Abstract
Background:
The inferior frontal lobe is an important area of the brain involved in the stress response, and higher activation with acute mental stress may indicate a more severe stress reaction. However, it is unclear if activation of this region with stress correlates with angina in individuals with CAD.
Methods:
Individuals with stable CAD underwent acute mental stress testing using a series of standardized speech/arithmetic stressors in conjunction with high resolution positron emission tomography imaging of the brain. Blood flow to the inferior frontal lobe was evaluated as a ratio compared to whole brain flow for each scan. Angina was assessed with the Seattle Angina Questionnaire’s angina-frequency subscale at baseline and 2 years follow-up.
Results:
We analyzed 148 individuals with CAD (mean age (SD) 62 (8) years; 69% male, and 35.8% African American). For every doubling in the inferior frontal lobe activation, angina frequency was increased by 13.7 units at baseline ( 13.7, 95% CI 6.3, 21.7, p=0.008) and 11.6 units during follow up ( 11.6, 95% CI 4.1, 19.2, p=0.01) in a model adjusted for baseline demographics. Mental stress-induced ischemia and activation of other brain pain processing regions (thalamus, insula and amygdala) accounted for 40.0% and 13.1% of the total effect of inferior frontal lobe activation on angina severity, respectively.
Conclusion:
Inferior frontal lobe activation with mental stress is independently associated with angina at baseline and during follow-up. Mental stress-induced ischemia and other pain processing brain regions may play a contributory role.
Keywords: angina pectoris, brain imaging, stress
Journal subject terms: Nuclear Cardiology and PET, Chronic Ischemic Heart Disease
Introduction
Angina pectoris is the hallmark symptom of coronary artery disease (CAD) and an important determinant of adverse outcomes, quality of life and healthcare costs in CAD.1, 2 Between 10% and 30% of patients with CAD remain symptomatic while receiving optimal medical therapy.3 Also, one third of patients who undergo coronary revascularization for stable CAD continue to experience angina symptoms.4-6 Therefore, despite optimal pharmacological and revascularization therapies, angina remains an important cause of disability and impaired quality of life in many individuals.
Recent evidence suggests psychological factors are stronger determinants of angina than the burden of CAD.7-11 In addition, the self-reported frequency of angina is related to the degree of myocardial perfusion defects induced by acute mental stress, but not by exercise stress testing.8 However, the central neurological mechanisms for the association between reported symptoms of angina and mental stress are unknown. Given the poor correlation between angina symptoms and extent of CAD, it is important to explore an alternative paradigm for angina as a neurocardiac phenomenon that originates in the brain, which is more in line with other pain syndromes.12
The inferior frontal lobe is an area of the brain known to be involved in emotional regulation and stress.13, 14 In a meta-analysis of brain imaging studies in humans, this region was consistently activated with psychological stressors.15 We have recently shown that CAD patients with mental stress-induced myocardial ischemia exhibit increased inferior frontal lobe perfusion in response to stress.16 Since the inferior frontal lobe is a key brain region involved in stress reactivity, study of its activation with an acute mental stress challenge may help to unmask functional abnormalities. Potential mechanisms include activation of central neurologic pain pathways and cardiac ischemic pathways (Figure 1). Therefore, we aimed to investigate the relationship between activation of this region with mental stress and angina in individuals with known CAD, as well as potential neurologic and ischemic mechanisms. We hypothesized that greater activation in the inferior frontal lobe during mental stress will be associated with angina, and in part mediated by myocardial ischemia and activation of pain perception pathways in the brain.
Figure 1. Central illustration showing the relationship between mental stress-induced activation of the inferior frontal lobe and self-reported anginal symptoms through the mental stress-induced ischemia and neurological pain receptor pathways.
