Abstract
The human mind is equally fluent in thoughts that involve self-generated mental content as it is with information in the immediate environment. Previous research has shown that neural systems linked to executive control (i.e. the dorsolateral prefrontal cortex) are recruited when perceptual and self-generated thoughts are balanced in line with the demands imposed by the external world. Contemporary theories (Smallwood and Schooler, 2015) assume that differentiable processes are important for self-generated mental content than for its regulation. The current study used functional magnetic resonance imaging in combination with multidimensional experience sampling to address this possibility. We used a task with minimal demands to maximise our power at identifying correlates of self-generated states. Principal component analysis showed consistent patterns of self-generated thought when participants performed the task in either the lab or in the scanner (ICC ranged from 0.68 to 0.86). In a whole brain analyses we found that neural activity in the ventromedial prefrontal cortex (vMPFC) increases when participants are engaged in experiences which emphasise episodic and socio-cognitive features. Our study suggests that neural activity in the vMPFC is linked to patterns of ongoing thought, particularly those with episodic or social features.
Keywords: Ongoing thought, Ventromedial prefrontal cortex, Memory
Highlights
-
•
We identified neural regions associated with patterns of self-generated thought.
-
•
Ventromedial prefrontal cortex (vMPFC) activity during episodic social cognition.
-
•
VMPFC may play a role in episodic and social features of self-generated thought.
1. Introduction
Human cognition is not always tethered to events taking place in the real world. Often, particularly when external demands are low (Giambra, 1995; Smallwood et al., 2003; Teasdale et al., 1993), we engage in thoughts with few links to events in the "here-and-now", a phenomena often studied under the umbrella term of mind-wandering (Smallwood and Schooler, 2006, 2015). These patterns of self-generated thoughts have links to both beneficial aspects of cognition, such as creativity (Baird et al., 2012; Gable et al., 2019) and goal-planning (Medea et al., 2018). Self-generated states also have links to detrimental aspects of cognition such as affective disturbance (Poerio et al., 2013; Smallwood et al, 2007) or lapses in attention (McVay and Kane, 2009; Smallwood et al., 2008). Since self-generated thought has an important influence on well-being (Mooneyham and Schooler, 2013), over the last decade understanding the neural mechanisms underlying different aspects of ongoing thought has emerged as an important question in the field of cognitive neuroscience (Christoff et al., 2016; Smallwood and Schooler, 2015).
Contemporary accounts of self-generated thought (Smallwood, 2013) hypothesise that the emergence of self-generated states depend on both processes that support the representations on which the mental content is based, as well as more general systems that are linked to regulating the occurrence of these states. This enables the thoughts to be maximally aligned to the current environmental demands (Andrews-Hanna et al., 2014; Smallwood, 2013; Smallwood and Andrews-Hanna, 2013). Consistent with this perspective Turnbull and colleagues used a paradigm which alternated between a demanding and non-demanding task context, a design which optimises the chance to identify the neural systems involved in the contextual control of patterns of ongoing thought (Turnbull et al., 2019a). This study used a paradigm that switched between a simple choice reaction time with a more difficult 1-back decision that relied on working memory, and this routinely leads to reductions in off-task thought in the easy task (Smallwood et al, 2009). Turnbull and colleagues found a region of dorsolateral prefrontal cortex was linked to the facilitation of on-task thoughts during the more difficult working memory task, and the expression of off-task thoughts when task demands reduced. This result highlights that brain regions linked to executive control are recruited when attention is regulated in line with contextual demands, however, it does not directly contribute to our understanding of the processes that differentiate between different types of experience.
In our prior study we found evidence that regions of lateral parietal cortex were important for maintaining attention on the task in hand (Turnbull et al., 2019a). Although this pattern demonstrates the neural systems recruited during a focus on external goals, it leaves open which systems support mental content that is self-generated. It is often hypothesised that memory systems which play an important role in self-generating mental content may be linked to episodic or mnemonic features since these thoughts often have temporal and/or self-relevant features (Smallwood and Schooler, 2015). Consistent with this perspective, individuals who are better at tasks relying on memory tend to report more off-task thoughts (Poerio et al., 2017; Wang et al., 2019), while the functional architecture/activity of neural regions linked to memorial processes are predictive of individual differences in patterns of ongoing thought (Ellamil et al., 2016; Ho et al., 2019; Smallwood et al., 2016). Furthermore, studies of dementia/lesions that target regions that are hypothesised to play a role in memorial or episodic processes impacts on the ability to self-generate mental content, including the hippocampus (McCormick et al., 2018), parahippocampus (O’Callaghan et al., 2019), ventromedial prefrontal cortex (Bertossi et al., 2016), and the temporal pole (Irish et al., 2012; Irish and Piguet, 2013).
