Abstract
Purpose
Evaluation of PnPP-19 safety and efficacy in reducing the intraocular pressure (IOP) of animals with healthy (normotensive) and ocular hypertensive eyes. PnPP-19 is a synthetic peptide designed from Phoneutria nigriventer spider toxin PnTx2-6.
Methods
Toxicity tests used chicken chorioallantoic membranes. Electroretinograms (ERGs) were recorded before and after administration of different doses of PnPP-19 on the eyes of Wistar rats. Histological sections of corneas and retinas were prepared. The efficacy of PnPP-19 in reducing IOP was evaluated for normotensive and ocular hypertensive animals using a tonometer. Ocular hypertension was induced in the right eye through injection of hyaluronic acid (HA) into the anterior chamber. ERG was recorded before and after glaucoma induction. The eyes were enucleated, and the corneas and retinas were histologically evaluated.
Results
PnPP-19 showed no toxicity, being safe for ocular application. A single topical instillation of one eye drop of the peptide solution was able to reduce IOP, both in healthy and ocular hypertensive rats, for 24 hours, without eliciting any apparent toxicity. PnPP-19 is a nitric oxide inducer and the results suggest that it may improve the conventional outflow of aqueous humor (AH), preventing the progression of optic nerve degeneration.
Conclusions
PnPP-19 has great potential to emerge as a promising drug for the treatment of ocular hypertension.
Translational Relevance
We regard our findings as exciting progress in translational glaucoma research, combining drug discovery, natural product research, and pharmacology, which may contribute to the establishment of new therapies for the treatment of this disease.
Keywords: glaucoma, nitric oxide, glaucoma medications
Introduction
Glaucoma is a chronic ocular disorder that causes progressive damage to the optic nerve. This disease results in visual field loss and irreversible blindness and has a multifactorial etiology,1,2 In 2010, approximately 60 million people were affected by glaucoma worldwide and this number might reach 79.6 million in 2020 and 112 million in 2040.3–5
The most studied risk factor for glaucoma is increased intraocular pressure (IOP), which damages the optic nerve.6 This increase in IOP is mediated by a reduction in the outflow of aqueous humor (AH) through the trabecular meshwork (TM), the juxtacanalicular tissue, and Schlemm's canal, which are the conventional outflow pathways.7,8 A reduced AH drainage rate through the conventional outflow system is a consequence of abnormal histologic or anatomic changes in some eye structures, leading to two major types of glaucoma: primary angle-closure glaucoma and primary open-angle glaucoma (POAG), the latter being the most common form of glaucoma.9
To facilitate the understanding of the events that lead to glaucoma, different experimental models of pressure-induced optic nerve damage have been developed. Cautery and occlusion of the episcleral / extraorbital vein, injection of hypertonic saline into AH collecting veins, and intracameral injection of hyaluronic acid (HA) are some models.7,10–12 The intracameral injection of HA seem consistent with POAG and support its use as a model of ocular hypertension in rats.12
Topical prostaglandin analogs are the first therapeutic options for glaucoma treatment. These drugs act mainly by improving the AH drainage through the unconventional or uveoscleral outflow pathway. Second-line agents comprise β-adrenergic and α-adrenergic blockers, carbonic anhydrase inhibitors, and cholinergic agonists, which are generally used when there is intolerance or contraindication to prostaglandin analogs.13
Nitric oxide (NO) has gained great attention recently as a potential new target for the treatment of glaucoma, and the evidence that NO plays an important role in IOP regulation, both in healthy and diseased eyes, has been shown.14 The biologic effects of NO could simultaneously mediate increased AH drainage through the conventional outflow and also protect the optical nerve from further injury.5,15,16
In 2017, the US Food and Drug Administration (FDA) has approved latanoprostene bunod ophthalmic solution for reducing IOP in patients with open-angle glaucoma or ocular hypertension. It was the first NO-donating drug approved by the FDA and now several others are under clinical development.14
The NO is an intracellular signaling molecule that is well-known for its key role in vasodilation, through its action on smooth muscle cells. NO mediates various eye effects, including IOP maintenance under physiological conditions. The IOP-lowering effects of NO donors have been shown in preclinical and clinical studies, by initially altering cell volume and contractility in the conventional outflow tissues. Studies in animals confirmed that NO-induced IOP lowering is mediated predominantly by an increase in the conventional outflow.15 However, it is important to note that the topical administration of an NO donor could produce positive or negative effects on outflow resistance and IOP based on the dose administered and location of the action.17,18
Therapeutic peptides have shown great promise as novel agents in the treatment of ocular diseases. These molecules offer several advantages, such as high potency, low unspecific binding, low toxicity, and little drug-drug interaction.19
PnPP-19 is a 19-amino-acids residues synthetic peptide designed from Phoneutria nigriventer spider toxin PnTx2-6. This peptide is a non-naturally occurring molecule, non-toxic, and engineered from non-contiguous domains of the native toxin. PnPP-19 is capable of enhancing the erectile function, improving relaxation of isolated strips of murine penile corpus cavernosum (cc) ex vivo, as well as potentiates the erection in vivo. The relaxation of cc is mediated by the activation of NO synthases leading to NO production, downstream activation of soluble guanylate cyclase, and cyclic guanosine monophosphate (cGMP) signaling.20
Considering that NO is important for IOP regulation, this study aimed at evaluating the safety and efficacy of PnPP-19 in reducing IOP in animals with healthy (normotensive) and hypertensive eyes.
Methods
Test Compounds
PnPP-19 was synthesized at Watsonbio (Houston, TX) and PnPP-19-FITC was synthesized at GenOne (Rio de Janeiro, RJ, Brazil). The peptide (4 mg) was dissolved in 1 mL of saline and 80 µg/eye (1.6 mM) were applied, as an eye drop (one drop was instilled). This dose was decided based on a previous study that evaluated the toxicity of PnPP-19 and its efficacy in healthy (normotensive) rats (unpublished data). One drop of Bimatoprost (Medley, Campinas, SP, Brazil) 0.3 mg/mL (equivalent to 8.8 µg/eye – 1 mM) was used. As vehicle, one drop of saline (0.9% NaCl) was used. The drop volume was the same for PnPP-19 and vehicle groups (i.e. 20 µL).