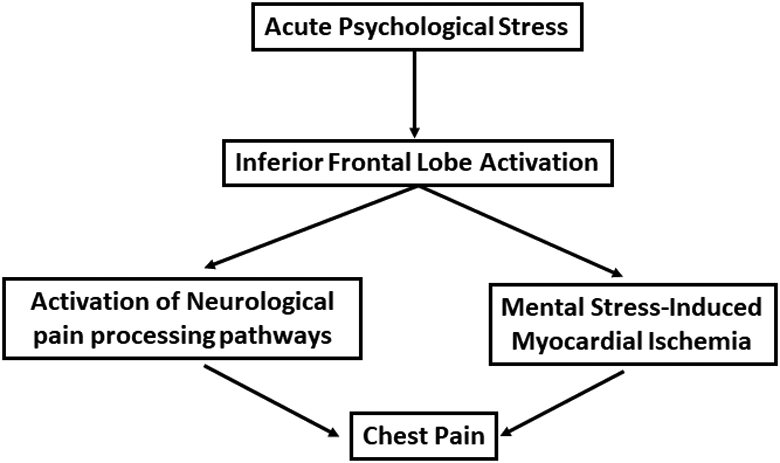
Neurological pain processing pathways include activation of thalamus, insula and amygdala
Methods
Study Cohort
The data that support the findings of our study are available from the corresponding author on reasonable request. Between June 2011 and August 2014, individuals with confirmed stable CAD were recruited from Emory-affiliated hospitals and clinics as part of the Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) as described elsewhere.17 CAD was defined based on a positive nuclear stress test, previous cardiac catheterization showing atherosclerosis, history of prior myocardial infarction, or a history of percutaneous coronary intervention or coronary artery bypass grafting. Participants were excluded if they: had a recent episode of unstable angina, decompensated heart failure within one week, were pregnant, had a systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 110 mm Hg on the day of the test, had a history of alcohol or substance abuse or severe psychiatric disorders including schizophrenia, psychotic depression, or bipolar disorder in the past year, or had chronic steroid use above 1.5 milligram per day. Data regarding the sociodemographic characteristics, medical history, and medication history of all participants were collected using standard questionnaires, and were verified using medical records. All patients provided written informed consent, and the study was approved by the Emory University Institutional Review Board (Supplemental Figure 1).
Study measures
Sociodemographic factors (age, sex, race, education, and current smoking), medical history (hypertension, hyperlipidemia, diabetes, obesity, previous myocardial infarction, previous revascularization), and list of medications taken were assessed using standardized questionnaires. Depressive symptoms were assessed using the Beck Depression Inventory (BDI) scale,18 and post-traumatic stress disorder symptoms were assessed using the post-traumatic stress disorder symptom checklist.19 Trait anxiety was measured with the State-Trait Anxiety Inventory (STAI).20 The level of distress of participants was evaluated at baseline and immediately after the mental stress test using the Subjective Units of Distress Scale (SUDS) during both PET imaging and the myocardial perfusion imaging procedures.21 The Duke Activity Status Index (DASI) was used to evaluate the functional capacity of study subjects.22
Angina symptoms were assessed at baseline and also at 2 years follow-up using the Seattle Angina Questionnaire’s (SAQ) angina-frequency subscale, which measures frequency of angina and use of nitroglycerin for chest pain over the previous 4 weeks.23 Scores for angina frequency are rescaled into on a 100-point scale, with 100 representing no chest pain and 0 representing angina occurring ≥ 4 times/day. The minimal clinically-significant change in SAQ scale is a change of 10 points.23 The SAQ has been validated in different populations and correlates with electronic daily diary entries for angina and nitroglycerin use.23, 24 Participants were divided into categories of angina frequency based on SAQ scores, defined as absent (score 100), monthly (score 61 to 99), weekly (score 31 to 60), and daily (score 0 to 30). Since only 3 participants (1.9%) reported daily angina, those with daily or weekly angina were combined into a single category for analysis.
Mental stress testing
All participants underwent mental stress testing using a standardized protocol in conjunction with both myocardial perfusion imaging and High Resolution Positron Emission Tomography (HR-PET) brain imaging, as previously described.17, 25 The mental stress protocols were done with the same protocols on two separate days and within 2 weeks from each other. The rate pressure product changes with mental stress were highly correlated (r=0.88) between the cardiac perfusion imaging and the brain PET imaging mental stress protocols.
HR-PET brain imaging
The HR-PET device has a spatial resolution of 2-mm which provides significantly higher sensitivity than conventional PET cameras.26 Mental stress testing was performed by trained staff using mental arithmetic and public speaking. Each individual would undergo eight brain scans with two scans during each of the 2 control (counting aloud and recalling a neutral event) and 2 scans for the two stress (arithmetic and public speaking) conditions. Each task lasted for approximately 2 min, and participants were scanned during each task. Subjects were injected with 20mCi of O-15 water 10 seconds after the beginning of each task to assess cerebral blood flow. Details regarding the mental stress testing protocol have been previously described.17 Readers of the brain imaging results were not aware of other clinical characteristics.
Myocardial perfusion imaging
Each subject underwent 3 nuclear 99 m-Tc sestamibi-gated single-photon emission computed-tomography (SPECT) scans at rest, during mental stress and during conventional stress testing (with exercise or pharmacological stress depending on walking capacity). Two experienced nuclear cardiologists performed visual interpretation of the SPECT scans blinded to both clinical and brain imaging data. Discrepancies in interpretation of images were resolved by consensus. The extent and the severity of perfusion defects were quantified at rest and during stress using a 17-segement model,27 where the severity of the defect was quantified using a 5-point scale from normal (score = 0) to absent perfusion (score = 4). These scores were then summed across the 17 segments yielding a total score for rest and each stress condition. Presence of ischemia was defined as a summed total score of ≥ 2 in any segment, or worsening of a preexisting impairment of at least 2 points in a single segment, or worsening of at least 1 point in 2 or more contiguous segments.28 Presence of scar tissue was determined by a summed total score of ≥ 4 at rest and no worsening during stress. Ejection fraction, systolic and diastolic volume during stress testing were also recorded. During mental stress testing of the cardiac imaging day, each participant was also asked if they were experiencing any chest pain.