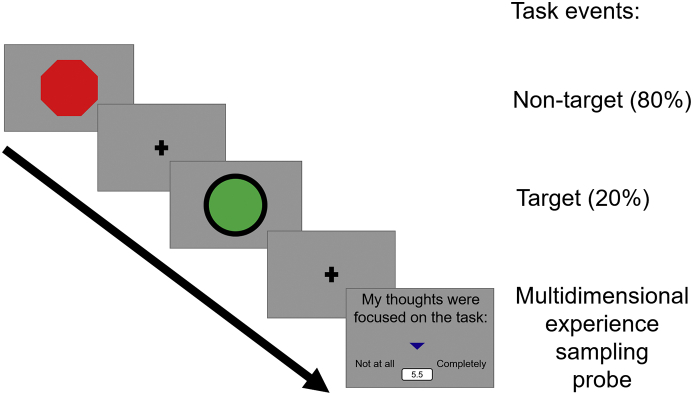
It is possible that in our prior study (Turnbull et al., 2019a) we failed to find evidence for neural systems linked to the expression of self-generated thought in a whole brain search because our design was optimised to identify how individuals regulate their experiences in line with external demands, necessitating a within-participant manipulation of task demands. In the current study, we assessed the neural basis behind different types of experience in a similar sized sample of participants as in our prior study (Turnbull et al., 2019a). Unlike our prior study, we focused only on a single task with minimal attentional demands and used a comparable number of experience sampling probes. We hoped this would produce more sensitive estimates of both the different patterns of ongoing thoughts that individuals focus on when task demands are low, and that it would increase our chance of detecting the involvement of regions implicated in self-generated mental content. The task design is summarised in Fig. 1. In order to provide an empirical constraint to our interpretation of the result, we examined the functional properties of any clusters revealed by our analysis by performing a formal meta-analysis using Neurosynth (Yarkoni et al., 2011).
Fig. 1.
Task paradigm used in this study. Participants were asked to respond to infrequent green circles. At intermittent intervals we asked the individuals to describe the contents of their experience using Multidimensional Experience Sampling (MDES).
2. Methods
2.1. Participants
One hundred and seven participants took part in this study. Ninety-one participants participated in a behavioural session (67 females; mean age: 23.38 years, standard deviation: 4.53 years, age range: 19–40 years). Sixty-two participants participated in a scanning session (41 females; mean age: 23.29 years; standard deviation: 4.51 years, age range: 18–39 years). After excluding participants 57 remained for fMRI data analysis (due to technical difficulties or excess movement). Forty-six participants participated in both the behavioural and scanning session. All participants had normal/corrected vision, and had no history of psychiatric or neurological illness. All scanning participants were right handed. This cohort was acquired from the undergraduate and postgraduate student population at the University of York. The study was approved by the local ethics committee at the York Neuroimaging Centre and University of York Psychology Department. All volunteers provided informed written consent and received monetary compensation or course credit for their participation.
2.2. Task paradigm
Participants were instructed to attend to the centre of the screen, they viewed target and non-target stimuli to which they responded only to target stimuli (mean stimulus presentation duration 1000 ms). A run of the task was 13 minutes and contained eight instances of experience sampling probes when participants answered one of each question (see the next section for details of the experience sampling technique). A question was presented for 4 s maximum on the screen based on the average response time from previous studies, followed by a 500 ms fixation cross. The rest of the time in a run was allocated to two kinds of experimental trials: target and non-target. In target trials (“go trials”) a green circle was randomly presented (20% of the experiment trials) and participants were required to make a response -a single key or button press was required. In non-target (“no-go”) trials a red octagon was presented (80% of the experiment trials) and no behavioural response was required. An experimental trial was fixed at 3000 ms. The inter-stimulus-intervals (ISI) consisted of a fixation cross and was jittered (1500–2500 ms). The stimulus was presented on screen for 500–1500 ms until a response was made. Once a response was captured, a fixation cross appeared on the screen for the remaining time. This task was designed to require minimal cognitive demand since these conditions facilitate the occurrence of self-generated thought at a level that is comparable to rest (Smallwood et al., 2009). The task is presented schematically in Fig. 1. In the scanner participants completed three runs of the task whereas in the behavioural session they completed one run of the task. Written instructions were presented at the start of each run.