Evaluation of PnPP-19 Toxicity Through Hen's Egg Test in Chorioallantoic Membrane Test
Hen's egg test in chorioallantoic membrane (HET-CAM) was performed as described by Ref. 21 and adapted by Ref. 22. The fertilized eggs were incubated at 37 ± 1°C and 60 ± 1% relative humidity, for 10 days. Then, the eggshells were opened and the inner membranes were carefully removed. PnPP-19 (40, 80, 160, and 320 µg) were applied to CAM and the membranes were observed during 300 seconds. Saline (0.9% NaCl) was used as a negative control and sodium hydroxide (NaOH 0.1M) as a positive control (N = 10).
The onset time of bleeding, lysis, and coagulation reactions was noted and the ocular irritation index (OII) was calculated by the following Equation 1:
| (1) |
Where h is the time in seconds of the onset of hemorrhage, l = lysis, and c = coagulation, during 300 seconds. The ratio was multiplied by a factor indicating the impact on vascular damage by the effect observed.21
Animals
Wistar male rats (7 to 10 weeks old) were used in these studies. The animals were obtained at the animal facility of Pharmacy School at Universidade Federal de Minas Gerais (UFMG, Belo Horizonte, Brazil) housed under controlled conditions (temperature 27 ± 5°C, luminosity 12 hours light-dark) without restriction of water or food. All procedures were approved by the ethics committee of the university (Processes 203/2018 and 171/2019). A total of 84 Wistar rats were used in this study. The experiments were carried out in accordance with the statement of the ARVO for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health (NIH) guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Electroretinography Tests
Electroretinography (ERG) tests were performed in accordance with the International Society of Clinical Electrophysiology guidelines.23 ERGs were performed 1, 7, and 15 days after the administration of one drop (20 µl) of PnPP-19 (40, 80, 160 µg/eye). As a control, we used the same eyes before the administration of the peptide solution. All ERGs were recorded after 12 hours of dark adaptation, using an Espion e2 electrophysiology system and a Ganzfeld LED stimulator ColorDome (Diagnosys LLC, Littleton, MA). Before ERG recordings, the animals were anesthetized via intraperitoneal (IP) injection with ketamine/xylazine 90:10 mg/kg, the pupils were dilated using one drop of 0.5% tropicamide, and the eyes topically anesthetized with 0.5% proxymetacaine hydrochloride.
For visual responses, bipolar contact-lens electrodes were placed on both corneas and needle electrodes were inserted into the back. Impedance value was set to less than 5 kΩ at 25 Hz for each electrode. The scotopic ERG protocol was recorded as proposed by Ref. 24 (N = 10).
The analysis of the ERG recordings consisted in measuring the amplitude and implicit time of scotopic a- and b-waves during exposure to 0.01 (rods response) or 3.0 cd.s/m2 (combined responses of cones and rods) as previously described.24–26
Induction of Glaucoma and IOP Elevation
Rats were intraperitoneally anesthetized with ketamine/xylazine (90:10 mg/kg) and a topical anesthetic (0.5% proxymetacaine hydrochloride) was instilled into their eyes. Glaucoma was induced according to the protocol described by Moreno et al. adapted by Franca et al.12,27 Thirty µL of an aqueous solution of HA (10 mg/mL) were injected in the right eye, into the anterior chamber near the corneal-scleral limbus, using a hypodermic needle (22 G). This procedure was performed once a week, on the same day and time for 3 weeks. Treatments for glaucomatous groups by instillation started after confirmation of elevated intraocular pressure (3 weeks after the first injection of HA).
For the treatments, the rats were randomly separated into the following groups: ocular hypertensive treated with vehicle (NaCl 0.9%); ocular hypertensive treated with PnPP-19; ocular hypertensive treated with Bimatoprost; ocular hypertensive treated with PnPP-19 + Bimatoprost; and normotensive animals that received vehicle (N = 6 for each group).
IOP Monitoring
IOP was evaluated, at the same time, in normotensive and hypertensive eyes by an experienced tonometrist, using a TonoPen Vet (Reichert, NY) that was calibrated before use. Non-sedated animals were carefully contained for the measurements. Four IOP readings were taken for each eye (with a standard error of < 5%), and their average was considered as the IOP value.
Nitrite Evaluation
NO level was indirectly determined by measuring the concentration of nitrite using the Griess methodology.28 PnPP-19 or vehicle was topically administered in healthy rats. After 2 hours, the animals were euthanized and their eyes were collected (N = 6). Lens and retina were extracted. The remaining tissues from each animal were homogenized in 100 µL of saline. The samples were then centrifuged at 4°C (5000 g, 10 minutes), and 30 µL of the homogenate were applied in duplicate to a microliter plate well, followed by 30 µL of Griess reagent (0.2% [w/v] naphthyleneethylene diamine and 2% [w/v] sulfanilamide in 5% [v/v] phosphoric acid). After 10 minutes at room temperature, the absorbance was measured with a microplate reader (Tecan Infinite00 PRO, Meilen, Switzerland) at a wavelength of 540 nm. NO2- standard reference curves were made with sodium NO2- in saline at 20, 15, 12.5, 10, 5, and 1.5 µM. The detection limit of the assay was ∼ 1.5 µM in distilled water. The total amount of protein found in the eye tissue was estimated by NanoDrop 2000 Spectrophotometer (Thermo Scientific Madison, WI) and the nitrite release was normalized per µg of protein.
PnPP-19-FITC Permeation Through the Cornea
To confirm that PnPP-19 can permeate the cornea, three healthy rats received the peptide conjugated with FITC or vehicle in a lightless condition. After 2 hours, the IOP was checked and the animals were euthanized using an overdose of anesthetic with IP injection of 270/30 mg/kg of ketamine and xylazine, respectively. The eyes were removed and submitted to histological analysis. Images were obtained using a confocal microscope (LSM 880; Zeiss Jena, Aalen, Germany) with a 20× objective, scale bar = 50 µm. FITC was excited at 490 nm with emission at 526 nm. The FITC quantification of fluorescence was measured using the Image J software (National Institutes of Health, Bethesda, MD). At least 10 randomly selected fields of every section were used to assess relative fluorescence intensity, which was analyzed according to the number of pixels.
