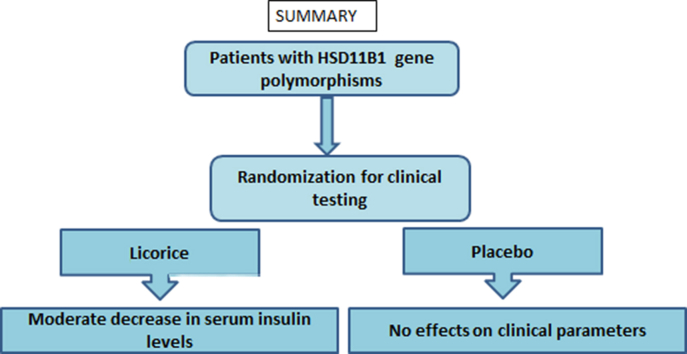
Abstract
The positive association of HSD11B1 gene polymorphism with type 2 diabetes (T2D) and prediabetic conditions has been revealed. In the current study, we assessed the effectiveness of licorice on the clinical profile of the patients with HSD11B1 gene polymorphism. Licorice (Glycyrrhiza Glabra) is a competitive inhibitor of 11 beta-hydroxysteroid dehydrogenase 1 (11β-HSD1) enzyme and has been traditionally reported as an anti-ulcer, anti-pyretic, anti-thirst, anti-inflammatory, hypoglycemic and hypolipidemic agent. The aim of the study was to assess the effectiveness of licorice on the clinical profile of participants with HSD11B1 gene polymorphism. The study was performed using diabetic patients with HSD11B1 gene polymorphism. Biochemical and anthropometric parameters were measured using standard diagnostic tools. Fourteen patients were divided into two groups by simple randomization, Licorice group (treated with 750 mg licorice/day for three weeks), and placebo group (treated with 750 mg placebo/day for three weeks). Investigations were repeated at the end of three weeks. Licorice showed a significant reduction in serum insulin levels (p = 0.03). There was no significant change in any other clinical parameters either by licorice or placebo. Conclusively, licorice moderately improves serum insulin levels in patients with HSD11B1 gene polymorphism. From our pilot study, the safety of licorice is confirmed at a dose of 750 mg/day. However, the study can be repeated at a higher dose to show its effectiveness and safety.
Keywords: 11beta-hydroxysteroid dehydrogenase, Licorice, Simple randomization
Graphical abstract
1. Introduction
Overexpression of 11β-HSD1 is involved in the onset of obesity, insulin resistance, diabetes and prediabetic conditions [1]. HSD11B1 gene that encodes 11 beta hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme, influences the circulating cortisol levels, insulin levels, blood sugar, lipid profile and other associated components [2]. HSD11B1 polymorphisms have shown positive association with type 2 diabetes and prediabetes conditions such as metabolic syndrome in the South Indian population [3]. Blood sugar and lipid levels are higher in patients with raised 11β-HSD1 levels. 11 beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme inhibition has been recommended as an ideal therapeutic target for the treatment of type 2 diabetes (T2D) [4].
Licorice is a natural inhibitor of 11β-HSD1, with hypoglycaemic and hypolipidemic properties [5]. In Ayurveda, it is used as a haemostatic, diuretic, bronchodilator, laxative, as an anti-ulcer, anti-pyretic, anti-inflammatory agent [6]. The mechanism of inhibition is by competitive inhibition of 11β-HSD1 and pretranslational inhibition of 11β-HSD1 [7].
Animal studies have shown that the licorice significantly decrease serum lipid levels [5,8,9]. In fifteen healthy patients, Armanini et al. showed that the consumption of licorice reduced body fat mass [10]. In another study of Fuhrman et al. licorice (0.1 g/day for four weeks) decreased plasma blood sugar and lipid levels [11].
No reports are available in literature regarding the impact of licorice on the patients with the HSD11B1 gene polymorphism. Consequently, we assessed these issues in the reduction of endpoints in patients with HSD11B1 gene polymorphisms with the 11β-HSD1 inhibitor Licorice study.
