Abstract
Upper airway patency to airflow and the occurrence of obstructive sleep apnea involve a complex interplay between pharyngeal anatomy and synergic co-activation of peri-pharyngeal muscles. In previous studies we observed large differences in the response to sleep-associated flow limitation between the genioglossus and other (non-GG) peri-pharyngeal muscles. We hypothesized that similar differences are present also during wakefulness. In the present study we compared the response to inspiratory loading of the genioglossus electromyogram and four other peri-pharyngeal muscles. Studies were performed in eight obstructive sleep apnea patients, seven age-matched healthy subjects and five additional younger subjects. Electromyogram activity was evaluated over a range of negative oesophageal pressures and expressed as % of maximal electromyograms. In healthy subjects, the slope response to inspiratory loading (electromyogram/pressures) was similar for the genioglossus and non-GG muscles studied. However, the electromyogram responses were significantly higher in the young subjects compared with older subjects. In contrast, in the obstructive sleep apnea patients, the electromyogram/pressure response of the non-GG muscles was similar to that of the age-matched healthy subjects, whereas the slope response of the genioglossus electromyogram was significantly higher than non-GG muscles. We conclude that both age and the presence of obstructive sleep apnea affect the response of peri-pharyngeal muscles to inspiratory loading. In patients with obstructive sleep apnea the genioglossus seems to compensate for mechanical disadvantages, but non-GG muscles apparently are not included in this neuromuscular compensatory mechanism. Our current and previous findings suggest that attempts to improve obstructive sleep apnea with myofunctional therapy should put added emphasis on the training of non-GG muscles.
Keywords: genioglossus, geniohyoid, pharyngeal dilator muscles, styloglossus, upper airway muscles
1 |. INTRODUCTION
The human pharyngeal airway is surrounded by highly compliant soft tissues. Therefore, active forces of pharyngeal dilator muscles are required to maintain patency to airflow during inspiration (Remmers, deGroot, Sauerland, & Anch, 1978). During wakefulness, reflex activation of upper airway dilator muscles prevents pharyngeal collapse (Malhotra, Fogel, Edwards, Shea, & White, 2000). During sleep, this protective mechanism is disrupted, leading to airway obstruction (White, 2005). Reduction in dilator muscle activity with the onset of sleep (Eckert, White, Jordan, Malhotra, & Wellman, 2013; Fogel et al., 2005; Sauerland & Harper, 1976) and diminution of the reflex response of the genioglossus (GG) to negative pressure and chemical drive (Eckert, McEvoy, George, Thomson, & Catcheside, 2007; Pillar et al., 2000; Stanchina et al., 2002) during sleep has been well documented. It is suggested that pharyngeal collapsibility during sleep and the resulting obstructive sleep apnea (OSA) syndrome is a result of sleep-associated decrease in muscle tone, primarily that of the GG, the main tongue protrusor.
However, it is well documented that the GG electromyogram tends to increase gradually during hypopneas and apneas, but this enhanced activity usually fails to restore pharyngeal patency before arousal (Berry, McNellis, Kouchi, & Light, 1997; Dotan, Pillar, Schwartz, & Oliven, 2015; Jordan et al., 2007; McGinley et al., 2008; Patil et al., 2007; Remmers et al., 1978; Younes et al., 2012). Applying suitable methodology, we have recently demonstrated that towards the end of prolonged flow limitation, before arousal, GG activity may increase to levels more than twice than that observed while awake, without alleviating flow limitation and restoring unobstructed breathing (Dotan et al., 2013; Oliven et al., 2018). In contrast, the activity of other peri-pharyngeal muscles remained well below their level observed during wakefulness. We suggested that alteration in co-activation during sleep of peri-pharyngeal muscles is likely to play a central role in the failure of muscular mechanisms to maintain pharyngeal patency during sleep in anatomically predis-posed subjects. Based on these findings, the functional mechanism leading to OSA is not only the delayed and often insufficient increase in the activity of the main tongue protrusor, the GG. Rather, inadequate recruitment of the other “accessory” skeletal muscles that need to act in concert with the GG to prevent pharyngeal collapse, may be equally important.
Although multiple studies have evaluated the pattern of control of the GG during wakefulness and sleep, knowledge about control of the activity of other dilator muscles in humans is rather scarce. Considering their central role in maintaining pharyngeal patency, and the finding that GG activity does not necessarily represent the activity of other dilator muscles, we compared in the present study the response of peri-pharyngeal muscles to inspiratory loads with that of the GG, in patients with OSA and healthy subjects.
2 |. METHODS
2.1 |. Subjects
Eight patients with OSA and 12 healthy subjects, all diagnosed in a full-night sleep study in the Technion Sleep Laboratory, were recruited for this study. Patients with any disease that could prevent the insertion of intramuscular electrodes and an oesophageal pressure transducer, including nasal polyps or choanal obstruction for other causes, any tonsillar or pharyngeal disease, hypersensitivity to local anaesthesia, or treatment with anticoagulants, were excluded. All studies were performed in the respiratory research laboratory of the Bnai-Zion Medical Centre. The aims and potential risks of the study were explained, and informed consent was obtained from all subjects. The study was approved by the institutional Human Investigations Review Board. Other data from these subjects have previously been published (Oliven et al., 2018).
