 7d). To rule out a delayed onset of any lung phenotypes in the (E15.5-E18.5)-Tgf-β1 CKO mutant animals, we also collected and analyzed lungs from 2-month-old animals (Fig. 7f-i). The size of the mutant and control lungs was similar (Fig. 7f, g), H&E staining did not show any obvious alterations to the lung parenchyma (Fig. 7h, i) and the differentiation of the epithelial cells was unchanged (data not shown).
Fig. 7.
Deletion of Tgf-β1 at later gestational stages led to progressively weaker lung phenotypes. a–e Deletion of the Tgf-β1 gene at E11.5 and E13.5 was associated with lung hypoplasia. Meanwhile, Tgf-β1 deletion at the E15.5 stage did not produce a gross lung phenotype (n = 3). f–i E15.5 pregnant females were fed doxycycline chow until birth of the pups. Lungs were collected from 2 months old, control and mutant animals for analysis. Tgf-β1 knockout at the E15.5 stage did not affect postnatal lung growth nor its alveolarization (n = 4)
The Tbx4-rTta transgene is not active in endothelial cells and pericytes at the E15.5 stage [20], and branching morphogenesis ends at around E16.5. Thus, the lack of a lung phenotype in (E15.5-E18.5)-Tgf-β1 CKO fetuses further suggests that Tgf-β1 is crucial for the early lung branching and that its function is most likely mediated through endothelial cells and pericytes.
Discussion
Lung development is finely orchestrated by the precise spatial-temporal expession of key morphogenic factors. Insufficiency or overexpression of these molecules lead to fetal lung abnormalities or altered adult lung response to environmental stimuli and toxins [28, 33].
Lung hypoplasia is caused by the incomplete development of lung which, consequently, fails to reach the normal size [40]. Its prevalence is estimated to be 7–26% of all neonatal autopsies, and it is thus a major documented cause of death of human newborns [41]. Disturbances of the bronchopulmonary vasculature, reduced airway branching morphogenesis, chest hypoplasia, and diaphragmatic defects are among the most frequent clinical causes of lung hypoplasia [42-44]. Although lung hypoplasia is predominantly observed in infants and children, its occurrence in adult patients has also been reported [45, 46].
The molecular mechanisms responsible for the development of primary lung hypoplasia are still under investigation. Genetic deletion of Fgf9, Fgf10, Adam17, Nfib, Shh, and Ctgf has been associated with lung hypoplasia in mice [47-52]. The TGF-β signaling pathway has several critical functions in embryonic and postnaltal lung development and adult lung regeneration after injury [3].
The embryonic lung expresses all three Tgf-β ligands. We deleted the Tgf-β1 gene specificaly in the embryonic lung mesenchyme [28] and found that mutant E18.5 fetuses diplayed severe bilateral hypolasia (Fig. 1). This was unexpected since conventional Tgf-β1 knockout did not display a specific lung phenotype. It is reasonable to hypothesize that when Tgf-β1 is deleted in the entire embryo, unknown signals may be activated to initiate compensatory mechanisms in these embryos. Given the ability of Tgf-β1 to signal remotely in an endocrine manner, the compensatory mechanism could entail the delivery of Tgf-β1 ligand from the mother to mutant fetuses through the placenta [17]. In our system, the deletion of Tgf-β1 is tissue specific, therefore, we were able to uncover a previously unknown role of the TGF-β signaling pathway in embryonic lung development.
In our mouse model, the mutation is highly penetrant, as <3% of the mutant embryos did not show the described lung phenotype. We speculate that this rescue effect was likely caused by either Tgf-β1 delivery to the embryonic lung from other embryonic or extraembryonic tissues in an endocrine manner or compensation by other Tgf-β ligands. Although, inefficient activation and expression of the Tbx4-rtTA transgene in those few embryos could also be a possibility.
In humans, one in every thousand newborns have primary congenital lung hypoplasia. Idiopathic lung hypoplasia may be caused by suboptimal expression of certain transcriptional factors and growth factors or associated with other syndromes or congenital abnormalities [53]. In our mouse model, lung hypoplasia seems to be caused by the combination of increased cell apoptosis with reduced lung-branching morphogenesis (Figs. 3 and 4). The increase in cell death in the mutant lung was somewhat predictable, as the role of the TGF-β signaling pathway in survival of several cell types has been documented before [54, 55]. However, the branching morphogenesis phenotype and its rescue by culturing the embryonic lungs in vitro on polycarbonate support was quite surprising.