Data Analysis
Analysis of HR-PET images was performed using statistical parametric mapping (SPM12) software, as previously described.29, 30 Images were realigned to the mean image of the study session, smoothed, and then normalized onto a standard brain template from the Montreal Neurological Institute. This method would allow evaluation of regional blood flow relative to the total brain blood flow. HR-PET imaging analysis was performed by creating individual contrast maps to determine changes in regional blood flow (Blood FlowNet = Blood FlowMental Stress Task – Blood FlowControl Task). The two mental stress tests showed an excellent overlap which was indicated by a Dice coefficient of 0.85. Therefore, the net change in blood flow was an average of the mental stress tasks minus the average of the control tasks. In the next step, a custom mask was applied to the contrast image to constrain regional blood flow to the bilateral inferior frontal lobes. Similar contrast images were also constructed to evaluate the regional blood flow to bilateral thalamus, insula, and amygdala. Individual subject responses (Blood FlowNet) were extracted from the masked contrast image and averaged across non-zero voxels.
Baseline characteristics of the population were compared according to increasing levels of angina frequency and severity using the analysis of variance for continuous, normally distributed variables or the chi-square test for categorical variables. For our main analysis, we constructed multivariable linear regression models to examine the association between inferior frontal lobe activation (outcome variable) and presence of angina. Models were adjusted for sociodemographic and lifestyle characteristics (age, sex, race, education, and current smoking). We used the base-2 logarithm of the brain activation values to examine the effect of doubling of the brain activation on angina frequency as each unit difference in the log- transformed brain activation represents a doubling of the score. We did not find any association between angina and any of the comorbidities or other medications and therefore these factors were not included in the multivariable regression models to avoid overfitting. We tested for multicollinearity using the Variance Inflation Factor and confirmed that none of the covariates entered in our model met criteria for collinearity. We performed a mediation analysis with bootstrapping (1,000 bootstrap samples and a 95 percent confidence interval) to test the statistical effects of mental stress-induced ischemia and other pain processing regions on estimates of stress-induced inferior frontal lobe activity on angina frequency.31 The other pain processing regions as identified in the literature included in the mediation analysis were activation of thalamus, insula and amygdala.32 Finally, in order to characterize the contribution of each variable that was associated with angina, relative importance analysis was performed, to quantify the relative importance of variables for their association with angina in regression analysis. This method would allow us to understand what variables contributed the most to angina.33
Results
A total of 148 individuals with CAD were enrolled with mean (SD) age 62 (8) years; 69% were male, and 36% were African American. Participants reporting higher angina frequency were younger, more often African-American, and higher rates of mental stress-induced ischemia (Table 1). Symptoms of depression, post-traumatic stress disorder, and anxiety as well as use of antidepressants and nitrates, were also more common in participants reporting higher angina frequency (Table 1). Also, participants reporting higher angina frequency were found to have higher levels of distress (SUDS score) immediately following mental stress. Similarly, the DASI score which is reflective of functional capacity of the participants was worse among those reporting higher angina frequency (Table 1). There were no other significant differences in the angina frequency subgroups in demographics, medical history, or severity of CAD. Use of beta-blockers and calcium channel blockers were highest in the group reporting daily or weekly angina but these differences were not statistically significant. No associations were found between the severity of angina symptoms with the presence of ischemia during conventional stress testing (P=0.21).
Table 1.