2. Materials and methods
2.1. Study design and selection criteria
This study is a randomized, double blind, placebo controlled, parallel arm, phase 2 clinical testing to compare the effectiveness of treatment with licorice and the treatment with matching placebo. Newly diagnosed T2D patients, aged 18 to 75, male or female, with HSD11B1 gene polymorphism willing to provide written informed consent before participating in the trial were included. The T2D was confirmed before assessing HSD11B1 gene polymorphism based on the clinical symptoms, fasting blood sugar (FBS) ≥ 126 mg/dL, postprandial (PPBS) and random blood sugar (RBS) ≥ 200 mg/dL, glycated hemoglobin (HbA1c) level ≥ 6.5%, following the criteria of the American Diabetes Association. The cases with type 1 diabetes, malignancy, acute infections, inflammation, endocrine disorders, patients on insulin therapy, and chemotherapeutic agents, old, sick and pregnant patients were excluded.
2.2. Patients
All participants were recruited from the inpatient and outpatient department of Kasturba Medical College, Mangalore. Patients of our previous study who showed positive association of HSD11B1 gene polymorphism with T2D and prediabetic conditions were used for licorice study [3]. The study protocol was approved by the Manipal University Ethics Committee (IEC no. ECR/191/Inst/KL/2013) and the trial has been registered in the Clinical trial registry of India (CTRI/2015/09/006171).
2.3. Licorice intervention
The standardized preparation of solid licorice root powder enclosed in the hard gelatin capsule was procured from the Himalaya Drug company whose products comply with standards of Ayurvedic pharmacopoeia of India. Each capsule contains 250 mg of licorice root powder extract. The company has quality management system for physiochemical standardization of raw material, quality assessment, and purity analysis under the directives of the department of AYUSH, certified with GLP, GMP (Good Manufacturing Practices), issued by the Licensing Authority, Directorate of Indian Systems of Medicine. Hydroalcoholic extract method involves crushing of the dried roots and rhizomes and extraction with 70% v/v ethanol, concentration of the hydroalcoholic extract under reduced pressure and evaporation in air to get a dry powder to be enclosed in the hard gelatin capsule [12]. Standardization and determination of their phytochemical profile is performed by HPTLC (High Performance Thin Layer Chromatography, and MS (Mass Spectrometry).
2.4. Intervention study procedure
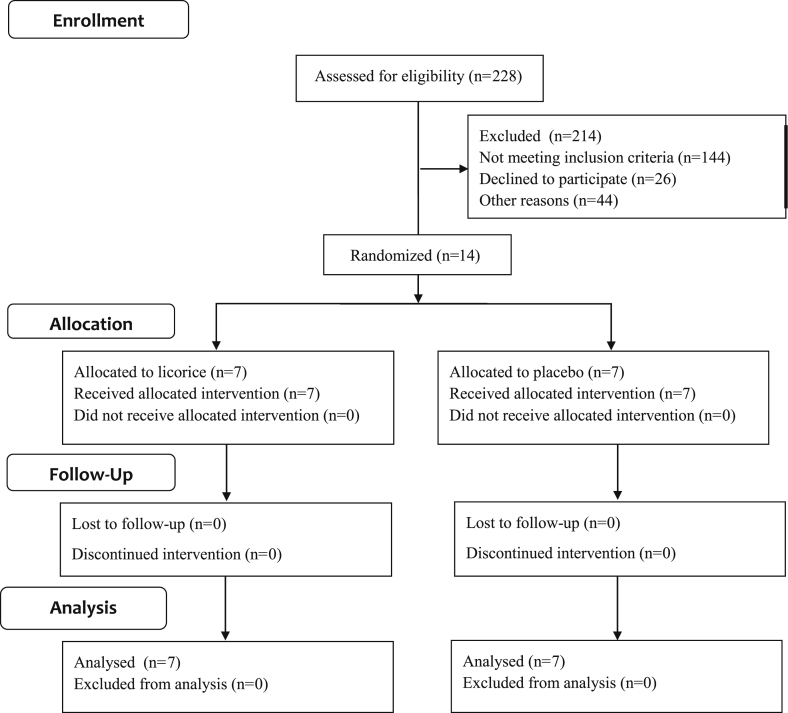
Fourteen T2D patients with HSD11B1 gene polymorphism were randomized arbitrarily into licorice and placebo groups by simple block randomization. All the participants received either Licorice or placebo in a dose of three capsules (250 mg) thrice daily, for three weeks. All participants underwent clinical examination and evaluation of weight, waist circumference (WC), blood pressure, fasting blood sugar (FBS), lipid levels (total cholesterol (TC), HDL-cholesterol, LDL-cholesterol, triglycerides (TG)), serum cortisol, serum insulin, serum sodium (Na+) and potassium (K+) levels on entry. Serum electrolyte levels (Sodium and potassium) and blood pressure were monitored at weekly intervals. All participants continued their routine diets during the study. Adverse effects if any were recorded. Clinical investigations were repeated at the end of 3 weeks to observe improvements in clinical profile. The consort flow diagram illustrates the progress through the phases of this study (Fig. 1).