The observation that lung-branching morphogenesis was diminished in our mutant embryos, contrasts with previous findings that showed that the TGF-β signaling pathway has a negative impact on lung-branching morphogenesis [56-58]. We postulate that a minimum level of activation of the TGF-β signaling is critical for the healthy growth of the embryonic lung tissue, by providing for the appropriate vascular development and mechanical support via synthesis of critical extracellular matrix proteins. Reduced expression of Tgf-β ligands in human infants with severe lung hypoplasia and in rats have been described before [53, 59].
Culturing the mutant embryonic lungs on polycarbonate membrane was enough to rescue the branching phenotype (Fig. 5). When lungs are cultured on polycarbonate membranes, oxygen reaches the cells through the thin air-liquid-interface. An intact vascular system is therefore not required for normal branching of the embryonic lung in vitro [60]. Havrilak et al. demonstrated that the lack of an intact endothelium does not interfere with lung branching morphogenesis in vitro [31]. Furthermore, the membrane itself provides anchoring support that allows the lung to attach and grow over time. TGF-β signaling is crucial for the development and functioning of the vascular system [61-64] as well as the synthesis of the extracellular matrix [65-68]. Therefore, we speculate that lung hypoplasia in mutant lungs could be partially caused by reduced and abnormal vascularization of the lung parenchyma, insufficient extracellular matrix development, or a combination of both. These phenomena would explain why culturing mutant lungs in vitro would rescue the branching phenotype in the mutant lungs. Whole mount staining for the endothelial marker Pecam1 revealed that the vascular bed in the mutant E12.5 lungs was immature (Fig. 5). This finding further reinforced our theory that the vascular system is involved in the genesis of the lung hypoplasia in Tgf-β1 CKO embryos.
Taken together, our data suggest that Tgf-β1 expression in the embryonic mesenchyme is necessary for the lung cell survival, proper branching morphogenesis and vasculature development, as well as activation of the FGF10 signaling pathway in the embryonic lung. However, further studies will be necessary to tease out the cause-effect relationship details of these observations.
To gather insights into the molecular mechanisms responsible for the lung hypoplasia, we utilized an unbiased approach which detected changes of phosphorylation in 226 signaling proteins in the mutant lungs compared with wild-type lungs (Fig. 6). For the scope of this manuscript, we focused on those proteins whose phosphorylation signal was strong in both mutant and control lungs and for which the signal difference was clear to the naked eye. Three of the five targets identified (Fgfr1, Hrs, and P85) feed directly or indirectly into the FGF/FGFR signaling pathway. Phosphorylation on the tyrosine 334 of Hrs was reduced in the mutant lungs. Hrs/Hgs is a crucial component of endosome formation and membrance receptor recycling after ligand binding. In Drosophila, the Hrs/Stam complex is required for efficient FGFR signaling [69]. Phosphorylation of Fgfr1 was reduced in the mutant E12.5 lungs. Most Fgf ligands bind to Fgfr1 with mild to strong affinity. For example, even though Fgf10 is known to strongly bind to Fgfr2, it also binds to Fgfr1 but with lower affinity [35, 36]. Therefore, in the mutant lungs, reduced phosphorylation of Fgfrl was most likely caused by the diminished expression of the Fgf10 gene.
Phoshorylation on the tyrosine 467 of P85 was increased after deletion of Tgf-β1 in the embryonic lung mesenchyme. Phosphoinositide-3-kinase is composed of a dimer regulatory (P85) and catalytic (P110) subunit [70]. Pi3k activates Akt and Rac1 to stimulate branching of mammary gland, epithelial cells, and neurons, as well as vascular sprouting [38, 71]. Fgfr2 is the main receptor for Fgf10, and once phosphorylated, binds to P85 which could be crucial for the Fgfr endocytosis and signaling regulation [37].
The other two proteins whose phosphoryation was affected in mutant lung were Vav2 and c-Kit. Vav2, a guanine nucleotide exchange factor, is expressed in most tissues and is especially critical for the proper development and functioning of endothelium, wherein it is essential for the activation of Rac1 during endothelial cell migration [72, 73].
Tgf-β1 appears to negatively regulate c-Kit phorphorylation on tyrosine 721. In the E11.5 embryonic lung, c-Kit is expressed by the mesenchymal cells located around the epithelial bud, while at later stages (E15.5-E17.5) it is strongly expressed by vascular wall cells [74]. TGF-β signaling also increases the expression of c-Kit in hepatocellular carcinoma cells [75] and arrests the proliferation of c-Kit positive cells in CD34 + /CD38− human bone marrow cells [76]. The biological implications of the increased phosphorylation of the c-Kit protein in the mutant lung is unclear and will be investigated in future studies.
