Abstract
A photoinduced palladium-catalyzed 1,2-carbofunctionalization of conjugated dienes has been developed. This mild modular approach, which does not require employment of exogeneous photosensitizers and external oxidants, allows for efficient and highly regio- and stereoselective synthesis of a broad range of allylic amines from readily available 1,3-dienes, alkyl iodides, and amines. Employment of O- and C-nucleophiles toward oxyalkylation and dialkylation products was also demonstrated. A putative π-allyl palladium radical-polar crossover path is proposed as a key event in this three-component coupling process. The utility of this protocol is highlighted by its application for derivatization of several amine-containing drugs.
1,3-Dienes are readily available versatile building blocks, which enjoy numerous applications in synthesis,1 material science,2 and medicinal chemistry.3 Difunctionalization of 1,3-dienes enables rapid access to complex molecules in one step and is therefore highly sought after.4 In particular, 1,2-carboamination of dienes represents an efficient synthetic route to allylic amines, which are valuable synthons,5 as well as important motifs in bioactive molecules (Scheme 1a). However, only isolated examples have been reported to date, with the vast majority being two-component annulative reactions proceeding at elevated temperatures (Scheme 1b).6 As a result, not only the choice of substrates but also the diversity of obtained products is limited. A multicomponent reaction (MCR) in which the carbon and nitrogen functionalities are introduced independently would therefore be an appealing method to address current limitations and allow for expedient synthesis of structurally diverse allylic amines. Herein, we report a light-induced palladium-catalyzed three-component coupling7 of 1,3-dienes, alkyl iodides and amines that occurs under mild conditions (Scheme 1c). This transformation is proposed to involve a putative π-allyl hybrid palladium radical intermediate A. The latter, upon radical-polar crossover step, is converted into a “traditional” π-allyl palladium intermediate B,8 which is then trapped with an amine to deliver the reaction products.
Scheme 1.
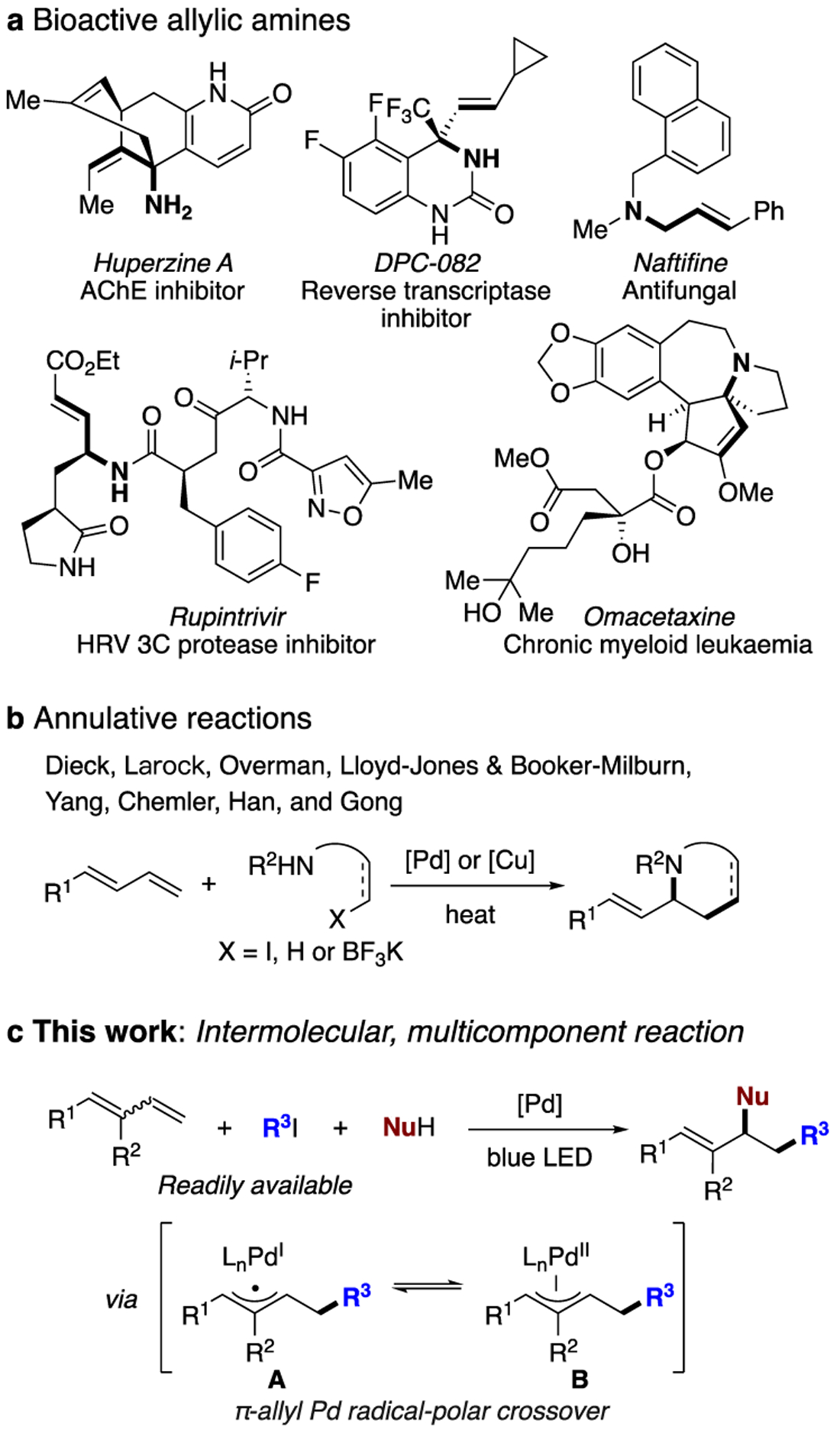
Background and Proposed 1,2-Carboamination of 1,3-Dienes
In recent years, the photoinduced palladium catalysis has become a highly emerging area of research.9 It has been established by our group and others that hybrid palladium radical species can be generated via cleavage of C(sp3)– and C(sp2)–X (X = Cl, Br, I, CO2NPhth, OTf) bonds in the presence of photoexcited palladium complexes. Formation of these species was crucial for achieving various processes, including remote desaturation,10 alkyl Heck reactions,11 and other transformations.12 Keeping in mind a facile addition of hybrid alkyl radical palladium species across the double bond,11 we envisioned that analogous addition at conjugated diene would also be feasible, thereby leading to a putative hybrid π-allyl palladium radical species A that would exist in equilibrium with the classical π-allyl complex B. The anticipated aminoalkylation product would be delivered upon nucleophilic attack by amine at the latter (Scheme 1c).