2.2 |. Electromyogram
An electromyogram (EMG) was recorded via pairs of Teflon-coated hook-wire electrodes after submucosal or subdermal injection of0.5 ml lidocaine 2%, as previously described (Dotan et al., 2015; Oliven et al., 2018). The GG and two additional muscles were studied in each subject. Electrodes were inserted: into the anterior body of the GG, near its insertion into the mandible; into the styloglossus (SG), at the left rim of the tongue, as posteriorly as possible; into the geniohyoid (GH), midway between the chin and the hyoid bone, 2–3 cm under the skin; into the sterno-cleido-mastoid (SCM), 3–4 cm above its insertion into the sternum; and into the sternohyoid (SH), 2 cm above its insertion into the sternum. All electrodes were positioned under direct vision. Electrodes were inserted into the GH, SCM and SH during volitional muscle contraction and based on ultrasound evaluation in preliminary studies. Adequate position of all electrodes was verified by short bursts of 40 Hz electrical stimulation (Dynex III, Medtronic Inc, Minneapolis, MN, USA), applied via the inserted electrodes, demonstrating tongue protrusion (GG) and retraction (SG) or visual and palpable muscle contraction (other muscles) during stimulation. EMG signals were amplified using P122 amplifiers (Grass Technologies, Warwick, RI, USA). Raw EMG signals were filtered (30–1000 Hz), rectified and processed with leaky integrators with a time constant of 100 ms to yield a moving-time-averaged EMG envelop. All EMG signals were expressed as % of the maximal value obtained for each muscle (see below).
2.3 |. Recording procedures
Subjects breathed through a pneumotachometer connected to a Validyne ±2 cmH2O pressure transducer. Mouth pressure (Pm) was monitored with a catheter connected to a side port of a mouthpiece. Intrathoracic pressure was measured with a Millar catheter (Millar Inc. Houston, TX, USA), inserted through the nose and positioned in the oesophagus (Pes). Analogue to digital acquisition of all parameters was performed at 1000 Hz for monitoring and data storage on a digital polygraphic data acquisition system (LabVIEW, National Instruments, Austin TX, USA).
2.4 |. Experimental procedure
About 30 min after electrode insertion, after the effect of local anaesthesia waned, subjects were seated at a designated short tubing system, an external nose seal was placed on them and the subjects breathed through a mouthpiece connected to the pneumotachometer. A distal valve separated the inspiratory from the expiratory tubing, and a small inflatable balloon placed in the inspiratory tube was used to create variable inspiratory resistance. Inspiratory resistance was increased intermittently. Subjects were asked to perform a wide range of inspiratory efforts, while observing the pressure tracings on a screen, and attempted to perform larger and smaller negative pressures, in random order, as instructed. Each time, subjects performed a single inspiratory effort, lasting for about 2 s during each partial obstruction of the inspiratory port, and were instructed to maintain a constant negative pressure during this time. The balloon was deflated after each inspiratory effort, and the subjects could breathe normally for three to six breaths until the next inspiratory partial obstruction. Stronger inspiratory efforts during obstructions were associated with larger (negative) pressure levels, reaching down to Pm and Pes of −40 to −50 cmH2O. Multiple manoeuvres were performed, with 5–10 cmH2O pressure differences between manoeuvres. Each pressure level was recorded two to four times, whereas EMGs, Pm, Pes and airflow were recorded continuously.
Thereafter, the nose seal and mouthpiece were removed, and maximal EMG levels (EMGmax) were obtained as previously described (Dotan et al., 2015; Oliven et al., 2018). After adequate explanations and preliminary trials, subjects were instructed to perform maximal tongue-thrust against the front teeth, maximal tongue protrusion and maximal tongue thrust against the cheek manoeuvres, to obtain EMGmax for the GG and the SG. Jaw opening and head flexion against resistance were used to obtain EMGmax values for the GH and neck muscles. Each manoeuvre was repeated three to four times, and for each subject and muscle the highest EMG recorded in one of these manoeuvres was considered EMGmax.
2.5 |. Data analysis
Peak EMG and peak (negative) Pes, obtained during resistive breathing, were used to construct individual EMG:Pes plots for every subject and every muscle. These EMG:Pes slopes, as well as the derived level of each EMG at a Pes of −25 cmH2O (the middle of the Pes range of −10 to −40 cmH2O obtained for all subjects, EMGp-25), were expressed as % of EMGmax. These parameters were used to compare the respiratory responses of the different muscles and subject groups to inspiratory loading.
All data are presented as mean ± SD; the t-test (unpaired) and Mann–Whitney test were used to compare group data with and without normal distribution; p < 0.05 was considered as statistically significant.
3 |. RESULTS
The anthropometric and sleep laboratory data of the subjects that participated in this study are given in Table 1. The OSA patients were eight males with severe OSA (AHI >45). The healthy subjects were five young men (age 20–23 years) without OSA (AHI <5) and seven men with AHI <10 (considered to be free of noteworthy OSA) who had an age range similar to that of the OSA patients.
TABLE 1.
