Abstract
Nicotine is the primary psychoactive chemical in both traditional and electronic cigarettes (e-cigarettes). Nicotine levels in both traditional cigarettes and e-cigarettes are an important concern for public health. Nicotine exposure due to e-cigarette use is of importance primarily due to the addictive potential of nicotine, but there is also concern for nicotine poisoning in e-cigarette users. Nicotine concentrations in e-liquids vary widely. Additionally, there is significant genetic variability in rate of metabolism of nicotine due to polymorphisms of CYP2A6, the enzyme responsible for the metabolism of approximately 80% of nicotine. Recent studies have shown CYP2A6 activity is also reduced by aromatic aldehydes such as those added to e-liquids as flavoring agents, which may increase nicotine serum concentrations. However, the impacts of flavored e-liquids on CYP2A6 activity are unknown. In this study, we investigated the impact of three flavored e-liquids on microsomal recombinant CYP2A6. Microsomal recombinant CYP2A6 was challenged at e-liquid concentrations ranging up to 0.125% (v/v) and monitored for metabolic activity using a probe molecule approach. Two e-liquids exhibited dose-dependent inhibition of CYP2A6 activity. Mass spectrometry was conducted to identify flavoring agents in flavored e-liquids that inhibited CYP2A6. Microsomal recombinant CYP2A6 was subsequently exposed to flavoring agents at concentrations ranging from 0.03 μM to 500 μM. Cinnamaldehyde and benzaldehyde were found to be the most potent inhibitors of microsomal CYP2A6 of the flavoring agents tested, with identified IC50 values of 1.1 μM and 3.0 μM, respectively. These data indicate certain aromatic aldehyde flavoring agents are potent inhibitors of CYP2A6, which may reduce nicotine metabolism in vivo. These findings indicate an urgent need to evaluate the effects of flavoring agents in e-cigarette liquids on the pharmacokinetics of nicotine in vivo.
Keywords: Cytochrome 2A6, nicotine, e-cigarette, flavorings, aldehydes
Graphical Abstract
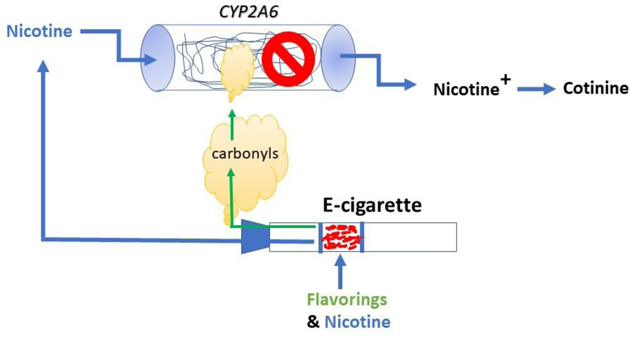
INTRODUCTION
Cytochrome P450 2A6 (CYP2A6) is one of the 60 members of the cytochrome P450 superfamily of drug-metabolizing enzymes found in humans. As is the case for other members of the cytochrome P450 superfamily, CYP2A6 is predominantly found in the liver, where it comprises approximately 4–13% of CYP450s expressed there2. However, CYP2A6 and its close relative, CYP2A13, have also been identified in the respiratory system3, 4.
CYP2A6 is responsible for the metabolism of approximately 30 therapeutic molecules, including drugs such as halothane and methoxyflurane5. However, the enzyme is best known for its role in nicotine oxidative metabolism6. It is responsible for approximately 80% of the metabolism of nicotine7. Nicotine is the primary psychoactive chemical of traditional and electronic cigarettes (e-cigarettes) and is metabolized by CYP2A6 into the intermediate metabolite nicotine-delta 1’(5’)-iminium ion, which is then converted to cotinine, primarily by aldehyde oxidase (Figure 1)8.
Figure 1. Primary nicotine metabolic pathway.
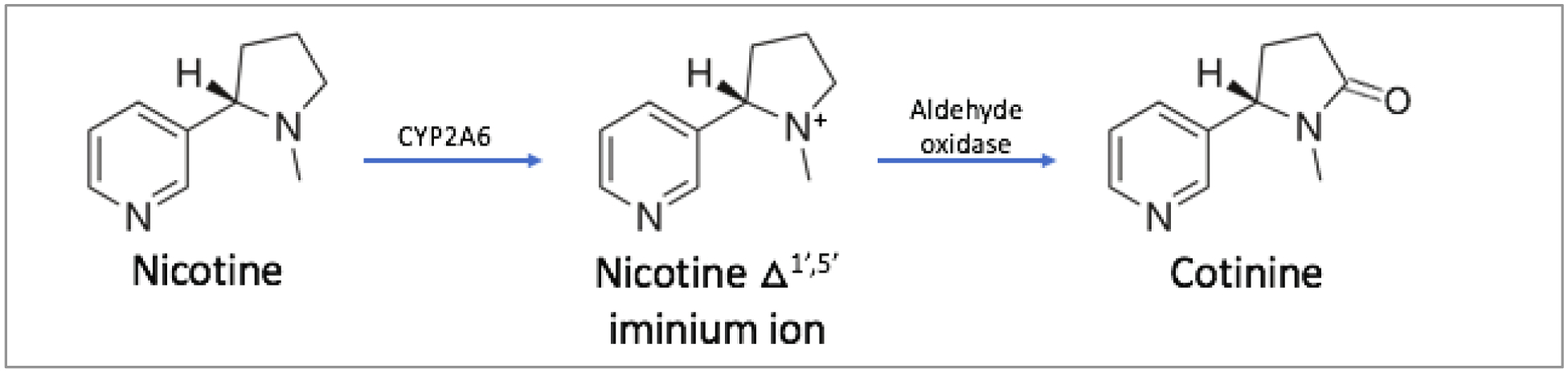
Nicotine is metabolized by CYP2A6 and aldehyde oxidase to form cotinine. Nicotine initially undergoes metabolic oxidation by CYP2A6 to form the intermediate metabolite nicotine-delta 1’(5’)-iminium ion, which is in equilibrium with 5’-hydroxynicotine. The intermediate metabolite is then metabolized by aldehyde oxidase to cotinine.
CYP2A6 is prone to inhibition by both pharmaceuticals and environmental chemicals. Inhibitors of CYP2A6 include methoxsalen, tranylcypromine, and a host of organosulfur compounds9–11. The effects of such perturbations of activity of CYP2A6 are especially important to the metabolism of nicotine. For example, a clinical study identified elevated levels of nicotine blood concentrations in individuals smoking mentholated cigarettes due to inhibition of nicotine metabolism by CYP2A6 and also glucuronidation12.
While the impact of CYP2A6 inhibition on nicotine serum levels is known, the impact of inhibition of CYP2A6 activity and decreased nicotine metabolism on smoking behavior and health effects has not been fully elucidated. A 1998 study found that individuals lacking a functioning CYP2A6 gene were less likely to become nicotine-dependent tobacco users, and on average smoked fewer cigarettes than subjects with a fully functional CYP2A6 gene13. Additionally, a larger epidemiologic study in southern China found reduced activity CYP2A6 genotypes were associated with a lower number of cigarettes smoked, delayed initiation of becoming a regular smoker, but paradoxically, decreased success of smoking cessation14, 15. A study involving the pharmacological inhibition of CYP2A6 found subjects given the CYP2A6 inhibitor methoxsalen smoked fewer cigarettes and used less tobacco product, as indicated by reduced levels of carbon monoxide10. Conversely, reduced CYP2A6 activity has been identified as a risk factor for addiction in adolescents, potentially due to rapid reinforcement of the psychoactive effects of nicotine16. Additional research is needed to elucidate the role of CYP2A6 activity on smoking behavior.