Histological Analysis
After ERG recordings or ocular hypertension induction and treatments, the animals were euthanized and eye tissues were processed for light microscopy. Eyes were enucleated and fixed in Davidson's solution (10% neutral phosphate-buffered formalin, 95% ethanol, glacial acetic acid, and ultrapure water 2:3:1:3, respectively). Samples were included in paraffin and 4-µm-thick sections were performed, stained with hematoxylin and eosin. The investigators taking the histological images masked to the treatment given to the eyes. Retinal ganglion cell (RGCs) were counted along a 200 µm linear region of the ganglion cell layer (GCL) either side of the optic nerve head (N = 6).
Data Analysis
ERG curve patterns were analyzed using Shapiro-Wilk test followed by Kruskal-Wallis and post-test of Dunn, being the mean and standard deviation calculated. The difference between efficacy of the different treatments was calculated using 2-way ANOVA followed by Bonferroni post-test. For nitrite concentration evaluation the Student t test for nonpaired data was used. The means ± SEM are shown for the number of independent experiments indicated in the figure descriptions. GraphPadPrism software was used and P values < 0.05 were regarded as statistically significant.
Results
Toxicity of PnPP-19 using HET-CAM Assay
PnPP-19, 0.9% NaCl, or 0.1 M NaOH were tested for their irritation potential in the CAM and the irritation score was measured after 300 seconds.
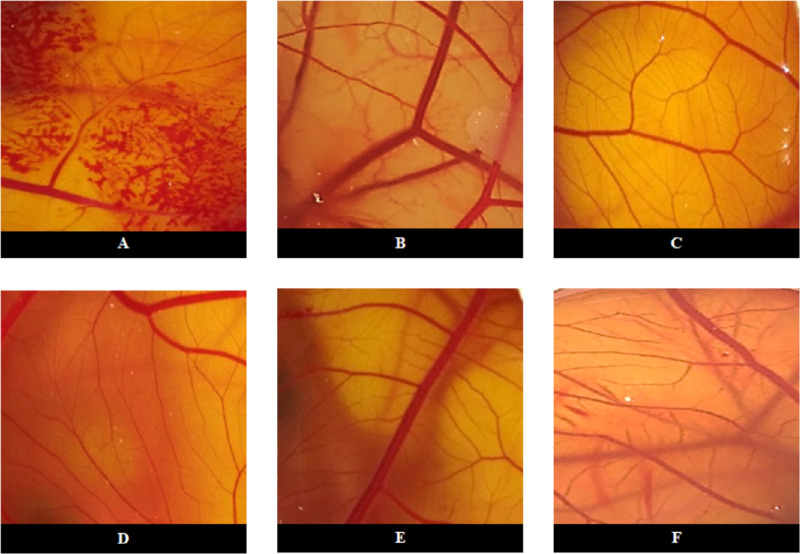
Regarding the positive control (0.1 M NaOH), initial lesions were observed in the first 30 seconds, and it was possible to observe bleeding and rosette coagulation (Fig. 1). The positive control score was 20.11 ± 0.32, which is adequate for a positive control, as it is severely irritating (Table). Negative control and PnPP-19 at all concentrations tested showed no signs of vascular response (see Fig. 1), and the cumulative mean scores calculated for the test were ≤ 0.9, which classifies PnPP-19 as non-irritant (see Table).
Figure 1.
Microscopy analysis of the effect of PnPP19 on CAM vessels. Images of HET-CAM after 300 seconds of exposure to: (A) 0.1M NaOH (positive control); (B) 0.9% NaCl (negative control) and treatment with PnPP-19 eye drops at the following concentrations: (C) 40 µg; (D) 80 µg; (E) 160 µg, and (F) 320 µg.
Table.
Ocular Irritation Index Scores for Different Doses of PnPP-19, Administered in Eye Drops
| Tested Solution | OII ± SEM | Irritancy Classification |
|---|---|---|
| 0.1 M NaOH | 20.11± 0.32 | a SI |
| 0.9% NaCl | ≤ 0.9 ± 0.0 | b NI |
| PnPP-19 (40 µg) | ≤ 0.9 ± 0.0 | NI |
| PnPP-19 (80 µg) | ≤ 0.9 ± 0.0 | NI |
| PnPP-19 (160 µg) | ≤ 0.9 ± 0.0 | NI |
| PnPP-19 (320 µg) | ≤ 0.9 ± 0.0 | NI |
Severely irritant (N = 10).
Non-irritant.
Electroretinography Evaluation
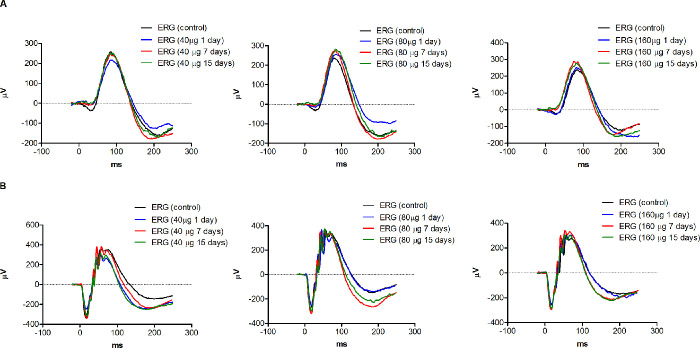
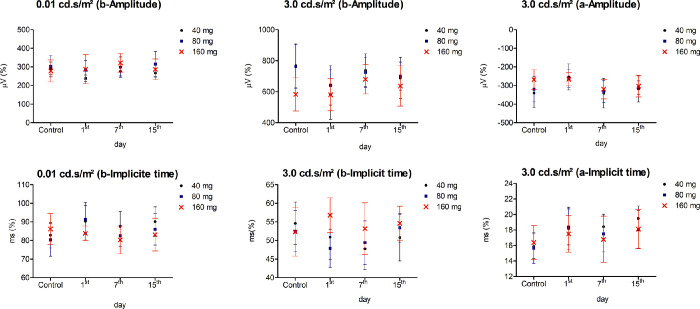
To investigate the safety of PnPP-19 for ocular application, we evaluated, through ERG, the effects of this peptide on the rat's vision. No differences in the pattern of ERG curves were observed for the eyes treated with PnPP-19 after 1, 7, and 15 days (Fig. 2). No changes were observed in amplitude or implicit time of a- and b-waves at the scotopic condition (Fig. 3), corroborating with the pattern of ERG curves (see Fig. 2).