Anthropometric and sleep data of the study subjects. AHI and SO2 data were obtained from conventional sleep studies performed before the current study
| Healthy | |||
|---|---|---|---|
| OSA (n = 8) | Older (n = 7) | Young (n = 5) | |
| AHI | 58.4 ± 9.4 | 7.6 ± 2.0*** | 3.8 ± 1.2*** |
| BMI | 33.5 ± 4.8 | 27.2 ± 3.5* | 23.6 ± 1.5** |
| Age | 53.6 ± 8.7 | 52.6 ± 10.0 | 21.2 ± 0.4*** |
| Lowest SO2 | 75.4 ± 8.5 | 90.4 ± 2.1*** | 93.8 ± 1.1** |
| %time SO2<90% | 18.9 ± 19.1 | 0.2 ± 0.5** | 0* |
Note. OSA, obstructive sleep apnea; AHI, apnea/hypopnea index; SO2, oxygen saturation; Pcrit, critical pressure.
p < 0.05,
p < 0.01,
p < 0.001 for comparison with OSA.
In each subject we recorded three EMGs. The GG-EMG, recorded near the insertion into the mandible, was obtained in all 20 subjects. In three of the subjects (two healthy and one OSA), only two EMGs were recorded. In another three subjects, one of the electrodes dislodged before EMGmax could be obtained. Therefore, in addition to the GG, 15 and 19 peri-pharyngeal muscle EMGs were available for analysis in the OSA and the healthy subjects, respectively (Table 2).
TABLE 2.
Peri-pharyngeal muscles evaluated in the obstructive sleep apnea (OSA) and healthy young and older subjects
| Healthy | |||
|---|---|---|---|
| OSA n = 8 | Young n = 5 | Older n = 7 | |
| Genioglossus | 8 | 5 | 7 |
| Styloglossus | 2 | 4 | 1 |
| Geniohyoid | 5 | 2 | 3 |
| Sternohyoid | 4 | - | 4 |
| Sterno-cleido-mastoid | 4 | 3 | 2 |
Table 3 presents averaged Pes and EMG data obtained during quiet breathing. OSA patients produced significantly more negative Pes than both young and older healthy subjects. OSA patients had similar peak GG-EMG activity to that observed in young healthy subjects, and both had higher peak GG-EMG activity than older healthy subjects. In contrast, the activity of non-GG muscles in OSA patients was similar to the average activity observed in age-matched healthy subjects, and both had significantly lower activity than that found in young healthy subjects. Differences between GG and non-GG muscles were not statistically significant in either group during quiet resting breathing.
TABLE 3.
Average peak oesophageal deflections and electromyogram activity observed during quiet breathing in the healthy subjects and obstructive sleep apnea patients
| Healthy subjects | |||
|---|---|---|---|
| Young | Older | OSA | |
| Pes (cmH2O) | 7.6 ± 1.2* | 7.1 ± 1.2** | 12.2 ± 2.9 |
| EMG GG (%max) | 7.6 ± 2.7*** | 5.2 ± 1.9 | 6.9 ± 3.5*** |
| EMG non-GG (%max) | 9.6 ± 4.4 | 3.9 ± 3.3**** | 4.5 ± 1.6***** |
OSA: obstructive sleep apnea; Pes: oesophageal pressure; EMG: electromyogram; GG: genioglossus.
p < 0.05,
p < 0.01 for comparison of Pes of healthy subjects and OSA patients.
p < 0.05 for comparison of GG EMG of young healthy subjects and OSA patients with older healthy subjects.
p < 0.01,
p < 0.001 for comparison of non-GG EMG of older healthy subjects and OSA patients with young healthy subjects.
Breathing through increasing inspiratory resistance triggered increasing respiratory efforts, as recognized by progressively more negative Pm and Pes during inspiration (Figure 1). Pm and Pes decreased similarly during inspiration, indicating that during wakefulness the pharynx did not collapse even in the face of highly negative intra-luminal pressures (B). Increasing respiratory efforts (i.e. increasingly negative pressures) were associated with increasing inspiratory EMG activity (B) in all muscles studied. EMG activity and pressures changed linearly, enabling construction of a linear peak inspiratory EMG versus peak Pes plot (C) for each subject and each muscle evaluated. These slopes were calculated as percentage of EMGmax(A).
FIGURE 1.