While our approach has some limitations such as the inability to determine whether these phosphorylation sites are activators or inibitors, our data deliver some important insights on how Tgf-β1 may modulate embryonic lung development. Furthermore, it strengthens the link between the Tgf-β1 cytokine and the FGF signaling pathway and lung vascular system development.
In vitro studies showed that both wortmannin and PD173074 halted lung branching and growth [77], and when combined together, the inhibitory effect was markedly increased (Fig. 6). Therefore, alteration of the activity of Pi3k and Fgfr1 may be critical for the development of the lung hypoplasia in our mouse model.
Deletion of Tgf-β1 in the lung at the E15.5 gestational stage did not alter the embryonic lung development, while its deletion at E11.5 and E13.5 was still associated with lung hypoplasia (Fig. 7). Branching morphogenesis terminates at E16.5, suggesting that Tgf-β1 may be involved in early rather than late branching process. In the E15.5 lung, the Tbx4-rtTA transgene is mostly active in myofibroblasts, but not in endothelial cells and pericytes [20]. Therefore, the lack of a lung phenotype in (E15.5-E18.5)-Tgf-β1 CKO fetuses further suggests that the ability of the Tgf-β1 gene to drive proper lung developmment involves the vascular system. It also suggests that lung hypoplasia and the halted FGF10 signaling pathway are likely caused by the immature lung vasculature.
In conclusion, by using a mesenchymal lung specific Cre mouse line, we were able to uncover new important insights on how Tgf-β1 regulates proper embryonic lung development. Our mouse model of lung hypoplasia could be utilized to decipher novel genes and signaling pathways important for the molecular pathogenesis of lung hypoplasia, which could potentially reveal novel molecular targets for the treatment of this disease in human infants.
Acknowledgements
MA was supported by NHLBI (NIH R01HL126705, NIH R01HL145064) and the American Heart Association (Grant-in-Aid: 17GRNT33650018); WS was supported by NHLBI (NIH R21HL109932); DW was supported by the NHLBI LungMAP (NIH UO1HL12268). GT was supported by Webb Foundation.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information The online version of this article (https://doi.org/10.1038/s41374-019-0256-3) contains supplementary material, which is available to authorized users.
References
- 1.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125: 754–65. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 5.Saito A, Horie M, Micke P, Nagase T. The role of TGF-beta signaling in lung cancer associated with idiopathic pulmonary fibrosis. Int J Mol Sci. 2018;19:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2018;56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito A, Horie M, Nagase T. TGF-beta signaling in lung health and disease. Int J Mol Sci. 2018;19:2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Zhuang F, Liu YH, Xu B, Del Moral P, Deng W, et al. TGF-beta receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur Respir J. 2008;32:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Bonniaud P, Fabre A, Frossard N, Guignabert C, Inman M, Kuebler WM, et al. Optimising experimental research in respiratory diseases: an ERS statement. Eur Respir J. 2018;51: 1702133. [DOI] [PubMed] [Google Scholar]
- 11.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124: 2659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo SH, Cunningham MC, Arabshahi B, Gruss JS, Grant JH, 3rd. The transforming growth factor-beta 3 knock-out mouse: an animal model for cleft palate. Plast Reconstr Surg. 2001;108:938–48. discussion 949–51 [DOI] [PubMed] [Google Scholar]
- 13.Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–21. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Heisterkamp N, Groffen J, Zhao J, Warburton D, Kaartinen V. TGF-beta3-null mutation does not abrogate fetal lung maturation in vivo by glucocorticoids. Am J Physiol. 1999;277:L1205–13. [DOI] [PubMed] [Google Scholar]
- 16.Bottinger EP, Letterio JJ, Roberts AB. Biology of TGF-beta in knockout and transgenic mouse models. Kidney Int. 1997;51: 1355–60. [DOI] [PubMed] [Google Scholar]
- 17.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–54. [DOI] [PubMed] [Google Scholar]
- 18.Boucherat O, Nadeau V, Berube-Simard FA, Charron J, Jeannotte L. Crucial requirement of ERK/MAPK signaling in respiratory tract development. Development. 2014;141:3197–211. [DOI] [PubMed] [Google Scholar]