In recent years, there has been a surge in the use of novel tobacco products like e-cigarettes17–20. In contrast to traditional cigarettes, e-cigarettes heat e-cigarette liquid, termed e-liquid, using a heated coil, which then aerosolizes the e-liquid for inhalation21. E-liquids are primarily composed of humectants propylene glycol (PG) and vegetable glycerin (VG)22. However, e-liquids also contain nicotine and various chemicals, termed flavoring agents, added to enhance the taste of the e-liquid23. While many of these flavoring agents are considered safe for ingestion, their potential for toxicity when inhaled has not been well-studied. With over 7,000 e-liquid flavors on the market, there is substantial variability in flavoring agents used and their concentrations24. Many of these flavoring agents are commonly found in food and routinely ingested25. The limited number of inhalation studies of flavoring agents that are available indicate that while they may be safe via ingestion, there is potential for harm when inhaled. For instance, the flavoring agent diacetyl, used as a flavoring agent in buttered popcorn, was found to cause Bronchiolitis Obliterans Syndrome when inhaled by manufacturing workers26. While many of the combustion products found in traditional cigarette smoke are not found in e-cigarette smoke, studies have found increased harmful aldehydes in smoke from flavored e-liquids compared to smoke from unflavored e-liquids27.
Limited research has been conducted on the potential for flavored e-liquids to affect nicotine pharmacokinetics. What little research that has been conducted suggests certain e-liquids flavoring agents may reduce CYP2A6 activity, and subsequently nicotine metabolism. A study on rational design of CYP2A6 inhibitors by Tani et al. found three aromatic aldehydes to be the most potent of CYP2A6 inhibitors tested28. As many e-liquid flavoring agents are aromatic aldehydes, we believe there is potential for flavored e-liquids to impact CYP2A6 activity. Additionally, a limited number of aromatic aldehydes used as flavoring agents have been investigated for effects on cytochrome P450 activity. Cinnamaldehyde is an aromatic aldehyde commonly used as an e-liquid flavoring agent29. Cinnamaldehyde was found to be an inhibitor of multiple cytochrome P450s, with CYP2A6 and CYP2E1 being the most prone to inhibition30. Furthermore, a study conducted by Rahnasto et al. on benzaldehyde derivatives found moderate to strong levels of inhibition of CYP2A6 by the flavoring chemicals31. However, e-liquid flavors are oftentimes due to a complex mixture of flavoring agents rather than just a single compound32. Therefore, we believe the study of potential perturbations of CYP2A6 of e-liquids should be conducted using the e-liquid in addition to individual flavoring agents. While a select number of individual flavoring agents have been screened for inhibition of CYP2A6, we were not able to identify any existing studies that elucidate the impact of e-liquids themselves on CYP2A6 activity. Based on flavors often found in e-liquids, we hypothesized various e-liquids may inhibit the enzyme CYP2A6 to varying degrees with the potential to reduce the metabolism of nicotine. We examined this hypothesis by testing a limited number of e-liquids using a cell-free CYP2A6 enzyme system.
EXPERIMENTAL PROCEDURES
E-Liquids and Reagents.
Three flavored e-liquids were purchased from three separate vape shops. To ensure possible variations in nicotine concentrations did not affect experiments, all selected e-liquids were labeled by the manufacturers as nicotine-free, which was later confirmed using gas chromatography-mass spectrometry. E-liquids were selected to test dissimilar flavor profiles to increase representativeness of e-liquids available on the marketplace. Additionally, when possible, “best-selling” flavors were selected. The e-liquid High Caliber Flamethrower was purchased from the online retailer “myvaporstore.com”. Strawberry Poptart was purchased from a local vape shop (The Vapor Girl, Chapel Hill, NC). Reds Apple Watermelon was purchased at a separate local vape shop (Local Liquids, Chapel Hill, NC). PG/VG ratios of selected e-liquids ranged from 30/70 to 55/45. E-liquids were stored away from light and in glass bottles prior to experimental use. The following flavoring compounds were purchased from Sigma-Aldrich (St. Louis, MO): food grade trans-cinnamaldehyde (≥95% pure), GC-grade benzaldehyde (≥99% pure), GC-grade isoamyl acetate (≥99% pure), GC-grade ethyl-vanillin (≥98.5% pure), and GC-grade vanillin (≥99% pure). A mixture of 60% PG and 40% VG obtained from Thermofisher Scientific (Thermo Fisher Scientific, Waltham, MA) was utilized as a vehicle control for e-liquids.
Preparation of E-Liquids and Flavoring Agents.
1.25% (v/v) stock solutions of e-liquids were prepared in Thermo Fisher Vivid® reaction buffer II prior to experimental use. Working solutions were diluted in 0.5X Vivid® reaction buffer II. Because test compounds are diluted with other reagents upon addition to a well, test compounds are prepared at 2.5x screening concentration using 0.5X Vivid® reaction Buffer II. E-liquids were screened for CYP2A6 inhibition at seven separate concentrations ranging up to 0.125% (v/v) using a 4-fold serial dilution scheme. 2.5 mM stock solutions of individual flavoring agents were prepared in Thermo Fisher Vivid® reaction buffer II. Flavoring agents identified in e-liquids using mass spectrometry and two other common e-liquid flavoring agents were screened for CYP2A6 inhibition at concentrations up to 500 μM using a 4-fold serial dilution scheme.
Mass Spectrometry.
Qualitative mass spectrometry was conducted on e-liquids to identify flavoring agents in e-liquids and vehicle control using gas chromatography-mass spectrometry (GC-MS). Qualitative e-liquid analysis was performed on a Bruker EVOQ 456 gas chromatograph-triple quadrupole mass spectrometer using an Agilent DB-5MS capillary column (30 m, 0.25 mm ID, 0.25 μM film) and helium carrier gas. Injections of 1 μL were performed using a Bruker CP-8400 autosampler with an injector temperature of 270 °C and a split ratio of 50:1. The GC oven was programmed with a 12.5 min temperature gradient (60–250 °C), and the transfer line and electron ionization source were held at 250 °C. Samples were prepared by diluting 10 μL of e-liquid in 1 mL of methanol (optima grade) and vortexing for 30 seconds. Full-scan mass spectra were acquired from m/z 40–500. Compound identification was performed using the NIST 2014 mass spectral database and Bruker MS Data Review software.
For Flamethrower and Strawberry Poptart, quantitative MS was conducted to quantify flavoring agents using the same instrument conditions utilized for qualitative mass spectrometry. Concentrations were determined by standard addition. E-liquids were diluted in methanol (optima grade) with quantitative standards. Full-scan mass spectra were acquired using the same instrument method mentioned previously. Peak areas of cinnamaldehyde, vanillin, and ethyl vanillin were integrated for quantification.
Microsomal Recombinant Human Cytochrome P450 2A6 Activity Screening.
Vivid® recombinant human cytochrome P450 2A6 microsomes, Vivid® microsomal recombinant human cytochrome b5, NADP+, 3-cyanocoumarin, 3-cyano 7-hydroxycoumarin, and Vivid® Regeneration System consisting of glucose-6-phosphate and glucose-6-phosphate dehydrogenase, were obtained from Thermofisher Scientific (Waltham, MA). Trans-2-phenylcyclopropylamine hydrochloride (tranylcypromine) was purchased from Sigma (Sigma-Aldrich, St. Louis, MO).
To screen e-liquids and flavoring agents for inhibition of CYP2A6, ThermoFisher Scientific Vivid® fluorescence-based CYP2A6 inhibition screening kits were utilized at room temperature, minimizing volatilization of e-liquid flavoring agents. Assays were carried out in Costar medium binding 96-well black polystyrene plates (Fisher Scientific, Waltham, MA). Tranylcypromine, a potent CYP2A6 inhibitor, was utilized as positive control at 100 μM. Vivid® regeneration system and Vivid® microsomal recombinant CYP2A6 Baculosomes® at 10 nanomolar were dispensed to each well containing test chemical for an incubation time of 10 minutes. After 10-minute incubation, the non-fluorescent CYP2A6 substrate 3-cyanocoumarin and NADP+ were added to each well at 10 μM and 30 μM, respectively. CYP2A6 activity was monitored by measuring the production of fluorescence produced by the metabolism of 3-cyanocoumarin into 3-cyano-7-hydroxycoumarin, its fluorescent metabolite, using a BMG LabTech CLARIOstar microplate reader (Cary, NC). Fluorescence due to of 3-cyano-7-hydroxycoumarin was measured in kinetic mode every minute for 60 minutes at excitation λ=415, emission λ=460.