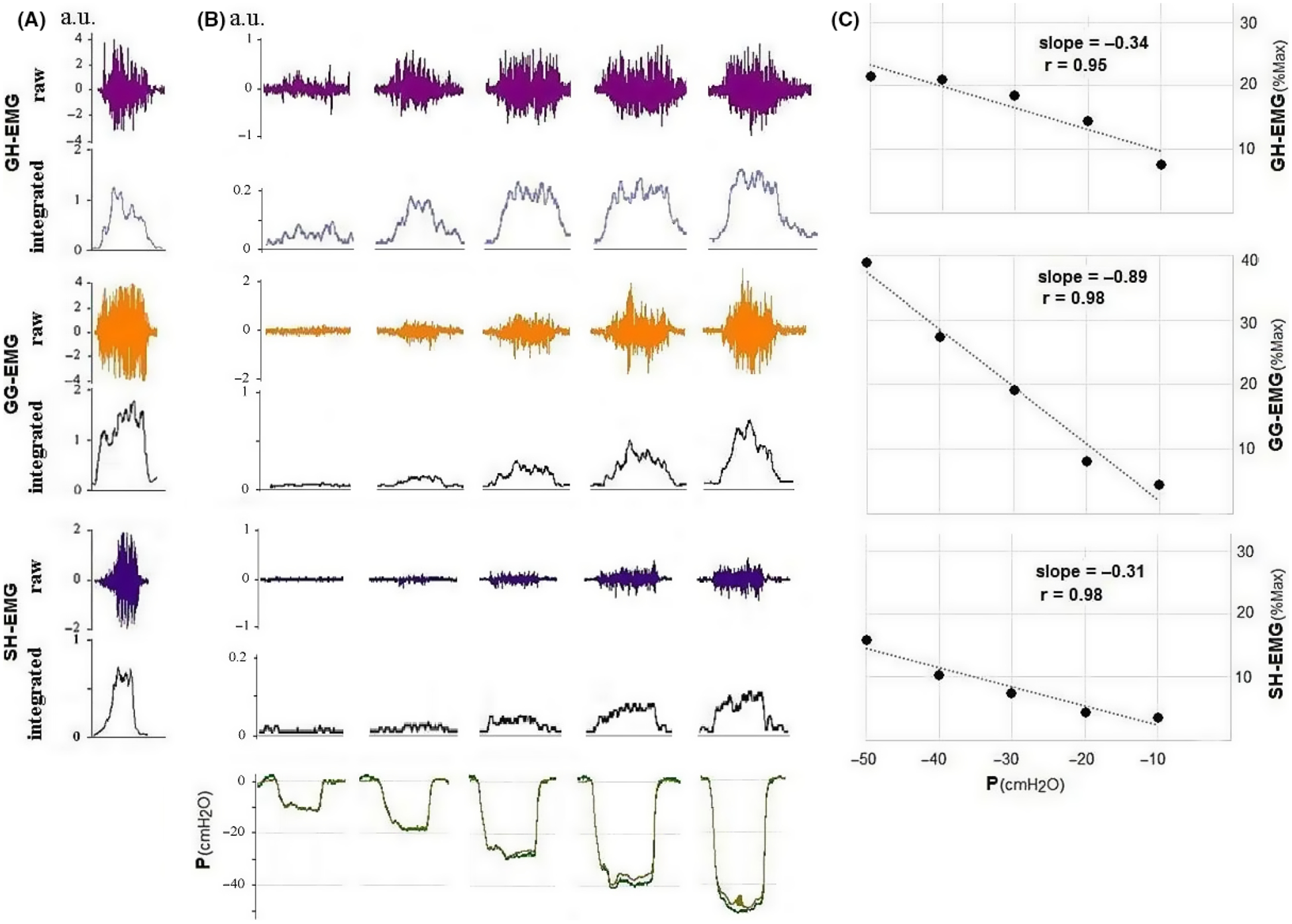
Tracings and slope (EMG/Pes) of one of the OSA patients. The slope of the GG-EMG versus Pes is substantially steeper than that of the two other muscles. EMG, electromyogram; Pes, oesophageal pressure; OSA, obstructive sleep apnea; GH, geniohyoid; GG, genioglossus; SH, sternohyoid; P, pressure; R, correlation coefficient; a.u., arbitrary units. Note the different y-scale of EMGmax.
The correlations of the EMG/Pes slopes ranged between 0.8 and 0.99, with mean r = 0.96 ± 0.04 and 0.94 ± 0.05 for GG and non-GG muscles, respectively. The average correlations for the four non-GG muscles were also similar (average range of r for the single muscles = 0.93–0.95). The slopes and EMGp-25 data obtained for the SG, GH, SCM and SH (non-GG muscles), calculated for all subjects (OSA and healthy) together, are presented in Table 4. Because the numbers of each muscle evaluated in each of the three groups (young healthy, older healthy and OSA) were insufficient to allow meaningful statistical evaluation, these muscles were grouped together, and the data for the GG were compared with those for non-GG muscles across the subject groups.
TABLE 4.
Slopes (EMG/Pes) and peak EMG activity at Pes −25 cmH2O of the peri-pharyngeal muscles evaluated (non-GG muscles) in addition to the GG
| Slope (EMG%max/cmH2O) | EMGP−25 (%max) | |
|---|---|---|
| SG (n = 7) | −0.73 ± 0.39 | 21.30 ± 8.83 |
| GH (n = 10) | −0.39 ± 0.31 | 12.46 ± 10.25 |
| SCM (n = 9) | −0.52 ± 0.22 | 17.12 ± 6.78 |
| SH (n = 8) | −0.48 ± 0.36 | 11.30 ± 6.8 |
| all non-GG (n = 34) | −0.51 ± 0.35 | 15.86 ± 11.59 |
Note. Mean ± SD data were calculated for all patients together (healthy + OSA). There was no significant difference between the parameters of the non-GG muscles.
EMG: electromyogram; Pes: oesophageal pressure; GG: genioglossus; SG: styloglossus; GH: geniohyoid; SCM: sterno-cleido-mastoid; SH: sternohyoid; OSA: obstructive sleep apnea. EMGP-25: peak EMG at Pes −25 cmH2O.