2.3. Multidimensional Experiential sampling (MDES)
Participant ongoing thought was measured using multidimensional experience sampling (MDES). When a probe occurred participants were asked how much their thoughts were focused on the task, followed by 12 randomly shuffled questions about their thoughts (Table 1). All questions were rated on a scale of 1–10. Within one run of the Go/No-go task, participants completed 8 sets of MDES probes yielding a total of 8 probes per individual in the behavioural session and 24 probes per individual in the scanning session. Two participants had one run dropped due to technical issues, leaving them with 16 probes overall.
Table 1.
Multidimensional experience sampling questions used to sample thoughts in the current study.
| Dimension | Statement | Scale_low | Scale_high |
|---|---|---|---|
| Task | My thoughts were focused on the task I was performing: | Not at all | Completely |
| Future | My thoughts involved future events: | Not at all | Completely |
| Past | My thoughts involved past events: | Not at all | Completely |
| Self | My thoughts involved myself: | Not at all | Completely |
| Person | My thoughts involved other people: | Not at all | Completely |
| Emotion | The emotion of my thoughts was: | Negative | Positive |
| Modality | My thoughts were in the form of: | Images | Words |
| Detail | My thoughts were detailed and specific: | Not at all | Completely |
| Deliberate | My thoughts were: | Spontaneous | Deliberate |
| Problem | I was thinking about solutions to problems (or goals): | Not at all | Completely |
| Diverse | My thoughts were: | One topic | Many topics |
| Intrusive | My thoughts were intrusive: | Not at all | Completely |
| Source | My thoughts were linked to information from: | Environment | Memory |
2.4. Procedure
In the behavioural session participants completed a single 13-min run of the Go/No-go task with MDES. In the scanning session participants completed three, 13-min functional runs of the Go/No-go task with MDES while undergoing fMRI. The scanner session took around 1 h and 15 min of which the scanning took ~45 min, this was separated into three blocks.
2.5. fMRI acquisition
All MRI scanning was carried out at the York Neuroimaging Centre. Structural and functional scans were acquired using a Siemens Prisma 3T MRI Scanner with a 64-channel phased-array head coil. Structural data were acquired using a T1-weighted (MPRAGE) whole-brain scan (TR = 2300 ms, TE = 2.26 ms, flip angle = 8°, matrix size = 256 x 256, 176 slices, voxel size = 1 x 1 x 1 mm). Functional data were collected using a gradient-echo EPI sequence with 54 bottom-up interleaved axial slices (TR = 3000 ms, TE = 30 ms, flip-angle = 80°, matrix size = 80 x 80, voxel size = 3 x 3 x 3 mm, 267 vol) covering the whole brain.
2.6. Data pre-processing
Five participants were excluded for incorrect number of volumes being collected or excess movement (mean framewise displacement (Power et al., 2014) > 0.3 mm and/or more than 15% of their data affected by motion). Functional and structural data were pre-processed and analysed using FMRIB’s Software Library (FSL, version 5.0.1, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FEAT/). Individual T1-weighted structural images were extracted using BET (Brain Extraction Tool). Functional data were pre-processed and analysed using the FMRI Expert analysis Tool (FEAT). Individual participant analysis involved motion correction using MCFLIRT and slice-timing correction using Fourier space time-series phase-shifting. After co-registration to the structural images, individual functional images were linearly registered to the MNI-152 template using FMRIB’s Linear Image Registration Tool (FLIRT). Registration from high resolution structural to standard space was then further refined using FNIRT nonlinear registration. Functional images were spatial smoothed using a Gaussian kernel of FWHM 6 mm, underwent grand-mean intensity normalisation of the entire four-dimensional dataset by a single multiplicative factor, and had high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50s).
2.7. Principal component analysis
Analysis of the MDES data was carried out in SPSS (Version 25, 2019). Principal component analysis (PCA) was applied to the scores from the 13 experience sampling questions comprising the probes for each participant in each testing environment (lab and scanner) separately. This was applied at the trial level in the same manner as in our prior studies (Konishi et al., 2017; Ruby et al., 2013a; Ruby et al., 2013b; Smallwood et al., 2016; Turnbull et al., 2019b). Specifically, we concatenated the responses of each participant for each trial into a single matrix and employed a PCA with varimax rotation. We performed this analysis separately for each session (behavioural and scanning) in order to examine the similarity in the solutions produced across each situation.