To this end, we commenced our study with examining the reaction of 1-phenylbutadiene (1), (trimethylsilyl)methyl iodide (2), and N-methylbenzylamine (3). Expectedly, the major challenge was to suppress the expected side reactions, including Heck reaction (5), hydroamination (6), and amine alkylation (7) processes. After extensive screening of reaction parameters,13 we were delighted to obtain 4a in 80% isolated yield (Table 1, entry 1). Similarly to our previous studies,11a the combination of Pd salt with Xantphos ligand proved to be the most efficient catalytic system. This reaction appeared to be insensitive to the stereochemistry of diene, as employment of a 1:1.2 mixture of (E)/(Z) isomers of 1 did not lead to a significant change of the yield (entry 2). Control experiments indicated that both visible light irradiation and palladium catalyst were necessary (entries 3, 4). Without silver triflimide (entry 5), the reaction was significantly less efficient due to the formation of substantial amounts of radical dimerization products (see Scheme 4b and discussion, therein). Likewise, the reaction efficiency was substantially lower in the absence of the base (entry 6). Employment of a bromo analog of 2 was much less efficient (24%), whereas the chloro counterpart did not react under these conditions at all.
Table 1.
Optimization of Reaction Conditionsa
 | |||
|---|---|---|---|
| Entry | Deviation from standard conditions | 4:5:6:7b | Yield of 4ac |
| 1 | None | 98:1:0.5:0.5 | 89% (80%) |
| 2 | (E)/(Z)-1 (1:1.2) | 96:1:1:2 | 83% (75%) |
| 3 | 45 or 80 °C (dark) | 8:0:0:92 | Trace |
| 4 | Without PdCl2 | 4:0:0:96 | Trace |
| 5 | Without AgNTf2 | 91:5:2:2 | 48%d |
| 6 | Without Cs2CO3 | 62:29:7:2 | 44% |
0.2 mmol scale; (E)-1:2:3 = 1:1.3:1.3.
Determined by GC/MS using pentadecane as internal standard; 4a:4b > 20:1.
Determined by 1H NMR using dibromomethane as internal standard; isolated yields given in parentheses.
Formation of substantial amounts of radical dimerization products was observed.
Scheme 4.

Mechanistic Studies
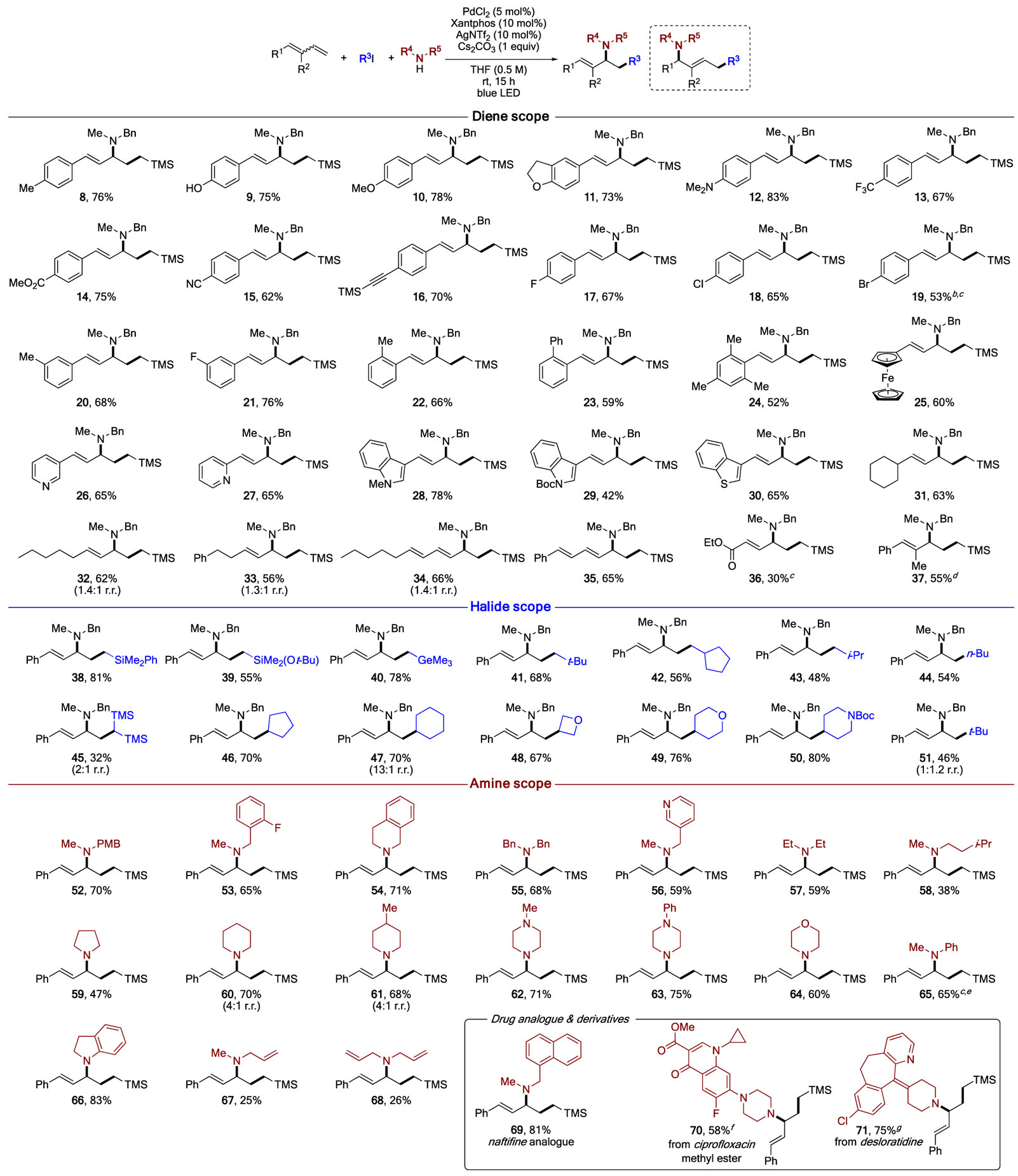
With the optimized conditions in hand, we examined the generality of this methodology (Table 2). First, the diene scope was tested. It was found that 1-aryl and hetaryl substituted dienes are all capable partners for this transformation. Thus, electron-rich dienes reacted smoothly to give the corresponding allyic amines in good yields (8–12). Notably, in the case of a phenol-containing moiety (9), the formation of a potential competing carboalkoxylation product was not observed, thus indicating high chemoselectivity of this reaction. Electron-deficient aryl groups were also tolerated, thus delivering the expected products in moderate to good yields (13–15). Alkyne and halide functionalities were also compatible with this transformation (16–19). Aminoalkylation of dienes possessing substituents at meta- and ortho-positions of the arene proceeded uneventfully producing the reaction products 20–24. Ferrocenyl and heteroaryl groups were also amenable to this catalytic process, affording the corresponding products in moderate to good yields (25–30). Alkyl-substituted dienes were also reactive under these reaction conditions. Selective 