- 19.Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech Dev. 1999;86: 125–36. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Menke DB, Jiang M, Chen H, Warburton D, Turcatel G, et al. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol. 2013;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turcatel G, Millette K, Thornton M, Leguizamon S, Grubbs B, Shi W, et al. Cartilage rings contribute to the proper embryonic tracheal epithelial differentiation, metabolism, and expression of inflammatory genes. Am J Physiol Lung Cell Mol Physiol. 2017;312:L196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishtiaq Ahmed AS, Bose GC, Huang L, Azhar M. Generation of mice carrying a knockout-first and conditional-ready allele of transforming growth factor beta2 gene. Genesis. 2014;52: 817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, El Agha E, Turcatel G, Chen H, Chiu J, Warburton D, et al. Mesenchymal adenomatous polyposis coli plays critical and diverse roles in regulating lung development. BMC Biol. 2015;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sala FG, Del Moral PM, Tiozzo C, Alam DA, Warburton D, Grikscheit T, et al. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development. 2011;138:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Liu H, Meyer C, et al. Transforming growth factor-beta (TGF-beta)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem. 2013;288:30708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–9. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. [DOI] [PubMed] [Google Scholar]
- 29.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. [DOI] [PubMed] [Google Scholar]
- 31.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol. 2003;258:185–200. [DOI] [PubMed] [Google Scholar]
- 32.Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140:3731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, et al. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan T, Volckaert T, Chanda D, Thannickal VJ, Langhe SP. Fgf10 signaling in lung development, homeostasis, disease, and repair after injury. Front Genet. 2018;9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francavilla C, Rigbolt KT, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, et al. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell. 2013;51:707–22. [DOI] [PubMed] [Google Scholar]
- 38.Carter E, Miron-Buchacra G, Goldoni S, Danahay H, Westwick J, Watson ML, et al. Phosphoinositide 3-kinase alpha-dependent regulation of branching morphogenesis in murine embryonic lung: evidence for a role in determining morphogenic properties of FGF7. PLoS ONE. 2014;9:e113555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen PT, Tsunematsu T, Yanagisawa S, Kudo Y, Miyauchi M, Kamata N, et al. The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br J Cancer. 2013;109:2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laudy JA, Wladimiroff JW. The fetal lung. 2: Pulmonary hypoplasia. Ultrasound Obstet Gynecol. 2000;16:482–94. [DOI] [PubMed] [Google Scholar]
- 41.Husain AN, Hessel RG. Neonatal pulmonary hypoplasia: an autopsy study of 25 cases. Pediatr Pathol. 1993;13:475–84. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura Y, Harada K, Yamamoto I, Uemura Y, Okamoto K, Fukuda S, et al. Human pulmonary hypoplasia. Statistical, morphological, morphometric, and biochemical study. Arch Pathol Lab Med. 1992;116:635–42. [PubMed] [Google Scholar]
- 43.Aghabiklooei A, Goodarzi P, Kariminejad MH. Lung hypoplasia and its associated major congenital abnormalities in perinatal death: an autopsy study of 850 cases. Indian J Pediatr. 2009;76: 1137–40. [DOI] [PubMed] [Google Scholar]
- 44.Mirchandani LV, Alam A, Iyer A, Kutty JT. Rare case of unilateral hypoplasia of lung with associated ventricular mass in an adult. J Clin Diagn Res. 2016;10:OD05–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Luca G, Petteruti F, Lerro A, Luciano A, Contaldo A, Pepino P. A case of lung hypoplasia in adulthood. Asian Cardiovasc Thorac Ann. 2010;18:493–4. [DOI] [PubMed] [Google Scholar]
- 46.Katsenos S, Antonogiannaki EM, Tsintiris K. Unilateral primary lung hypoplasia diagnosed in adulthood. Respir Care. 2014;59: e47–50. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Wessels A, Chen J, Phelps AL, Oatis J, Tint GS, et al. Late gestational lung hypoplasia in a mouse model of the Smith-Lemli-Opitz syndrome. BMC Dev Biol. 2004;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–6. [DOI] [PubMed] [Google Scholar]
- 49.Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128: 2095–106. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J, Chen H, Peschon JJ, Shi W, Zhang Y, Frank SJ, et al. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev Biol. 2001;232:204–18. [DOI] [PubMed] [Google Scholar]
- 51.Grunder A, Ebel TT, Mallo M, Schwarzkopf G, Shimizu T, Sippel AE, et al. Nuclear factor I-B (Nfib) deficient mice have severe lung hypoplasia. Mech Dev. 2002;112:69–77. [DOI] [PubMed] [Google Scholar]
