Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been established as a cause of severe alveolar damage and pneumonia in patients with advanced Coronavirus disease (COVID-19). The consolidation of lung parenchyma precipitates the alterations in blood gases in COVID-19 patients that are known to complicate and cause hypoxemic respiratory failure. With SARS-CoV-2 damaging multiple organs in COVID-19, including the central nervous system that regulates the breathing process, it is a daunting task to compute the extent to which the failure of the central regulation of the breathing process contributes to the mortality of COVID-19 affected patients. Emerging data on COVID-19 cases from hospitals and autopsies in the last few months have helped in the understanding of the pathogenesis of respiratory failures in COVID-19. Recent reports have provided overwhelming evidence of the occurrence of acute respiratory failures in COVID-19 due to neurotropism of the brainstem by SARS-CoV-2. In this review, a cascade of events that may follow the alterations in blood gases and possible neurological damage to the respiratory regulation centers in the central nervous system (CNS) in COVID-19 are related to the basic mechanism of respiratory regulation in order to understand the acute respiratory failure reported in this disease. Though a complex metabolic and respiratory dysregulation also occurs with infections caused by SARS-CoV-1 and MERS that are known to contribute toward deaths of the patients in the past, we highlight here the role of systemic dysregulation and the CNS respiratory regulation mechanisms in the causation of mortalities seen in COVID-19. The invasion of the CNS by SARS-CoV-2, as shown recently in areas like the brainstem that control the normal breathing process with nuclei like the pre-Bötzinger complex (pre-BÖTC), may explain why some of the patients with COVID-19, who have been reported to have recovered from pneumonia, could not be weaned from invasive mechanical ventilation and the occurrences of acute respiratory arrests seen in COVID-19. This debate is important for many reasons, one of which is the fact that permanent damage to the medullary respiratory centers by SARS-CoV-2 would not benefit from mechanical ventilators, as is possibly occurring during the management of COVID-19 patients.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Neuroinvasive, Brain, Cerebrospinal Fluid, CSF, Central Nervous System, CNS, Neurotropic, Transcribrial, Olfactory, Neuron
A. Introduction
COVID-19 caused by SARS-CoV-2 has resulted in an alarming rate of morbidity and mortality worldwide. Patients with severe COVID-19 (Figure 1A arrows) are known to develop an extensive disease of the lung parenchyma like lobar pneumonia requiring hospitalization.1,50 There have been reports of patients with mild COVID-19 who have significant blood gas alteration and chest computed tomography (CT) scan findings without significant symptoms,2 which is alarming. The reason for the latter is that the patients without symptoms but an ongoing COVID-19 along with organ damage can complicate rapidly without giving the diagnosis and treatment a chance to save their lives.3 The alteration of PO2 and PCO2 in COVID-19 is complex4 and difficult to comprehend when compared to conventual viral cases of pneumonia. The reason for the added complexity of the alteration of PO2 and PCO2 in COVID-19 is the concurrent renal, gastrointestinal, and