We read with interest the article by Batisse et al.1 describing 11 possible cases of symptomatic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) reinfection. However, the intervening period of ‘clinical cure’ was not confirmed by a negative SARS-CoV-2 PCR test result. Similarly, Lafaie et al.2 reported three elderly patients who were infected with SARS-CoV-2 who recovered ‘clinically’ then were readmitted with ‘new’ coronavirus disease 2019 (COVID-19) symptoms, again with no SARS-CoV-2 PCR negative result between the two COVID-19 episodes. This SARS-CoV-2 reinfection phenomenon is indeed one of the many ongoing debates during the present COVID-19 pandemic and it is still unclear to what extent this is due to true reinfection, or possible persistent low level infection.3 , 4
Part of the problem is that there is no well-defined, consensus definition or criteria for deciding what constitutes true SARS-CoV-2 reinfection. Here we present 6 cases of hospitalised patients or staff with likely SARS-CoV-2 reinfection based on objective laboratory-based criteria and rationales.
From our diagnostic laboratory database, we searched for a pattern of SARS-CoV-2 POS-NEG-POS polymerase chain reaction (PCR) results in any COVID-19 case, then extracted and tabulated the details of the clinical episodes for each patient fitting these initial search criteria.
The SARS-CoV-2 PCR assay that we were using during this time was the commercial AusDiagnostics SARS-CoV-2 PCR kit (AusDiagnostics UK Ltd., Chesham, England) with a manufacturer's stated sensitivity and specificity of 97.7% (90.8–100%) and 99.4% (97.4–100%), respectively. The SARS-CoV-2 IgG antibody testing was performed using the commercial DiaSorin Liaison SARS-CoV-2 S1/S2 IgG assay (Diasorin Ltd., Kent, England) with a stated manufacturer's sensitivity and specificity of 97% (86.8%−99.5%) and 98.5% (97.6%−99.1%), respectively.
Patients or staff meeting the following criteria were included in this possible SARS-CoV-2 reinfection cohort:
-
-
an initial SARS-CoV-2 PCR-confirmed acute coronavirus disease 2019 (COVID-19) illness
-
-
followed by clinical recovery and discharge with at least one negative SARS-CoV-2 PCR result
-
-
followed by a confirmed SARS-CoV-2 PCR positive result (with or without symptoms) at least 28 days after the previous SARS-COV-2 PCR result
These criteria were based on the findings that in most COVID-19 cases, viral shedding reaches a minimum by day 28 after an initial acute SARS-CoV-2 infection.5 It is still unclear how protective SARS-CoV-2 IgG antibodies are in the convalescent period, and how long any such protection may last. Such antibodies start to rise 5–10 days post-onset of infection, peaking by days 12–15,6 and will contain a proportion of neutralising antibodies,7 making any SARS-CoV-2 reinfection very unlikely within this period. Hence, we assumed that SARS-CoV-2 reinfection cannot occur within the first 28 days post-illness onset. As patients move beyond 28 days post-illness onset, SARS-CoV-2 IgG antibodies gradually wane,8 increasing the possibility that SARS-CoV reinfection may occur.
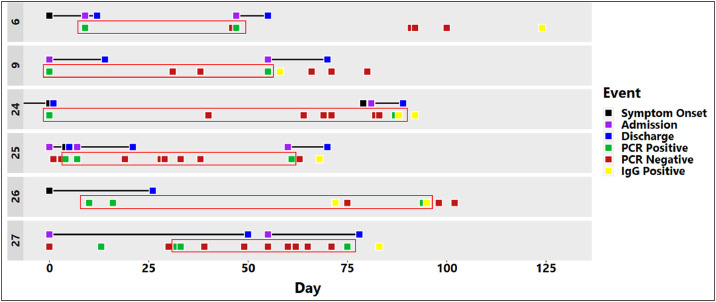
We identified 6 patients or staff that fit the criteria above (Table 1 , Fig. 1 ). All 6 cases had at least one SARS-CoV-2 IgG antibody test (Abbott Architect SARS-CoV-2 IgG assay; Abbott, Maidenhead, UK). Cases 24 and 26 had two SARS-CoV-2 IgG antibody tests, all of which were positive.
Table 1.
Characteristics and timeline of possible COVID-19 reinfection cases.