Baseline Characteristics of the Participants Reporting Angina Frequency
| Characteristic | Angina frequency | p-value | ||
|---|---|---|---|---|
| Absent N= 94 |
Monthly N=35 |
Daily or weekly N=19 |
||
| Demographics | ||||
| Age, Mean (SD) | 64 (7) | 59 (8) | 57 (8) | <0.001 |
| Men, N (%) | 70 (74.5) | 21 (60.0) | 11 (57.9) | 0.093 |
| African American, N (%) | 23 (24.5) | 17 (48.0) | 13 (68.4) | 0.001 |
| Years of school, Mean (SD) | 15 (3) | 13 (4) | 13 (2) | 0.011 |
| Current smoker, N (%) | 11 (11.7) | 8 (22.9) | 4 (21.1) | 0.28 |
| Medical factors | ||||
| Hypertension, N (%) | 68 (72.3) | 29 (82.9) | 16 (84.2) | 0.49 |
| Hyperlipidemia, N (%) | 78 (83.0) | 25 (71.4) | 18 (94.7) | 0.082 |
| Diabetes, N (%) | 25 (26.6) | 18 (51.4) | 7 (36.8) | 0.074 |
| Obesity, N (%) | 77 (81.9) | 27 (77.1) | 15 (78.9) | 0.49 |
| Previous MI, N (%) | 35 (37.2) | 12 (34.3) | 6 (31.6) | 0.71 |
| Previous revascularization, N (%) | 47 (50) | 16 (45.7) | 9 (47.4) | 0.81 |
| Gensini score, Mean (SD) | 39.3 (54.6) | 32.1 (44.1) | 29.0 (32.3) | 0.42 |
| Stress-induced ischemia, N (%) | ||||
| Conventional (exercise/pharmacologic) | 34 (34.0) | 18 (51.4) | 7 (36.8) | 0.21 |
| Mental | 19 (20.2) | 12 (34.3) | 7 (36.8) | 0.02 |
| Summed difference score, Mean (SD) | 2.6 (4.6) | 2.9 (3.6) | 2.1 (2.8) | 0.81 |
| Presence of scar, N (%) | 17 (18.1) | 4 (11.4) | 2 (10.6) | 0.33 |
| Ejection Fraction, Mean (SD) | 68.4 (15.3) | 63.1 (14.7) | 72.3 (12.5) | 0.12 |
| End Systolic volume, Mean (SD) | 44.3 (56.1) | 60.0 (58.2) | 34.2 (31.3) | 0.19 |
| End Diastolic volume, Mean (SD) | 118.8 (65.4) | 134.7 (75.6) | 108 (42.0) | 0.31 |
| Psychological factors | ||||
| BDI-II score, Mean (SD) | 7.77 (6.8) | 19.31 (12.4) | 21.95 (13.5) | <0.001 |
| PCL score, Mean (SD) | 26.27 (10.6) | 36.37 (13.8) | 44.65 (17.8) | <0.001 |
| STAI Anxiety-Trait score, Mean (SD) | 32.7 (11.0) | 42.2 (11.3) | 44.8 (12.2) | <0.001 |
| Mental stress with brain PET scan | ||||
| Baseline SUDS score, Mean (SD) | 15.2 (18.0) | 17.4 (20.5) | 16.6 (18.9) | 0.78 |
| Post-mental stress SUDS score, Mean (SD) | 15.7 (11.0) | 20.9 (7.9) | 26.2 (17.2) | 0.009 |
| Change in SUDS score with stress, Mean (SD) | 0.5 (3.4) | 2.1 (7.5) | 9.9 (10.1) | 0.01 |
| Mental stress with myocardial perfusion | ||||
| Baseline SUDS score, Mean (SD) | 10.3 (15.2) | 16.6 (27.4) | 17.2 (22.2) | 0.29 |
| Post-mental stress SUDS score, Mean (SD) | 17.3 (18.1) | 29.7 (33.6) | 39.7 (34.0) | 0.007 |
| Change in SUDS score with stress, Mean (SD) | 6.0 (2.1) | 13.0 (6.7) | 22.5 (5.8) | 0.002 |
| DASI score, Mean (SD) | 43.9 (12.1) | 31.2 (17.7) | 22.3 (11.5) | <0.001 |
| Medications, N (%) | ||||
| Antidepressants | 29 (30.9) | 10 (29.4) | 12 (63.2) | 0.043 |
| ACE inhibitors | 44 (46.8) | 14 (41.2) | 8 (42.1) | 0.67 |
| Aspirin | 83 (88.3) | 30 (88.2) | 16 (84.2) | 0.88 |
| Beta blocker | 70 (74.5) | 23 (67.6) | 15 (78.9) | 0.38 |
| Statin | 86 (91.5) | 29 (85.3) | 18 (94.7) | 0.25 |
| Calcium channel blockers | 18 (19.1) | 8 (23.5) | 5 (26.3) | 0.72 |
| Nitrates | 15 (16.0) | 10 (29.4) | 8 (42.1) | 0.02 |
| Increase in angina frequency during follow-up, N (%) | 16 (21.3) | 11 (40.7) | 1 (9.1) | 0.03 |
SD: standard deviation, BDI-II : Beck Depression Inventory scale, PCL: Post-traumatic stress disorder symptom checklist, STAI: State- Trait Anxiety Inventory, ACE: angiotensin converting enzyme
As shown in Figure 2A, compared to participants without angina, both groups reporting monthly angina symptoms or weekly/daily symptoms were found to have higher inferior frontal lobe activation to mental stress at both baseline and after 2 years of follow-up (P<0.001). Those reporting angina during mental stress testing with cardiac perfusion imaging also had higher inferior frontal lobe activation (1.43 ± 0.42) compared to individuals who did not have active chest pain (1.19 ± 0.14) during mental stress testing (P=0.03). These individuals also had fewer years of education and higher BDI-II and PCL scores (Supplemental Table 1). In addition, those who reported angina during mental stress-testing, were found to have higher distress levels measured by SUDS evaluated immediately after mental stress with both cardiac perfusion imaging and brain PET imaging compared to those who did not have active chest pain with mental stress testing (Table 1). Similarly, the group experiencing angina during stress testing with cardiac perfusion imaging had higher increases in SUDS score during mental stress testing compared to those who did not experience chest pain with mental stress testing (Supplemental Table 1).