adrenal damage that are known contributors toward the maintenance of blood pH and blood gases and act as buffers to combat any alteration in these parameters that may occur.5 An alarming, but yet unexplored, component in COVID-19 is the damage to the neurons in the central nervous system (CNS) that has been reported to commence during the disease in some patients6−8 (Figure 1). Additionally, it is easy to compute the complexity that ongoing damage to respiratory regulating neurons in CNS would add to the aforementioned pulmonary damage in COVID-19. Also worrying are the reports of acute respiratory failure in 45–65% of cases in COVID-19 in which the patients lost spontaneous breathing, necessitating the use of ventilators, and eventually died.7 Some of the patients with COVID-19 that have been reported to recover from pneumonia, but could not be weaned from invasive mechanical ventilation,9 and need to be investigated for SARS-CoV-2 neurotropism that can deteriorate and prove to be fatal.6,7 The occurrence of these spontaneous (autonomic) breathing control failures early in COVID-19 is alarming and possibly reflective of the damaging effect of SARS-CoV-2 on the CNS nuclei that control normal involuntary breathing mechanics. SARS-CoV-2 probably invades the brain via axonal transport and transneuronal spread from the olfactory nerves on to the rhinencephalon, finally reaching the brainstem causing the irreversible respiratory failure seen in severe COVID-19, typically characterized by lack of dyspnea.3,9,10 Healthcare professionals should be heralded for the occurrence of acute respiratory arrest in early COVID-19, with some cases reflecting milder clinical evidence of hypoxia with accompanying hypocapnia and show no sense of breathlessness.3 Failure to comprehend and investigate the neurotropic potential of the virus,6,7,9 which has proven to be neurotoxic10−12 to the neurons in the brainstem,7,9 can result in more fatalities in COVID-19, by the time this clinically overt feature of SARS-CoV-2 gets investigated and draws our attention. In COVID-19, the respiratory symptoms are the most common, and neurogenic breathing failure is suspected in cases that have been reported.7,9,10,13 SARS-CoV-2 has challenged our complex computing ability to understand its pathogenesis. Along with this, notions such as COVID-19 being a mere flu, which like influenza and other viral pneumonia does not need to be worried about, has weakened our ability to stay ahead of the curve with this deadly virus. It is only after the huge number of deaths worldwide now that we are understanding the notorious and noxious nature of this virus.
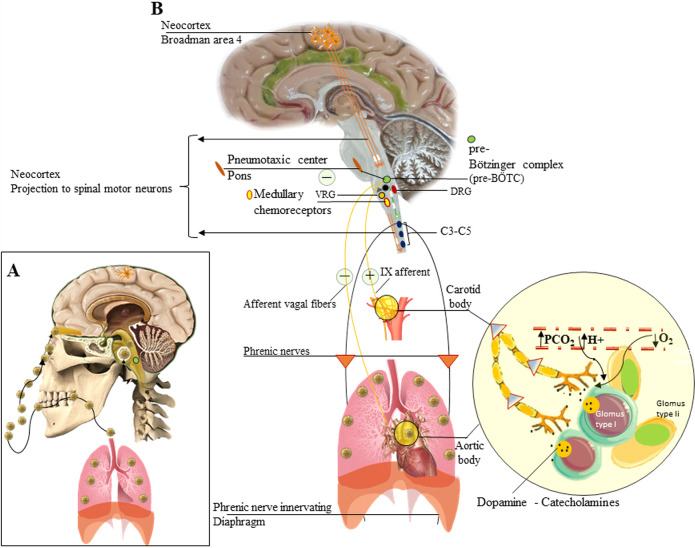
Figure 1.
Regulation of respiration in humans and the effects of SARS-CoV-2 on CNS. Route of acquisition of SARS-CoV-2 (A) that spreads to lungs29 and olfactory mucosa and olfactory bulb from where it can spread to the CNS via retrograde neuronal transfer, CSF, and direct damage to the frontal lobe. The breathing process in humans is regulated by CNS centers (B) that are located in brainstem (gray) and cerebral cortex (neocortex). The spread of the virus to the CNS includes the brainstem.9,20 The damage caused in the medulla oblongata in the Bötzinger complex (pre-BÖTC) (green circle) can result in respiratory arrest and failure necessitating the use of ventilators. The neural connections and transmission from peripheral chemoreceptors (yellow circle) are shown.