1,2-addition was observed with a secondary alkyl substituent (31), whereas mixtures of 1,2- and 1,4-adducts were isolated for the primary counterparts (32–33), probably due to steric reasons. In addition, this MCR can be performed with trienes to obtain the corresponding dienamines 34–35. A lower yield from diene ester (36) was obtained, presumably due to the electronic mismatch between the silylmethyl radical and electron-deficient diene ester. Importantly, 1,2-disubstituted butadiene also proved to be a viable substrate to produce trisubstituted alkenyl amine 37. A higher catalyst loading, however, was required to achieve a reasonable yield for this substrate.
Table 2.
Scope of Three-Component 1,2-Carboamination of 1,3-Dienesa
|
0.4 mmol scale; isolated yields.
NMR yield.
0.2 mmol scale.
10 mol % PdCl2, 20 mol % Xantphos and 20 mol % AgNTf2 were used.
10 mol % Pd(OAc)2, 20 mol % Xantphos and 20 mol % AgNTf2 were used.
THF/MeOH = 1:1 (0.25 M).
THF/DCM = 2:1 (0.33 M).
Next, the scope of alkyl iodides has been evaluated. Primary alkyl iodides bearing α-silyl or germyl substituents reacted efficiently to furnish the coupling products 38–40 in reasonable to good yields. Reactions employing primary alkyl iodides gave diminished yields (41–44), presumably due to the stronger C–I bond and thus lower reactivity,14 as well as due to the competing alkylation reaction of the amine. In contrast, different secondary alkyl iodides reacted well producing the corresponding allylic amines 45–50 in good yields. Due to the steric reasons, employment of bulky tert-butyl iodide, however, produced a nearly equimolar mixture of 1,2- and 1,4-adducts (51). This reaction was found to be very general with the respect to the secondary amine used. Thus, benzylamines readily participated in this reaction, delivering the allylic amines 52–55 in moderate to good yields. An amine containing a picolyl moiety (56) was also found to be effective. Aliphatic acyclic amines also reacted, though with somewhat lower efficiency (57–58). Reactions with various amine heterocycles, including pyrrolidine, piperidine, piperazine, and morpholine, proceeded smoothly to furnish the target allylic amines in good yields (59–64), presumably due to higher nucleophilicity15 in comparison to that of their acylic counterparts. Remarkably, reactions of less nucleophilic N-Me-aniline and indoline were successful, as well (65–66). Importantly, employment of allylic amines led to the corresponding di- and triallylamines, albeit in low yields (67–68). Notably, N-methyl-1-naphthyl amine underwent smooth reaction to give 69, which represents an alkylated analogue of the drug naftifine. The scope of this transformation was also tested in a more complex setting. Hence, a secondary amine moiety of ciprofloxacin and desloratadine drugs was successfully converted into allylic amine functionality (70–71).
Importantly, 1,3-dienes containing substituents at C2 and/or C3 positions were also proven competent reaction partners. However, in these cases, 1,4-aminoalkylation products 73 and 75 were obtained selectively (Scheme 2).16
Scheme 2.
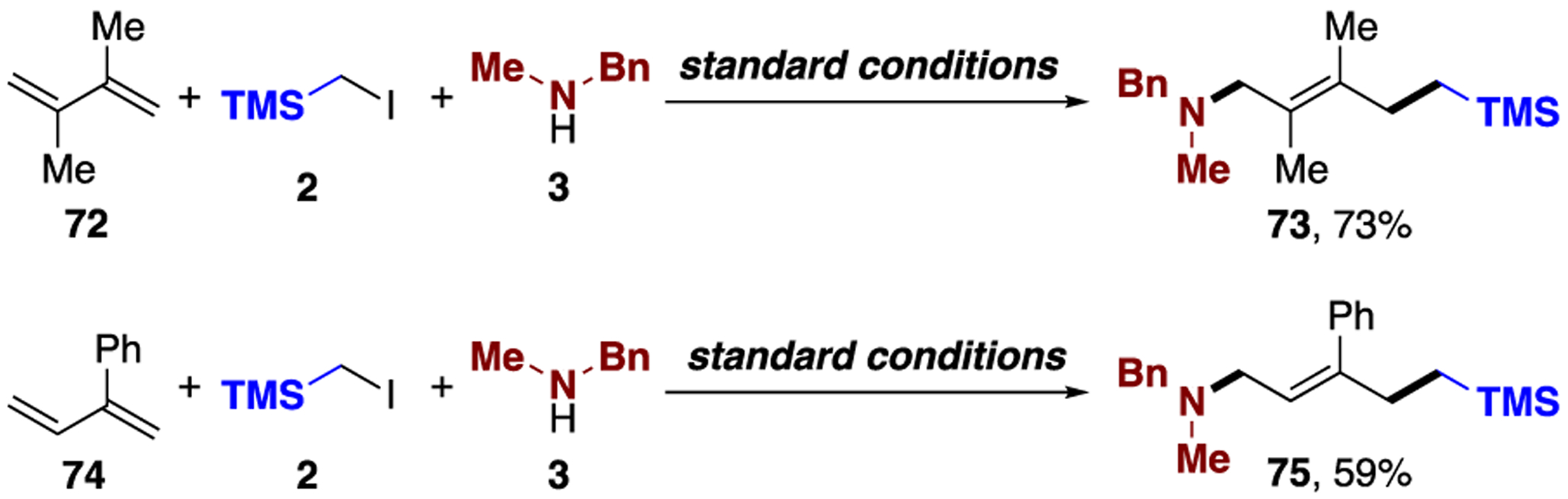
Examples of 1,4-Carboamination of 1,3-Dienesa
a0.4 mmol scale; isolated yields.