2.8. fMRI analysis
Task-based analyses were carried out using FSL. A model was set up including 6 explanatory variables (EVs) as follows: EVs 1 and 2 modelled time periods in which participants responded to target and non-target trials. EV 3 modelled activity 6s prior to each MDES probe. EVs 4, 5 and 6 modelled the 3 thought components, with a time period of 6s prior to the MDES probes and the scores for the related task-component as a parametric regressor. EVs were mean-centred within each run and no thresholding was applied to the EVs. Standard and extended motion parameters were included as confounds. This was convolved with a hemodynamic response function using FSL’s gamma function. We chose to use the same 6s interval as used in Turnbull et al., 2019b . Contrasts were included to assess brain activity that related to each component of thought during the 6s period prior to the probe. For thoughts, main effects (positively or negatively related to thoughts in both trials) were included. The three runs were included in a fixed level analysis to average across the activity threshold within an individual. Group level analyses followed best practice (Eklund et al., 2016). Specifically we used FLAME, as implemented by FSL, applied cluster-forming threshold of Z = 3.1, and corrected these at p < .05 (corrected for family-wise error rate using random field theory). Brain figures were made using MRICroGL (V2.1.49-0, 2019) and SurfIce (V2, 2019). Meta-analytic decoding used Neurosynth (Yarkoni et al., 2011) to find terms most commonly associated with our neural maps in the literature. This platform collects and synthesises results from many different research studies, and identifies the terms associated most often with each region of the brain.
2.9. Data and code availability statement
Multidimensional Experience Sampling data is available upon request from the authors via email. All unthresholded maps produced in these analyses are freely available on NeuroVault. These can be found in the “A role for ventromedial prefrontal cortex in self-generated episodic social cognition” collection:
https://identifiers.org/neurovault.collection:6069.
The code for the Go/No-go task paradigm is freely available at:
https://vcs.ynic.york.ac.uk/hw1012/go_nogo_experience_sampling/tree/master/.
Ethical approval conditions do not permit public sharing of raw data as participants have not provided sufficient consent. Data and analysis scripts can be accessed by contacting the Research Ethics and Governance Committee for the York Neuroimaging Centre or the corresponding author, Delali Konu. Data will be accessible upon request within accordance with General Data Protection Regulation (GDPR).
3. Results
3.1. Behavioural results
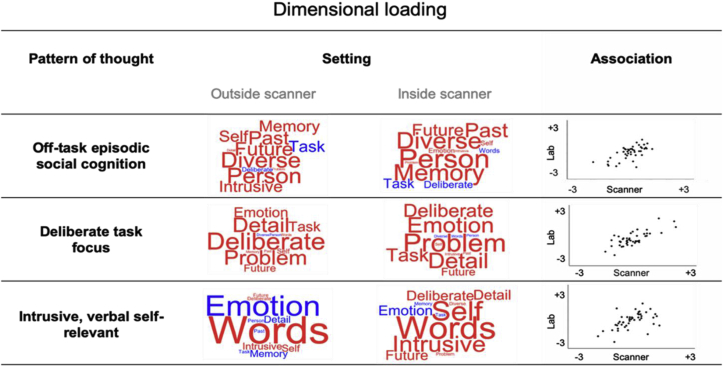
In the scanning session 24 (8 MDES per run) probes were collected with 1456 observations in total. In the behavioural session 8 probes were collected with 728 observations in total. Table 2 presents the average response to these questions in terms of means and standard deviations. A principal component analysis was conducted on the 13 questions comprising the MDES probes for each session, one for the behavioural session, and one for the scanning session, both using varimax rotation. This was done to describe the underlying structure of the participant responses about their thoughts in a compact low dimensional manner. For the scanning session results revealed 3 components with eigenvalues greater than 1, which was also suggested by the scree plot (Fig. 1, Supplementary Materials). The components extracted accounted for a total of 47.41% of the variance (component 1 = 20.47%, component 2 = 15.33% and component 3 = 11.61%). The first component represents a dissociation between diverse episodic and social content and thoughts about the tasks. The second component describes a focus on task relevant problem solving, that is detailed and deliberate, and of positive emotional valence. The third component represents thoughts that are intrusive, in the form of words, related to the self, and negative in tone. These components are presented as word clouds in Fig. 2. For the behavioural session a 3 factor solution was also apparent in the scree plot (Fig. 1, Supplementary Materials). The PCA was run with a 3 factor selection variable; 44.82% of variance being accounted for (component 1 = 21.65%, component 2 = 14.06% and component 3 = 9.10%). It is apparent in Fig. 2 that these components identified show very similar patterns as were present in the scanning session.
Table 2.