- 52.Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn. 2008;237:485–93. [DOI] [PubMed] [Google Scholar]
- 53.Wu CS, Chen CM, Chou HC. Pulmonary hypoplasia induced by oligohydramnios: findings from animal models and a population-based study. Pediatr Neonatol. 2017;58:3–7. [DOI] [PubMed] [Google Scholar]
- 54.Frassanito MA, De Veirman K, Desantis V, Di Marzo L, Vergara D, Ruggieri S, et al. Halting pro-survival autophagy by TGFbeta inhibition in bone marrow fibroblasts overcomes bortezomib resistance in multiple myeloma patients. Leukemia. 2016;30: 640–8. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y, et al. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Rep. 2016;6:32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J, Sime PJ, Bringas P Jr., Gauldie J, Warburton D. Adenovirus-mediated decorin gene transfer prevents TGF-beta-induced inhibition of lung morphogenesis. Am J Physiol. 1999;277(2 Pt 1):L412–22. [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Sime PJ, Bringas P Jr., Tefft JD, Buckley S, Bu D, et al. Spatial-specific TGF-beta1 adenoviral expression determines morphogenetic phenotypes in embryonic mouse lung. Eur J Cell Biol. 1999;78:715–25. [DOI] [PubMed] [Google Scholar]
- 58.Serra R, Pelton RW, Moses HL. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development. 1994;120:2153–61. [DOI] [PubMed] [Google Scholar]
- 59.Chen MF, Gray KD, Prentice MA, Mariano JM, Jakowlew SB. Human pulmonary acinar aplasia: reduction of transforming growth factor-beta ligands and receptors. Pediatr Res. 1999;46: 61–70. [DOI] [PubMed] [Google Scholar]
- 60.Havrilak JA, Shannon JM. Branching of lung epithelium in vitro occurs in the absence of endothelial cells. Dev Dyn. 2015;244: 553–63. [DOI] [PubMed] [Google Scholar]
- 61.Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D'Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE. 2009;4:e5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perrella MA, Jain MK, Lee ME. Role of TGF-beta in vascular development and vascular reactivity. Miner Electrolyte Metab. 1998;24:136–43. [DOI] [PubMed] [Google Scholar]
- 63.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–27. [DOI] [PubMed] [Google Scholar]
- 64.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci USA. 2003;100: 15859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hinz B The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015;47: 54–65. [DOI] [PubMed] [Google Scholar]
- 66.Roberts AB, McCune BK, Sporn MB. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992;41:557–9. [DOI] [PubMed] [Google Scholar]
- 67.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–9. [DOI] [PubMed] [Google Scholar]
- 68.Obreo J, Diez-Marques L, Lamas S, Duwell A, Eleno N, Bernabeu C, et al. Endoglin expression regulates basal and TGF-beta1-induced extracellular matrix synthesis in cultured L6E9 myoblasts. Cell Physiol Biochem. 2004;14:301–10. [DOI] [PubMed] [Google Scholar]
- 69.Chanut-Delalande H, Jung AC, Baer MM, Lin L, Payre F, Affolter M. The Hrs/Stam complex acts as a positive and negative regulator of RTK signaling during Drosophila development. PLoS ONE. 2010;5:e10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fruman DA. Regulatory subunits of class IA PI3K. Curr Top Microbiol Immunol. 2010;346:225–44. [DOI] [PubMed] [Google Scholar]
- 71.Zhu W, Nelson CM. PI3K signaling in the regulation of branching morphogenesis. Biosystems. 2012;109:403–11. [DOI] [PubMed] [Google Scholar]
- 72.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Valle-Perez B, Martinez VG, Lacasa-Salavert C, Figueras A, Shapiro SS, Takafuta T, et al. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285:10748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinkai T, Shinkai M, Pirker MA, Montedonico S, Puri P. Spatial and temporal patterns of c-kit positive cells in embryonic lungs. Pediatr Surg Int. 2011;27:181–5. [DOI] [PubMed] [Google Scholar]
- 75.Rojas A, Zhang P, Wang Y, Foo WC, Munoz NM, Xiao L, et al. A positive TGF-beta/c-KIT feedback loop drives tumor progression in advanced primary liver cancer. Neoplasia. 2016;18: 371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morita N, Yamamoto M, Tanizawa T. Correlation of c-kit expression and cell cycle regulation by transforming growth factor-beta in CD34+CD38− human bone marrow cells. Eur J Haematol. 2003;71:351–8. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Ito T, Udaka N, Okudela K, Yazawa T, Kitamura H. PI3K-AKT pathway mediates growth and survival signals during development of fetal mouse lung. Tissue Cell. 2005;37:25–35. [DOI] [PubMed] [Google Scholar]