Comparing the muscular response of the GG and the other non-GG muscles, in young and older healthy subjects, it became apparent that the pattern of peri-pharyngeal muscle activation in the presence of obstruction to airflow is age dependent (Table 5): although each age group demonstrated similarities in the pattern of GG and non-GG activation, the younger subjects responded with significantly stronger recruitment of the muscles, resulting in significantly steeper slopes and higher EMGP-25 values than for the older healthy subjects. The difference between peak GG-EMG between young and older healthy subjects did not reach significance at Pes of −25 cmH2O, but the difference was significant at Pes = −40 cmH2O(38.9 ± 12.1 versus 22.3% ± 7.7%max for the young and older subjects, respectively, p < 0.05).
TABLE 5.
Comparison of the response of the GG and non-GG peri-pharyngeal muscles to external partial occlusion of the inspiratory airway in young and older healthy subjects
| Healthy subject | |||
|---|---|---|---|
| Young | Older | OSA | |
| EMGslope (EMG%max/cmH2O) | |||
| GG | −0.95 ± 0.37 | −0.47 ± 0.29* | −0.89 ± 0.48 |
| All non-GG | −0.87 ± 0.31 | −0.40 ± 0.24** | −0.37 ± 0.27*** |
| SG | −0.99 | −0.25 | −0.46 |
| GH | −0.92 | −0.40 | −0.18 |
| SCM | −0.67 | −0.32 | −0.49 |
| SH | — | −0.49 | −0.47 |
| GG | 22.7 ± 7.3 | 15.3 ± 6.4 | 19.1 ± 10.9 |
| All non-GG | 26.7 ± 14.0 | 12.0 ± 8.9* | 10.9 ± 0.27*** |
| EMGP−25 (%max) | |||
| SG | 26.1 | 16.9 | 27.7 |
| GH | 17.6 | 18.1 | 7.0 |
| SCM | 23.8 | 13.8 | 13.7 |
| SH | — | 9.1 | 11.5 |
GG: genioglossus; EMG: electromyogram; OSA: obstructive sleep apnea; SG: styloglossus; GH: geniohyoid; SCM: sterno-cleido-mastoid; SH: sternohyoid. EMGP-25: peak EMG at Pes −25 cmH2O.
p < 0.05,
p < 0.01 for comparison of young and older subjects.
p < 0.01 for comparison of young and OSA patients.
Figure 2 depicts the EMG to Pes relationship of the GG and non-GG muscles of the three groups (young healthy, older healthy, OSA). It can be seen that the OSA patients responded to external loading differently to both the other groups: they increased their GG slope similar to the young healthy subjects, resulting in an almost significant difference to the slope of the older subjects (p = 0.074); however, the non-GG muscles were recruited like those of the age-matched older subjects, resulting in a highly significant lower mean slope and EMGp-25 compared with the young healthy subjects (p < 0.001 and p < 0.005, respectively). Accordingly, OSA patients were the only group whose GG and non-GG muscles were activated differently in the face of external partial airway obstruction, resulting in a lower non-GG slope (−0.89 ± 0.48 versus −0.37 ± 0.20 EMG% max/cmH2O, respectively, p = 0.005) and EMGp-25 (19.1 ± 10.9 versus 10.9 ± 5.9 cmH2O, respectively, p < 0.05).
FIGURE 2.
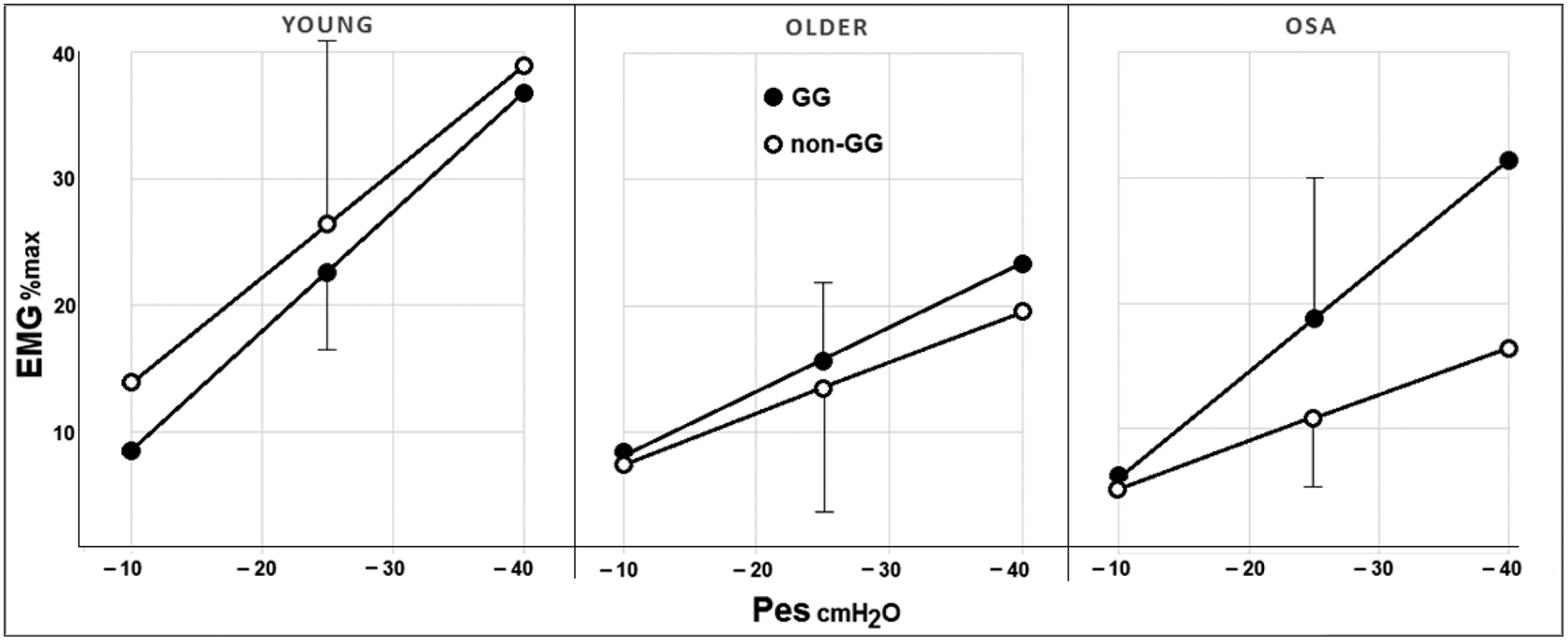
Comparison of mean slope of the GG and non-GG muscles’ response to increasingly negative Pes levels in healthy young and older subjects and in patients with OSA. EMG, electromyogram; GG, genioglossus; Pes, oesophageal pressure; OSA, obstructive sleep apnea
4 |. DISCUSSION
In this study, patients with OSA and healthy subjects performed a range of short inspiratory actions while breathing against high inspiratory resistance, and the EMG response of several peri-pharyngeal muscles during these manoeuvres was evaluated. We found that in OSA patients, the GG-EMG increased substantially more than other peri-pharyngeal muscles during increasing inspiratory actions. In healthy subjects, on the other hand, the response of the GG was similar to that of the other non-GG muscles. However, the response of all peri-pharyngeal muscles, including the tongue muscles, was significantly higher in younger than in older subjects.