Figure 2. Mental Stress-Induced Inferior Frontal Lobe activation according to angina frequency levels at baseline (A) and changes during 2 years of follow-up (B).
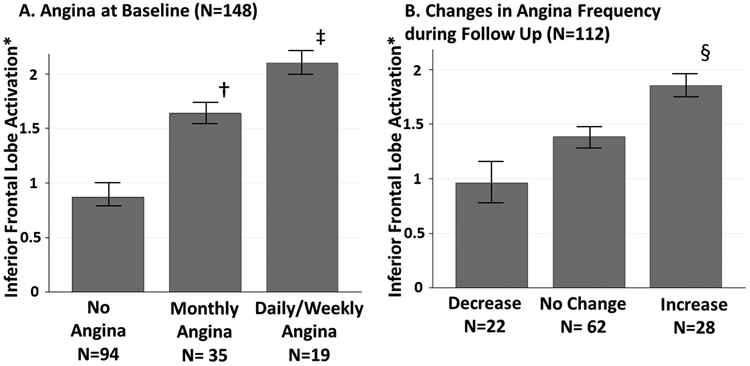
* net difference ml−1* min −1*100mg−1
a indicating P-value= 0.03 comparing those with monthly angina to the group with no angina
b indicating P-value- 0.009 comparing those with daily/weekly angina to the group with no angina
c indicating P-value- 0.01 comparing those with daily/weekly angina to the group with no angina
A total of 112 individuals completed the SAQ at 2 years of follow up. The characteristics of these individuals were not significantly different from the baseline cohort (Supplemental Table 2). Twenty eight individuals (24.1%) reported an increase in the frequency of angina episodes during follow up (Figure 2B). These individuals were found to have a higher mean inferior frontal lobe activation with mental stress at baseline compared to those who reported a decrease in chest pain frequency (1.82 ± 0.15 vs 0.92 ± 0.28, P=0.01, Figure 2B). There were no significant differences in the baseline demographics and clinical characteristics of the group who reported an increase in angina frequency compared to those with no change or improvement in chest pain symptoms (Supplemental Table 3).
In addition to angina, inferior frontal lobe activation during mental stress was shown to be higher among those with mental stress-induced ischemia compared to those without ischemia (1.46 ± 0.26 vs 1.02 ± 0.24, P=0.01). After adjusting for sociodemographic and lifestyle characteristics, presence of mental stress induced ischemia was positively associated with inferior frontal lobe activation (=0.202, 95% CI 0.09-0.41, P=0.009). There was also a significant positive association between mental stress-induced inferior frontal lobe activation with other regions involved in pain processing, including the thalamus, insula and amygdala (Supplemental Figure 2).
After adjusting for sociodemographic and lifestyle characteristics, SAQ scores were negatively associated with inferior frontal lobe activation (P=0.01). For every doubling in the inferior frontal lobe activation, angina frequency was increased by 13.71 units, after adjustment for the factors above ( 13.7, 95% CI 6.3, 21.7, p=0.008). Also, as shown in Supplemental Figure 3, there was a positive linear association between changes in SAQ during 2 years of follow up and mental stress-induced inferior frontal lobe activation at baseline. After adjusting for the above factors, every doubling in inferior frontal lobe activation was associated with 11.6 units increase in angina frequency score during follow-up ( 11.6, 95% CI 4.1, 19.2, p=0.01). These changes in SAQ scores are clinically meaningful given that the minimal clinically-significant change in SAQ scores is a change of 10 units. Formal mediation analyses showed that mental stress-induced ischemia and activation of other pain processing brain regions accounted for 40.0% and 13.1% of the total effect of inferior frontal lobe activation on angina severity at baseline, respectively. (Supplemental Figure 4).
Our relative importance analysis model included all variables that were significantly associated with presence of angina on bivariate analysis. As shown in Figure 3, inferior frontal lobe activation was the most important correlate of angina, contributing to 36.5% of the variance in the model.