Figure 2.
ERG curve in scotopic conditions. The amplitude and implicit time values were analyzed (A) 0.01 and (B) 3.0 cd.s/m2) after: 1, 7, and 15 days of exposure to PnPP-19 (40, 80, and 160 µg). As a control, we used the eyes before the administration of the peptide solution (N = 10).
Figure 3.
Mean ± standard derivation of the amplitude and implicit time. The amplitude (microvolts - µV) and implicit time (millisecond - ms) values of a- and b-waves in scotopic conditions (0.01 and 3.0 cd.s/m2) after PnPP-19 treatment. As a control we used the eyes before exposing to the peptide. ERG curve patterns were analyzed using Shapiro-Wilk test followed by Kruskal-Wallis and post-test of Dunn (N = 10).
Histology of Rat Cornea and Retina Cross-Sections
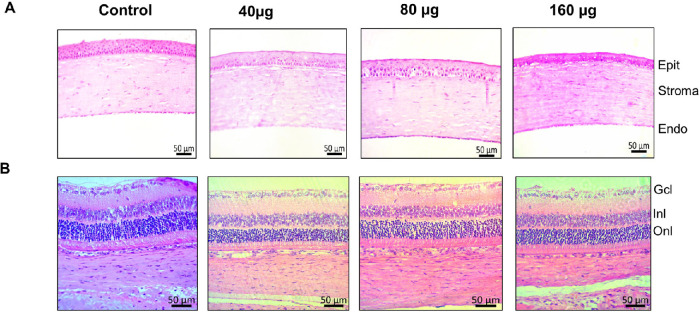
PnPP-19 was nontoxic to the cornea or retina at all concentrations tested, and no morphological changes were observed after histological analysis. Photomicrographs in Figure 4 show no effects 15 days after PnPP-19 instillation.
Figure 4.
PnPP-19 does not alter cornea and retina morphology. Sequence of illustrative photographs of histological layers of the cornea (A) and retina (B) after PnPP-19 instillation in the doses of 40, 80, and 160 µg and Control (N = 6). Cornea layers: epithelium (Epit), stroma, endothelium (Endo). Retina layers: ganglion cell layer (Gcl), inner nuclear layer (Inl), and outer nuclear layer (Onl). Digital images were obtained with a 20× objective using a microscope (Apotome.2, Zeiss, Germany).
PnPP-19 Reduces IOP in Animals
We evaluated the in vivo efficacy of topically instilled PnPP-19 or vehicle in healthy (normotensive) rat eyes. PnPP-19 showed a reduction in IOP compared to the vehicle, after 2, 4, 5, 6, and 24 hours of treatments. Subsequently, after PnPP-19 administration, IOP decreased around 20% compared to the IOP before PnPP-19 administration and was significantly different from vehicle (Fig. 5). PnPP-19 did not alter median arterial pressure values (data not shown).
Figure 5.
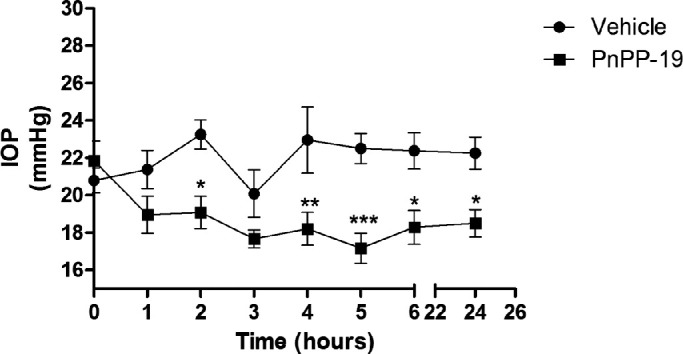
PnPP-19 (80 µg/eye) reduces IOP in normotensive (healthy) rats. Comparison between PnPP-19 and Vehicle. Results are expressed in mm Hg (N = 6). Asterisks represent statistical difference compared to control * P < 0.5; ** P < 0.01; *** P < 0.001, 2-way ANOVA with Bonferroni post-test.
FITC-Conjugated PnPP-19 (PnPP-19-FITC) was Able to Permeate Cornea and Reduce IOP of Rats After Topical Administration
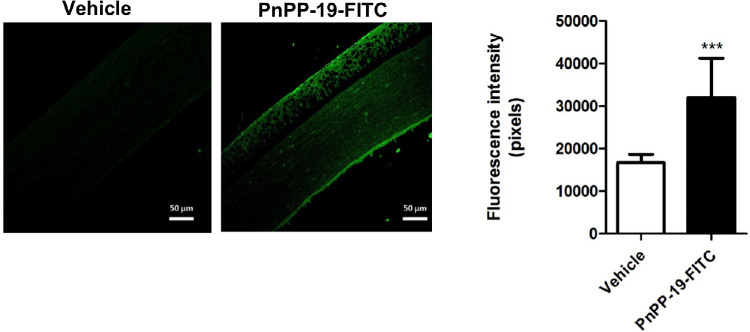
Fluorescence results indicate that FITC-PnPP-19 was able to permeate the cornea and reduce the IOP from 24.26 ± 1.67 mm Hg (vehicle) to 19.88 ± 2.43 mm Hg, in healthy rats. One drop of FITC-PnPP-19 or saline was instilled 2 hours before IOP measure (Fig. 6).
Figure 6.
Permeation of PnPP-19 through the cornea. Fluorescence promoted by PnPP-19-FITC showed that this peptide was able to permeate through the cornea (N = 3). Asterisks represent statistical difference compared to vehicle *** P <0.001, 2-way ANOVA with Bonferroni post-test.