Mean and standard deviations of each question type for each session.
|
Question |
Behavioural session |
Scanning session |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Deliberate | 5.04 | 2.66 | 4.90 | 2.65 |
| Detail | 5.52 | 2.52 | 5.45 | 2.66 |
| Diverse | 4.32 | 2.58 | 4.19 | 2.48 |
| Emotion | 6.12 | 1.91 | 6.39 | 2.09 |
| Future | 4.88 | 2.65 | 4.71 | 2.76 |
| Intrusive | 4.56 | 2.44 | 4.44 | 2.49 |
| Modality | 4.87 | 2.82 | 4.53 | 2.80 |
| Past | 4.29 | 2.43 | 4.06 | 2.45 |
| Person | 4.19 | 2.63 | 4.32 | 2.75 |
| Problem | 5.27 | 2.67 | 4.75 | 2.79 |
| Self | 5.37 | 2.69 | 5.77 | 2.77 |
| Source | 5.32 | 2.76 | 4.69 | 2.83 |
| Task | 6.22 | 2.52 | 6.34 | 2.57 |
Fig. 2.
Patterns of thought identified in this study. The word cloud shows the loadings identified through the independent application of principal component analysis (PCA) to two different data sets (inside and outside the scanner). The colour of the word describes the direction of the relationship (red = positive, blue = negative) and the size of the item reflects the magnitude of the loading. The scatter plots in the grey subpanel show the correlations across the 46 individuals who participated in both sessions. In both cases we selected three components based on the scree plot and applied varimax rotation to the dimensions.
A proportion of our participants from the scanning session (n = 46) took part in both sessions of the study and we assessed the relationship between the individual participant loadings on each component in the behavioural lab and in the scanner. Only participants who completed both scanning and behavioural sessions were included in this analysis. Results showed strong clear positive associations between all three components (Off-task episodic thought, r(46) = 0.652, p < .001, two-tailed, Intra Class Coefficient (ICC) = 0.79, p < .001; Deliberate task focus, r(46) = 0.753, p < .001, ICC = 0.860, p < .001; Intrusive verbal self-relevant thought, r (46) = 0.526, p < .001, ICC = 0.68, p < .001). Together these analyses show that the application of PCA to the MDES data yields consistent dimensions of experience that are consistent across individuals. These correlations are presented in the form of scatterplots in Fig. 2.
3.2. Association between experience and neural activity
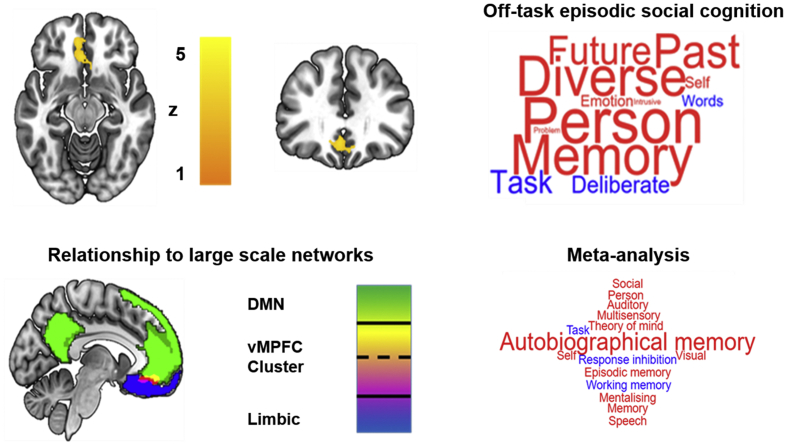
Our next analysis examined whether any of the patterns of thought identified in our analyses are linked to reliable changes in neural activity. We performed a multiple regression in which spatial maps describing the associations between neural activity for each PCA for each of the 24 experience sampling probes for each individual were the dependent measures. Following the recommendations we controlled for family wise error using a cluster forming threshold of Z = 3.1, p < .05 FEW (Eklund et al., 2016). Results established that the ventromedial prefrontal cortex (vMPFC) was significantly more activated as participants endorsed increasingly off-task episodic social cognition experiences (PCA 1, Fig. 3). We contextualized this result in the context of a whole brain by overlaying this region with the set of large-scale networks from Yeo and colleagues (Yeo et al., 2011). This determined that the vMPFC region fell at the intersection of the default mode (green) and limbic networks (blue). Finally, to embed this result in a functional context we decoded this spatial map using Neurosynth and the resulting meta-analysis is presented in the form of a word cloud. No significant whole-brain results were found for either component 2 or 3 even at a lower threshold (Z = 2.3).
Fig. 3.
Association between ventromedial prefrontal cortex (vMPFC) activity and patterns of episodic social cognition. A region of vMPFC (BA 11) showed a positive correlation with increasing reports of off-task episodic thought. This region fell at the intersection of the limbic and default mode networks as defined by Yeo and colleagues (Yeo et al., 2011). A meta-analysis of the most likely functional associations using Neurosynth is presented in the form of a word cloud. In the word clouds the colour represents the likelihood of the association with the term (red = positive, blue = negative) and the font size describes the magnitude.