Previous investigators found that GG-EMG during wakefulness is higher in patients with OSA than in healthy control subjects (Mezzanote, Tangel, & White, 1992), apparently representing a compensatory neuromuscular mechanism for maintaining patency in a more collapsible airway. GG-EMG was slightly (but statistically significantly) higher in OSA, as compared with age-matched healthy subjects, even during quiet breathing. This difference is not explained solely by the more negative Pes observed in OSA patients during resting breathing, as it was much more evident at higher levels of respiratory effort, when comparisons were performed at equal Pes. In addition, in OSA patients, the GG was activated substantially more than non-GG muscles at higher levels of respiratory efforts. Both the activity of the non-GG muscles during quite breathing and their recruitment during increasing loading were similar in OSA and age-matched healthy subjects and lower than in young healthy subjects, consistent with age-related rather than disease-related effects on the pattern of activation of these muscles.
Although activation of the GG was similar to that of the other peri-pharyngeal muscles in both age groups of the healthy subjects, the activities recorded in the young subjects were substantially and significantly higher than those of the older ones. Both muscle recruitment (slope) and the mid-range level of activity (EMGp-25) were higher in the young subjects during increased respiratory effort. This augmented response may be a consequence of the higher reflex response to negative pressures described in younger healthy subjects (Malhotra et al., 2006). Older subjects also exhibit a lower GG-EMG response to hypoxaemia (Tafil-Klawe & Klawe, 2003). One may speculate that, in addition to age-related mechanical changes (Malhotra et al., 2006), differences in the intensity of tongue and other peri-pharyngeal muscle activation during obstruction to airflow may contribute to the increased prevalence of OSA in older subjects.
The relationship between control of upper airway muscles during wakefulness and sleep is unclear. During wakefulness responses to negative intra-pharyngeal pressure are triggered by local mechano-reflexes, in addition to the central drive (Lo et al., 2007). During sleep, muscle tone decreases (Sauerland & harper, 1976) and the response to these reflexes is abolished or greatly reduced (Eckert et al., 2007; Stanchina et al., 2002). Despite these differences the mode of dilator muscle activation during wakefulness appears to be relevant to its recruitment during sleep, and therefore to sleep apnea. For example, patients with OSA have abnormal compensatory responses to inspiratory resistive loading when awake (Greenberg & Scharf, 1993; Rajagopal, Abbrecht, & Tellis, 1984). A similar response was observed in healthy offspring of OSA patients, suggesting that underlying abnormalities in neural control manifested during wakefulness may play a role in the aetiology of OSA (Pillar, Schnall, Peled, Oliven, & Lavie, 1997). Also, several studies have indicated that training of the tongue and other peri-pharyngeal muscles (myofunctional therapy), leading to conditioning and strengthening of these muscles, can ameliorate sleep apnea (Camacho et al., 2015; Guimarães, Drager, Genta, Marcondes, & Lorenzi-Filho, 2009). Accordingly, the pattern and magnitude of peri-pharyngeal muscle activation observed in this study during wakefulness may be relevant also to control mechanisms and, therefore, the tendency for airway collapse during sleep. We have found that flow limitation during sleep triggers marked increases in GG-EMG to levels substantially above those observed during wakefulness, whereas other peri-pharyngeal muscles showed only minimal increases, to levels well below those observed while awake (Dotan et al., 2015; Oliven et al., 2018). This difference in response during sleep is similar to the larger increase in GG-EMG (as compared with non-GG muscles) observed in the current study during obstructed breathing while awake.