Fig. 1.
CONSORT Flow Diagram.
2.5. Primary and secondary outcome measure
The primary end-points in this study were the decrease of baseline weight, blood sugar, lipid levels, serum insulin and cortisol levels. The licorice safety and toxicity profiles were secondary end points.
3. Statistical analysis
All statistical analyses were performed using SPSS version 20 for windows (SPSS, Chicago, Illinois, USA) and SNPstat Software [13]. A Paired t-test was used to compare the improvement in clinical parameters by the licorice and placebo. The sample size was calculated using the formula n= (2∗ (Zα+ Z β)2∗ σ2)/d2 at 95% confidence interval, 80% power, based on the study of Fuhrman et al. [11] with the effect size of 14 and standard deviation of 9. All the values are represented as a mean ± standard deviation. The p values less than 0.05 were considered statistically significant.
4. Results
4.1. Clinical profile after licorice and placebo intervention
The licorice showed significant reduction in the serum insulin levels (p = 0.03) in participants with HSD11B1 gene polymorphism (Table 1). However, there was no improvement in other clinical parameters by licorice. Placebo did not significantly change any clinical parameters in participants with HSD11B1 gene polymorphism.
Table 1.
Effect of licorice and placebo on clinical parameters of T2D patients with HSD11B1 gene polymorphism.
| Parameters | Licorice treated group |
Placebo treated group |
||||
|---|---|---|---|---|---|---|
| Before trial | After trial | p | Before trial | After trial | p | |
| Weight (kg) | 69.28 ± 11.18 | 68.38 ± 10.28 | 0.132 | 65.72 ± 6.78 | 66.04 ± 6.87 | 0.483 |
| WC (cms) | 89.07 ± 16.22 | 88.35 ± 15.73 | 0.601 | 85.57 ± 3.99 | 87.5 ± 3.92 | 0.161 |
| FBS (mg/dL) | 97.28 ± 14.56 | 101 ± 12.19 | 0.62 | 103.71 ± 11.2 | 111.14 ± 17.08 | 0.089 |
| TC (mg/dL) | 183.85 ± 29.61 | 186.28 ± 33.88 | 0.699 | 195.85 ± 45.97 | 194.1 ± 51.55 | 0.853 |
| HDL (mg/dL) | 51.11 ± 10.21 | 52.14 ± 9.88 | 0.482 | 50.18 ± 12.77 | 48.8 ± 16.28 | 0.483 |
| LDL (mg/dL) | 117.55 ± 30.21 | 124.52 ± 37.78 | 0.279 | 128.3 ± 48.17 | 131.31 ± 51.66 | 0.706 |
| TG (mg/dL) | 94.57 ± 37.84 | 90.14 ± 25.68 | 0.547 | 144.7 ± 64.26 | 130.57 ± 45.02 | 0.396 |
| SBP mmHg | 121.71 ± 11.85 | 118.57 ± 13.45 | 0.481 | 129.28 ± 10.17 | 128.57 ± 24.10 | 0.925 |
| DBP mmHg | 81.14 ± 9.08 | 77.28 ± 9.74 | 0.236 | 77.14 ± 7.55 | 77.14 ± 7.55 | 1.00 |
| K+ nmol/L | 4.4 ± 0.3 | 4.6 ± 0.42 | 0.141 | 4.44 ± 0.43 | 4.38 ± 0.45 | 0.577 |
| Na+ nmol/L | 140 ± 2.08 | 139.14 ± 1.21 | 0.225 | 139.66 ± 2.8 | 139.33 ± 1.03 | 0.765 |
| Serum Insulin uIu/mL | 16.70 ± 6.22 | 12.42 ± 3.80 | 0.03∗ | 20.25 ± 6.42 | 19.09 ± 6.77 | 0.73 |
| Serum cortisol ng/mL | 82.75 ± 48.87 | 81.98 ± 41.76 | 0.957 | 104.54 ± 52.49 | 111.04 ± 51.29 | 0.541 |
Data are shown as mean Standard deviation. ∗Significant p value < 0.05. WC, waist circumference; FBS, fasting blood sugar; TC, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; K+, serum potassium; Na+, serum sodium.
4.2. Presentation of side effects
During the three-week treatment, patients on licorice showed signs of gastro intestinal (GI) (burping, flatulence), oedema and musculo-skeletal (back pain) pain. Two patients on placebo complained of excess hunger and weight gain (Table 2).