B. The Pulmonary Disease and Its Sequelae in COVID-19
The damage caused by SARS-CoV-2 at the level of gaseous exchange in the lungs causes exudative and organized diffuse alveolar damage.14 There are reports in COVID-19 that severely hypoxaemic patients, may present quite differently from one another: normally breathing (“silent” hypoxemia) or remarkably dyspnoeic; quite responsive to nitric oxide or not; deeply hypocapnic or normo/hypercapnic; and either responsive to prone position or not. Therefore, the same disease presents itself with impressive nonuniformity.15 The ongoing exudation and fibrosis in the terminal bronchioles and alveolar walls thicken the gaseous barrier further leading to profound hypoxia and threatening hypoxemic-respiratory failure.14,16 It has been observed that many patients with a severe drop in PO2 remain asymptomatic initially3,15 due to a possible compensation by an increase in the rate of breathing that comes into play by the neurogenic mechanism (detailed below)5 to combat hypoxia resulting from a poor diffusion of O2 across the alveolar barrier. A possible explanation for such severe hypoxemia occurring in a compliant lung is the possible loss of lung perfusion and hypoxic vasoconstriction. It is important to mention here that PCO2 initially in the course of COVID-19 remains at near-normal levels,15,17 possibly because CO2, being more permeable across the alveolar barrier compared to O2,5 manages to diffuse out into the fluid-filled alveoli early in COVID-19.
C. Multiorgan Diseases Contributing toward the Alteration in Blood Gases in COVID-19
It is pertinent here to bear in mind that a complex picture of multiorgan involvement17 could evolve in advanced COVID-19, affecting the blood gases, after adrenal damage (secreting aldosterone and cortisol), gastrointestinal damage (H+; −HCO3– ion secretion), coagulation abnormalities, and metabolic derangements like lactate production from cellular damage. Taken into consideration, these factors (Figure 2) must be contributing to the respiratory failures seen in COVID-19.6 In the end-organ failure sate in COVID-19, a vicious cycle of tissue damage, metabolic acidosis, and blood gas alteration seems to be the key leading to respiratory failure in COVID-19, until fatal incidences like pulmonary embolism, cardiac ruptures, or stroke do not do the same.
Figure 2.
Cascade of acquisition and organ transmission of SARS-CoV-2. SARS-CoV-2 after accessing the body via eyes and mouth (top two boxes at left) can opt for a retrograde neuronal route to enter the brainstem. The virus that enters the nose in its upper part is known to infect the olfactory mucosa from where it can take a transcribrial route to the olfactory bulb (OB) that has been recently reported to show distortions in COVID-19. From the OB, it can infect the frontal lobe of the brain. Alternatively, from entry into CSF from the subacrhinoid spaces around olfactory nerves, it can spread to the rest of the CNS. SARS-CoV-2 after being inhaled reaches the lungs where it causes pneumonia resulting in hypoxemic respiratory failure and death. Note that other pathways leading to death detailed in the lower half of the figure. Importantly, the dissemination of SARS-CoV-2 to the brainstem and CNS via CSF, retrograde neuronal, and transcribrial routes can initiate an early onset of respiratory arrest by damaging medullary centers of breathing resulting in death.
D. The Central Regulation of Breathing
D1. Central Regulation of Breathing under Physiological Conditions
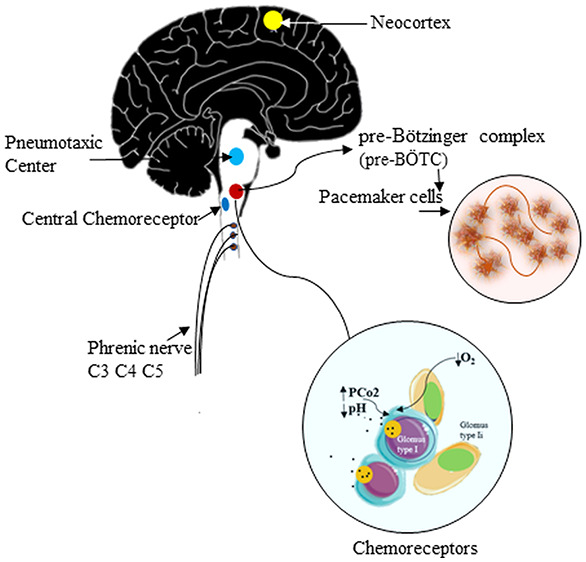
The breathing process under physiological conditions by the CNS is an involuntary (autonomous) process enforced by pacemaker cells in the pre-Bötzinger complex (pre-BÖTC) on either side of the medulla oblongata in the brainstem (Figure 1B green circle). These neurons produce rhythmic discharges5,18 that reach the phrenic nerve motor neurons (Figure 1B). Besides, dorsal (DRG) and ventral (VRG) groups of respiratory neurons are present in the medulla, and they are known to project to the pre-BÖTC pacemaker neurons (Figure 1B DRG-VRG). The rhythmic discharges of the pre-BÖTC pacemaker neurons are modified by a pneumotaxic center (nucleus parabrachialis), which