Statistical Analyses.
Data were generated from experiments conducted on three separate days, and were performed in duplicate each day. E-liquid dilutions were formed immediately prior to experiment each day. When appropriate, data were compared to PG/VG vehicle control and positive inhibition control (100 μM tranylcypromine). Fluorescence in the presence of 100 μM tranylcypromine is considered the maximal percent inhibition for microsomal recombinant CYP2A6. Fluorescence for each well was calculated using the equation listed in Equation 1 at 60-minute incubation time.
Equation 1. Percent inhibition equation.
A is the relative fluorescence units observed in the presence of the test compound, B is the relative fluorescence units observed in the presence of known CYP2A6 inhibitor (100 μM tranylcypromine), while C is the relative fluorescence units observed in the PG/VG vehicle control. All fluorescence values used in Equation 1 are at 60-minute incubation time.
Statistical analyses were conducted using GraphPad Software Inc. Prism 8.0.1 (San Diego, CA). Results are expressed as means +/− standard error of the mean (SEM) collected from six individual replicates. Unless otherwise stated, statistical significance is determined as results having a p-value of <0.05. p-values are calculated in GraphPad Prism 8.0.1 using a two-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test to compare case samples to appropriate controls.
The half maximal inhibitory concentration (IC50) of CYP2A6 was calculated for each e-liquid and individual flavoring agent. 95% confidence intervals of IC50 values are listed after each calculated IC50. IC50 values for each e-liquid and flavoring agent were calculated using a four-parameter variable slope function in GraphPad Prism 8.0.1.
RESULTS
Certain Flavored E-Liquids Inhibit Microsomal Recombinant CYP2A6 Activity.
To determine whether e-liquids and e-liquid flavoring agents perturb the activity of CYP2A6, we exposed microsomal recombinant CYP2A6 to PG/VG (vehicle control) or e-liquids in a 96-well plate format, and monitored the production of 3-cyano 7-hydroxycoumarin, the fluorescent metabolite of 3-cyanocoumarin. The vehicle control, 60/40 PG/VG, at 0.125% (v/v) did not impact the activity of microsomal recombinant CYP2A6 (Figure 2). In contrast, at 0.125% (v/v), the highest concentration tested, Apple Watermelon exhibited an average CYP2A6 inhibition of 25.8% relative to maximal inhibition identified utilizing 100 μM tranylcypromine. As illustrated in Figure 3, lower concentrations of Apple Watermelon exhibited negligible microsomal recombinant CYP2A6 inhibition. Strawberry Poptart exhibited near maximal inhibition of CYP2A6 at 0.125% and 0.031% (v/v), and exhibited an average inhibition of 61.5% and 22.7% of microsomal recombinant CYP2A6 at 0.0078% and 0.0019%, respectively. Flamethrower exhibited the most potent inhibition of microsomal recombinant CYP2A6. Near-complete inhibition of microsomal recombinant CYP2A6 was identified down to 0.0078% (v/v). Flamethrower exhibited microsomal recombinant CYP2A6 inhibition of 79.8%, 54.7%, and 22.7% of maximal inhibition at 0.0019% (v/v), 0.00048% (v/v), and 0.00012% (v/v), respectively (Figure 3). Additional control experiments were conducted to ensure dose-dependent decrease in fluorescence observed by e-liquid incubation was due to inhibition of microsomal recombinant CYP2A6 rather than a non-inhibitory mechanism or perturbation in assay conditions. To ensure pH of assay conditions was not altered by e-liquid flavoring agents, the pH of 0.125% (v/v) e-liquid in 0.5X Vivid® Buffer II was measured for each e-liquid. Alterations of the pH of 0.5X Vivid® Buffer II was determined to not have been adversely affected by the presence of any e-liquid at 0.125% (v/v). Background fluorescence was also measured for each e-liquid and was determined to have a negligible effect for each e-liquid (data not shown).
Figure 2.
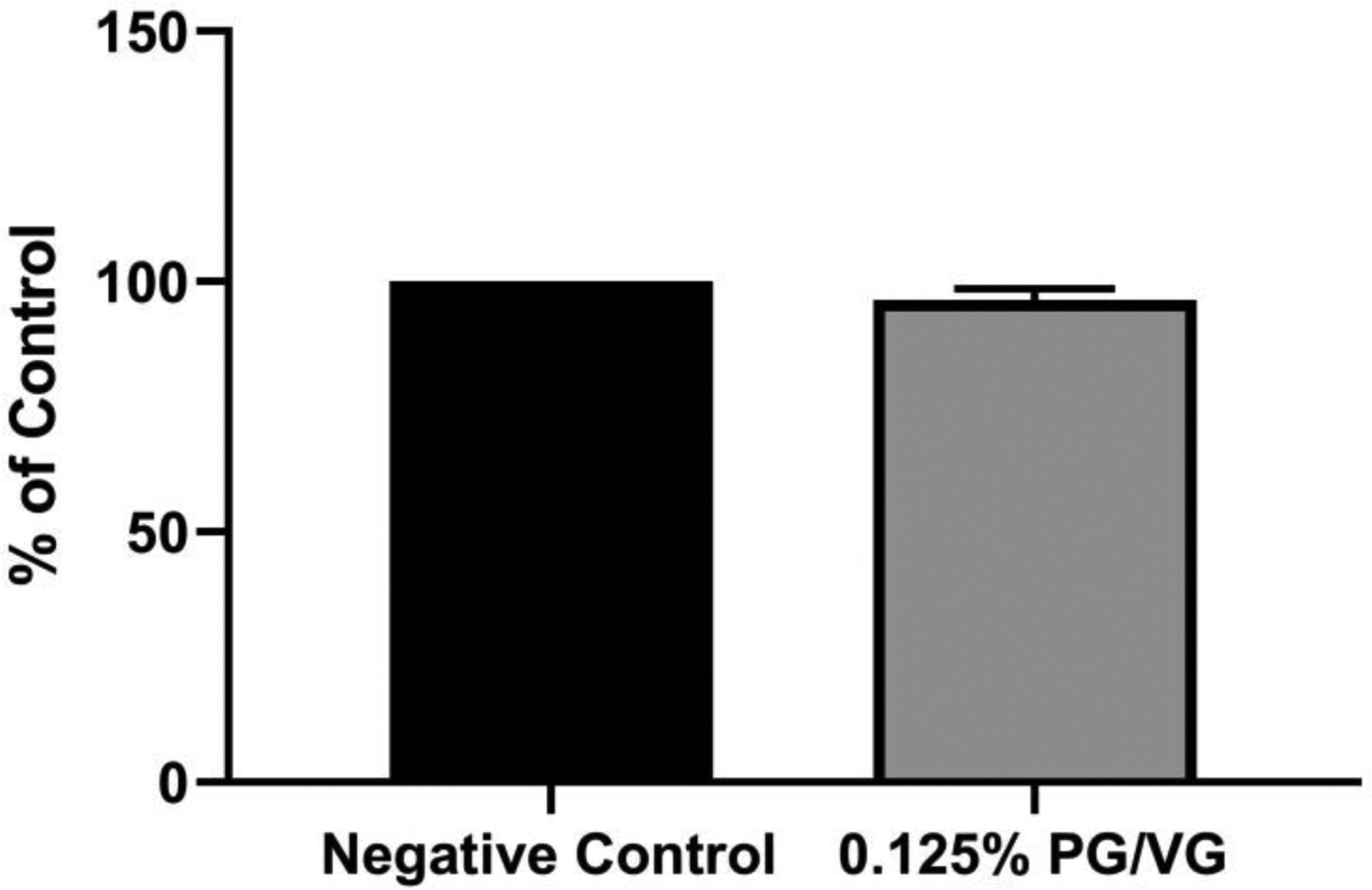
PG/VG effect on microsomal recombinant CYP2A6 activity. 0.125% (v/v) PG/VG (vehicle control) did not significantly decrease activity of microsomal recombinant CYP2A6 at 60-minute incubation time relative to negative control. n=3, mean +/− SEM. Ratio paired t-test identified no significant differences in fluorescence by 0.125%(v/v) PG/VG.
