Abstract
The small molecule ONC201 was identified in a screen for compounds that would induce expression of the gene encoding tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) in tumors and thus cause an autocrine- or paracrine-induced death in tumor cells. Two Research Articles in this issue of Science Signaling by Ishizawa et al. and Kline et al. describe how ONC201 can also trigger cytotoxicity by inducing a stress response. The mechanisms of the stress response induced differ between hematological malignancies and solid tumors, highlighting the complexity of ONC201-induced toxicity and raising intriguing issues of tissue-specific pathways activated by the drug.
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) is a member of the TNF family of ligands that can initiate apoptosis through engagement of its death receptors (DR), DR4 and DR5 (1–3). TRAIL selectively induces apoptosis of various tumor cells and transformed cells, but not most normal cells, and therefore has garnered intense interest as a promising antitumor agent. Despite its robust killing effect in tumor cells in vitro and in animal models, TRAIL ligands and DR agonistic antibodies have shown limited efficacy in clinical trials (4–6).
To overcome the lack of activity of exogenous TRAIL ligands, Allen et al. screened a library of small molecules to identify those that induce the expression of the gene encoding TRAIL in tumor cells and thereby activate DRs through an autocrine or paracrine mechanism (Fig. 1) (7). This screen identified ONC201 [TRAIL-inducing compound 10 (TIC10), also known as NSC350625], which induces dual inhibition of the kinases AKT and MEK, resulting in dephosphorylation of the transcription factor FOXO3a. Dephosphorylated FOXO3a translocates from the cytoplasm into the nucleus, where it binds to the promoter of the gene encoding TRAIL to up-regulate its transcription. ONC201 induces TRAIL-mediated apoptosis in various cell lines and promotes tumor regression by inducing apoptosis in multiple xenograft tumor model systems of solid tumors, including colon, breast, and brain tumors. With additional preferable features such as stability, oral bioavailability, and penetration of the blood–brain barrier, ONC201 is being investigated as an antitumor therapeutic agent (8, 9) and is currently in early-phase clinical testing for various human malignancies.
Fig. 1. Structure and mechanism of action of ONC201.
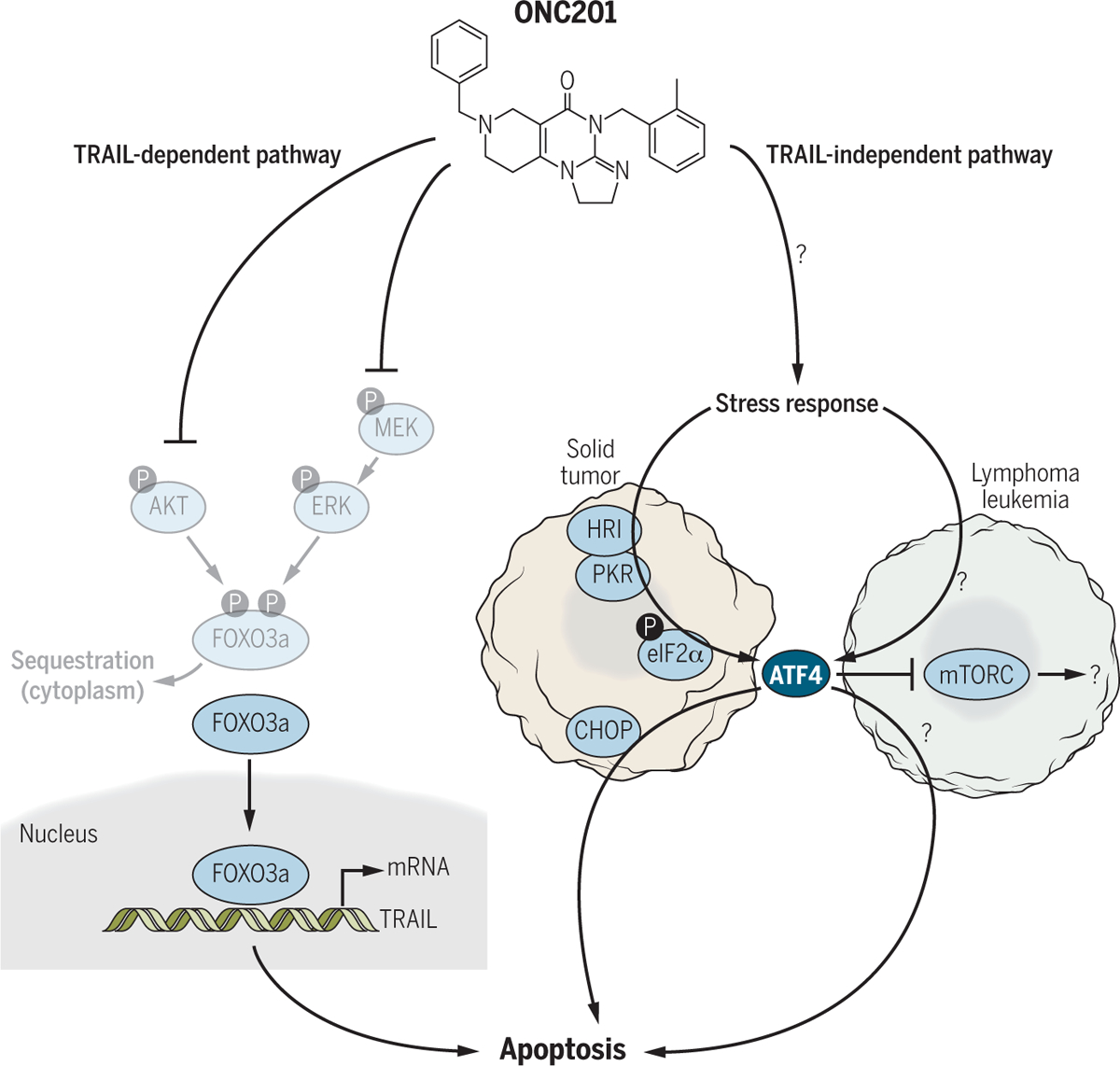
ONC201 induces apoptosis through TRAIL-dependent and TRAIL-independent pathways. In the TRAIL-dependent pathway, ONC201 inhibits AKT and MEK, resulting in the dephosphorylation and nuclear translocation of FOXO3a, which increases transcription of the gene encoding TRAIL. The cells then die by activation of the TRAIL DRs. In the TRAIL-independent pathway, ONC201 induces a stress response by an unknown mechanism. Although the mechanisms of the stress response that are activated appear to be different between solid tumors and hematological malignancies, ATF4 appears to be a common player in these tumor types.