Moreover, the use of O- and C-nucleophiles, such as phenol (76) and malonate derivatives (78), respectively, led to the 1,2-carbofunctionalization products in reasonable yields (Scheme 3).
Scheme 3.
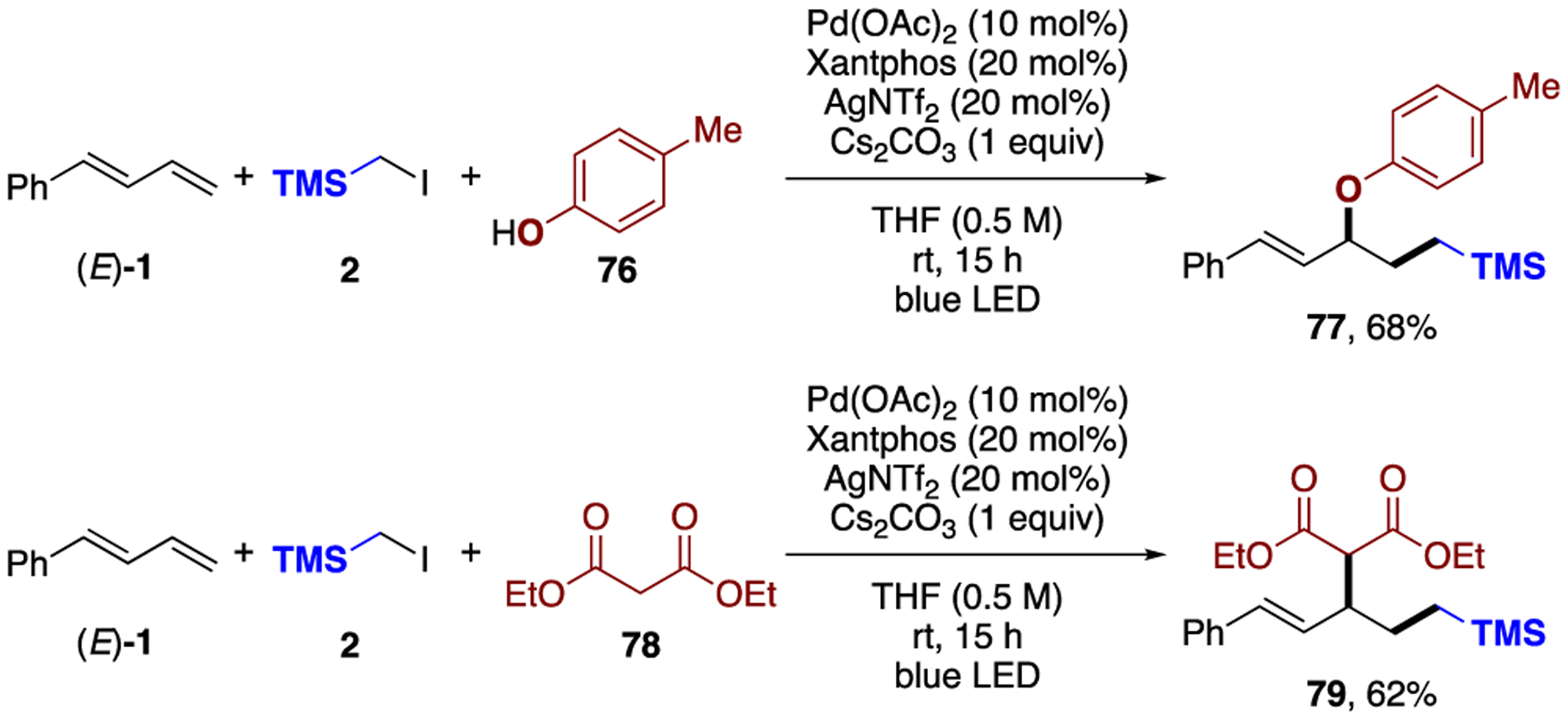
Employment of O- and C-Nucleophilesa
a0.2 mmol scale; isolated yields.
Naturally, we were eager to elucidate the mechanism of this novel transformation. We believe that under these reaction conditions, an interplay between hybrid radical and ionic palladium intermediates takes place. The involvement of radical intermediates was unambiguously supported by the following experiments. Thus, initially formed alkyl radicals from alkyl iodide 2 were trapped by 2,2,6,6-tetramethyl-piperidine-N-oxyl radical (TEMPO) in 22% yield (Scheme 4a, 8011a). Next, the intermediacy of the allylic radicals was uncovered in the reaction performed without silver triflimide. In that case, the reaction produced a substantial amount of allylic radical dimerization17 products 81 (Scheme 4b, Table 1, entry 5). A beneficial effect of accessing more electrophilic π-allyl palladium entities in reactions with nucleophiles by employing weakly coordinating anions is well documented.18 Accordingly, we presumed that employment of triflimide would not only render the Pd-complex more electrophilic but also potentially shift the proposed equilibrium between π-allyl radical- and π-allyl ionic Pd entities to the right (cf. Scheme 1c, A ⇆ B). To validate this assumption, we synthesized model π-allyl palladium complexes bearing a Xantphos ligand19 with iodide (82) and triflimide (83) counterions and subjected them to the reaction with TEMPO under blue light irradiation (Scheme 4c). In both cases,20 the product of allyl radical trapping (84)17c,21 was observed in good yields; however, the reaction of 83 occurred at a slower rate as compared to that of complex 82.13 Control experiments under the thermal conditions showed that the reaction of 82 with TEMPO still proceeded albeit much slower, while complex 83 was completely nonreactive under such conditions. These results support our hypothesis that a triflimide anion stabilizes the π-allyl Pd complex against homolysis upon irradiation with blue light or thermal conditions, thereby suppressing the undesired radical dimerization process.