may play a role in switching between inspiration and expiration5 (Figure 1B), in the pons and afferents in the vagus nerve from receptors in the airways and lungs. A rise in the PCO2 or H+ ion concentration of arterial blood or a drop in its PO2 increases the rhythmic discharge activity in the medulla oblongata (Figure 1B) and vice versa.5 The effects of variations in serum chemistry on the rhythmic discharge of pre-BÖTC are mediated via respiratory chemoreceptors: the carotid and aortic bodies19 (Figure 1B yellow circles) and the central chemoreceptor area in the medulla (Figure 1B oval yellow area). Each carotid and aortic body contains isles of two types of cells, glomus type I and glomus type II cells (supporting cells), surrounded by fenestrated sinusoidal capillaries (Figure 1B big yellow circle). The glomus type I is closely associated with glossopharyngeal nerve afferent nerve (CN-IX) endings and is excited by hypoxia-induced inhibition of O2-sensitive potassium K+ channels), and the transmitter involved appears to be dopamine, which excites the nerve endings by way of D2 dopaminergic receptors.5,19 The glomus type I receptors in the carotid/aortic bodies gets stimulated, with increased afferent nerve discharges, by a rise in the PCO2 or H+ concentration of arterial blood or a decline in its PaO2 below 55–60 mmHg. Therefore, the stimulatory effects of hypoxia on ventilation are not manifested until they become strong enough.
Besides, even though spontaneous breathing is not usually a conscious phenomenon, both inspiration and expiration are under voluntary control.5 The pathways for voluntary control pass from the neocortex (Figure 1B Brodman’s area 4, orange neurons) to the motor neurons innervating the respiratory muscles, without influencing the medullary neurons (Figure 1B orange lines with white arrows).
D2. Disorders in Central Regulation of Breathing in COVID-19
The occurrence of spontaneous breathing in COVID-19 patients with hypoxia and normo/hypocapnia point toward a retained ability of the pre-BÖTC neuronal discharge. Damage in the above zone is expected to compromise the process of autonomic breathing extending into the medula, as has been reported in COVID-19 patients with damage extending into the medulla oblongata.7,9 Besides, recent reports are consistent with the involvement of the brainstem and especially of the respiratory center in COVID-19 patients.9,20 Patients with COVID-19 often develop respiratory failure 8–14 days after symptom onset, with “silent hypoxemia” and a high respiratory rate,3 which explains the hypocapnia hinted at above. The hypoxia in COVID-19 leading to stimulation of the pre-BÖTC via chemoreceptor area is expected to cause an increased respiratory rate and depth that has been commonly reported in COVID-19 patients.3,9 On the contrary, the patients with profound hypoxia seemingly appear to be asymptomatic, with apparent cyanosis with slight exertions lead to collapse, and the need for ventilatory support for breathing.3 An increased rate of breathing, as high as 38/mins, profound hypoxia with low PCO2 levels, without any dyspnea until late in the disease course is what is seen in COVID-19 and needs to be explained.3,21 Damage to vagal receptors in the lungs and perhaps mechanoreceptors in the respiratory muscles involved in breathing to the sensorimotor cortex might explain the absence of the sensation of dyspnea. Also, loss of the ability to breathe spontaneously as has been reported in a variable number of COVID-19 cases6,7 is alarming and needs to be investigated. If SARS-CoV-2 is considered a neurotropic virus, as has been elucidated recently in many reports,6−10 an early attack of the SARS-CoV-2 on CNS involving the pre-BÖTC and DRG by the spreading along glossopharyngeal and vagal afferents from chemoreceptors to the medullary centers is expected,20 and an investigation into this route may help explain the loss of the ability to breathe spontaneously in patients with COVID-19. The fact that patients retain the ability to breathe voluntarily in early COVID-19 indicates that the pathways from the neocortex to the muscles of respiration via the spinal motor neurons (Figure 1B orange lines with white arrows) remain functional.