In this issue, two groups report mechanisms for the antitumor effect of ONC201 that are independent of TRAIL transcription (Fig. 1). Ishizawa et al. found that ONC201 induced apoptosis in hematological malignancies (mantle cell lymphoma and acute myeloid leukemia), including those resistant to other targeted inhibitors (10). They found that the mechanism previously identified in solid tumors (induction of TRAIL-mediated death) was not operational in leukemia and lymphoma cells. The effect of ONC201 in these malignancies did not require caspase-8 activation or FOXO3a-dependent transcription of TRAIL. Gene expression profiling analysis revealed that ONC201 induced endoplasmic reticulum (ER) stress–related or integrated stress response (ISR)–related genes [specifically, activating transcription factor 4 (ATF4), C/EBP-homologous protein (CHOP), growth arrest and DNA damage–inducible protein (GADD34), and Tribbles homolog 3 (TRIB3)]. During ER stress, the ER is functionally disrupted by the accumulation of misfolded or unfolded proteins. ER stress is caused by depleted Ca2+ concentrations, oxidative stress, hypoxia, or glucose deprivation, conditions often encountered in pathological conditions such as malignancies, neurodegenerative diseases, and viral infections. To overcome these perturbations and restore ER homeostasis, ER stress activates a signaling network called the unfolded protein response (UPR). Under overwhelming ER stress, however, the UPR promotes apoptosis (11, 12). The ISR is a similar cytoprotective mechanism that maintains cellular proteostasis and is induced by ER stress, amino acid starvation, hypoxia or ischemia, oxygen and glucose deprivation, or viral infection (13, 14). Therefore, Ishizawa et al. tested whether ONC201 induced either the UPR or the ISR. In the classic ER stress signaling pathway, the eukaryotic translation initiation factor 2 alpha (eIF2a)–ATF4-CHOP signaling pathway plays a major role (11, 15). However, Ishizawa et al. found that ONC201-induced ATF4 up-regulation was independent of eIF2a phosphorylation; instead, it was induced through a posttranscriptional mechanism in hematological malignancies. Because eIF2a phosphorylation was not involved with the anticancer effect of ONC201, Ishizawa et al. proposed calling this mechanism an atypical ISR. They also found that ONC201-induced apoptosis was mediated by, at least in part, ATF4, but not CHOP. They further observed that ONC201-induced ATF4 resulted in the inhibition of mammalian target of rapamycin complex (mTORC).