Table 2.
Number of patients in licorice and placebo groups with presentation of side effects.
| Side effects | Licorice group (n) | Placebo group (n) |
|---|---|---|
| GI side effects (Burping, flatulence) and oedema | 1 | 0 |
| Musculo-skeletal pain (Back pain and left side body pain) | 2 | 0 |
| Excess hunger | 0 | 1 |
| Weight gain | 0 | 1 |
5. Discussion
Herbal and Ayurveda therapies are unlikely to have the adverse effects of conventional treatments for T2D and have been widely researched, especially in India. Here, in our study the Ayurveda capsules of drug licorice (Himalaya Drug Company, Mangalore, Karnataka), was evaluated for use in diabetic patients having HSD11B1 gene polymorphism. We found a significant decrease in serum insulin level by licorice. Similar decrease in serum insulin levels were observed in mice by the study of Sil et al. and Kamisoyama et al. [14,15]. In our study, licorice did not significantly improve any other clinical parameters. Contradictory to this, licorice brought significant decrease in FBS (p < 0.01), TC (p < 0.01). LDL (p < 0.01), and TG (p < 0.01) in 12 hypercholesteraemic Israeli patients [11]. However, their study population was different, which may be the reason for discrepancy.
Licorice is a natural 11β-HSD1 inhibitor. No studies have reported the effect of licorice in Indian population. To the best of our knowledge, this is the first study on the effectiveness of licorice on patients with HSD11B1 gene polymorphism. In our previous studies, we observed positive association of HSD11B1 gene polymorphism with T2D, prediabetic conditions, and their clinical profile [3,16,17]. Therefore, we assessed the influence of the licorice on clinical profile of patients with HSD11B1 gene polymorphism. In our study, 71% of cases on licorice showed marginal weight reduction compared to 14% of cases on placebo. Reduction in body weight has been previously reported in high fat diet induced obese rats by Eu et al. [18]. Hypertension is considered to be the common side effect of licorice. However, 86% cases of our study on licorice showed decrease in BP. A study by Fuhrman et al. also showed decrease in SBP in hypercholesteraemic patients treated with licorice [11]. In another study, Sharma et al. studied the effect of licorice in combination with 23 other Ayurveda formulation on T2D Indian patients for 3 months and the patients showed improvement in clinical profile from second month onwards [19]. However, it’s uncertain that the improvement was mediated by licorice alone, as it was administered with other formulation. The knowledge of the consequences of licorice-anti-diabetic drug interactions is essential so that a unique combination of the licorice and modern medicine could be achieved [20]. Currently, there is limited clinical evidence to support serious licorice–drug interactions, especially the interaction with anti-diabetic drugs. There has been a report of the inhibitory effect of licorice–warfarin interaction on the hepatic microsomal enzyme system and risk of blood clotting [21]. In another study licorice induced hypokalemia in one patient presenting digoxin toxicity [22]. However, our study participants were not on digoxin and did not show hypokalemia. Table 3 shows probable effects of licorice interaction with known drugs.