4. Discussion
Our study set out to determine neural regions that support patterns of self-generated thought. Using a well powered experimental design we recorded neural function using fMRI while participants performed a task with low cognitive demands. We also recorded self-reported descriptions of the individuals’ experience along multiple dimensions. We used these data to identify regions whose activity was associated with reports of low dimensional representations of the experience sampling data generated by principal component analysis (broadly corresponding to “patterns of thought”). Decomposing our experience sampling data gathered during scanning, revealed three patterns: episodic social cognition, a state of deliberate, detailed task-focus and a pattern of unpleasant verbal self-relevant thoughts. We found a similar factor structure in a control experiment conducted in the lab, as well as common loadings across individuals, establishing the stability of our experience sampling measures. Our neuroimaging analysis identified that a pattern of episodic social cognition was associated with enhanced neural activity within the ventromedial prefrontal cortex (vMPFC). This study, therefore, establishes neural activity in the vMPFC is recruited during periods of self-generated thought with episodic and social features.
Although our study establishes the vMPFC is recruited during complex patterns of self-generated thoughts, our data does not specify which precise aspect of ongoing thought this neurocognitive association reflects. The vMPFC plays an important role in a number of different aspects of cognition as can be seen in the meta-analysis of this region we performed using Neurosynth (Yeo et al., 2011). This analysis highlighted the terms “autobiographical memory” and “memory” as the functional terms most commonly used to interpret activity in this area, but also included aspects of social cognition (“self”, “person”, “theory of mind”) and sensory features (“visual”, “auditory” and “multisensory”). Our data shows that patterns of thoughts can also have heterogeneous features, with the component linked to the vMPFC highlighting multiple features including episodic qualities (“memory”, “future” and “past”) and socio-cognitive features (“self and “person”). This dimension was also linked to “diverse”, underlining that this pattern of thought has complex features. The broad pattern captured by our decomposition of experience sampling data, coupled with the complex functional landscape of the vMPFC, makes it difficult to delineate the specific relationship between activity in the ventromedial prefrontal cortex and patterns of ongoing thought. However, there are a number of candidate accounts within the literature that are worth considering. One line of work suggests that the vMPFC plays a role in complex episodic or social processes, given a role in autobiographical memory (Benoit et al., 2014), self/social cognition (D’Argembeau et al., 2007; Kelley et al., 2002; Macrae et al., 2004) and mental time travel (D’Argembeau, 2013). A role of vMPFC in episodic or social cognitive processes could account for the prominence of temporal and social terms in the associated pattern of thought. Other studies have highlighted the role of the vMPFC in reward-based decision making (Lin et al., 2016; Weilbacher and Gluth, 2017). It is possible, therefore, that the observed association with activity in vMPFC indicates the hypothesised motivational component to off-task thought (Seli et al., 2015). Studies of affective disturbances also implicate the vMPFC (Oakes et al., 2017) and a prior study demonstrated that this region contained information regarding emotional features of both task-based and naturally occurring emotional states, albeit ones that were related to memories from the past (Tusche et al., 2014). Finally, it is possible that the vMPFC plays a more general role in self-generated experiences perhaps facilitating their elaboration through a role in associate inferences based on memory (Spalding et al., 2018). Clearly given the complex nature of ongoing thought patterns, and the heterogeneous role of the vMPFC, further work is needed to elucidate the functional significance of ventromedial prefrontal activity during self-generated thought.
Finally, it is important to bear in mind that our study is correlational, and this feature of our design limits the ability of our paradigm to address causal relationships. In this context, lesion studies could be important for profitably exploring the functional relationship between the vMPFC and patterns of ongoing thought in a more precise manner (Bertossi et al., 2016).
In conclusion, our study set out to identify the neural correlates of patterns of self-generated mental contents that transcends the here-and-now. We found that a pattern of ongoing thought that was most focused on episodic social cognition rather than the external task was linked to increased activity in a region of the ventromedial prefrontal cortex. Since states of self-generated thought are linked to both beneficial and detrimental aspects of psychological functioning (Mooneyham and Schooler, 2013), our study highlights the ventromedial prefrontal cortex as relevant to understanding the influences that self-generated experiences play in well-being and happiness.
CRediT authorship contribution statement
Delali Konu: Data curation, Formal analysis, Investigation, Project administration, Visualization, Writing - original draft, Writing - review & editing. Adam Turnbull: Data curation, Formal analysis, Methodology, Software, Writing - original draft. Theodoros Karapanagiotidis: Data curation, Formal analysis, Software, Writing - original draft. Hao-Ting Wang: Methodology, Software, Writing - original draft. Lydia Rebecca Brown: Data curation, Investigation, Project administration. Elizabeth Jefferies: Conceptualization. Jonathan Smallwood: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing - original draft, Writing - review & editing.
