Abstract
Study Objectives:
We sought to estimate the impact of sentinel nodes in gynecologic oncology on fellowship training and discuss potential solutions.
Design:
Retrospective multi-institution cohort, II-2
Setting:
Three tertiary cancer referral cancer centers
Patients:
Patients with endometrial and vulvar cancer undergoing lymph node evaluation.
Interventions:
Patient history and fellow case volumes were evaluated retrospectively for type of lymph node assessment.
Measurements:
Minimally invasive endometrial cancer and vulvar cancer fellow case volumes in three large institutions were reviewed, and average annual volumes calculated for each clinical gynecologic oncology fellow. For vulvar cancer, probabilities of sentinel lymph node mapping and laterality of lesions were estimated from the literature. For endometrial cancer, estimates of lymphadenectomy rates were determined using probabilities calculated from our historic database and from review of the literature.
Main Results:
Modeling the approaches to lymphadenectomy in endometrial cancer (full, selective, sentinel), 100% versus 68% versus 24% respectively of patients would require complete pelvic lymphadenectomy and 100% versus 34% versus 12% would require para-aortic lymphadenectomy. In vulvar cancer, rates of inguinal femoral lymphadenectomy are expected to drop from 81% of unilateral groins to only 12% of groins.
Conclusions:
Sentinel lymph node biopsy for endometrial and vulvar cancer will play an increasing role in practice, and coincident with this will be a dramatic decrease in pelvic, para-aortic and inguinofemoral lymphadenectomies. The declining numbers will require new strategies to maintain competency in our specialty. New approaches to surgical training and continued medical education will be necessary to ensure adequate training for fellows and young faculty across gynecologic surgery.
Keywords: vulvar cancer, endometrial cancer, sentinel lymph nodes, surgical training
Precis:
Sentinel node excision is rapidly replacing full lymphadenectomy in gynecologic oncology and will have significant impact on trainee experience and volume.
Introduction
Multiple recent papers and editorials have stressed the importance of high volume surgeons in gynecologic surgical outcomes [1–5]. As surgical fields evolve and adopt new, less invasive techniques, training programs must also adapt teaching methods to provide the experience needed to train proficient surgeons. New gynecologic oncology fellows graduate from residency with less surgical experience to start their advanced training and receive less training in their fellowships [6–7]. Maintaining proficiency in now rarer procedures is a challenge for surgical specialties [8]. One such surgical procedure is lympadenectomy which is a principle component of cancer staging. To the advantage of our patients, advances in surgical technique have decreased the need for routine full lymphadenectomy (LND). As seen in breast cancer and melanoma, sentinel lymph node (SLN) evaluation is replacing full LND in patients with endometrial, cervical, and vulvar cancer.
The approach to lymph node assessment in endometrial cancer has evolved throughout the years. Routine LND initially was advocated by many [9–11], however large studies have failed to demonstrate therapeutic benefit of such an approach in early stage disease [12,13]. Alternative approaches which rely on tumor characteristics to select those at highest risk of lymph node involvement have evolved to minimize the number of patients undergoing LND [14–17]. SLN evaluation has more recently been applied to endometrial cancer [18–24]. This approach is gaining acceptance and the most recent National Comprehensive Cancer Network (NCCN) guidelines for endometrial cancer include the option of SLN evaluation in lieu of full lymphadenectomy.
Similarly, the GOG173 and GROINSS-V studies examined the feasibility and safety of SLN biopsy in vulvar squamous cell cancers [25–27]. These studies confirmed the safety and reliability of SLN biopsy in unifocal vulvar cancers less than 4 cm, reducing the need for full inguinal femoral LND in the majority of patients with vulvar cancer. NCCN guidelines support the use of SLN evaluation in vulvar cancer and this is becoming the standard of care approach in most centers.
The objective of this study is to model the impact of SLN approach for vulvar and endometrial cancer on surgical training volumes. We also outline suggestions to offset the impact of decreasing volumes on surgical proficiency with thoughtful strategies to augment surgical and anatomic training.
Materials and Methods:
IRB approval was obtained for this study. Mayo Clinic, Memorial Sloan Kettering Cancer Center (MSKCC), and MD Anderson Cancer Center (MDACC), reviewed their fellow reported case volumes from 2012-2015 and determined the total number of minimally invasive endometrial cancer cases and early vulvar cancer cases that were performed per fellow and in each institution. Mean annual numbers were used to estimate the number of cases performed per year per fellow. The number of clinical fellows at each institution used to calculate cases/year was Mayo Clinic (n=4), MSKCC (n=6) and MDACC (n=6).