Table 3.
Licorice-drug interactions.
Since there is a great individual variation in the susceptibility to licorice, it is difficult to determine precisely the dose of licorice required [24]. In previous studies, side effects of oedema and hypertension were observed in those patients whose daily intake of licorice was in the range from 1.5 to 250 gm/day [24]. Half of the lower limit of this dose was used in our study.
Since this is the pilot comparative study and considering patient’s safety as well as acceptability, we used smaller sample size and shorter duration. Except for serum insulin levels, licorice did not improve any clinical parameters. However, the study needs to be replicated with the larger sample size and longer study duration to study the effectiveness of licorice. The study with higher doses of licorice is necessary with precautions as the current dose decreases only insulin but not FBG in Indian population with HSD11B1 gene polymorphism.
According to Ayurveda our study participants showing HSD11B1 polymorphism belong to the Kapha constituent [25,26]. In colder climates, licorice may induce nasal congestion, sinusitis, running nose, in patients with kapha constitution. However, in normal climatic conditions and in other prakritis, licorice may prevent nasal sinusitis, severe acute respiratory syndrome, and even the coronavirus by preventing fusion of the virus with the membrane of the host cell [27,28]. Thus future studies must include patients showing HSD11B1 polymorphisms, exhibiting pitta body constitution, and pitta symptoms, as licorice may provide better results in these conditions according to Ayurveda. Since the anti-inflammatory effect of licorice is confirmed [29], it is also worthwhile to assess even the inflammatory markers like CRP, IL-6, and TNF- α in the T2D patients who generally show higher levels of inflammation in their bodies [30]. While licorice is indispensable against various ulcers and infections, the glycyrrhizin component of licorice may show aldosterone-like effects if consumed at higher dose [31]. Removal of the glycyrrhizin compound will eliminate the risk altogether [31].
From our study, safety of licorice for pilot study is confirmed and the study can be repeated with the higher dose. Many pharmaceutical companies are developing potential 11β-HSD1 inhibitors to treat obesity and T2D. Studies in mice have yielded promising results with reduction in body weight and FBS in obese and diabetic mice strains [18,32]. While human studies using novel 11β-HSD1 inhibitors are awaited, in this study we have used the licorice as a ‘prototype’ to assess the clinical effects of 11β-HSD1 inhibition on metabolic profile of patients with HSD11B1 gene polymorphism.
6. Conclusion
In conclusion, licorice moderately improves serum insulin levels in patients with HSD11B1 gene polymorphism. From our pilot study, safety of licorice is confirmed at a dose of 750 mg/day. However, the study can be repeated at a higher dose to show its effectiveness and safety.
Source(s) of funding
Research Society for the Study of Diabetes in India (ch#77723/3.2.11), M. Vishwanathan Diabetes Centre, Chennai and Department of Science and Technology India (DST/INSPIRE fellowship/2012/598) financially supported this study.
Conflict of interest
None.