Figure 3.
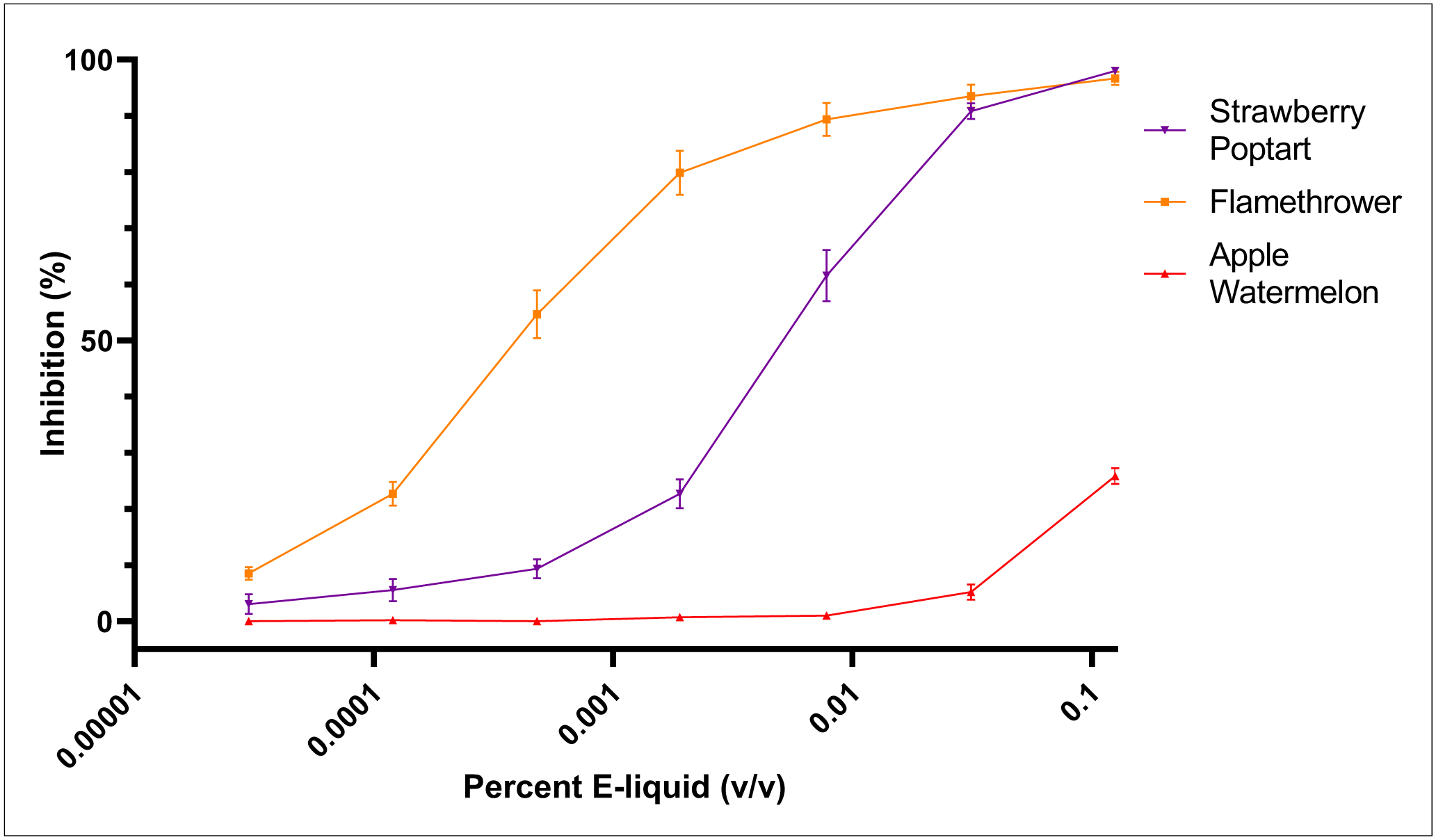
E-liquid inhibition of microsomal recombinant CYP2A6 at 60 minutes. E-liquids were screened for CYP2A6 inhibition at concentrations ranging from 0.000031% to 0.125% (v/v). Flamethrower exhibited inhibition of microsomal recombinant CYP2A6 at concentrations as low as 0.00012%, while Strawberry Poptart exhibited inhibition of microsomal recombinant CYP2A6 at 0.0019% (v/v). Apple Watermelon exhibited only limited inhibition at 0.125% (v/v). Percent inhibition calculated from the PG/VG baseline. Mean +/− SEM, n=3.
To ensure reduced presence of the fluorescent metabolite by Flamethrower and Strawberry Poptart was due to microsomal recombinant CYP2A6 inhibition and not due to flavoring agent interaction with the metabolite, we incubated the fluorescent metabolite, 3-cyano 7-hydroxycoumarin, at 0.5 μM in 0.5% (v/v) of each e-liquid for 60 minutes at room temperature. There was no significant decrease of fluorescence of the metabolite by either Flamethrower or Strawberry Poptart. This indicates that flavored e-liquids and flavoring agents do not interact with 3-cyano 7-hydroxycoumarin to reduce fluorescence at the designated wavelength (data not shown).
The IC50 was calculated using the seven concentrations tested. As seen in Table 1, due to insufficient microsomal recombinant CYP2A6 inhibition, an IC50 was not calculable for Apple Watermelon. The IC50 of Strawberry Poptart and Flamethrower were calculated as 0.0058% (v/v) (0.0048%, 0.0069%), and 0.00038% (v/v) (0.00025%, 0.00052%), respectively. IC50 values are summarized in Table 1.
Table 1.
Summary of Inhibition of Microsomal Recombinant CYP2A6 by Selected E-Liquids at 60 Minutes.a
| Lowest Concentration Tested | Highest Concentration Tested | E-Liquid Name | IC50 of 3-cyano coumarin 7-hydroxylation | IC50 95% confidence interval (lower limit-upper limit) |
|---|---|---|---|---|
| 0.000031% | 0.125% | Apple Watermelon | >0.125% | -- |
| 0.000031% | 0.125% | Strawberry Poptart | 0.0058% | 0.0048–0.0069% |
| 0.000031% | 0.125% | Flamethrower | 0.00038% | 0.00025–0.00052% |
For screening of e-liquids impact on microsomal recombinant CYP2A6 activity, each concentration was screened on three separate days in duplicate (n=3).
Qualitative Mass Spectrometry.