The approaches to lymph node assessment in endometrial cancer were classified as follows: A) routine complete pelvic and para-aortic LND for all patients, B) selective pelvic and para-aortic LND based on primary tumor factors, and C) SLN (Figure 1). When approach A (routine systematic pelvic and para-aortic LND) is performed, all patients undergo pelvic and para-aortic LND [7]. In approach B, need and extent of LND was based on tumor characteristics at the time of surgery. Approach C (SNL) will result in pelvic and possible para-aortic LND for those patients without sentinel node mapping. When SLN did not map, we assumed approach B would be applied and patients would undergo a pelvic and para-aortic LND based on primary tumor factors. The number of single sided pelvic and para-aortic LND that would be performed using each approach was estimated. Four assumptions are made in the model: 1) all patients are candidates for LND regardless of age, BMI or other co-morbidities, 2) lymph node evaluation was for staging, excluding cases with pre-operatively enlarged lymph nodes on CT scan, 3) rates of non-mapping for SLN is equally distributed across grades and depths of invasion, and 4) when a positive SLN is identified, no further LND is performed.
Figure 1:
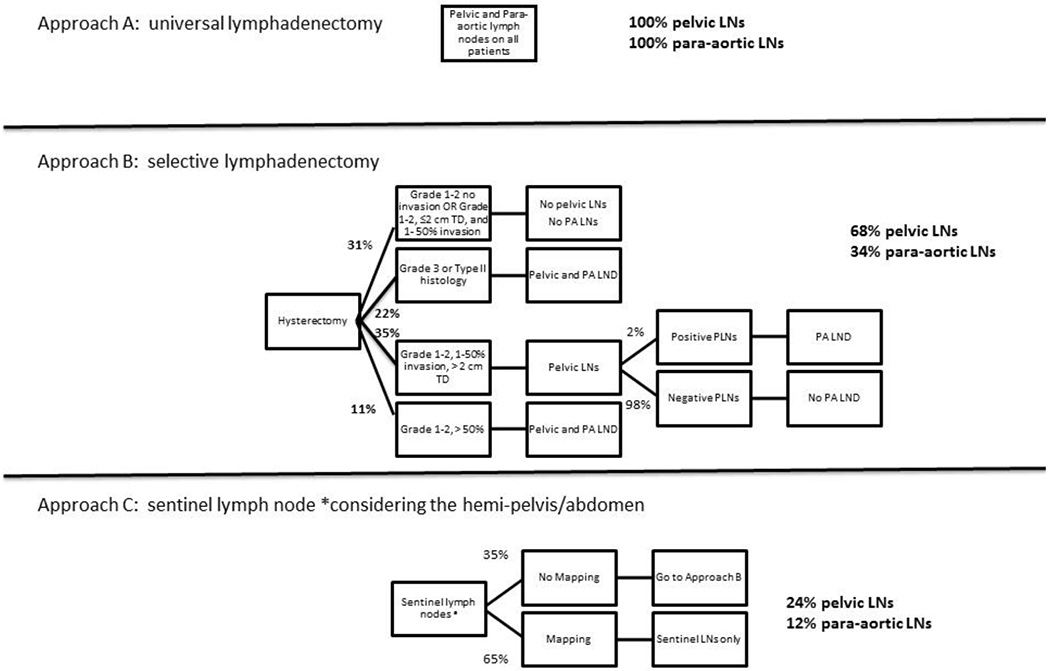
3 approaches to lymph node evaluation in endometrial cancer and resultant rates of full lymphadenectomy. Approach A is universal lymphadenectomy, Approach B uses tumor pathologic features including grade, tumor diameter, and depth of myometrial invasion to determine need for pelvic and para-aortic lymphadenectomy, and Approach C used sentinel lymph nodes, assuming a 50% bilateral mapping rate and 80% unilateral mapping rate.
For vulvar cancer, two approaches to LND were considered: 1) full inguinal femoral LND and 2) SLN biopsy (Figure 2). Rates of lymph node mapping and rates of bilateral mapping were estimated from the literature and the number of single sided inguinal femoral LND was estimated for each approach [25–27]. Assumptions in the model are 1) all candidates for radical vulvectomy are candidates for SLN biopsy, 2) no patients have palpable or image-positive inguinal lymph nodes, 3) lateral lesions drain unilaterally and midline lesions drain bilaterally, and 4) if a positive SLN is identified, no further LND is carried out.