Based on the results of the aforementioned studies, the following reaction mechanism is proposed (Scheme 5). First, photoexcited LnPd0 undergoes a single electron transfer (SET) process with alkyl iodide to produce hybrid alkyl palladium radical species C,11 which adds to the terminal position of diene to produce radical species A that exists in equilibrium with π-allyl complex B. In the presence of a triflimide anion, this equilibrium is shifted toward complex B. A subsequent attack of the amine at the latter delivers the carbofunctionalization product and regenerates the palladium catalyst.22
Scheme 5.
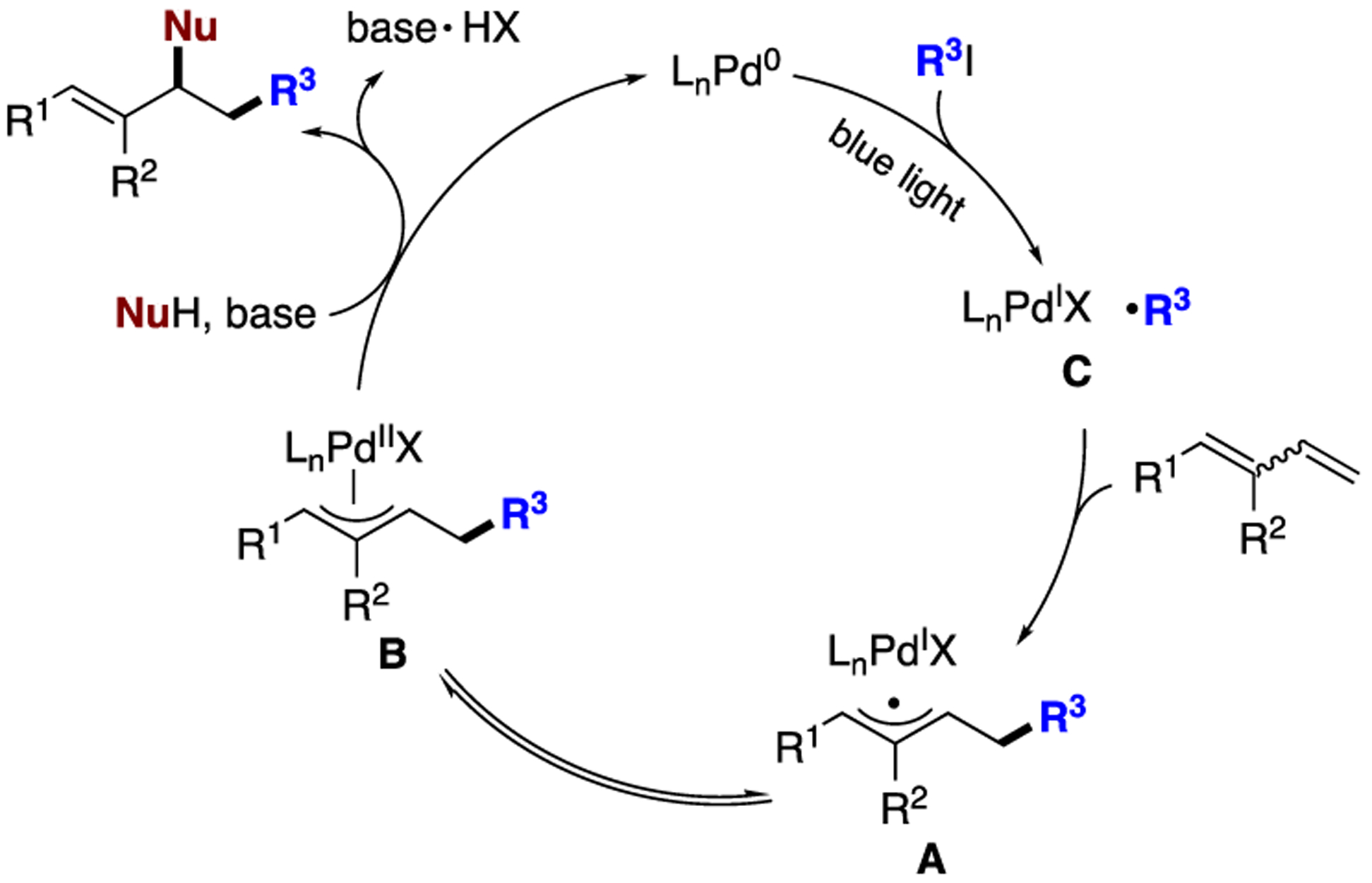
Proposed Mechanism
In conclusion, the first visible-light-induced protocol for the intermolecular aminoalkylation, oxyalkylation, and dialkylation of 1,3-dienes has been developed. This mild multicomponent coupling reaction, which utilizes readily available reaction partners with broad functional group compatibility, does not require employment of exogeneous photosensitizers or external oxidants. It can be used for the late-stage derivatization of complex molecules and drugs. Preliminary mechanistic studies suggest that the radical-polar crossover path is crucial for the success of this reaction. It is expected that this modular, general, and mild method for synthesis of densely functionalized alkenes will find use in organic synthesis and drug discovery.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (GM120281), National Science Foundation (CHE-1936422), and Welch Foundation (Chair, AT-0041) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c03993.