PnPP-19 Increases Nitrite Levels in Homogenized Eye Surface of Rats
It is known that PnPP-19 increases NO release in different tissues.20,29 Our results showed that PnPP-19 stimulated an increase in nitrite production in healthy rat's eye tissues (48.70 ± 1.19 [NO2-] nmol/mg of protein) compared to the vehicle (31.01 ± 0.38 [NO2-] nmol/mg of protein; Fig. 7).
Figure 7.
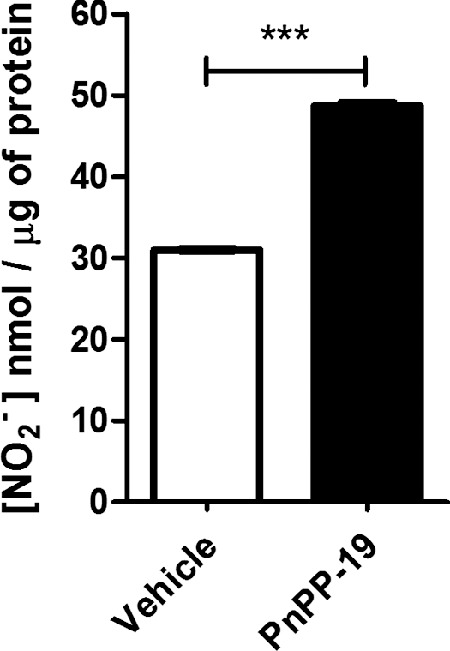
Effect of PnPP-19 instillation on nitrite concentration (NO2-) in the homogenized eye tissues. The tissues of the normotensive (healthy) eyes were collected 2 hours after local instillation of vehicle (saline) or PnPP-19 (80 µg/eye). Each column represents the mean ± SEM (N = 6). *** P < 0.001, the Student t test for nonpaired data.
The Action of PnPP-19 on Ocular Hypertension Animals
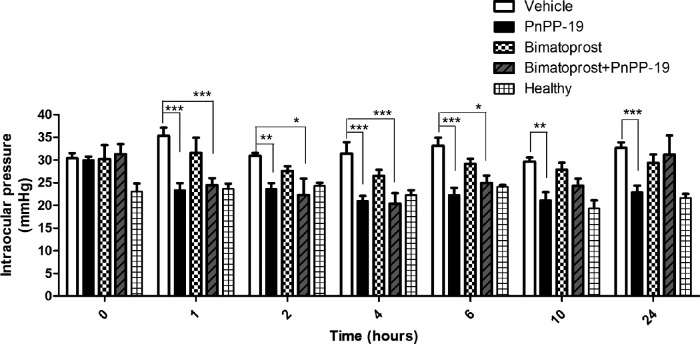
PnPP-19 ability to reduce IOP was evaluated in an ocular hypertension experimental model induced by intraocular injection of HA. After these intraocular injections, there was a significant increase in the IOP in all groups that received HA. The IOP of the vehicle group was 30.41 ± 2.69 and the healthy group was 23.03 ± 2.7 being significantly different with P > 0.01, in time 0. Once the establishment of ocular hypertension, the group treated with PnPP-19 significantly reduced IOP compared to vehicle group, at all times tested. It is noteworthy that, after 24 hours of a single instillation of one drop of the peptide or vehicle (22.9 ± 3.6 vs. 32.6 ± 3.5 mm Hg, respectively, P < 0.001) the IOP remained low for peptide-treated groups and their IOP values were similar to those of the healthy group, at all points (Fig. 8).
Figure 8.
PnPP-19 reduces IOP in rats with ocular hypertension. Comparison between treated hypertensive eyes with Vehicle (saline – NaCl 0.9%), PnPP-19 (80 µg/eye), Bimatoprost (8.8 µg/eye), PnPP-19 + Bimatoprost (PnPP-19 at 80 µg/eye plus Bimatoprost at 8.8 µg/eye) and healthy eyes. Results expressed in mm Hg (N = 6). Asterisks represent statistical difference in relation to the untreated * P < 0.5; ** P < 0.01; *** P < 0.001, 2-way ANOVA with Bonferroni post-test.
The PnPP-19 treated group also had lower IOP in comparison with Bimatoprost, a commercially antiglaucomatous drug (22.9 ± 3.6 vs. 29.3 ± 2.8 mm Hg, respectively, P < 0.001) after 24 hours of a single instillation. When PnPP-19 + Bimatoprost were co-administered in glaucomatous eyes, there was a significant reduction of IOP, which lasted 6 hours (Fig. 8).
PnPP-19 Promotes Neuroprotection in the Retina of Ocular Hypertension Rats
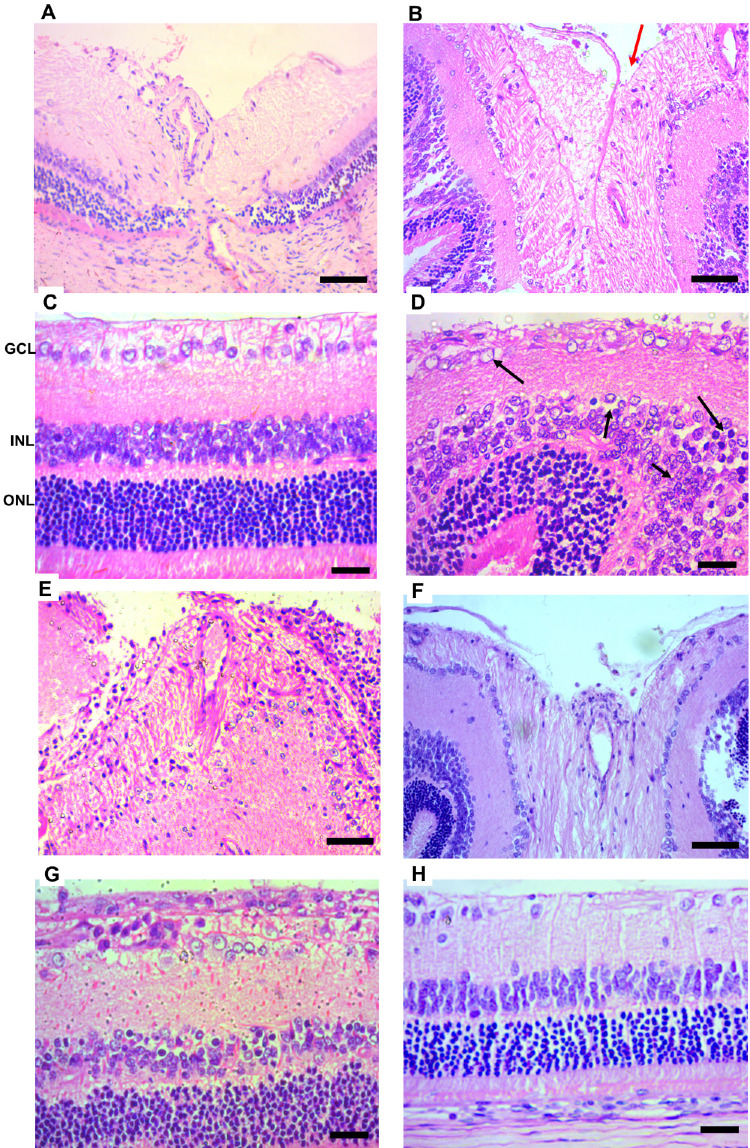
Histological analyses of the eyes of ocular hypertensive rats showed an increase in optic nerve excavation (red arrows) due to the loss of RGCs axons and also a large reduction in optic nerve neural fibers (Figs. 9B, 9E, and 9F). The treatment with PnPP-19 reduced these damages if compared to the vehicle-treated glaucoma and Bimatoprost group (Figs. 9E, 9H).