Figure 3. Significant correlates of angina at baseline ranked by importance*.
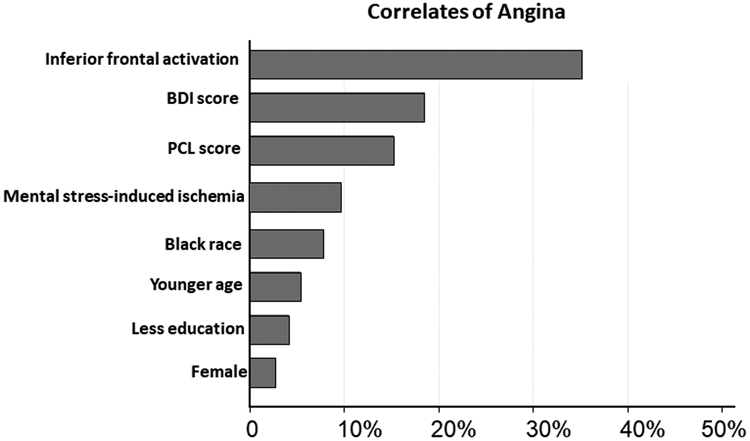
Angina assessed by Seattle Angina Questionnaire.
*No contribution found with conventional myocardial ischemia
BDI : Beck Depression Inventory scale, PTSD: Post-traumatic stress disorder symptom checklist, less education indicates fewer years of formal education by self-report
Discussion
To our knowledge, the present study is the first to show that among individuals with CAD, greater mental stress-induced activation in the inferior frontal lobe region is positively associated with self-reported angina severity. We found a dose-response relationship between stress-induced inferior frontal lobe activation and angina frequency. There was also a significant positive association between inferior frontal lobe activation during stress and the magnitude of change in angina frequency after 2 years of follow-up, suggesting that brain-related changes predict worsened future angina as well. Finally, we showed that mental stress-induced ischemia and other brain regions associated with pain processing (thalamus, insula and amygdala) partly mediate the relationship between inferior frontal lobe activation and angina severity. These findings may indicate important clinical implications in the management of angina by linking it, for the first time, to stress responses in the brain.
Our findings that focus on brain-related changes with stress complement previous work that has focused on subjective stress measures as key determinants of angina. A key advantage to our findings is that our measures are objective and imaging-based. This may, to a certain extent, provide the underlying neurophysiological basis for previous studies examining angina and depression, post-traumatic stress disorder symptoms, anxiety, and perceived stress.7, 34, 35 When evaluated together in the same model, activation of the inferior frontal lobe was the most important correlate of angina, followed by psychological factors. Most importantly, all of these factors, and not traditional risk factors or conventional ischemia, explained angina severity in our relative importance model. This has potential important clinical implications when considering the clinical evaluation of chest pain in patients with CAD, for which stress testing and coronary angiography are the predominant tests ordered. An important additional consideration is that angina itself may also lead to higher stress levels, as evidenced by higher subjective distress scores in our participants who experienced chest pain during mental stress testing. However, accounting for subjective distress scores after mental stress did not change the results.
Although our findings that conventional ischemia did not associate with angina are unexpected from the perspective of everyday clinical practice, other studies have also found that exercise stress ischemia and angina history are not associated.9-11 However, our finding that mental stress ischemia associates with angina has been previously noted in the literature.8 Our mediation model may help to understand the mechanisms through which mental stress ischemia may lead to increased angina, although more work is needed.
Our study builds upon previous research that has implicated the inferior frontal lobe in processing physical and psychological pain, as well as the overall fear of having pain. 15, 36-40Our research also suggests potential downstream neurobiological consequences to increased inferior frontal activation, including increased mental stress-induced myocardial ischemia,16 which accounts for 40 percent of its effect on angina frequency. Other downstream neurological activations may also help to explain higher angina frequency, including the thalamus, insula and amygdala, which collectively explained 13% of the effect.32, 41 Overall, previous research on the relationship of this region with pain and neurobiological mechanisms warrant further research on interventions that can modify these pathways, such as neurofeedback, deep brain stimulation, and transcranial magnetic brain stimulation.42-44
The present study has a number of limitations. First, we used a standardized experimental protocol to induce psychological stress in participants with stable CAD. While this protocol offers an objective manner of measuring stress effects, it may not reflect multiple and various naturally occurring real-life stressors; this may lead to underestimation of the effect size. Second, angina symptoms were assess retrospectively using a questionnaire, rather than using a prospective diary documentation of angina symptoms. However, this method is clinically relevant since it is similar to the way health care professionals collect information about patients’ symptoms during clinical visits. The SAQ scale is also a well validated tool for assessment of presence and severity of angina, and because we used the questionnaire before mental stress testing, it is unlikely that the responses were biased by the testing itself. Third, information on other subsets of the SAQ including the quality of life measures was not available in our study. However, functional capacity data were available, which help to assess important downstream determinants of angina, neurocardiac dysfunction, and ischemic heart disease. Fourth, similar stress protocols were used for mental stress testing for cardiac perfusion imaging and brain PET imaging which could increase the possibility of stress-retest effect. However, the reaction to both mental stress protocols were highly correlated with each other which suggests a similar stress reactivity to both protocols. Finally, the low temporal resolution of PET imaging modality used in our study, limited our ability to perform network analysis and therefore understand the temporal relationship between inferior frontal lobe activation with other brain regions during mental stress. Future studies using other modalities such as functional magnetic resonance imaging could help us understand dynamic relationships of other brain regions with the inferior frontal lobe during mental stress.