The primary objective of conducting qualitative mass spectrometry on the selected flavored e-liquids was to identify specific flavoring agents within each liquid that may be responsible for inhibition of microsomal recombinant CYP2A6. The e-liquids were analyzed in non-targeted scan mode. If listed below, each identified flavoring agent was confirmed using Agilent GC-MS National Institute of Standards and Technology (NIST) mass spectral library. Retention time as well as R-match scores, based on retention time and mass spectral patterns, are also listed for each identified flavoring agent. Chromatograms are shown in Figure 4A–D. As expected, PG (1.8 min) and VG (4.3 min) were detected in all e-liquids, including the vehicle control. Apple Watermelon also contained triglyceride 1,2,3-triacetoxypopane (6.7 min, R-match: 850), commonly known as triacetin (Figure 4B). Triacetin is a humectant and flavoring agent commonly found in e-liquids33. Strawberry Poptart contained triacetin (6.7 min, R-Match: 873), vanillin (7.2 min, R-Match: 896), and ethyl vanillin (7.6 min, R-Match: 877) (Figure 4C). Additional peaks were visible in the Strawberry Poptart chromatogram but were not identifiable with high confidence using the NIST library without the use of analytical standards. The most plausible matches for the peaks are benzyl alcohol (4.3 min) and acetaldehyde (5.9 min). Flamethrower contained cinnamaldehyde (6.4 min, R-Match: 929) (Figure 4D). Additional peaks were visible in the in the Flamethrower chromatogram but were not identifiable with high confidence using the NIST library without the use of analytical standards. The most plausible matches for the peaks at 8.4 and 9.9 minutes are cinnamyl alcohols. Trans-cinnamaldehyde had some matching; however, the trans-cinnamaldehyde analytical standard eluted considerably earlier than 8.4 minutes and the mass spectra of the two unidentified peaks at 8.4 and 9.9 minutes did not resemble the mass spectra of the mass spectra of the trans-cinnamaldehyde standard. Furthermore, R-match scores for the two unidentified peaks were higher for a cinnamyl alcohol than trans-cinnamaldehyde. Therefore, we believe the unidentified peaks are more likely a cinnamyl alcohol. Qualitative mass spectrometry was also conducted to ensure no nicotine was present in e-liquids labeled as nicotine-free.
Figure 4.
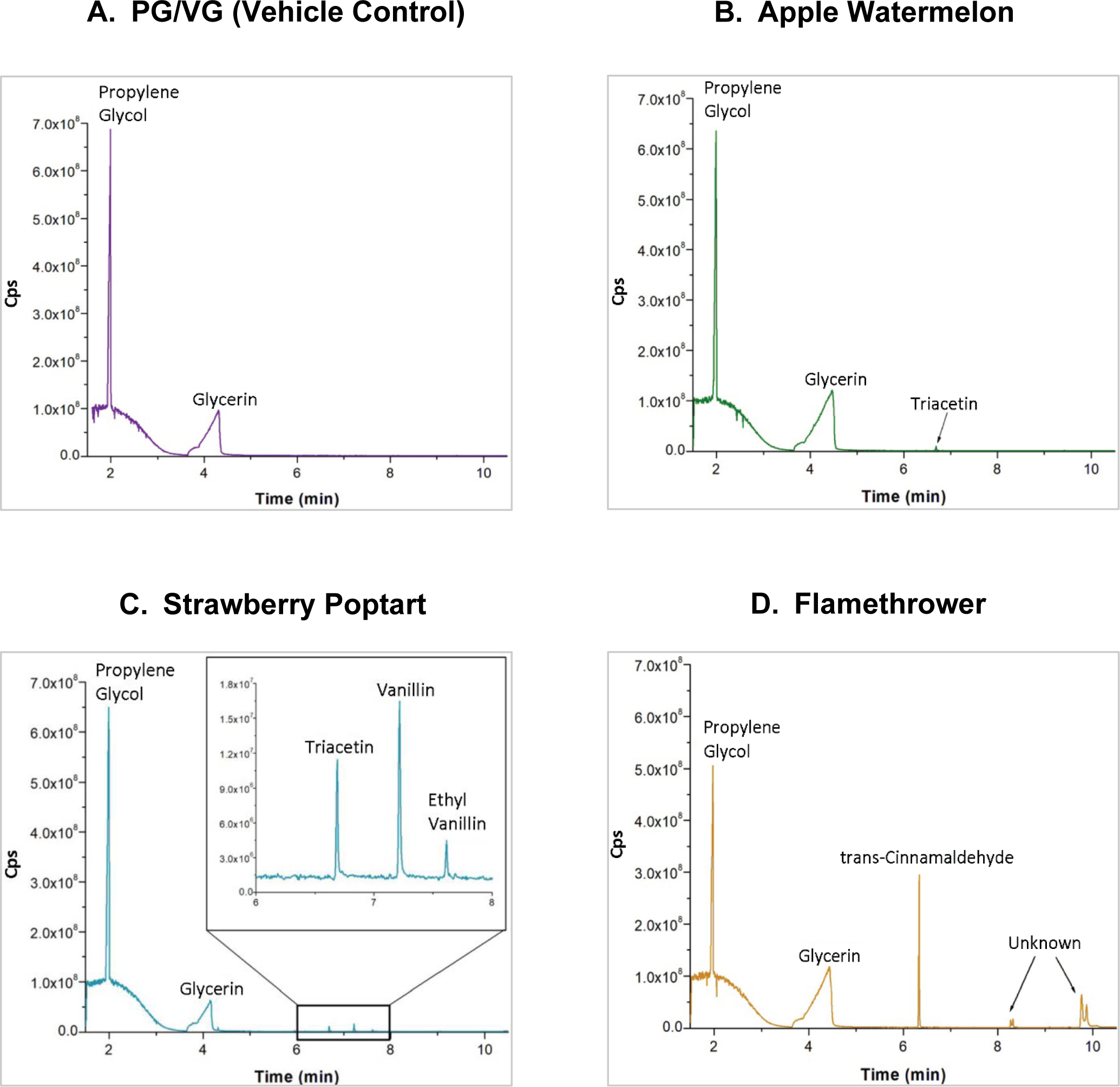
Chromatograms of PG/VG (vehicle control) and selected flavored e- liquids. A. PG/VG vehicle control chromatogram peaks were identified as PG (1.8 min) and VG (4.3 min). B. Apple Watermelon chromatogram flavoring agent peak was identified as triacetin (6.7 min). C. Strawberry Poptart chromatogram flavoring agent peaks were identified as triacetin (6.7 min), vanillin (7.2 min), and ethyl vanillin (7.6 min). D. Flamethrower chromatogram flavoring agent peak was identified as and trans-cinnamaldehyde (6.4 min).
Certain E-Liquid Flavoring Agents Inhibit Microsomal Recombinant CYP2A6 Activity.
To examine what specific flavoring agents may impact microsomal recombinant CYP2A6 activity, and thereby potentially affecting the metabolism of nicotine in e-cigarette users, we subsequently screened the three flavoring agents identified using qualitative mass spectrometry in e-liquids that inhibited microsomal recombinant CYP2A6. In addition to cinnamaldehyde, vanillin, and ethyl vanillin, two other flavoring agents that are commonly found in e-liquids, benzaldehyde and isoamyl acetate, were screened for inhibition of microsomal recombinant CYP2A634–36. Isoamyl acetate, the only non-aldehyde screened for impacts to microsomal recombinant CYP2A6 activity, was selected for comparison. E-liquid flavoring agents were screened for inhibition of CYP2A6 at eight concentrations ranging from 0.03 μM to 500 μM. As triacetin was present in an e-liquid (Apple Watermelon) that had a negligible effect on microsomal recombinant CYP2A6 activity, it was not screened for inhibition of microsomal recombinant CYP2A6 activity.