Figure 9.
Histologic analysis of healthy and ocular hypertension rats. The vehicle-treated ocular hypertension animals exhibited an increase in the excavation of the optic nerve (red arrows) and increase in the number of cytoplasmic vacuolization in the GCL (black arrows) (B, D) if compared to healthy rats (A,C). Besides, the INL displays more edema, pyknotic nuclei, and cellular disorganization (black arrows) (D). The ONL exhibits a decreased cell number, and greater edema and cell disorganization compared with the healthy retina D. Treatment with PnPP-19 (E,G) reduced histologic damage if compared to vehicle-treated glaucoma B and D or Bimatoprost (F,H). Healthy A and C; ocular hypertension vehicle-treated B and D; ocular hypertension treated with PnPP-19 E and G Bimatoprost F and H. Retina layers: ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). Digital images were obtained using a microscope (Apotome.2, Zeiss, Germany) with a 10× and 20× objective, scale bar = 50 µm.
When compared to healthy retinas, ocular hypertensive vehicle-treated retinas (Figs. 9C and 9D) showed a decrease in cell number, an increase in cytoplasmic vacuolization (black arrow), and in the number of pyknotic nuclei in the GCL. The inner nuclear layer (INL) displayed more edema, pyknotic nuclei, and cellular disorganization (black arrows). The outer nuclear layer (ONL) exhibited a decreased cell number, greater edema, and cell disorganization compared with healthy retinas.
It was possible to observe that the increase in IOP probably contributed to the decrease in the number of RGCs (Fig. 10). Vehicle-treated ocular hypertensive animals had a reduction in retinal RGCs compared to PnPP-19-treated ocular hypertensive animals or Bimatoprost + PnPP-19 and Bimatoprost alone (see Fig. 10).
Figure 10.
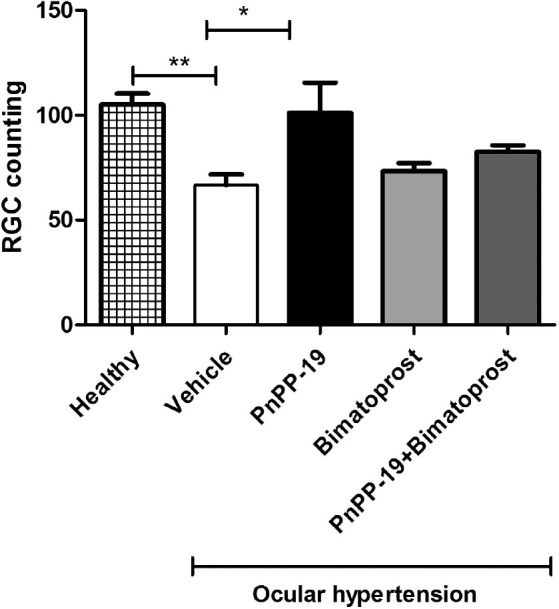
Increases in the IOP were accompanied by reductions in the number of RGCs (retinal ganglion cells). There was a smaller number of RGCs in the retinas of ocular hypertension animals compared to healthy rats. Ocular hypertension animals treated with PnPP-19 have more RGCs than vehicle treated ocular hypertension rats, and were not statistically different compared to healthy rats (N = 6). Asterisks represent statistical difference concerning the untreated * P < 0.05; ** P < 0.01, 2-way ANOVA with Bonferroni post-test.
Discussion
In the present work, we demonstrated that the synthetic peptide PnPP-19 is capable of reducing IOP in animals with healthy and hypertensive eyes, besides protecting against injury to the optic nerve, after topical administration as eye drops.
IOP is the critical risk factor for glaucoma, a neurodegenerative disease and frequent cause of blindness worldwide. POAG is associated with high IOP and is the leading cause of glaucomatous blindness, affecting 80 to 90% of patients with glaucoma. IOP reduction decreases the incidence rate and slows the progression of POAG.9,30 Treatment of POAG requires pharmacotherapy as soon as ocular hypertension is detected. Despite the availability of several anti-glaucoma medications, there is still an important medical need in this field.
In this context, the peptide PnPP-19 emerges as an interesting therapeutic option because, besides reducing IOP, it has a short polypeptide chain without disulfide bridges, which eases its synthesis. All these features strongly indicate its viability for use and commercialization. In addition, we showed herein that this peptide is not toxic or irritating when applied to the CAM. In contrast, as expected, NaOH solution was severely irritating, as presented in the OII score table (Table). This test is appropriate, because CAM is analogous to the human retina and its vasculature. Irritations at risk of vascular damage, such as bleeding, lysis, and coagulation, can be evaluated by HET-CAM test, which is optimal for the study of compound accuracy and safety for ocular application.31,32
Distinct ERG components are related to different retinal structures, so ERG is often used to monitor the functional integrity of the retina.33 ERG examination did not indicate any change by PnPP-19 even at high doses, suggesting safety and no internal retinal impairment (see Fig. 2, Fig. 3). The histopathological analysis showed that peptide-treated cornea and retina have the same morphology compared to control (see Fig. 4), indicating security for the use of the peptide in all concentrations tested.