In summary, we found a positive relationship between inferior frontal lobe activity with stress and reported angina. These findings suggest that altered brain reactivity to stress could possibly represent a key mechanism in chest pain perception in individuals with CAD. Mental stress ischemia and activation of pain regions of the brain are important potential mechanisms. Although more research is needed, these results may potentially shift the paradigm by which angina is evaluated by re-focusing clinical evaluation/management of psychological stress as adjunct to traditional cardiac evaluations.
Supplementary Material
Clinical Perspective.
Angina pectoris is the hallmark symptom of coronary artery disease and psychological factors such as mental stress are shown to be important determinants of angina. However, little is known about the brain mechanisms responsible for the association between angina and psychological stress. In this study, we investigated the role of the inferior frontal lobe which is an important brain region involved in emotional regulation and stress. Findings from our study showed that in patients with coronary artery disease, activation of the inferior frontal lobe in response to acute mental stress is associated with the severity of self-reported angina at baseline and also at 2 years of follow-up. Our data also showed that higher activation of this region is associated with worsening of self-reported angina during a 2 year follow-up period. Finally we demonstrated that myocardial ischemia induced by mental stress and activation of other brain pain processing regions (thalamus, insula and amygdala) play contributory roles in the association between inferior frontal lobe activation and angina.
Acknowledgments
Sources of Funding:
This work was supported by grants P01 HL101398, R01 HL109413, R01HL109413-02S1, R01HL125246, K24HL077506, K24 MH076955, UL1TR000454, KL2TR000455, K23HL127251, and T32HL130025 from the NIH.
Footnotes
Disclosures: None
References
- 1.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–10. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Peterson E The burden of angina pectoris and its complications [corrected]. Clin Cardiol. 2007;30:I10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–87. [DOI] [PubMed] [Google Scholar]
- 5.Holubkov R, Laskey WK, Haviland A, Slater JC, Bourassa MG, Vlachos HA, Cohen HA, Williams DO, Kelsey SF, Detre KM; Investigators NDRR. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J. 2002;144:826–33. [DOI] [PubMed] [Google Scholar]
- 6.Wijeysundera HC, Nallamothu BK, Krumholz HM, Tu JV and Ko DT. Meta-analysis: effects of percutaneous coronary intervention versus medical therapy on angina relief. Ann Intern Med. 2010;152:370–9. [DOI] [PubMed] [Google Scholar]
- 7.Hayek SS, Ko YA, Awad M, Del Mar Soto A, Ahmed H, Patel K, Yuan M, Maddox S, Gray B, Hajjari J, et al. Depression and chest pain in patients with coronary artery disease. Int J Cardiol. 2017;230:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimple P, Shah AJ, Rooks C, Douglas Bremner J, Nye J, Ibeanu I, Raggi P and Vaccarino V. Angina and mental stress-induced myocardial ischemia. J Psychosom Res. 2015;78:433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan MD, Ciechanowski PS, Russo JE, Spertus JA, Soine LA, Jordan-Keith K and Caldwell JH. Angina pectoris during daily activities and exercise stress testing: The role of inducible myocardial ischemia and psychological distress. Pain. 2008;139:551–61. [DOI] [PubMed] [Google Scholar]
- 10.Sheps DS, McMahon RP, Pepine CJ, Stone PH, Goldberg AD, Taylor H, Cohen JD, Becker LC, Chaitman B, Knatterud GL, et al. Heterogeneity among cardiac ischemic and anginal responses to exercise, mental stress, and daily life. Am J Cardiol. 1998;82:1–6. [DOI] [PubMed] [Google Scholar]
- 11.Ketterer MW, Bekkouche NS, Goldberg AD, McMahon RP and Krantz DS. Symptoms of anxiety and depression are correlates of angina pectoris by recent history and an ischemia-positive treadmill test in patients with documented coronary artery disease in the pimi study. Cardiovasc Psychiatry Neurol. 2011;2011:134040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira VH, Cerqueira JJ, Palha JA and Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166:30–7. [DOI] [PubMed] [Google Scholar]
- 13.Koch SBJ, Mars RB, Toni I and Roelofs K. Emotional control, reappraised. Neurosci Biobehav Rev. 2018;95:528–534. [DOI] [PubMed] [Google Scholar]
- 14.Dixon ML, Thiruchselvam R, Todd R and Christoff K. Emotion and the prefrontal cortex: An integrative review. Psychol Bull. 2017;143:1033–1081. [DOI] [PubMed] [Google Scholar]