The e-liquid flavoring agents were screened using the same assay conditions as e-liquids. Because flavoring agents were dissolved directly into Vivid® reaction buffer II prior to experimental use, no vehicle control was necessary. The fluorescence intensity in the absence of an inhibitor or test chemical was treated as the maximal microsomal recombinant CYP2A6-mediated 3-cyano 7-hydroxycoumarin formation. As data in Figure 5 illustrate, benzaldehyde and cinnamaldehyde were the most potent inhibitors of microsomal recombinant CYP2A6. Both benzaldehyde and cinnamaldehyde exhibited a strong dose-dependent microsomal recombinant CYP2A6 inhibition. An IC50 value of 1.1 μM (0.65 μM, 1.67 μM) was identified for cinnamaldehyde, indicating cinnamaldehyde is a potent inhibitor of microsomal recombinant CYP2A6. Benzaldehyde also appeared to be a potent inhibitor of microsomal recombinant CYP2A6, with an IC50 of 3.0 μM (1.56 μM, 5.37 μM). The two heavier aromatic aldehydes, vanillin and ethyl vanillin, exhibited significantly lower potency for microsomal recombinant CYP2A6 inhibition. Vanillin and ethyl vanillin exhibited a nearly identical dose-response curve, with near-maximal inhibition occurring only at 500 μM. The calculated IC50 values for vanillin and ethyl vanillin were 70.0 μM (46.5 μM, 164 μM) and 69.4 μM (57.0 μM , 89.3 μM), respectively. Isoamyl acetate, the sole non-aldehyde tested, exhibited negligible inhibition of microsomal recombinant CYP2A6 up to 500 μM, suggesting that aromatic aldehydes may be more apt to inhibit microsomal recombinant CYP2A6. Due to limited inhibition of microsomal recombinant CYP2A6, an IC50 was not calculable for isoamyl acetate. IC50 values of flavoring agents are summarized in Table 2.
Figure 5.
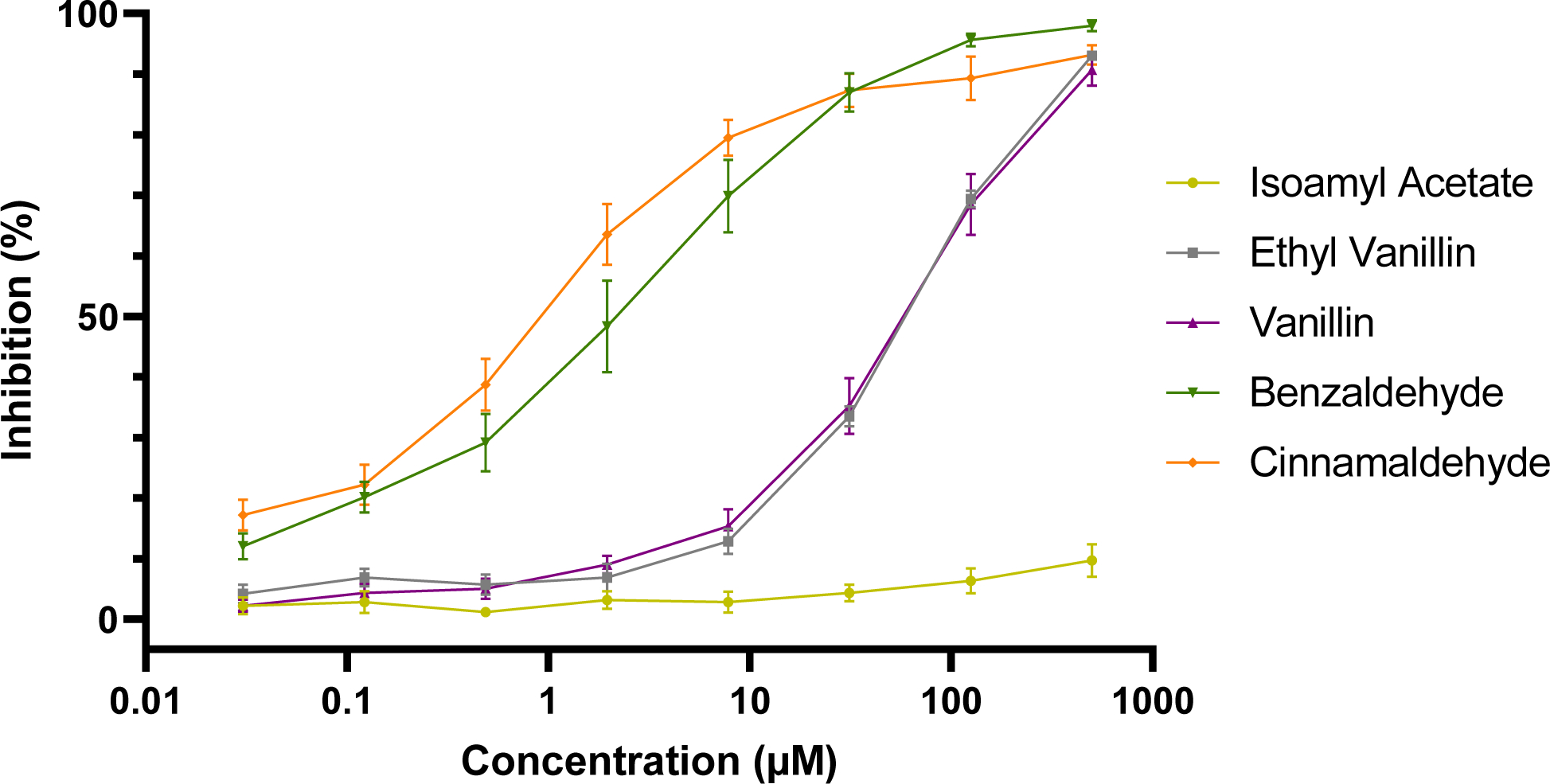
E-liquid flavoring agent inhibition of microsomal recombinant CYP2A6. Cinnamaldehyde and benzaldehyde exhibited the strongest dose-dependent inhibition of microsomal recombinant CYP2A6. Vanillin and ethyl vanillin exhibited a less potent inhibition of microsomal recombinant CYP2A6. Isoamyl acetate exhibited no inhibition at even the highest concentration. Each concentration was screened on three separate days in duplicate (n=3). Mean +/− SEM.
Table 2.
Summary of Inhibition of Microsomal Recombinant CYP2A6 by E-Liquid Flavoring Agents
| Lowest Concentration Tested |
Highest Concentration Tested | Flavoring Agent | IC50 of 3-cyano coumarin 7-hydroxylation | IC50 95% confidence interval (lower limit-upper limit) |
|---|---|---|---|---|
| 0.03 μM | 500 μM | Isoamyl Acetate | > 500 μM | -- |
| 0.03 μM | 500 μM | Vanillin | 70.0 μM | 46.5–164 μM |
| 0.03 μM | 500 μM | Ethyl vanillin | 69.4 μM | 57.0–89.3 μM |
| 0.03 μM | 500 μM | Benzaldehyde | 3.0 μM | 1.6–5.5 μM |
| 0.03 μM | 500 μM | Cinnamaldehyde | 1.1 μM | 0.65–1.7 μM |
Quantitative Mass Spectrometry.
Quantitative GC-MS analysis was used to identify the concentration of cinnamaldehyde in Flamethrower, as well as the concentration of vanillin and ethyl vanillin in Strawberry Poptart. GC-MS revealed a cinnamaldehyde concentration of 295.3 mM in Flamethrower. Vanillin in Strawberry Poptart was detected at 26.4 mM, while ethyl vanillin was found at 7.3 mM.
DISCUSSION
In the present study, purified microsomal recombinant CYP2A6 was used to evaluate the potential for e-liquids and common e-liquid flavoring agents to inhibit CYP2A6, the enzyme that metabolizes approximately 80% of nicotine in the body. Reduced activity of CYP2A6, whether reduced-function genotype or pharmacological inhibition, has been shown to alter serum concentrations of nicotine37, 38. The data presented here indicate certain e-liquids and common flavoring agents of e-liquids are capable of inhibiting microsomal recombinant CYP2A6, which may impact nicotine metabolism in vivo.
As flavored e-liquids are a complex mixture of chemicals, it was revealing to test the impact of the e-liquid as a whole rather than relying on findings of individual flavoring agents. A mixture may cause an effect that is notably different than the sum of effects of individual chemicals that comprise the mixture. The primary concern is that certain chemicals within the mixture may reduce or enhance the effect of other chemicals within the mixture, termed a cocktail effect.