D3. Pathogenesis of COVID-19 Caused by SARS-CoV-2
There are clear shreds of evidence that SARS-CoV-2 targets diverse organs and tissues (Figure 2) after entry into the human body. The route of entry of SARS-CoV-2 into the human body (Figure 1A) and further spread of the virus to the lungs (Figure 2 bottom left cascade), CNS (Figure 2 top right cascade), and the systemic circulation show the systemic derangements that are known to cause death in patients with organopathies that occur in COVID-19.
D3.1. Evidence of Access of SARS-CoV-2 to the Medulla Oblongata and Other Parts of CNS
There is a high suspicion that SARS-CoV-2 may be targeting pre-BÖTC and related important CNS zones that regulate the process of normal breathing6,7,9 require evidence of SARS-CoV-2 invading the brain (Figure 1B). Recent reports on the neurotropic effects of SARS-CoV-2 has shown that it affects the medulla oblongata20 and frontal lobe neurons in patients with COVID-19.22 Additionally, the fact that SARS-CoV-2 has been isolated from the CSF of COVID-19 affected patents23 has ended the debate on the neurovirulent potential of this virus. The hematogenous, retrograde neuronal transport and nasal routes across the cribriform plate6 (Figure 1A upward-directed arrow) have been proposed as routes the virus may take to gain entry to the CNS.6,24 Regardless of the route that SARS-CoV-2 adopts to reach the brain, the finding of SARS-CoV-2 affecting the medulla and brainstem respiratory centers7,9,20 is alarming. It is easy to compute that if the virus invades the brainstem in the early phases of COVID-19, the occurrences of respiratory failures due to damage in respiratory regulating centers can occur long before the hypoxemic influence comes into effect as has been reported recently.7 The details of the mechanism of neuronal injury remain murky, but a viral budding leading to eventual rupture of the neurons has been reported recently for SARS-CoV-2.22 If similar mechanisms of neurotoxicity do occur in medullary nuclei in COVID-19, partial damage to the pacemaker neurons in the pre-BÖTC area can lead to intervals of loss of autonomic breathing and evoke neurological manifestations as reported in COVID-19 affected individuals.7,24 The knowledge of the circuit of neural projections and synapses that regulate the process breathing is cardinal to the understanding of respiratory failures in general and that seen in COVID-19 in particular. The spinal motor neurons that act as a nuclear group for phrenic nerve, emerging as nerve roots, is located at cervical 3, 4, and 5 (C3, C4, and C5) (Figure 1B oval blue dots in the spinal cord). These motor neurons are regulated by descending pathways (Figure 1B green lines with white arrows) in the spinal cord crossing the level C1 and C2. Understandably, the involvement or damage to the phrenic nerve roots and motor nerves to the diaphragm and thoracic muscles involved in breathing axons in COVID-19 could completely cease respiratory function as has been recently reported.25 In the latter report, COVID-19 infection was confirmed by a SARS-CoV-2 positive PCR test result from a pharyngeal swab, and the patient remains on a ventilator respirator due to neuromuscular weakness.
D3.2. Correlating Syndromic COVID-19 with SARS-CoV-2 Mediated Damage to the Breathing Regulating Centers in the Brain
Relating the pulmonary sign and symptoms of patients with COVID-19 and the ongoing neurological deficits can help in uncovering the possible regions of the CNS affected by SARS-CoV-29,20,25 affecting the breathing process. For example, the ability of COVID-19 patients to retain control over voluntary breathing suggests that the neocortical projections of the brain to the spinal motor neurons (Figure 1B orange lines with white arrows) are spared in COVID-19. Additionally, dual damage of the pneumotaxic center and vagi is also expected to result in inspiratory spasms that resemble breath-holding in the inspiratory phase of breathing (apneusis) as has been reported in animal studies,5 though the patients with COVID-19 have an increase in respiratory rate,3 which as stated above appears to be more a response to hypoxia from impulses originating from carotid and aortic bodies (Figure 1B yellow circle).