- 15.Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC and Derntl B. Psychosocial versus physiological stress - Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, et al. Brain Correlates of Mental Stress-Induced Myocardial Ischemia. Psychosom Med. 2018;80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, et al. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YP and Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr. 2013;35:416–31. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard EB, Jones-Alexander J, Buckley TC and Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34:669–73. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, and Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970 [Google Scholar]
- 21.Benjamin CL, O'Neil KA, Crawley SA, Beidas RS, Coles M and Kendall PC. Patterns and predictors of subjective units of distress in anxious youth. Behav Cogn Psychother. 2010;38:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR and Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–4. [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M and Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 24.Kimble LP, Dunbar SB, Weintraub WS, McGuire DB, Fazio S, De AK and Strickland O. The Seattle angina questionnaire: reliability and validity in women with chronic stable angina. Heart Dis. 2002;4:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–4. [DOI] [PubMed] [Google Scholar]
- 26.Tuna U, Johansson J and Ruotsalainen U. Evaluation of analytical reconstruction with a new gap-filling method in comparison to iterative reconstruction in [(1)(1)C]-raclopride PET studies. Ann Nucl Med. 2014;28:417–29. [DOI] [PubMed] [Google Scholar]
- 27.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J and Santana C. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. J Nucl Cardiol. 2007;14:420–32. [DOI] [PubMed] [Google Scholar]
- 28.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, Ficaro EP, Freeman MR, Hendel RC, Jain D, et al. ; American Society of Nuclear C. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73. [DOI] [PubMed] [Google Scholar]
- 29.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R and Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittbrodt MT, Moazzami K, Lima BB, Alam ZS, Corry D, Hammadah M, Campanella C, Ward L, Quyyumi AA, Shah AJ, et al. Early childhood trauma alters neurological responses to mental stress in patients with coronary artery disease. J Affect Disord. 2019;254:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preacher KJ and Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. [DOI] [PubMed] [Google Scholar]
- 32.Rosen SD, Paulesu E, Wise RJ and Camici PG. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart. 2002;87:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeBreton JM and Tonidandel S. Multivariate relative importance: extending relative weight analysis to multivariate criterion spaces. J Appl Psychol. 2008;93:329–45. [DOI] [PubMed] [Google Scholar]
- 34.Jespersen L, Abildstrom SZ, Hvelplund A and Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–81. [DOI] [PubMed] [Google Scholar]
- 35.Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ, et al. Chest Pain and Mental Stress-Induced Myocardial Ischemia: Sex Differences. Am J Med. 2018;131:540–547 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casey KL, Lorenz J and Minoshima S. Insights into the pathophysiology of neuropathic pain through functional brain imaging. Exp Neurol. 2003;184 Suppl 1:S80–8. [DOI] [PubMed] [Google Scholar]
- 37.Palermo S, Benedetti F, Costa T and Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp. 2015;36:1648–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apkarian AV, Bushnell MC, Treede RD and Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. [DOI] [PubMed] [Google Scholar]
- 39.Godinho F, Faillenot I, Perchet C, Frot M, Magnin M and Garcia-Larrea L. How the pain of others enhances our pain: searching the cerebral correlates of 'compassional hyperalgesia'. Eur J Pain. 2012;16:748–59. [DOI] [PubMed] [Google Scholar]
- 40.Zandbelt BB, Bloemendaal M, Neggers SF, Kahn RS and Vink M. Expectations and violations: delineating the neural network of proactive inhibitory control. Hum Brain Mapp. 2013;34:2015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. [DOI] [PubMed] [Google Scholar]
- 42.Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, Mantione M, Ooms P, de Koning P, Vulink N, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–7. [DOI] [PubMed] [Google Scholar]
- 43.Kohl SH, Veit R, Spetter MS, Gunther A, Rina A, Luhrs M, Birbaumer N, Preissl H and Hallschmid M. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z, Jiang Z, Jiang B, McClure MA and Mu Q. High-Frequency Repetitive Transcranial Magnetic Stimulation Could Improve Impaired Working Memory Induced by Sleep Deprivation. Neural Plast. 2019;2019:7030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