Experimental procedures; analytical data for all new compounds (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c03993
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Büschleb M; Dorich S; Hanessian S; Tao D; Schenthal KB; Overman LE Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms. Angew. Chem., Int. Ed 2016, 55, 4156–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Valente A; Mortreux A; Visseaux M; Zinck P Coordinative Chain Transfer Polymerization. Chem. Rev 2013, 113, 3836–3857. [DOI] [PubMed] [Google Scholar]; (b) Zaikov GE Trends in Polymer Chemistry In Materials Chemistry: A Multidisciplinary Approach to Innovative Methods; Friedrich K, Zaikov GE; Haghi AK, Eds.; CRS Press: Boca Raton, FL, 2016. [Google Scholar]
- (3).Paik IH; Xie SJ; Shapiro TA; Labonte T; Sarjeant AAN; Baege AC; Posner GH Second Generation, Orally Active, Antimalarial, Artemisinin-Derived Trioxane Dimers with High Stability, Efficacy, and Anticancer Activity. J. Med. Chem 2006, 49, 2731–2734. [DOI] [PubMed] [Google Scholar]
- (4).For selected reviews on difunctionalization of 1,3-dienes, see:; (a) Xiong Y; Sun Y; Zhang G Recent Advances on Catalytic Asymmetric Difunctionalization of 1,3-Dienes. Tetrahedron Lett. 2018, 59, 347–355. [Google Scholar]; (b) Wu X; Gong L-Z Palladium(0)-Catalyzed Difunctionalization of 1,3-Dienes: From Racemic to Enantioselective. Synthesis 2019, 51, 122–134. [Google Scholar]
- (5).Skoda EM; Davis GC; Wipf P Allylic Amines as Key Building Blocks in the Synthesis of (E)-Alkene Peptide Isosteres. Org. Process Res. Dev 2012, 16, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) O’Connor JM; Stallman BJ; Clark WG; Shu AYL; Spada RE; Stevenson TM; Dieck HA Some Aspects of Palladium-Catalyzed Reactions of Aryl and Vinylic Halides with Conjugated Dienes in the Presence of Mild Nucleophiles. J. Org. Chem 1983, 48, 807–809. [Google Scholar]; (b) Larock RC; Berrios-Peña N; Narayanan K Palladium-Catalyzed Heteroannulation of 1,3-Dienes by Functionally Substituted Aryl Halides. J. Org. Chem 1990, 55, 3447–3450. [Google Scholar]; (c) Overman LE; Rosen MD Total Synthesis of (–)-Spirotryprostatin B and Three Stereoisomers. Angew. Chem., Int. Ed 2000, 39, 4596–4599. [PubMed] [Google Scholar]; (d) Houlden CE; Bailey CD; Ford JG; Gagné MR; Lloyd-Jones GC; Booker-Milburn KI Distinct Reactivity of Pd(OTs)2: The Intermolecular Pd(II)-Catalyzed 1,2-Carboamination of Dienes. J. Am. Chem. Soc 2008, 130, 10066–10067. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Xing D; Yang D Pd(II)-Catalyzed Intramolecular 1,2-Aminoalkylation of Conjugated 1,3-Dienes for the Synthesis of Pyrrolizidines. Org. Lett 2013, 15, 4370–4373. [DOI] [PubMed] [Google Scholar]; (f) Um C; Chemler SR Synthesis of 2-Aryl and 2-Vinylpyrrolidines via Copper-Catalyzed Coupling of Styrenes and Dienes with Potassium β-Aminoethyl Trifluoroborates. Org. Lett 2016, 18, 2515–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen S-S; Meng J; Li Y-H; Han Z-Y Palladium-Catalyzed Enantioselective Heteroannulation of 1,3-Dienes by Functionally Substituted Aryl Iodides. J. Org. Chem 2016, 81, 9402–9408. [DOI] [PubMed] [Google Scholar]; (h) Chen S-S; Wu M-S; Han Z-Y Palladium-Catalyzed Cascade sp2 C–H Functionalization/Intramolecular Asymmetric Allylation: From Aryl Ureas and 1,3-Dienes to Chiral Indolines. Angew. Chem., Int. Ed 2017, 56, 6641–6645. [DOI] [PubMed] [Google Scholar]; (i) Zhang T; Shen H-C; Xu J-C; Fan T; Han Z-Y; Gong L-Z Pd(II)-Catalyzed Asymmetric Oxidative Annulation of N-Alkoxyheteroaryl Amides and 1,3-Dienes. Org. Lett 2019, 21, 2048–2051. [DOI] [PubMed] [Google Scholar]; For an example of non-annulative aminomethylamination reaction, see:; (j) Liu Y; Xie Y; Wang H; Huang H Enantioselective Aminomethylamination of Conjugated Dienes with Aminals Enabled by Chiral Palladium Complex-Catalyzed C–N Bond Activation. J. Am. Chem. Soc 2016, 138, 4314–4317. [DOI] [PubMed] [Google Scholar]; When this manuscript was under original submission, a related work on light-induced two-component aminoalkylation of dienes has been published:; (k) Huang H-M; Koy M; Serrano E; Pflüger PM; Schwarz JL; Glorius F Catalytic Radical Generation of π-Allylpalladium Complexes. Nat. Catal 2020, 3, 393–400. [Google Scholar]