Several limitations of this study should be considered in interpreting our findings. In attempting to encompass a wide range of peri-pharyngeal muscles, the number of single muscles studied in each group of subjects was relatively small, hindering statistical comparison for each single muscle. More than 20 peri-pharyngeal and neck muscles are probably involved in the stabilization of the pharynx against collapse, and evaluation of each of these muscles in various subject groups is not practical. As the GG is known to be the most effective pharyngeal dilator (Odeh et al. l1995), we considered all other muscles to act as accessory muscles, and grouped them together. However, it is the balance between upper airway anatomy and coordinated pharyngeal dilation that is key to pharyngeal stability and there are likely to be multiple strategies that may result in effective muscle coordination. The pattern of activation of various dilator muscles is likely to be heterogeneous, and it is possible that under certain circumstances, one or more peri-pharyngeal muscles may be activated similarly to the GG in OSA patients. Also, the route of breathing may affect the magnitude and/or pattern of muscle response to inspiratory obstruction: EMG:Pes measurements were performed with an external resistive load and a mouthpiece, and oral breathing has been shown to reduce the GG response to hypoxaemia (Tafil-Klawe & Klawe, 2003). However, the GG-EMG response to negative pressure in the upper airway is not affected by the route of application of the negative pressures (Doherty, Cullen, Nolan, & McNicholas, 2008).
Another potential limitation of the current study is that the OSA and age-matched healthy subjects were not weight matched. Obesity has been shown to have an indirect effect on GG endurance, as well as on its fiber composition (Carrera et al., 2004). To address this shortcoming we removed the two most heavy OSA patients (body mass index [BMI] about 40), leaving a group of six OSA patients with similar BMI to the seven healthy age-matched subjects. The difference between GG and non-GG muscles’ response to respiratory loading in this OSA group remained significant (p < 0.02). The difference in the GG-EMG/Pes slope compared with the age and BMI-matched healthy subjects was borderline significant (p = 0.054, compared with p = 0.074 when the two most heavy OSA patients were included). On the other hand, there is no reason to assume that the lower BMI of the young healthy subjects contributed to their more vigorous muscular response to ventilatory loading.
In conclusion, we compared the acute EMG response of peri-pharyngeal muscles to external respiratory loads in young and older healthy subjects and in patients with OSA. We found that young subjects increased their peri-pharyngeal muscle activity with increasing respiratory efforts significantly more than older subjects. The increase in activity of the GG was similar to that of other muscles in both groups. In contrast, OSA patients increased the activity of the GG significantly more than that of the other peri-pharyngeal muscles studied. These findings indicate that various peri-pharyngeal muscles respond differently to upper airway obstruction (i.e. the GG does not represent the response of all peri-pharyngeal muscles). In addition to the physiological relevance, the present findings, in association with our findings during sleep (Dotan et al., 2015; Oliven et al., 2018), may also have clinical relevance. It appears that patients with OSA recruit the GG activity adequately, but fail to activate sufficiently their “accessory” non-GG dilator muscles. Obviously, intense contraction of the GG elicited by electrical stimulation can improve, at least partially, pharyngeal patency (Dotan et al., 2011). However, our current findings suggests that, in addition to improving GG muscle function, more emphasis should be given to training of non-GG peri-pharyngeal muscles, with the aim of improving co-activation of dilator muscles and contributing to pharyngeal stability during sleep. Accordingly, when considering myofunctional therapy for obstructive sleep apnea, therapeutic strategies should be considered to also activate and improve the function of non-GG muscles.
ACKNOWLEDGEMENT
This work was supported by United States-Israel Binational Science Foundation grant 2011491.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- Berry RB, McNellis MI, Kouchi K, & Light RW (1997). Upper airway anesthesia reduces phasic genioglossus activity during sleep apnea. American Journal of Respiratory and Critical Care Medicine, 156, 127–132. 10.1164/ajrccm.156.1.9608037 [DOI] [PubMed] [Google Scholar]
- Camacho M, Certal V, Abdullatif J, Zaghi S, Ruoff CM, Capasso R, & Kushida CA (2015). Myofunctional Therapy to Treat Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Sleep, 1(38), 669–675. 10.5665/sleep.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera M, Barbé F, Sauleda J, Tomás M, Gómez C, Santos C, & Agustí AG (2004). Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. European Respiratory Journal, 23, 425–429. 10.1183/09031936.04.00099404 [DOI] [PubMed] [Google Scholar]
- Doherty LS, Cullen JP, Nolan P, & McNicholas WT (2008). The human genioglossus response to negative airway pressure: stimulus timing and route of delivery. Experimental Physiology, 3, 288–295. 10.1113/expphysiol.2007.039677 [DOI] [PubMed] [Google Scholar]
- Dotan Y, Golibroda T, Oliven R, Netzer A, Gaitini L, Toubi A, & Oliven A (2011). Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. European Respiratory Journal, 38, 338–347. 10.1183/09031936.00125810 [DOI] [PubMed] [Google Scholar]