The IOP of healthy rats decreased around 20% after 2 hours of instillation of a single dose of PnPP-19, and this effect lasted for 24 hours (Fig. 5). This result highlights the effectiveness of this peptide, because if the desired IOP level is not achieved with a single dose or therapy, additional doses of medications may be included in the treatment regimen,13 which may trigger several adverse symptoms. Therefore, topical administration, with a low frequency of treatment, is a valuable and interesting resource to have, and is also advantageous for individuals who have difficulties with medication adherence.34
Recent evidence indicates that the NO signaling pathway plays an important role in ocular homeostasis, regulating AH drainage and, therefore, IOP. Contractile tissues form the anatomic ocular structures that regulate AH drainage. The TM cells are known to be highly contractile, analogous to vascular smooth muscle cells, in which the role of NO-cGMP signaling in endothelium-dependent relaxation is well understood.15
Studies in animals have shown that NO-induced IOP reduction is mainly mediated by an increase in conventional outflow caused by a relaxation of the TM cells, and probably by Schlemm's canal permeability increase. The Schlemm's channel cells rise endogenous NO generation in response to shear stress supporting the NO function in IOP homeostasis.35,36 In addition, the therapeutic potential of NO has recently been validated in patients with POAG.15
In the presence of oxygen, endogenous NO has a short half-life, which makes it difficult to be measured in biological systems. Intracellular NO can be easily oxidized to more stable compounds, such as nitrite (NO2-) and nitrate (NO3-).37 For this reason, nitrite concentration measurement is a good method for indirectly inferring if there is any change in NO levels after drug administration.
Topical administration of PnPP-19 increased nitrite levels in the ocular tissue from the anterior chamber, which may simultaneously improve conventional AH flow. Moreover, FITC-labeled PnPP-19 assay indirectly revealed that this peptide can permeate corneal layers reaching tissues of the anterior chamber. It is possible that PnPP-19 improves NO release, evidenced by an increase in nitrite concentration, which promotes contractility changes that corroborate with IOP reduction, but more evidence is needed to prove this action.
Previous studies have shown that chronic administration of HA by intracameral injections induces significant IOP elevation and damage in the retina, which seems consistent with some characteristics of POAG,12,38 in addition, it has been demonstrated that this model may be useful for pharmacological studies with drugs that affect the formation of aqueous humor or outflow or both,12,39 being relevant to test the PnPP-19 action. Our results show that the IOP increased 32% after the intraocular injections of HA and the reduction in IOP triggered by PnPP-19 was also effective in the ocular hypertension model we used. This finding is therapeutically relevant given the clinical routine, highlighting that, despite reducing IOP by around 30% after 24 hours of a single instillation, PnPP-19 did not change MAP as previously described.20,40
A commercial prostaglandin-analog antiglaucoma agent (Bimatoprost) was used to compare its effects with those of PnPP-19. IOP lowering remained for 24 hours after the treatment with a single topical instillation of PnPP-19. Bimatoprost, on the other hand, was not able to reduce IOP according to the established protocol (see Fig. 8910). It is possible that this lack of effect is associated with a difference in drug concentrations. However, Franca et al.27 obtained good results using 9 µg of Bimatoprost for the treatment of glaucoma in rats, a similar dose as the one used in this work, but it was administered in an ocular insert.
When co-administered Bimatoprost and PnPP-19, the effect improved, leading to a higher reduction in IOP values, compared to Bimatoprost alone. Similar to the results observed in this study, latanoprostene bunod, an NO-donating prostaglandin F receptor agonist, and was more effective than the reference compound, latanoprost, in lowering IOP. In this case, the dual-mode of action, combining prostaglandin F receptor activation and NO donation, increased AH outflow through both unconventional and conventional pathways, simultaneously.15 However, the interaction between PnPP-19 and Bimatoprost did not promote synergism in the effect, on the contrary, we observed that this interaction impaired the action of PnPP-19 because its effect was less if compared to the peptide alone. We believe that interaction between these drugs happened, and Bimatoprost may have impaired the binding of PnPP-19 to its target, and further investigation is needed to clarify this question.
Glaucoma is characterized as a slow degeneration of RGC followed by axon loss. It manifests as a progressive retinal nerve fiber layer thinning and optic nerve head cupping, which functionally results in a characteristic pattern of visual field loss.13
Therefore, at the end of the treatment, histological sections of the retina and the optic nerve area were evaluated to verify the RGC loss and optic nerve head cupping. As shown, the vehicle-treated group showed significant RGC loss, whereas in the PnPP-19-treated group the RGC number was similar to that of healthy animals, suggesting that the treatment with PnPP-19 also promoted neuroprotection. However, future experiments are needed to confirm this last result.
Conclusions
The present work shows that an NO-inducer peptide, PnPP-19, when topically administered as eye drops, reduces IOP after a single dose, both in normotensive and ocular hypertensive rats, without eliciting apparent toxicity. Our results suggest that PnPP-19 is, probably, improving the conventional AH outflow and directly preventing the progression of optic nerve degeneration. Thus, this synthetic peptide has great potential to emerge as a promising drug for the treatment of glaucoma, isolated or in combination with conventional drugs.
Acknowledgments
Supported by fellowships and grants that were awarded by the Brazilian University UFMG (Universidade Federal de Minas Gerais), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), INCT - Nanotecnologia Farmacêutica (Instituto Nacional de Ciência e Tecnologia), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), BIOZEUS Biopharmaceutical S.A., and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais).
A portion of this work was presented at the annual meeting of the North American Society of Toxinology - The Venom Week 2020, Gainesville, Florida, United States, Mar 2020.