The damage caused by SARS-CoV-2 to the central chemoreceptor does not explain the loss of spontaneous breathing, as they do not generate the pacemaker impulses for autonomic breathing, although it can strongly affect the breathing rate and depth. Partial neurotoxicity of the neurons in the pre-BÖTC region (Figure 1A,B green circle), which normally generates pacemaker impulses for autonomic breathing appears to be the most likely explanation for the episodes of the feeling of loss of spontaneous respirations experienced by the COVID-19 patients. A complete or substantial loss of the neurons in the pre-BÖTC region understandably would induce a neurogenic acute respiratory arrest despite the presence of moderate hypoxia and hypocapnia, as has been reported in COVID-19 patients with involvement of the brainstem at the level of the medulla oblongata.9,20,23 The question is, why do some patients survive, while others complicate to suffer acute respiratory failure and death? This can be best explained based on the degree of the quantitative loss of neurons in the pre-BÖTC region (Figure 1B green circle) and the ability of the neurons to compensate for a partial loss in the patients who survive the episode of medullary neurotoxicity in COVID-19. Although further research is needed to establish the cause(s) of nonhypoxemic respiratory failure in COVID-19, a direct effect of SARS-CoV-2 on the phrenic nerve roots25 or its nuclei at cervical three to five (C3–C5) (Figure 1B three oval dots below the medulla oblongata), paralysis of the diaphragm and damage of spinal motor neurons below C5 segments or a combination of these,25 can contribute to the syndromic respiratory failures seen in COVID-19.
E. Central Neurogenic Respiratory Failure and COVID-19
The concept of central neurogenic respiratory failure and its contribution to the fatalities reported in the last six months and the mortalities that are being reported daily in COVID-19 is yet to be understood completely. The involvement of brainstem by SARS-CoV-29,20 and phrenic and ventral nerve rootlets25 to the respiratory muscles are clear pieces of evidence toward neurogenic and neuromuscular damages that occur in COVID-19. It is important to mention here that in a full-blown case of COVID-19 distinguishing hypoxemic respiratory failure from exclusive respiratory arrests due damage to the brainstem pre-BÖTC and phrenic nerve nuclear group at C3–C5 is difficult if not impossible. A respiratory arrest that cannot be explained by the extent of lung damage in early onset COVID-19 and abnormal breathing patterns should be thoroughly investigated clinically, as it has been hinted previously that neuroinvasiveness by SARS-CoV-2 can occur early during COVID-196,7,24 and can be missed. The shortcut routes (Figure 1A, upward-directed arrow) that SARS-CoV-2 can opt across the cribriform plate to reach the olfactory bulb,6,26 cerebrospinal fluid (CSF), and frontal lobe of the brain22 can explain the early spread of SARS-CoV-2 to the brainstem to affect the breathing regulating centers. With a rapidly rising mortality in patients with COVID-19 exhibiting extrapulmonary manifestations,27 there is an urgent need to understand and diagnose the neurological symptoms early in the course of this disease.10,28 Additionally, the poor awareness of hypoxemia in COVID-19 patients may also be attributed to a possible defective CNS processing of the respiratory signals.29
F. Conclusion and Future Research
COVID-19 has clearly shown that as compared to the taxonomically related SARS-CoV-1, the SARS-CoV-2 is a more deadly pathogen. The transmissibility and multiorgan attack potential are two aspects where SARS-CoV-2 has shown earlier versions of zoonotic coronaviruses to be miniature pathogens. There is a need to remain ahead of the curve with the strategies that SARS-CoV-2 is implementing to cause fatalities in humans. The attack on the CNS in general and the brainstem, in particular, is just emerging. Future research to contain the spread of SARS-CoV-2 to the CNS will expectedly prove to be a daunting challenge for the scientific community, but for obvious reasons it could prove to be lifesaving.