- Dotan Y, Pillar G, Schwartz AR, & Oliven A (2015). Asynchrony of lingual muscle recruitment during sleep in obstructive sleep apnea. Journal of Applied Physiology, 118, 1516–1524. 10.1152/japplphysiol.00937.2014 [DOI] [PubMed] [Google Scholar]
- Dotan Y, Pillar G, Tov N, Oliven R, Steinfeld U, Gaitini L, … Oliven A (2013). Dissociation of EMG and mechanical response in sleep apnoea during propofol anaesthesia. European Respiratory Journal, 41, 74–84. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, McEvoy RD, George KE, Thomson KJ, & Catcheside PG (2007). Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. Journal of Physiology, 581(Pt 3), 1193–1205. 10.1113/jphysiol.2007.132332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A, & Wellman A (2013). Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. American Journal of Respiratory and Critical Care Medicine, 188, 996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, & Schory K, …, Pierce RJ (2005). The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. Journal of Physiology, 564(Pt 2), 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HE, & Scharf SM (1993). Depressed ventilatory load compensation in sleep apnea. Reversal by nasal CPAP. American Review of Respiratory Disease, 148, 1610–1615. 10.1164/ajrccm/148.6_Pt_1.1610 [DOI] [PubMed] [Google Scholar]
- Guimarães KC, Drager LF, Genta PR, Marcondes BF, & Lorenzi-Filho G (2009). Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. American Journal of Respiratory and Critical Care Medicine, 179, 962–966. 10.1164/rccm.200806-981OC [DOI] [PubMed] [Google Scholar]
- Jordan AS, Wellman A, Heinzer RC, Lo Y. l., Schory K, Dover L, …, White DP (2007). Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax, 62, 861–867. 10.1136/thx.2006.070300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eiker-mann M, … White DP (2007). Influence of wakefulness on pharyngeal airway muscle activity. Thorax, 62, 799–805. 10.1136/thx.2006.072488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA, & White DP (2000). Local mechanisms drive genioglossus activation in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 161, 1746–1749. 10.1164/ajrccm.161.5.9907109 [DOI] [PubMed] [Google Scholar]
- Malhotra A, Huang Y, Fogel RB, Lazic S, Pillar G, Jakab M, … White DP (2006). Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. American Journal of Medicine, 119(72), e9–e72. 10.1016/j.amjmed.2005.01.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, & Patil SP (2008). Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. Journal of Applied Physiology, 105, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanote WS, Tangel DJ, & White DP (1992). Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms). J. Clin. Invest, 89, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeh M, Schnall R, Gavriely N, & Oliven A (1995). Dependency of upper airway patency on head position: the effect of muscle contraction. Respiration Physiology, l00, 239–244. [DOI] [PubMed] [Google Scholar]
- Oliven R, Cohen G, Dotan Y, Somri M, Schwartz AR, & Oliven A (2018). Alteration in upper airway dilator muscle co-activation during sleep: comparison of patients with OSA and healthy subjects. Journal of Applied Physiology, 124, 421–429. [DOI] [PubMed] [Google Scholar]
- Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, & Smith PL (2007). Neuromechanical control of upper airway patency during sleep. Journal of Applied Physiology, 102, 547–556. 10.1152/japplphysiol.00282.2006 [DOI] [PubMed] [Google Scholar]
- Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, & White DP (2000). Upper airway muscle responsiveness to rising PCO2 during NREM sleep. Journal of Applied Physiology, 89, 1275–1282. [DOI] [PubMed] [Google Scholar]
- Pillar G, Schnall RP, Peled N, Oliven A, & Lavie P (1997). Impaired respiratory response to resistive loading during sleep in healthy offspring of patients with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 155, 1602–1608. 10.1164/ajrccm.155.5.9154864 [DOI] [PubMed] [Google Scholar]
- Rajagopal KR, Abbrecht PH, & Tellis CJ (1984). Control of breathing in obstructive sleep apnea. Chest, 85, 174–180. 10.1378/chest.85.2.174 [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, & Anch AM (1978). Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology, 44, 931–938. 10.1152/jappl.1978.44.6.931 [DOI] [PubMed] [Google Scholar]
- Sauerland EK, & Harper RM (1976). The human tongue during sleep. Electromyographic activity of the genioglossus muscle. Experimental Neurology, 51, 160–170. 10.1016/0014-4886(76)90061-3 [DOI] [PubMed] [Google Scholar]
- Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, & White DP (2002). Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. American Journal of Respiratory and Critical Care Medicine, 165, 945–949. 10.1164/ajrccm.165.7.2108076 [DOI] [PubMed] [Google Scholar]
- Tafil-Klawe M, & Klawe JJ (2003). Role of nose breathing in genioglossus muscle response to hypoxia in older and younger subjects. Journal of Physiology and Pharmacology, 54(Suppl 1), 48–54. [PubMed] [Google Scholar]
- White DP (2005). Pathogenesis of obstructive and central sleep apnea. American Journal of Respiratory and Critical Care Medicine, 172, 1363–1370. 10.1164/rccm.200412-1631SO [DOI] [PubMed] [Google Scholar]
- Younes M, Loewen AH, Ostrowski M, Laprairie J, Maturino F, & Hanly PJ (2012). Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. Journal of Applied Physiology, 112, 249–258. 10.1152/japplphysiol.00312.2011 [DOI] [PubMed] [Google Scholar]