To determine whether the primary flavoring agents are responsible for the inhibition of CYP2A6 by the flavored e-liquids, we utilized the quantitative mass spectrometry results of cinnamaldehyde in Flamethrower and the vanillin and ethyl vanillin levels in Strawberry Poptart. As illustrated in Table 3, we calculated what we would expect the IC50 to be for Flamethrower based on the measured IC50 of 1.1 μM identified for cinnamaldehyde in the microsomal recombinant CYP2A6 assay and the measured concentration of cinnamaldehyde in Flamethrower. Based on the measured cinnamaldehyde IC50 and the cinnamaldehyde concentration in Flamethrower of 295 mM, we would expect an IC50 of 0.00037% (v/v) for the e-liquid. This value is nearly identical to the measured IC50 value of 0.00038% (v/v) for Flamethrower. As illustrated in the microsomal recombinant CYP2A6 assay, this finding indicates the inhibition of CYP2A6 by Flamethrower is likely predominantly driven by the presence of cinnamaldehyde.
Table 3.
Identified Vs. Expected microsomal recombinant IC50 values of E-liquids.
| E-Liquid Flavoring | Primary Flavoring Agent | Measured IC50 of Primary Flavoring Agent | Concentration of Flavoring Agent in E-liquid | Expected IC50 of E- liquid Based on Flavoring IC50 and Concentration | Identified IC50 of E-liquid |
|---|---|---|---|---|---|
| Flamethrower | Cinnamaldehyde | 1.1 μM | 295 mM | 0.00037% | 0.00038% |
| Strawberry Poptart | Vanillin Ethyl vanillin | Vanillin: 70 μM Ethyl Vanillin: 69.4 μM | Vanillin: 26.4 mM Ethyl vanillin: 7.3 mM | 0.21% (additive) | 0.0058% |
To determine whether vanillin and ethyl vanillin were the flavoring agents primarily responsible for inhibiting microsomal recombinant CYP2A6 additively, we calculated what we would expect the IC50 for Strawberry Poptart to be based on the measured vanillin concentration of 26.4 mM and ethyl vanillin concentration of 7.3 mM, in conjunction with measured IC50 of 70 μM and of 69.4 μM, respectively. Based on the concentration and measured IC50 of the two primary flavoring agents, we would expect an IC50 of CYP2A6 for Strawberry Poptart to be 0.21% (v/v) if the chemicals acted on CYP2A6 additively (Table 3). This is markedly higher than the measured IC50 of 0.0058% (v/v) for Strawberry Poptart for microsomal recombinant CYP2A6. While additional experiments are necessary to identify the cause of the difference in potency, it is possible the chemicals that formed the unidentified peaks in the qualitative mass spectrometry chromatogram may also exhibit an inhibitory effect on microsomal recombinant CYP2A6. Additionally, the possibility of a synergistic effect on CYP2A6 inhibition between the chemicals must be investigated.
While we tested only a small fraction of e-liquids currently on the marketplace for inhibition of microsomal recombinant CYP2A6 activity, we believe our findings from five common e-liquid flavoring agents are relevant to a broad portion of e-liquids currently available on the market. All four of the flavoring agents that exhibited some extent of inhibition of microsomal recombinant CYP2A6 were aromatic aldehydes. The only non-aromatic aldehyde tested was isoamyl acetate, which had no impact on the activity of CYP2A6. While the number of e-liquids and flavoring agents screened in the present study was limited, CYP2A6 appears to be prone to inhibition by aromatic aldehydes. The mechanism of CYP2A6 inhibition by aromatic aldehydes is not known. However, interaction with a cysteine or lysine residue has been suggested as a possible mechanism of inhibition30. The two lower molecular weight aldehydes, benzaldehyde (MW: 106.12) and cinnamaldehyde (MW: 132.16), were the more potent of the aromatic aldehydes for CYP2A6 inhibition. The two vanillin derivatives, vanillin (MW: 152.15) and ethyl vanillin (MW: 166.16), were less potent than benzaldehyde and cinnamaldehyde.
The question of biological relevance is inherently important when interpreting results from an isolated enzyme system. E-liquid flavoring agents will be delivered concomitantly with nicotine via inhalation. Because CYP2A6, like the vast majority of cytochrome P450s, is predominantly found in the liver, e-liquid flavoring agents must first be readily absorbed into the bloodstream in order to interact with hepatic CYP2A6. While nicotine in e-cigarettes is readily absorbed through epithelial cells and into the bloodstream, there is limited information on the deposition, absorption, and distribution of the various e-liquid flavoring agents when inhaled, as well as limited data on the amount and site of deposition of e-cigarette aerosols particles in general.
Due to the limited data on absorption and distribution of the tested flavoring agents via inhalation, it is not currently feasible to determine what effect the inhalation of such flavored e-liquids may have on activity of hepatic CYP2A6 in vivo. Since many e-liquid flavoring agents are routinely ingested via diet, an argument has been made that the inhalation of such flavoring agents is unlikely to have a stronger impact on CYP2A6 activity than orally administered flavoring agents. However, e-cigarette aerosols are rapidly delivered to the respiratory tract when inhaled, and bioavailability may be altered when flavoring agents are inhaled as compared to ingested. Therefore, it is not appropriate to compare exposure of aromatic aldehydes via ingestion to exposure to aromatic aldehydes via inhalation. Additional research is necessary to determine the absorption of various e-liquid flavoring agents to the bloodstream in order to determine whether inhibition of CYP2A6 in vivo may impact nicotine metabolism.
CONCLUSIONS
The data presented here indicate certain e-liquids and a subset of common e-liquid flavoring agents inhibit microsomal recombinant CYP2A6 to varying degrees. While only preliminary, these findings may be relevant to current and emerging concerns regarding health impacts of nicotine in e-cigarettes as well as the impact of flavorings on nicotine pharmacokinetics. This study highlights the need for investigation of possible synergistic effect of chemicals in e-liquids on CYP2A6 inhibition, confirmation of the microsomal recombinant CYP2A6 finding in a cell-based system, as well as the need for additional research on absorption of e-liquid flavoring agents into the bloodstream from the respiratory tract.
ACKNOWLEDGMENTS
The authors would like to thank Dr. James Beaudoin and Dr. Kim Brouwer for their assistance on data analysis and manuscript feedback.
Funding
This research was funded by National Institutes of Health Grant Nos. R01 HL139369 and T32 ES007126, and the US EPA-UNC Toxicology Training Agreement CR-83591401-0.