| Case no. | Age (yrs) |
Sex | Ethnicity | Past medical history | 1st COVID-19 episode/ PCR POS result (Day 0) |
Treatment | Recovery/ discharge date (days since Day 0) |
Onset of second episode (days since Day 0) |
Symptoms | SARS-CoV-2 IgG (Day tested) |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 49 | F | Asian | Obesity, T1DM, epilepsy, previous sepsis due to obstructive pyelonephritis. | Fever, cough, myalgia, diarrhoea | IV AB, CS | 3 | 38 | Fever, low BP, treated for sepsis due to obstructive ureteric calculus but CT chest shows evidence of COVID19 and PCR positive. | Positive (Day 72) |
| 9 | 93 | M | White British | IgG multiple myeloma, AF, CCF, HTN, cognitive impairment | Lethargy, reduced appetite, diarrhoea | IV and PO AB | 14 | 55 | Cough, fever, dyspnoea | Positive (Day 58) |
| 24 | 82 | M | White British | AF, CCF with PPM/ICD, AAA, T2DM, lung cancer, AS | Cough, fever, sore throat, dyspnoea, new oxygen demand, haemoptysis | PO AB | 1 | 87 | Fever, cough, dyspnoea | Positive (Days 88, 92) |
| 25 | 86 | F | White British | IHD, HTN, HF, hypothyroidism, OA, PMR | Unresponsive episode, low BM | IV AB | 17 | 57 | Asymptomatic, admitted with fall and sternal fracture. Routine swab on admission. | Positive (Day 62) |
| 26 | 62 | F | White British | Healthcare staff | Cough, fever, dyspnoea | Home care | 16 | 84 | Asymptomatic, routine staff screening | Positive (Days 62, 85) |
| 27 | 83 | M | White British | T2DM, 1st degree AV block, bronchitis, GORD | Dyspnoea, new oxygen requirement | IV AB, O2 | 18 | 43 | Asymptomatic, routine swab prior to discharge. | Positive (Day 51) |
AAA: abdominal aortic aneurysm, AB: antibiotics, AF: atrial fibrillation, AS: aortic stenosis, AV: atrioventricular, CCF: congestive cardiac failure, CS: corticosteroids, GORD: gastro-oesophageal reflux disease, HF: heart failure, HTN: hypertension, ICD: implantable cardioversion device, IV: intravenous, OA: osteoarthritis, O2: oxygen, PMR: polymyalgia rheumatica, PO: oral, T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus.
Fig. 1.
Timeline of events for each patient. As the specific date of illness onset was unclear for several patients the first SARS-CoV-2 PCR positive result was used as time zero (Day 0). The image shows all the testing that was performed for each patient. The specific interval over which reinfection is likely to have occurred (SARS-CoV-2 PCR POS-POS) in each case is highlighted using red boxes.
For Case 24, these IgG tests were positive on days 88 and 92, with the first of these testing positive the day after the second positive PCR swab result. This indicates that the SARS-CoV-2 PCR positive swab was taken in the presence of SARS-CoV-2 IgG antibodies, as these typically take 5–10 days to appear.6 This apparent SARS-CoV-2 reinfection in Case 24 was symptomatic. Similarly, for Case 26, the SARS-CoV-2 IgG was positive on samples taken on days 62 and 85, again indicating that the second positive PCR result (on day 84) must have occurred in the presence of SARS-CoV-2 IgG antibodies. Reinfection in Case 26 was asymptomatic. This was a staff member whose testing had been conducted as part of the routine screening for staff who worked on immunosuppressed patient wards. The same argument can be applied to Cases 9 (symptomatic reinfection), 25 (asymptomatic reinfection) and 27 (asymptomatic reinfection), where the second SARS-CoV-2 PCR and SARS-CoV IgG positive results occurred within 5–10 days of each other (Table 1, Fig. 1). We did not culture the second COVID-19 episode SARS-CoV-2 PCR positive swabs to check for virus viability. Batisse et al.,1 however, did find viable SARS-CoV-2 in one out of two patient samples tested during their second COVID-19 episodes.
Thus while SARS-CoV-2 reinfection is a possibility for any of these 6 cases, we are the most confident of Cases 24 and 26 being true cases of SARS-CoV-2 reinfection, as they exhibited the largest interval (87 and 84 days, respectively) between their two COVID-19 episodes. Also, their two positive SARS-CoV-2 IgG antibody tests showed that antibodies were present after the first and persisted through to the second COVID-19 episode, and were therefore less likely to be a false positive finding.
Reinfection with the four known human seasonal coronavirus infections has been described, even in the presence of pre-existing coronavirus antibodies, and is not unusual.9 However, ‘reactivated’, ‘relapsed’ or ‘latent’ infection seems less likely and is not yet described for the family of coronaviruses.10
Our SARS-CoV-2 reinfection criteria are not perfect and will inevitably be refined as new findings accumulate. Yet our small case series here indicates that symptomatic and asymptomatic SARS-CoV-2 reinfection can occur in the presence of SARS-CoV-2 IgG antibodies. Further studies are needed to determine to what extent SARS-CoV-2 shedding and transmission occur during symptomatic and asymptomatic reinfection.
References
- 1.Batisse D., Benech N., Botelho-Nevers E. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020 doi: 10.1016/j.jinf.2020.06.073. Jun 30S0163-4453(20)30454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafaie L., Célarier T., Goethals L. Recurrence or Relapse of COVID-19 in older patients: a description of three cases. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16728. 1 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan L., Xu D., Ye G. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkcaldy R.D., King B.A., Brooks J.T. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA. 2020;323:2245–2246. doi: 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Long Q.X., Liu B.Z., Deng H.J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 7.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 8.Seow J., Graham C., Merrick B., et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv 2020.07.09.20148429.
- 9.Edridge A.W.D., Kaczorowska J.M., Hoste A.C.R., Bakker M., Klein M., Jebbink M.F., et al. Coronavirus protective immunity is short-lasting. Posted 16 June 2020. medRxiv 2020.05.11.20086439; doi: 10.1101/2020.05.11.20086439. [DOI] [PubMed]
- 10.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]