- (7).For recent examples on radical-triggered three-component coupling reactions, see:; (a) Gockel SN; Buchanan TL; Hull KL Cu-Catalyzed Three-Component Carboamination of Alkenes. J. Am. Chem. Soc 2018, 140, 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buquoi JQ; Lear JM; Gu X; Nagib DA Heteroarene Phosphinylalkylation via a Catalytic, Polarity-Reversing Radical Cascade. ACS Catal. 2019, 9, 5330–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lear JM; Buquoi JQ; Gu X; Pan K; Mustafa DN; Nagib DA Multi-Component Heteroarene Couplings via Polarity-Reversed Radical Cascades. Chem. Commun 2019, 55, 8820–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For reactions involving addition of alkyl radicals to π-allyl Pd(II) species, see:; (a) Lang SB; O’Nele KM; Tunge JA Decarboxylative Allylation of Amino Alkanoic Acids and Esters via Dual Catalysis. J. Am. Chem. Soc 2014, 136, 13606–13609. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lang SB; O’Nele KM; Douglas JT; Tunge JA Dual Catalytic Decarboxylative Allylations of α-Amino Acids and Their Divergent Mechanisms. Chem. - Eur. J 2015, 21, 18589–18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For reviews, see:; (a) Chuentragool P; Kurandina D; Gevorgyan V Catalysis by Visible Light Photoexcited Palladium Complexes. Angew. Chem., Int. Ed 2019, 58, 11586–11598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D; Chuentragool P; Gevorgyan V Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kancherla R; Muralirajan K; Sagadevan A; Rueping M Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends Chem. 2019, 1, 510–523. [Google Scholar]; (d) Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G Visible Light-Induced Palladium-Catalysis in Organic Synthesis. Chem. Lett 2019, 48, 181–191. [Google Scholar]
- (10).(a) Parasram M; Chuentragool P; Sarkar D; Gevorgyan V Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers. J. Am. Chem. Soc 2016, 138, 6340–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, Auxiliary-Enabled Photoinduced Pd-Catalyzed Remote Desaturation of Aliphatic Alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chuentragool P; Parasram M; Shi Y; Gevorgyan V General, Mild, and Selective Method for Desaturation of Aliphatic Amines. J. Am. Chem. Soc 2018, 140, 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) Kurandina D; Parasram M; Gevorgyan V Visible Light-Induced Room-Temperature Heck Reaction of Functionalized Alkyl Halides with Vinyl Arenes/Heteroarenes. Angew. Chem., Int. Ed 2017, 56, 14212–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang G-Z; Shang R; Cheng W-M; Fu Y Irradiation-Induced Heck Reaction of Unactivated Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2017, 139, 18307–18312. [DOI] [PubMed] [Google Scholar]; (c) Kurandina D; Rivas M; Radzhabov M; Gevorgyan V Heck Reaction of Electronically Diverse Tertiary Alkyl Halides. Org. Lett 2018, 20, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang G-Z; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Decarboxylative Heck Reaction of Aliphatic N-(Acyloxy)phthalimides at Room Temperature. Org. Lett 2018, 20, 888–891. [DOI] [PubMed] [Google Scholar]; (e) Koy M; Sandfort F; Tlahuext-Aca A; Quach L; Daniliuc CG; Glorius F Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light. Chem. - Eur. J 2018, 24, 4552–4555. [DOI] [PubMed] [Google Scholar]; (f) Kancherla R; Muralirajan K; Maity B; Zhu C; Krach PE; Cavallo L; Rueping M Oxidative Addition to Palladium(0) Made Easy through Photoexcited-State Metal Catalysis: Experiment and Computation. Angew. Chem., Int. Ed 2019, 58, 3412–3416. [DOI] [PubMed] [Google Scholar]; (g) Chuentragool P; Yadagiri D; Morita T; Sarkar S; Parasram M; Wang Y; Gevorgyan V Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3)–H Sites of Alcohols. Angew. Chem., Int. Ed 2019, 58, 1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).For selected examples, see:; (a) Zhou W-J; Cao G-M; Shen G; Zhu X-Y; Gui Y-Y; Ye J-H; Sun L; Liao L-L; Li J; Yu D-G Visible-Light-Driven Palladium-Catalyzed Radical Alkylation of C–H Bonds with Unactivated Alkyl Bromides. Angew. Chem., Int. Ed 2017, 56, 15683–15687. [DOI] [PubMed] [Google Scholar]; (b) Ratushnyy M; Parasram M; Wang Y; Gevorgyan V Pd-Catalyzed Atom Transfer Radical Cyclization at Remote Unactivated C(sp3)-H Sites Enabled by Hydrogen Atom Transfer of Hybrid Vinyl Pd-Radical Intermediates. Angew. Chem., Int. Ed 2018, 57, 2712–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang G-Z; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Direct C–H Alkylation of Heteroarenes with Tertiary and Secondary Alkyl Bromides. Synthesis 2018, 50, 2908–2914. [Google Scholar]; (d) Cheng W-M; Shang R; Fu Y Irradiation-Induced Palladium-Catalyzed Decarboxylative Desaturation Enabled by a Dual Ligand System. Nat. Commun 2018, 9, 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Koy M; Bellotti P; Katzenburg F; Daniliuc CG; Glorius F Synthesis of All-Carbon Quaternary Centers by Palladium-Catalyzed Olefin Dicarbofunctionalization. Angew. Chem., Int. Ed 2020, 59, 2375–2379. [DOI] [PubMed] [Google Scholar]; (f) Ratushnyy M; Kvasovs N; Sarkar S; Gevorgyan V Visible Light-Induced Palladium-Catalyzed Generation of Aryl Radicals from Aryl Triflates. Angew. Chem., Int. Ed 2020, DOI: 10.1002/anie.201915962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).See Supporting Information for details.