Figure 2:
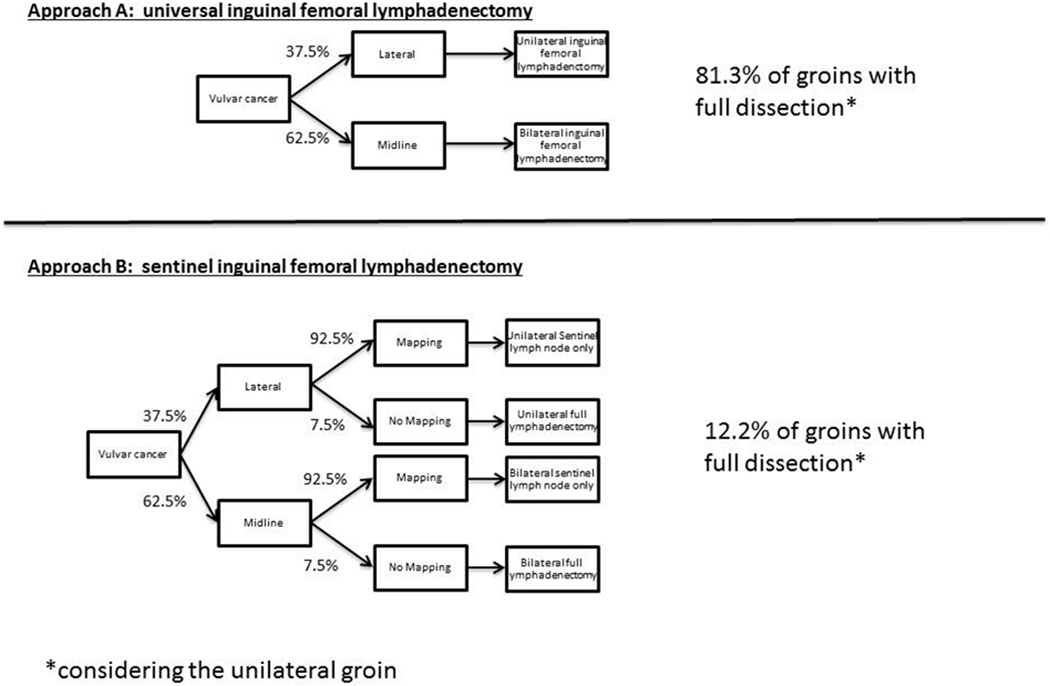
2 approaches to lymph node evaluation in vulvar cancer and resultant rates of full lymphadenectomy. Approach A is universal lymphadenectomy, and depending on laterality of mass, 81% of groins will require lymphadenectomy. Approach B is sentinel lymphadenectomy.
Results:
As described above for endometrial cancer, approach A will result in 100% of patients having pelvic and para-aortic lymphadenectomy. In a simplified selective lymphadenectomy approach as described in Figure 1, LND is omitted in patients with grade 1-2 disease without myometrial invasion or with ≤50% myometrial invasion and tumors ≤2 cm in size. Grade 3 endometriod, type II histology or > 50% myometrial invasion underwent full pelvic and para-aortic LND. All other patients underwent pelvic LND with reflex para-aortic LND. A historical database including 1184 patients without stage IV disease who had surgery from 1999-2008 was reviewed to determine rates of LND when using tumor characteristics. When approach B (selective LND based on primary tumor factors) is performed, 68% of patients will undergo bilateral pelvic LND. In addition 34% will undergo para-aortic LND [15, 28]. Approach C is sentinel lymph node approach. A review of the literature shows an overall mapping rate of 51-94%, with higher rates with the use of indocyanine green (ICG) dye and increased experience [18,20, 21,26–36]. Using the most conservative estimates, a unilateral SLN will be identified in 80% of hemi-pelvises with bilateral mapping seen in 50% of cases. This will result in 35% of possible hemi-pelvises without mapping. In non-mapping cases, if the uterine factor approach was applied (Approach B), this will lead to a 24% rate of single-sided pelvic LND and 12% of patients will undergo para-aortic lymphadenectomy.
The approaches to lymph node assessment in vulvar cancer are shown in Figure 2. When approach A (full inguinal femoral LND) is applied, 100% of midline lesions have bilateral LND while in lateral lesions, ipsilateral LND is performed. In GROINNS-V, 37.5% of tumors were lateral and 62.5% were bilateral, so 37.5% of patients would require full unilateral lymphadnectomy and 62.5% would require bilateral lymphadenectomy [18]. Therefore for approach A, this results in full inguinal femoral LND in 81.3% of groins when considering lateral lesion will not require lymphadenectomy. If the SLN approach is applied (Approach B), only 12.2% of groins are fully dissected after taking into account a 7.5% failed mapping rate from GOG173[25]. Figure 3 summarizes the rates of lymphadenectomy for endometrial and vulvar cancer by approach.