Disclosure: C.N. da Silva, Biozeus (F); L.F.N. Dourado, Biozeus (F); M.E. de Lima, Biozeus (F); A. da Silva Cunha-Jr, Biozeus (F)
References
- 1. Weinreb RN, Tee Khaw P. Primary open-angle glaucoma. Lancet. 2004; 363P: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 2. Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 3. Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 5. Aliancy J, Stamer WD, Wirostko B. A review of nitric oxide for the treatment of glaucomatous disease. Ophthalmol Ther. 2017; 6: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costa VP, Harris A, Stefánsson E, et al.. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res. 2003; 22: 769–805. [DOI] [PubMed] [Google Scholar]
- 7. Morrison JC, Cepurna WO, Johnson EC. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp Eye Res. 2015; 141: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison JC, Fraunfelder FW, Milne ST, Moore CG. Limbal microvasculature of the rat eye. Investig Ophthalmol Vis Sci. 1995; 36: 751–756. [PubMed] [Google Scholar]
- 9. Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: a unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm. 2015; 95(Pt B): 173–181. [DOI] [PubMed] [Google Scholar]
- 10. Morrison JC, Moore CG, Deppmeier LMH, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997; 64: 85–96. [DOI] [PubMed] [Google Scholar]
- 11. Shareef SR, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma SC. Chronic ocular hypertension following episcleral venous occlusion in rats. Exp Eye Res. 1995; 61: 379–382. [DOI] [PubMed] [Google Scholar]
- 12. Moreno MC, Aldana Marcos HJ, Croxatto JO, et al.. A new experimental model of glaucoma in rats through intracameral injections of hyaluronic acid. Exp Eye Res. 2005; 81: 71–80. [DOI] [PubMed] [Google Scholar]
- 13. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014; 311: 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garhöfer G, Schmetterer L. Nitric oxide: a drug target for glaucoma revisited. Drug Discov Today. 2019; 24: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 15. Cavet ME, Vittitow JL, Impagnatiello F, Ongini E, Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Investig Ophthalmol Vis Sci. 2014; 55: 5005–5015. [DOI] [PubMed] [Google Scholar]
- 16. Wareham LK, Buys ES, Sappington RM. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide. 2018; 77: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58: 99–105. [DOI] [PubMed] [Google Scholar]
- 18. Funk R, Gehr J, Rohen J. Short-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the Albino rabbit. Curr Eye Res. 1996; 15: 87–93. [DOI] [PubMed] [Google Scholar]
- 19. Mandal A, Pal D, Agrahari V, Trinh HM, Joseph M, Mitra AK. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018; 126: 67–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva CN, Nunes KP, Torres FS, et al.. PnPP-19, a synthetic and nontoxic peptide designed from a phoneutria nigriventer toxin, potentiates erectile function via NO/cGMP. J Urol. 2015; 194: 1481–1490. [DOI] [PubMed] [Google Scholar]
- 21. Gilleron L, Coecke S, Sysmans M, et al.. Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. Toxicol Vitr. 1996; 10: 431–446. [DOI] [PubMed] [Google Scholar]
- 22. da Silva CN, da Silva FR, Dourado LFN, et al.. A new topical eye drop containing LyeTxI-b, a synthetic peptide designed from a lycosa erithrognata venom toxin, was effective to treat resistant bacterial keratitis. Toxins (Basel). 2019; 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCulloch, DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol Ophthalmol. 2015; 130: 1–12. [DOI] [PubMed] [Google Scholar]
- 24. Toledo CR, Pereira VV, Dourado LFN, Paiva MRB, Silva-Cunha A. Corosolic acid: antiangiogenic activity and safety of intravitreal injection in rats eyes. Doc Ophthalmol. 2019; 138: 181–194. [DOI] [PubMed] [Google Scholar]
- 25. Perlman I. Relationship between the amplitudes of the b wave and the a wave as a useful index for evaluating the electroretinogram. Br J Ophthalmol. 1983; 67: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown KT. The electroretinogram: its components and their origins. Vision Res. 1968; 8: 633–677. [DOI] [PubMed] [Google Scholar]
- 27. Franca JR, Foureaux G, Fuscaldi LL, et al.. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: in vitro and in vivo evaluation. PLoS One. 2014; 9: e95461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126: 131–138. [DOI] [PubMed] [Google Scholar]
- 29. Freitas ACN, Silva GC, Pacheco DF, et al.. The synthetic peptide PnPP-19 induces peripheral antinociception via activation of NO/cGMP/KATP pathway: role of eNOS and nNOS. Nitric Oxide. 2017; 64: 31–38. [DOI] [PubMed] [Google Scholar]
- 30. Heijl A. Glaucoma treatment: by the highest level of evidence. Lancet. 2015; 385: 1264–1266. [DOI] [PubMed] [Google Scholar]
- 31. Vargas A, Zeisser-Labouèbe M, Lange N, Gurny R, Delie F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev. 2007; 59: 1162–1176. [DOI] [PubMed] [Google Scholar]
- 32. Abdelkader H, Ismail S, Hussein A, Wu Z, Al-Kassas R, Alany RG. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen's egg chorioallantoic membrane and excised bovine cornea models. Int J Pharm. 2012; 432: 1–10. [DOI] [PubMed] [Google Scholar]
- 33. Perlman I. Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc Ophthalmol. 2009; 118: 3–28. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Huang W, Zhang X. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol. 2018; 96: e277–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cavet ME, Decory HH. The role of nitric oxide in the intraocular pressure lowering efficacy of latanoprostene bunod: review of nonclinical studies. J Ocul Pharmacol Ther. 2018; 34: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashpole NE, Overby DR, Ethier CR, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm's canal cells. Investig Ophthalmol Vis Sci. 2014; 55: 8067–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA. 1993; 90: 8103–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012; 2012: 692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benozzi J, Nahum LP, Campanelli JL, Rosenstein RE. Effect of hyaluronic acid on intraocular pressure in rats. Investig Ophthalmol Vis Sci. 2002; 43: 2196–2200. [PubMed] [Google Scholar]
- 40. Nunes da Silva C, Nunes KP, De Marco Almeida F, et al.. PnPP-19 peptide restores erectile function in hypertensive and diabetic animals through intravenous and topical administration. J Sex Med. 2019; 16: 365–374. [DOI] [PubMed] [Google Scholar]