- (14).For some reductive potential of activated and inactivated primary alkyl halides, see:; Isse AA; Lin CY; Coote ML; Gennaro A Estimation of Standard Reduction Potentials of Halogen Atoms and Alkyl Halides. J. Phys. Chem. B 2011, 115, 678–684. [DOI] [PubMed] [Google Scholar]
- (15).Kanzian T; Nigst TA; Maier A; Pichl S; Mayr H Nucleophilic Reactivities of Primary and Secondary Amines in Acetonitrile. Eur. J. Org. Chem 2009, 2009, 6379–6385. [Google Scholar]
- (16).For a recent example of rhodium-catalyzed 1,4-carboamination, see:; Pinkert T; Wegner T; Mondal S; Glorius F Intermolecular 1,4-Carboamination of Conjugated Dienes Enabled by Cp*RhIII-Catalyzed C–H Activation. Angew. Chem., Int. Ed 2019, 58, 15041–15045. [DOI] [PubMed] [Google Scholar]
- (17).For examples of dimerization of allyl radicals, see:; (a) Sasaoka S; Yamamoto T; Kinoshita H; Inomata K; Kotake H Palladium-Catalyzed Coupling of Allylic Acetates with Zinc. Chem. Lett 1985, 14, 315–318. [Google Scholar]; (b) Millan A; Campana AG; Bazdi B; Miguel D; Alvarez de Cienfuegos L; Echavarren AM; Cuerva JM Ti/Pd Bimetallic Systems for the Efficient Allylation of Carbonyl Compounds and Homocoupling Reactions. Chem. - Eur. J 2011, 17, 3985–3994. [DOI] [PubMed] [Google Scholar]; (c) Xuan J; Zeng T-T; Feng Z-J; Deng Q-H; Chen J-R; Lu L-Q; Xiao W-J; Alper H Redox-Neutral α-Allylation of Amines by Combining Palladium Catalysis and Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed 2015, 54, 1625–1628. [DOI] [PubMed] [Google Scholar]; For other Pd-catalyzed reactions of allyl radicals, see:; (d) Campana AG; Bazdi B; Fuentes N; Robles R; Cuerva JM; Oltra JE; Porcel S; Echavarren AM Divergent Titanium-mediated Allylations with Modulation by Nickel or Palladium. Angew. Chem., Int. Ed 2008, 47, 7515–7519. [DOI] [PubMed] [Google Scholar]; (e) Millan A; Martin-Lasanta A; Miguel D; Alvarez de Cienfuegos L; Cuerva JM Ti/Pd-Promoted Intramolecular Michael-type Addition of Allylic Carboxylates to Activated Alkenes. Chem. Commun 2011, 47, 10470–10472. [DOI] [PubMed] [Google Scholar]
- (18).(a) Åkermark B; Åkermark G; Hegedus LS; Zetterberg K Amination of π-Allylpalladium Chloride Complexes. A Mechanistic Study. J. Am. Chem. Soc 1981, 103, 3037–3040. [Google Scholar]; (b) Cantat T; Genin E; Giroud C; Meyer G; Jutand A Structural and Kinetic Effects of Chloride Ions in the Palladium-Catalyzed Allylic Substitutions. J. Organomet. Chem 2003, 687, 365–376. [Google Scholar]; (c) Pratihar S; Marek J; Roy S Mono Cationic Palladium(II): Synthesis, Characterization and Catalytic Activity in Suzuki Coupling. Inorg. Chim. Acta 2011, 372, 362–366. [Google Scholar]; (d) Cadu A; Tšupova S; Hashmi ASK Silver Triflimide In Encyclopedia of Reagents for Organic Synthesis; Charette A, Bode J, Rovis T, Shenvi R, Eds.; John Wiley & Sons, Ltd. [Google Scholar]
- (19).Johns AM; Utsunomiya M; Incarvito CD; Hartwig JF A Highly Active Palladium Catalyst for Intermolecular Hydroamination. Factors that Control Reactivity and Additions of Functionalized Anilines to Dienes and Vinylarenes. J. Am. Chem. Soc 2006, 128, 1828–1839. [DOI] [PubMed] [Google Scholar]
- (20).For examples of reactions of π-allyl palladium complexes under UVA light, see:; (a) Muzart J; Pale P; Pete J-P Preparation of Conjugated Carbonyl Compounds by Photolysis of η3-Allylpalladium Complexes. J. Chem. Soc., Chem. Commun 1981, 0, 668–669. [Google Scholar]; (b) Vermeersch G; Marko J Photoreactivity of η3-Allylpalladium Complexes Studied by CIDNP. J. Chem. Soc., Perkin Trans 2 1986, 383–389. [Google Scholar]; (c) Crozet MP; Muzart J; Pale P; Tordo P Photolyse des Complexes η3–Allylpalladium: Étude Par Résonnance Para-magnétique Électronique des Nitroxydes Allyliques Formés en Présence de Nitrosodurène. J. Organomet. Chem 1983, 244, 191–200. [Google Scholar]
- (21).For examples of trapping cinnamyl radicals with TEMPO, see:; (a) Song F; Wang F; Guo L; Feng X; Zhang Y; Chu L Visible-Light-Enabled Stereodivergent Synthesis of E- and Z- Configured 1,4-Dienes by Photoredox/Nickel Dual Catalysis. Angew. Chem., Int. Ed 2020, 59, 177–181. [DOI] [PubMed] [Google Scholar]; (b) Yasu Y; Koike T; Akita M Visible Light-Induced Selective Generation of Radicals from Organoborates by Photoredox Catalysis. Adv. Synth. Catal 2012, 354, 3414–3420. [Google Scholar]; (c) Xu B; Troian-Gautier L; Dykstra R; Martin R; Gutierrez O; Tambar UK Photocatalyzed Diastereoselective Isomerization of Cinnamyl Chlorides to Cyclopropanes. J. Am. Chem. Soc 2020, 142, 6206–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Although generation of alkyl radicals by light irradiation of EDA complexes of alkyl halides with amines is known, the possibility of such a metal-free scenario for this transformation was ruled out based on the fact that the reaction did not proceed in the absence of a palladium catalyst (Table 1, entry 4). For light-induced radical generation from EDA complexes, see:; (a) Sun X; Wang W; Li Y; Ma J; Yu S Halogen-Bond-Promoted Double Radical Isocyanide Insertion under Visible-Light Irradiation: Synthesis of 2-Fluoroalkylated Quinoxalines. Org. Lett 2016, 18, 4638–4641. [DOI] [PubMed] [Google Scholar]; (b) Wang Y; Wang J; Li G-X; He G; Chen G Halogen-Bond-Promoted Photoactivation of Perfluoroalkyl Iodides: A Photochemical Protocol for Perfluoroalkylation Reactions. Org. Lett 2017, 19, 1442–1445. [DOI] [PubMed] [Google Scholar]; (c) Tang X; Studer A Alkene 1,2-Difunctionalization by Radical Alkenyl Migration. Angew. Chem., Int. Ed 2018, 57, 814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Herraiz AG; Suero MG A Transition-Metal-Free & Diazo-Free Styrene Cyclopropanation. Chem. Sci 2019, 10, 9374–9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