Figure 3: Decreaseing rates of lymphadenectomy with advances in surgical approach to lymph node assessment in endometrial and vulvar cancer.
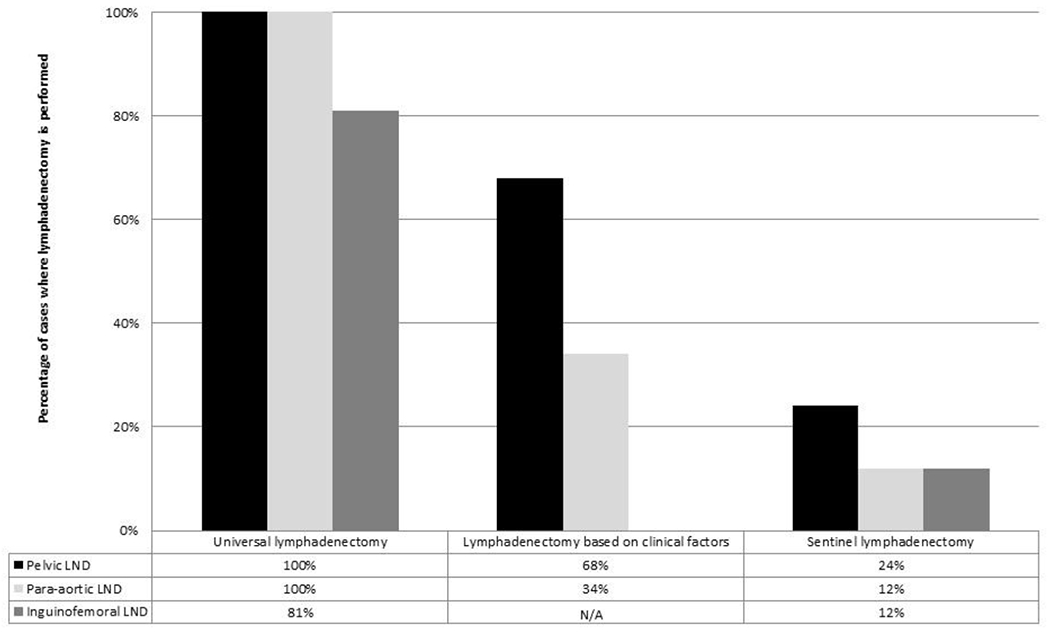
Summary of rates of full lymphadenectomy depending on approach to lymph node assessment for pelvic, para-aortic, and inguinofemoral lymph node basins.
Case volumes by year were calculated for each fellow across institutions using fellow case logs. For endometrial cancers, there was an average of 35 (range 28-50) cases per year per fellow. The theoretical number of pelvic and para-aortic lymphadenectomies per fellow per year by approach is reported in Table 1. With a sentinel node approach, the number of unilateral pelvic lymphadenectomy decreases from 70 cases to 17 cases per fellow per year, and the number of para-aortic lymphadenectomy cases drops to 8 cases per fellow per year. An average of 4 (range 3-10) relevant vulvar cancer cases existed per fellow per year across institutions. With the application of sentinel nodes approach, the number of unilateral full groin dissection drops to 1 case per fellow per year from 7.
Table 1:
Theoretical numbers of full lymphadenectomy in gynecologic cancer per fellow oer year (considering the hemi-pelvis or unilateral groin as the unit)
| Endometrial Cancer | Vulvar Cancer | ||
|---|---|---|---|
| Average cases per fellow per year | 35a | 4a | |
| Pelvic | para-aortic | inguinofemoral | |
| Surgical Approach | |||
| Universal lymphadenectomy | 70 | 70 | 7 |
| Lymphadenectomy based on clinical factors | 48 | 24 | N/A |
| Sentinel Lymphadenectomy | 17 | 8 | 1 |
as reported in fellow case logs
Discussion
Staging for many gynecologic cancers using a SLN approach is becoming increasingly accepted and will likely become standard of care in the near future. Just as minimally invasive techniques for hysterectomy, this will have great benefit for our patients, but will impact the surgical experience and proficiency of gynecologic oncologists in practice and the training of fellows. Our results demonstrate significant reductions in the number of these complex procedures using a primary SLN strategy. To ensure continued availability of expertise across our specialty, we should develop strategies to minimize the impact of these changes. Options include alterations to training for fellows, availability of continued surgical education for practicing gynecologic oncologists, augmentation of mentoring relationships of new staff in first jobs and triage for selected cases to experienced surgeons/centers.