Footnotes
This work was supported by European Research Council awarded to JS (WANDERINGMINDS - 646927).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116977.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316(1):29. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Mrazek M.D., Kam J.W., Franklin M.S., Schooler J.W. Inspired by distraction: mind wandering facilitates creative incubation. Psychol. Sci. 2012;23(10):1117–1122. doi: 10.1177/0956797612446024. [DOI] [PubMed] [Google Scholar]
- Benoit R.G., Szpunar K.K., Schacter D.L. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc. Natl. Acad. Sci. U.S.A. 2014;111(46):16550–16555. doi: 10.1073/pnas.1419274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E., Tesini C., Cappelli A., Ciaramelli E. Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia. 2016;90:12–24. doi: 10.1016/j.neuropsychologia.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Christoff K., Irving Z.C., Fox K.C., Spreng R.N., Andrews-Hanna J.R. Mind-wandering as spontaneous thought: a dynamic framework. Nat. Rev. Neurosci. 2016;17(11):718. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front. Hum. Neurosci. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A., Ruby P., Collette F., Degueldre C., Balteau E., Luxen A. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cognit. Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M., Fox K.C., Dixon M.L., Pritchard S., Todd R.M., Thompson E., Christoff K. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage. 2016;136:186–196. doi: 10.1016/j.neuroimage.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Gable S.L., Hopper E.A., Schooler J.W. When the muses strike: creative ideas of physicists and writers routinely occur during mind wandering. Psychol. Sci. 2019;30(3):396–404. doi: 10.1177/0956797618820626. [DOI] [PubMed] [Google Scholar]
- Giambra L.M. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Conscious. Cognit. 1995;4:1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Ho N.S.P., Wang X., Vatansever D., Margulies D.S., Bernhardt B., Jefferies E., Smallwood J. Individual variation in patterns of task focused, and detailed, thought are uniquely associated within the architecture of the medial temporal lobe. Neuroimage. 2019;202:116045. doi: 10.1016/j.neuroimage.2019.116045. [DOI] [PubMed] [Google Scholar]
- Irish M., Addis D.R., Hodges J.R., Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain. 2012;135:2178–2191. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- Irish M., Piguet O. The pivotal role of semantic memory in remembering the past and imagining the future. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00027. ARTN 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. Finding the self? An event-related fMRI study. J. Cognit. Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Konishi M., Brown K., Battaglini L., Smallwood J. When attention wanders: pupillometric signatures of fluctuations in external attention. Cognition. 2017;168:16–26. doi: 10.1016/j.cognition.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Lin W.J., Horner A.J., Burgess N. Ventromedial prefrontal cortex, adding value to autobiographical memories. Sci. Rep. 2016;6 doi: 10.1038/srep28630. ARTN.2863010.1038/srep.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae C.N., Moran J.M., Heatherton T.F., Banfield J.F., Kelley W.M. Medial prefrontal activity predicts memory for self. Cerebr. Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- McCormick C., Rosenthal C.R., Miller T.D., Maguire E.A. Mind-wandering in people with hippocampal damage. J. Neurosci. 2018;38(11):2745–2754. doi: 10.1523/JNEUROSCI.1812-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay J.C., Kane M.J. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. J. Exp. Psychol. Learn. Mem. Cognit. 2009;35(1):196. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medea B., Karapanagiotidis T., Konishi M., Ottaviani C., Margulies D., Bernasconi A.…Smallwood J. How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Exp. Brain Res. 2018;236(9):2469–2481. doi: 10.1007/s00221-016-4729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham B.W., Schooler J.W. The costs and benefits of mind-wandering: a review. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale. 2013;67(1):11–18. doi: 10.1037/a0031569. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C., Shine J.M., Hodges J.R., Andrews-Hanna J.R., Irish M. Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116(8):3316–3321. doi: 10.1073/pnas.1818523116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes P., Loukas M., Oskouian R.J., Tubbs R.S. The neuroanatomy of depression: a review. Clin. Anat. 2017;30(1):44–49. doi: 10.1002/ca.22781. [DOI] [PubMed] [Google Scholar]