ABBREVIATIONS
- ANOVA
Analysis of variance
- CYP2A6
Cytochrome P450 2A6
- CYP2A13
Cytochrome P450 2A13
- CYP450
Cytochrome P450
- FEMA
The Flavors and Extracts Manufacturers Association
- GC-MS
Gas chromatography-mass spectrometry
- IC50
Half maximal inhibitory concentration
- mM
millimolar
- MW
Molecular weight
- NIST
National Institute of Standards and Technology
- PG
Propylene glycol
- SEM
Standard error of the mean
- μM
micromolar
- VG
Vegetable glycerin
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: DISCLAIMER
The research described in this article has been reviewed by the US Environmental Protection Agency Center for Public Health and Environmental Assessment and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
REFERENCES
- 1.Raunio H; Rahnasto-Rilla M, CYP2A6: genetics, structure, regulation, and function. Drug Metabol Drug Interact 2012, 27 (2), 73–88. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan S, Handbook of pharmacogenomics and stratified medicine. 2014; p 1.online resource (xxiii, 1093 pages). [Google Scholar]
- 3.Ding X; Kaminsky LS, Human Extrahepatic Cytochromes P450: Function in Xenobiotic Metabolism and Tissue-Selective Chemical Toxicity in the Respiratory and Gastrointestinal Tracts. Annu Rev Pharmacol Toxicol 2003, 43 (1), 149–173. [DOI] [PubMed] [Google Scholar]
- 4.Chiang HC; Wang CK; Tsou TC, Differential distribution of CYP2A6 and CYP2A13 in the human respiratory tract. Respiration 2012, 84 (4), 319–26. [DOI] [PubMed] [Google Scholar]
- 5.Raunio H; Rautio A; Pelkonen O, The CYP2A subfamily: function, expression and genetic polymorphism. IARC Sci Publ 1999, (148), 197–207. [PubMed] [Google Scholar]
- 6.Nakajima M; Yamamoto T; Nunoya K; Yokoi T; Nagashima K; Inoue K; Funae Y; Shimada N; Kamataki T; Kuroiwa Y, Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos 1996, 24 (11), 1212–7. [PubMed] [Google Scholar]
- 7.Tyndale RF; Sellers EM, Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Metab Dispos 2001, 29 (4 Pt 2), 548–52. [PubMed] [Google Scholar]
- 8.Williams DE; Shigenaga MK; Castagnoli N Jr., The role of cytochromes P-450 and flavin-containing monooxygenase in the metabolism of (S)-nicotine by rabbit lung. Drug Metab Dispos 1990, 18 (4), 418–28. [PubMed] [Google Scholar]
- 9.Xia X-Y; Peng R-X; Yu J-P; Wang H; Wang J, In vitro metabolic characteristics of cytochrome P-450 2A6 in Chinese liver microsomes. Acta pharmacologica Sinica 2002, 23 (5), 471–476. [PubMed] [Google Scholar]
- 10.Sellers EM; Kaplan HL; Tyndale RF, Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clin Pharmacol Ther 2000, 68 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K.-i.; Kamataki T, Screening Of Organosulfur Compounds as Inhibitors of Human CYP2A6. Drug metabolism and disposition 2001, 29 (7), 983–989. [PubMed] [Google Scholar]
- 12.Benowitz NL; Herrera B; Jacob P 3rd, Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther 2004, 310 (3), 1208–15. [DOI] [PubMed] [Google Scholar]
- 13.Pianezza ML; Sellers EM; Tyndale RF, Nicotine metabolism defect reduces smoking. Nature 1998, 393 (6687), 750. [DOI] [PubMed] [Google Scholar]
- 14.Audrain-McGovern J; Al Koudsi N; Rodriguez D; Wileyto EP; Shields PG; Tyndale RF, The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics 2007, 119 (1), e264–74. [DOI] [PubMed] [Google Scholar]
- 15.Liu T; David SP; Tyndale RF; Wang H; Zhou Q; Ding P; He Y-H; Yu X-Q; Chen W; Crump C; Wen X-Z; Chen W-Q, Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction (Abingdon, England) 2011, 106 (5), 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Koudsi N; O’Loughlin J; Rodriguez D; Audrain-McGovern J; Tyndale RF, The genetic aspects of nicotine metabolism and their impact on adolescent nicotine dependence. Journal of Pediatric Biochemistry 2010, 1 (02), 105–123. [Google Scholar]
- 17.King BA; Alam S; Promoff G; Arrazola R; Dube SR, Awareness and ever-use of electronic cigarettes among US adults, 2010–2011. Nicotine & tobacco research 2013, 15 (9), 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agaku IT; King BA; Husten CG; Bunnell R; Ambrose BK; Hu SS; Holder-Hayes E; Day HR, Tobacco product use among adults—United States, 2012–2013. MMWR. Morbidity and mortality weekly report 2014, 63 (25), 542. [PMC free article] [PubMed] [Google Scholar]
- 19.McMillen R; Maduka J; Winickoff J, Use of emerging tobacco products in the United States. Journal of environmental and public health 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu S-H; Gamst A; Lee M; Cummins S; Yin L; Zoref L, The use and perception of electronic cigarettes and snus among the US population. PloS one 2013, 8 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olmedo P; Goessler W; Tanda S; Grau-Perez M; Jarmul S; Aherrera A; Chen R; Hilpert M; Cohen JE; Navas-Acien A, Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect 2018, 126 (2), 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spindle TR; Talih S; Hiler MM; Karaoghlanian N; Halquist MS; Breland AB; Shihadeh A; Eissenberg T, Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug and alcohol dependence 2018, 188, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grana R; Benowitz N; Glantz SA, E-cigarettes: a scientific review. Circulation 2014, 129 (19), 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S-H; Sun JY; Bonnevie E; Cummins SE; Gamst A; Yin L; Lee M, Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco control 2014, 23 (suppl 3), iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klager S; Vallarino J; MacNaughton P; Christiani DC; Lu Q; Allen JG, Flavoring chemicals and aldehydes in e-cigarette emissions. Environmental science & technology 2017, 51 (18), 10806–10813. [DOI] [PubMed] [Google Scholar]
- 26.van Rooy FG; Rooyackers JM; Prokop M; Houba R; Smit LA; Heederik DJ, Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med 2007, 176 (5), 498–504. [DOI] [PubMed] [Google Scholar]
- 27.Khlystov A; Samburova V, Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environmental science & technology 2016, 50 (23), 13080–13085. [DOI] [PubMed] [Google Scholar]
- 28.Tani N; Juvonen RO; Raunio H; Fashe M; Leppänen J; Zhao B; Tyndale RF; Rahnasto-Rilla M, Rational design of novel CYP2A6 inhibitors. Bioorganic & medicinal chemistry 2014, 22 (23), 6655–6664. [DOI] [PubMed] [Google Scholar]
- 29.Clapp PW; Pawlak EA; Lackey JT; Keating JE; Reeber SL; Glish GL; Jaspers I, Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. American Journal of Physiology-Lung Cellular and Molecular Physiology 2017, 313 (2), L278–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan J; Oshiro T; Thomas S; Higa A; Black S; Todorovic A; Elbarbry F; Harrelson JP, Inactivation of CYP2A6 by the dietary phenylpropanoid trans-cinnamic aldehyde (cinnamaldehyde) and estimation of interactions with nicotine and letrozole. Drug Metabolism and Disposition 2016, 44 (4), 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahnasto M; Raunio H; Poso A; Juvonen R, More potent inhibition of human CYP2A6 than mouse CYP2A5 enzyme activities by derivatives of phenylethylamine and benzaldehyde. Xenobiotica 2003, 33 (5), 529–539. [DOI] [PubMed] [Google Scholar]
- 32.Tierney PA; Karpinski CD; Brown JE; Luo W; Pankow JF, Flavour chemicals in electronic cigarette fluids. Tobacco control 2016, 25 (e1), e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farley SM; Schroth KR; Grimshaw V; Luo W; DeGagne JL; Tierney PA; Kim K; Pankow JF, Flavour chemicals in a sample of non-cigarette tobacco products without explicit flavour names sold in New York City in 2015. Tobacco control 2018, 27 (2), 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behar RZ; Luo W; Lin SC; Wang Y; Valle J; Pankow JF; Talbot P, Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tobacco control 2016, 25 (Suppl 2), ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fetterman JL; Weisbrod RM; Feng B; Bastin R; Tuttle ST; Holbrook M; Baker G; Robertson RM; Conklin DJ; Bhatnagar A, Flavorings in tobacco products induce endothelial cell dysfunction. Arteriosclerosis, thrombosis, and vascular biology 2018, 38 (7), 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosmider L; Sobczak A; Prokopowicz A; Kurek J; Zaciera M; Knysak J; Smith D; Goniewicz ML, Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 2016, 71 (4), 376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benowitz NL; Swan GE; Jacob P III; Lessov-Schlaggar CN; Tyndale RF, CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clinical Pharmacology & Therapeutics 2006, 80 (5), 457–467. [DOI] [PubMed] [Google Scholar]
- 38.Sellers EM; Ramamoorthy Y; Zeman MV; Djordjevic MV; Tyndale RF, The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine & tobacco research 2003, 5 (6), 891–899. [DOI] [PubMed] [Google Scholar]