Acknowledgments
We are greatly thankful to all the participants in this study and Manipal University for their collaboration in this study. ND is grateful to DST for funding the study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Seckl J.R., Walker B.R. Minireview: 11β-Hydroxysteroid Dehydrogenase Type 1-A tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 2.Nair S., Lee Y.H., Lindsay R.H., Walker B.R., Tataranni B.A., Bogardus C. 11beta-Hydroxysteroid dehydrogenase Type 1: genetic polymorphisms are associated with Type 2 diabetes in Pima Indians independently of obesity and expression in adipocyte and muscle. Diabetologia. 2004;47:1088–1095. doi: 10.1007/s00125-004-1407-6. [DOI] [PubMed] [Google Scholar]
- 3.Devang N., Satyamoorthy K., Rai P.S., Nandini M., Rao Satish, Phani N.M. Association of HSD11B1 gene polymorphisms with type 2 diabetes and metabolic syndrome in South Indian population. Diabetes Res Clin Pract. 2017;131:142–148. doi: 10.1016/j.diabres.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Anderson A., Walker B.R. 11β-HSD1 inhibitors for the treatment of type 2 diabetes and cardiovascular disease. Drugs. 2013;73:1385–1393. doi: 10.1007/s40265-013-0112-5. [DOI] [PubMed] [Google Scholar]
- 5.Saleem M., Mohammad A., Al-Tameemi J., Sulaiman G.M. Biological study of the effect of licorice roots extract on serum lipid profile, liver enzymes and kidney function tests in albino mice. Afr J Biotechnol. 2011;10:12702–12706. [Google Scholar]
- 6.Hegde P.L., Harini A. 1st ed. vol. 1. Chaukhambha Publishers; New Delhi: 2011. Knowledge of gana explained by Charaka and Susruta; pp. 54–74. (A text book of Dravyaguna Vijnana). [Google Scholar]
- 7.Whorwood C.V., Donovan S.J., Wood P.J., Phillips D.I. Regulation of glucocorticoid receptor alpha and beta isoforms and type I 11beta-hydroxysteroid dehydrogenase expression in human skeletal muscle cells: a key role in the pathogenesis of insulin resistance? J Clin Endocrinol Metab. 2001;86:2296–2308. doi: 10.1210/jcem.86.5.7503. [DOI] [PubMed] [Google Scholar]
- 8.Alwash Y.S., Latif A.R., Bayati N.J. Effect of licorice extract on lipid profile in hypercholestermic male rabbits. QMJ. 2011;7:167–178. [Google Scholar]
- 9.Visavadiya N.P., Narasimhacharya A.V. Hypocholesterolaemic and antioxidant effects of Glycyrrhiza glabra (Linn) in rats. Mol Nutr Food Res. 2006;50:1080–1086. doi: 10.1002/mnfr.200600063. [DOI] [PubMed] [Google Scholar]
- 10.Armanini D., De Palo C.B., Mattarello M.J., Spinella P., Zaccaria M., Ermolao A. Effect of licorice on the reduction of body fat mass in healthy subjects. J Endocrinol Invest. 2003;26:646–650. doi: 10.1007/BF03347023. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman B., Volkova N., Kaplan M., Presser D., Attias J., Hayek T. Antiatherosclerotic effect of licorice extract supplementation on hypercholesterolemic pateints: increased resistance of LDL to atherogenic modifications reduced plasma lipid levels and decreased systolic blood pressure. Nutrition. 2002;18:268–273. doi: 10.1016/s0899-9007(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 12.Srikantam S., Arumugam G. Hydroalcoholic extract of licorice (Glycyrrhiza glabra L.) root attenuates ethanol and cerulein induced pancreatitis in rats. Asian Pac J Trop Biom. 2019;9:424–433. [Google Scholar]
- 13.Sole X., Guino E., Valls J., Iniesta R., Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 14.Sil R., Ray D., Chakraborty A.R. Glycyrrhizin ameliorates metabolic syndrome- induced liver damage in experimental rat model. Mol Cell Biochem. 2015;409:177–189. doi: 10.1007/s11010-015-2523-y. [DOI] [PubMed] [Google Scholar]