The companion report by Kline et al. characterized the early events (18 to 48 hours after exposure to ONC201) that precede the inactivation of AKT and ERK and subsequent up-regulation of TRAIL expression in various solid tumor cancer cell lines (16). They performed gene expression profiling in two colon cancer cell lines and found that the genes encoding ATF4 and CHOP and a subset of genes with binding sites for ATF4 and CHOP were up-regulated by ONC201. ATF4 and CHOP abundance was also increased in xenografts from mice treated with ONC201. Both ATF4 and CHOP played critical roles in ONC201’s mechanism of cytotoxicity in these solid tumors, contrary to the observations of Ishizawa et al., in which only ATF4 mediated ONC201-induced apoptosis in hematological malignancies. The two studies also revealed different mechanisms of ATF4 up-regulation. In contrast to Ishizawa et al., Kline et al. found that HRI (heme-regulated inhibitor kinase) or PKR (double-stranded RNA-activated protein kinase) phosphorylated eIF2a, leading to up-regulation of ATF4 and CHOP.
Both studies showed that the anticancer effects of ONC201 are mediated by the induction of stress-related genes. Although the upstream mechanism of precisely how ONC201 induced ATF4 was different in these two studies, ATF4 appeared to be a common driver of ONC201-induced apoptosis in solid and hematologic malignancies. Thus, ATF4 could be used as a pharmacodynamic marker of ONC201 efficacy. It is noteworthy that the anticancer effect of ONC201 was independent of AKT/ERK/FOXO3a transcriptional activation in the hematological malignancies, suggesting that the mechanism of action of ONC201 is tissue- or cancer type–specific.
Further studies are needed to identify the direct cellular targets of ONC201; to characterize the mechanism by which ONC201 inhibits AKT and ERK signaling and induces stress response–related genes, the mechanism by which ATF4 induces cell death; and to determine the implication of ONC201-induced down-regulation of mTORC1. It will also be important to determine the mechanisms of ONC201-mediated toxicity that are active in different cell types and what regulates these differences. These questions must be answered for effective clinical development of this drug. Nonetheless, ONC201 shows promise as a new drug for cancer therapy.
Acknowledgments:
Research in the Lipkowitz laboratory is supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Rahman M, Pumphrey JG, Lipkowitz S, The TRAIL to targeted therapy of breast cancer. Adv. Cancer Res 103, 43–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone RW, Frew AJ, Smyth MJ, The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 8, 782–798 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Salvesen G, Regulated cell death: Signaling and mechanisms. Annu. Rev. Cell Dev. Biol 30, 337–356 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Invest 125, 487–489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hollander MW, Gietema JA, de Jong S,Walenkamp AM, Reyners AK, Oldenhuis CN,de Vries EG, Translating TRAIL-receptor targeting agents to the clinic. Cancer Lett. 332, 194–201 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Abdulghani J, El-Deiry WS, TRAIL receptor signaling and therapeutics. Expert Opin. Ther. Targets 14, 1091–1108 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Allen JE, Krigsfeld G, Mayes PA, Patel L,Dicker DT, Patel AS, Dolloff NG, Messaris E,Scata KA, Wang W, Zhou JY, Wu GS,El-Deiry WS, Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med 5, 171ra17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JE, Krigsfeld G, Patel L, Mayes PA,Dicker DT, Wu GS, El-Deiry WS, Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol. Cancer 14, 99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talekar MK, Allen JE, Dicker DT, El-Deiry WS, ONC201 induces cell death in pediatric non-Hodgkin’s lymphoma cells. Cell Cycle 14, 2422–2428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizawa J, Kojima K, Chachad D, Ruvolo P,Ruvolo V, Jacamo RO, Borthakur G, Mu H,Zeng Z, Tabe Y, Allen JE, Wang Z, Ma W,Lee HC, Orlowski R, Sarbassov DD, Lorenzi PL,Huang X, Neelapu SS, McDonnell T, Miranda RN,Wang M, Kantarjian H, Konopleva M, Davis RE,Andreeff M, ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal 9, ra17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim I, Xu W, Reed JC, Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov 7, 1013–1030 (2008). doi: [DOI] [PubMed] [Google Scholar]
- 12.Tameire F, Verginadis II II, Koumenis C, Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy. Semin. Cancer Biol 33, 3–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly N, Gorman AM, Gupta S, Samali A, The eIF2a kinases: Their structures and functions. Cell. Mol. Life Sci 70, 3493–3511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD,Calfon M, Sadri N, Yun C, Popko B, Paules R,Stojdl DF, Bell JC, Hettmann T, Leiden JM,Ron D, An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Oyadomari S, Mori M, Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Kline CLB, Van den Heuvel APJ, Allen JE,Prabhu VV, Dicker DT, El-Deiry WS, ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2a kinases. Sci. Signal 9, ra18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