- Poerio G.L., Sormaz M., Wang H.-T., Margulies D., Jefferies E., Smallwood J. The role of the default mode network in component processes underlying the wandering mind. Soc. Cognit. Affect Neurosci. 2017;12(7):1047–1062. doi: 10.1093/scan/nsx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio G.L., Totterdell P., Miles E. Mind-wandering and negative mood: does one thing really lead to another? Conscious. Cognit. 2013;22(4):1412–1421. doi: 10.1016/j.concog.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F.J., Smallwood J., Engen H., Singer T. How self-generated thought shapes mood—the relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0077554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F.J., Smallwood J., Sackur J., Singer T. Is self-generated thought a means of social problem solving? Front. Psychol. 2013;4:962. doi: 10.3389/fpsyg.2013.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P., Cheyne J.A., Xu M., Purdon C., Smilek D. Motivation, intentionality, and mind wandering: implications for assessments of task-unrelated thought. J. Exp. Psychol. Learn. Mem. Cogn. 2015;41(5):1417–1425. doi: 10.1037/xlm0000116. [DOI] [PubMed] [Google Scholar]
- Smallwood J. Distinguishing how from why the mind wanders: a process–occurrence framework for self-generated mental activity. Psychol. Bull. 2013;139(3):519. doi: 10.1037/a0030010. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Andrews-Hanna J. Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Front. Psychol. 2013;4:441. doi: 10.3389/fpsyg.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J., Beach E., Schooler J.W., Handy T.C. Going AWOL in the brain: mind wandering reduces cortical analysis of external events. J. Cognit. Neurosci. 2008;20(3):458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Karapanagiotidis T., Ruby F., Medea B., De Caso I., Konishi M.…Jefferies E. Representing representation: integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J., Nind L., O’Connor R.C. When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Conscious. Cognit. 2009;18(1):118–125. doi: 10.1016/j.concog.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Smallwood J., O’Connor R.C., Sudbery M.V., Obonsawin M. Mind-wandering and dysphoria. Cognit. Emot. 2007;21(4):816–842. [Google Scholar]
- Smallwood J., Obsonsawin M., Baracaia S.F., Reid H., O’Connor R., Heim D. The relationship between rumination, dysphoria, and self-referent thinking: some preliminary findings. Imagin., Cognit. Pers. 2003;22(4):317–342. [Google Scholar]
- Smallwood J., Schooler J.W. The restless mind. Psychol. Bull. 2006;132(6):946. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W. The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- Spalding K.N., Schlichting M.L., Zeithamova D., Preston A.R., Tranel D., Duff M.C., Warren D.E. Ventromedial prefrontal cortex is necessary for normal associative inference and memory integration. J. Neurosci. 2018;38(15):3767–3775. doi: 10.1523/JNEUROSCI.2501-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J.D., Proctor L., Lloyd C.A., Baddeley A.D. Working-memory and stimulus-independent thought - effects of memory load and presentation rate. Eur. J. Cognit. Psychol. 1993;5(4):417–433. doi: 10.1080/09541449308520128. [DOI] [Google Scholar]
- Turnbull A., Wang H.T., Murphy C., Ho N.S.P., Wang X., Sormaz M.…Smallwood J. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-11764-y. ARTN 381610.1038/s41467-019-11764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A., Wang H.T., Schooler J.W., Jefferies E., Margulies D.S., Smallwood J. The ebb and flow of attention: between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. Neuroimage. 2019;185:286–299. doi: 10.1016/j.neuroimage.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Tusche A., Smallwood J., Bernhardt B.C., Singer T. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- Wang H.-T., Ho N.S.P., Bzdok D., Bernhardt B.C., Margulies D.S., Jefferies E., Smallwood J. bioRxiv; 2019. Neurocognitive Patterns Dissociating Semantic Processing from Executive Control Are Linked to More Detailed Off-Task Mental Time Travel; p. 765073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbacher R.A., Gluth S. The interplay of Hippocampus and ventromedial prefrontal cortex in memory-based decision making. Brain Sci. 2017;7(1) doi: 10.3390/brainsci7010004. ARTN.410.3390/brainsci7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–U695. doi: 10.1038/Nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Multidimensional Experience Sampling data is available upon request from the authors via email. All unthresholded maps produced in these analyses are freely available on NeuroVault. These can be found in the “A role for ventromedial prefrontal cortex in self-generated episodic social cognition” collection:
https://identifiers.org/neurovault.collection:6069.
The code for the Go/No-go task paradigm is freely available at:
https://vcs.ynic.york.ac.uk/hw1012/go_nogo_experience_sampling/tree/master/.
Ethical approval conditions do not permit public sharing of raw data as participants have not provided sufficient consent. Data and analysis scripts can be accessed by contacting the Research Ethics and Governance Committee for the York Neuroimaging Centre or the corresponding author, Delali Konu. Data will be accessible upon request within accordance with General Data Protection Regulation (GDPR).