Author Contributions
A.M.B. conceived the concept of the respiratory arrests seen in COVID-19 after reports of its occurences in current pandemic caused by SARS-CoV-2. A.M.B. performed a literature search and computed the mechanisms underlying central regulation of respiration and related them to the case reports and published studies on respiratory dysregulations seen in COVID-19. A.M.B. wrote the first draft of the manuscript, responded to the reviewers, and finalized the draft for submission.
This study did not receive any funding.
The author declares no competing financial interest.
References
- Vancheri S. G.; Savietto G.; Ballati F.; Maggi A.; Canino C.; Bortolotto C.; Valentini A.; Dore R.; Stella G. M.; Corsico A. G.; Iotti G. A.; Mojoli F.; Perlini S.; Bruno R.; Preda L. (2020) Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms [published online ahead of print, 2020 May 30]. Eur. Radiol. 1–9. 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.; Han X.; Jiang N.; Cao Y.; Alwalid O.; Gu J.; Fan Y.; Zheng C. (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20 (4), 425–434. 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottestad W.; Seim M.; Mæhlen J. O. (2020) COVID-19 with silent hypoxemia. Covid-19 med stille hypoksemi. Tidsskr. Nor. Laegeforen. 140 (7), 1. 10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- Millán-Oñate J.; Millan W.; Mendoza L. A.; Sánchez C. G.; Fernandez-Suarez H.; Bonilla-Aldana D. K.; Rodríguez-Morales A. J. (2020) Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann. Clin. Microbiol. Antimicrob. 19 (1), 16. 10.1186/s12941-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. E., Barman S. M., Brooks H. L., and Yuan J. X.-J. (2019) Ganong’s Review of Medical Physiology, 26th ed., McGraw-Hill Education, New York. https://accessmedicine.mhmedical.com/book.aspx?bookid=2525. [Google Scholar]
- Baig A. M.; Khaleeq A.; Ali U.; Syeda H. (2020) Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 11 (7), 995–998. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Li Y. C.; Bai W. Z.; Hashikawa T. (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 92, 552. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Liu T.; Yang N.; Han D.; Mi X.; Li Y.; Liu K.; Vuylsteke A.; Xiang H.; Guo X. (2020) Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Frontiers of Medicine 1–9. 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganelli F.; Vargas M.; Iovino A.; Iacovazzo C.; Santoro L.; Servillo G. (2020) Brainstem involvement and respiratory failure in COVID-19 [published online ahead of print, 2020 May 29]. Neurol Sci. 1, 3. 10.1007/s10072-020-04487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G. C.; Spencer P. S.; Reis J.; Buguet A.; Faris M.; Katrak S. M.; Láinez M.; Medina M. T.; Meshram C.; Mizusawa H.; Öztürk S.; Wasay M. (2020) The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 414, 116884. 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J.; Kremer S.; Merdji H.; Clere-Jehl R.; Schenck M.; Kummerlen C.; Collange O.; Boulay C.; Fafi-Kremer S.; Ohana M.; Anheim M.; Meziani F. (2020) Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 382 (23), 2268–2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benameur K.; Agarwal A.; Auld S. C.; Butters M. P.; Webster A. S.; Ozturk T.; Howell J. C.; Bassit L. C.; Velasquez A.; Schinazi R. F.; Mullins M. E.; Hu W. T. (2020) Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA. Emerging Infect. Dis. 1. 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G. C.; Reis J.; Spencer P. S.; Buguet A.; Öztürk S.; Wasay M.; (2020) COVID-19 international neurological registries. Lancet Neurol. 19 (6), 484–485. 10.1016/S1474-4422(20)30148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T.; Chong J.