- 15.Kamisoyama H., Honda K., Tominaga Y., Yokota S., Hasegawa S. Investigation of the anti-obesity action of licorice flavonoid oil in diet-induced obese rats. Biosci Biotechnol Biochem. 2008;72:3225–3231. doi: 10.1271/bbb.80469. [DOI] [PubMed] [Google Scholar]
- 16.Devang N., Satyamoorthy K., Rai P.S., Nandini M., Basu A., Phani M.N. Association of HSD11B1 rs12086634 and HSD11B1 rs846910 gene polymorphisms with polycystic ovary syndrome in South Indian women. Int J Diabetes Dev Ctries. 2018;38:p381–p386. [Google Scholar]
- 17.Devang N., Nandini N., Rao S., Adhikari P. HSD11B1 gene polymorphisms in type 2 diabetes and metabolic syndrome—do we have evidence for the association. Int J Diabetes Dev Ctries. 2016;36:95–102. [Google Scholar]
- 18.Eu C.H., Lim W.Y., Ton S.H., Bin Abdul Kadir K. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010;9:81. doi: 10.1186/1476-511X-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma R.K., Patki P.S. Double-blind, placebo-controlled clinical evaluation of an Ayurvedic formulation (GlucoCare capsules) in non-insulin dependent diabetes mellitus. J Ayurveda Integr Med. 2010;1:45–51. doi: 10.4103/0975-9476.59827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borse S.P., Singh D.P., Nivsarkar M. Understanding the relevance of herb-drug interaction studies with special focus on interplays: a prerequisite for integrative medicine. Porto Biomed J. 2019;4 doi: 10.1016/j.pbj.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck A., DeWitt B., Lukes A. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221–1227. [PubMed] [Google Scholar]
- 22.Harada T., Ohtaki E., Misu K., Sumiyoshi T., Hosoda S. Congestive heart failure caused by digitalis toxicity in an elderly man taking a licorice-containing Chinese herbal laxative. Cardiology. 2002;98:218. doi: 10.1159/000067316. [DOI] [PubMed] [Google Scholar]
- 23.Coxeter Peter D., Duke Colin C., Roufogalis Basil, McLachlan Andrew J. Liquorice-drug interactions. J Complement Med. 2003;2:40–43. [Google Scholar]
- 24.Stormer F.C., Reistad R., Alexander J. Glycyrrhizic acid in liquorice-evaluation of health hazard. Food Chem Toxicol. 1993;31:303–312. doi: 10.1016/0278-6915(93)90080-i. [DOI] [PubMed] [Google Scholar]
- 25.Ghodke Y., Joshi K., Patwardhan B. Traditional medicine to modern pharmacogenomics: Ayurveda prakriti type and CYP2C19 gene polymorphism associated with the metabolic variability. Evid Based Complement Alter Med. 2011:249528. doi: 10.1093/ecam/nep206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindaraj P., Nizamuddin S., Sharath S., Jyothi V., Rotti H., Raval R. Genome-wide analysis correlates Ayurveda prakriti. Sci Rep. 2015;5:15786. doi: 10.1038/srep15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun L., Cohen M. 3rd ed. Elsevier Australia; Chatswood, New South Wales: 2010. Herbs and natural supplements: an evidence-based guide. [Google Scholar]
- 28.Martin B.R., Reshamwala G., Short M. Treatment of a woman with Glycyrrhiza glabra for acute sinusitis: a case report. J Chiropr Med. 2018;17:268–274. doi: 10.1016/j.jcm.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang R., Yuan B.C., Ma Y.S., Zhou S., Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017;55:5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack Rena M., Donath Marc Y., LeRoith D., Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39:S244–S252. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- 31.Asl M.N., Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim W.Y., Chia Y.Y., Liong S.Y., Ton S.H., Kadir K.A., Husain S.N. Lipoprotein lipase expression, serum lipid and tissue lipid deposition in orally-administered glycyrrhizic acid-treated rats. Lipids Health Dis. 2009;8:31. doi: 10.1186/1476-511X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]