-M.; Nakajima N.; Sano M.; Yamazaki J.; Miyamoto I.; Nishioka H.; Akita H.; Sato Y.; Kataoka M.; Katano H.; Tobiume M.; Sekizuka T.; Itokawa K.; Kuroda M.; Suzuki T. (2020) Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19. Emerging Infect. Dis. 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski M.; Aberegg S. K. (2020) Using applied lung physiology to understand COVID-19 patterns [published online ahead of print, 2020 May 27]. Br. J. Anaesth. 10.1016/j.bja.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren H. K.; Kjøstolfsen G. H.; Aaløkken T. M.; Latif N.; Brekke H.; Lind A.; Hesstvedt L. (2020) A man in his nineties with fever and dry cough. En mann i 90-årene med feber og tørrhoste. Tidsskr. Nor. Laegeforen. 140 (6), 1. 10.4045/tidsskr.20.0218. [DOI] [PubMed] [Google Scholar]
- Dallan C.; Romano F.; Siebert J.; Politi S.; Lacroix L.; Sahyoun C. (2020) Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health 4, e21. 10.1016/S2352-4642(20)30164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C.; Ellenberger H. H.; Ballanyi K.; Richter D. W.; Feldman J. L. (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254 (5032), 726–729. 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E. M.; Salman S.; Nurse C. A. (2018) Sensory Processing and Integration at the Carotid Body Tripartite Synapse: Neurotransmitter Functions and Effects of Chronic Hypoxia. Front. Physiol. 9, 225. 10.3389/fphys.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfamante G.; Chiumello D.; Canevini M. P.; Priori A.; Mazzanti M.; Centanni S.; Felisati G. (2020) First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 1. 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- Xie J.; Tong Z.; Guan X.; Du B.; Qiu H.; Slutsky A. S. (2020) Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 46 (5), 837–840. 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A.; Bryce C.; Grimes Z.; Gordon R. E.; Reidy J.; Lednicky J.; Sordillo E. M.; Fowkes M. (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 92 (7), 699–702. 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.; Zhang M.; Gao J.; Wang J. (2020) Sars-Cov-2: Underestimated damage to the nervous system [published online ahead of print, 2020 Mar 24]. Travel Med. Infect Dis. 101642. 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M. (2020) Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 26 (5), 499–501. 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn T.; Dabitz R.; von Wernitz-Keibel T.; Aufenanger J.; Nowak-Machen M.; Janssen H. (2020) Acute Polyradiculoneuritis with Locked-in Syndrome in a Patient with Covid-19. J. Neurol. 267, 1883. 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T.; Lolli V.; Sadeghi N.; Rovai A.; Trotta N.; Taccone F. S.; Creteur J.; Henrard S.; Goffard J.-C.; De Witte O.; Naeije G.; Goldman S.; De Tiege X. (2020) Early postmortem brain MRI findings in COVID-19 non-survivors medRxiv. Neurology 1. 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- Kalkeri R.; Goebel S.; Sharma G. D. (2020) SARS-CoV-2 Shedding from Asymptomatic Patients: Contribution of Potential Extrapulmonary Tissue Reservoirs [published online ahead of print, 2020 May 13]. Am. J. Trop. Med. Hyg. 10.4269/ajtmh.20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. U.; Hanif M.; Ali M. J.; Haider M. A.; Kherani D.; Memon G. M.; Karim A. H.; Sattar A. (2020) Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front. Neurol. 11, 518. 10.3389/fneur.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. H.; Zhang Y.; Gui Y.; Wu S.; Li Y. C. (2020) The possible impairment of respiratory-related neural loops may be associated with the silent pneumonia induced by SARS-CoV-2. J. Med. Virol. 1. 10.1002/jmv.26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clker-Free-Vector-Images (pixabay.com). Body Human Expiration Free Photo (Figure 2, Picture B) https://www.needpix.com/photo/32553/body-human-expiration-diagram-lungs-anatomical-medicine-education-anatomy [Accessed 23 May 2020].