The decrease in case volume and opportunity for surgical training of complex procedures is not unique to our specialty. In obstetrics and gynecology, many residency programs struggle to meet minimum requirements for abdominal hysterectomy as practice patterns change. Many reports in general and gynecologic surgery have documented significant decline in open surgical cases with the introduction of laparoscopic approaches [37–39]. Type III radical hysterectomy for cervical cancer, urinary diversion [8], and exenteration are all less frequently performed today in gynecologic oncology. A survey of gynecologic fellows in 2004, showed that fellows over time have a lower probability of being able to independently perform certain procedures. This survey showed a trend toward fellows’ acquiring fewer surgical skills, with 69.8% of fellows feeling greater emphasis should be placed on surgical training [40].
Strategies to enhance surgical training need to move beyond total reliance on live-surgical hands-on experience, as illustrated by the present manuscript. These strategies can include augmented training in anatomy, use of simulation and cadaver labs, performance of mock procedures using cadavers, and more meaningful evaluation and feedback during live surgery. Research should focus on the ideal methodology; a one-size-fits-all approach will not work. For instance, robotic simulation has shown to be beneficial, however it may be more helpful for robotic set-up and arm placement and less useful for learning the intricacies of intra-caval tissue planes [41–42]. Enhanced training, including fresh frozen cadaver labs in surgical anatomy can better prepare learners to more efficiently master complex operations such as lymphadenectomy [43–44]. Advanced robotics and laparoscopic postgraduate courses are ideal to complement fellowship training, as they can focus on surgical techniques and anatomy.
As some procedures most certainly will become irrelevant procedures as fields advance, national educational goals should take into account the changes in surgical fields when developing milestones and competency-based evaluations. Rather than volume-based measures, competency-based measures should play a large role in structuring and evaluating training programs. Where volume targets still exist, they should be consistent with available numbers as outlined in this manuscript.
Strengths of this paper include using three large referral centers with fellowship training programs, each with 300+ endometrial cancer cases and 20-60 vulvar cancer cases each year. We used conservative estimates for single sided and bilateral mapping rates, knowing that as gynecologic oncologists increase their experience, mapping rates will be far higher [45]. Weaknesses include that this is a purely modeling paper and makes several assumptions regarding clinical approach and sentinel mapping. We have minimized this impact by basing estimates on actual case log volumes. The models do not take into account lymphadenectomies for grossly enlarged nodes or vulvar cancers > 4 cm in tumor diameter, nor does it include lymphadenectomy in the setting of a positive sentinel lymph node. These scenarios would increase lymphadenectomy rate from those reported.
Conclusion
In conclusion, we have highlighted an unavoidable reality in our surgical experience brought on by research and subsequent practice improvement. The decline in experience for pelvic, para-aortic and inguinal lymphadenectomy as a result of practice evolution calls for a change in our educational resources to maintain an expert-trained work force. While there are indisputable benefits to the sentinel node approach for our patients, special attention must be paid to the collateral impact. Continued ensured competence in our specialty in retroperitoneal and groin dissections will require the use of other tools such as simulation, cadaver labs, and careful case distribution in training. Determining metrics to measure competency will be important to gynecologic oncology fellowships to ensure that graduating fellows have the skills needed to be independent practitioners, understanding that surgical learning continues to occur after formal education.
Acknowledgements:
Dr. Leitao is supported in part by the MSK Cancer Center Support Grant P30 CA008748.
The authors report no external funding source for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare that they have no conflicts of interest and nothing to disclose.
The work presented here has been presented at the Western Association of Gynecologic Oncology in June 2016.
References
- [1].Walter Andrew. Every woman deserves a high-volume gynecologic surgeon, American Journal of Obstetrics and Gynecology. 216 (2016) 139.e1–3. 10.1016/j.ajog.2016.10.027 [DOI] [PubMed] [Google Scholar]
- [2].Mowat A Maher C, Ballard E. Surgical outcomes for low-volume versus high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. 215(2016) 21–33. 10.1016/j.ajog.2016.02.048 [DOI] [PubMed] [Google Scholar]
- [3].Tunitsky E Citil A, Ayaz R et al. Does surgical volume influence short-term outcomes of laparoscopic hysterectomy, American Journal of Obstetrics and Gynecology. 203 (2010) 24–30 10.1016/j.ajog.2010.01.070 [DOI] [PubMed] [Google Scholar]
- [4].Drukker L Hants Y, Farkash R et al. Impact of surgeon annual volume on short-term maternal outcome in cesarean delivery, American Journal of Obstetrics and Gynecology. 215 (2016), 85–92 10.1016/j.ajog.2016.03.028 [DOI] [PubMed] [Google Scholar]
- [5].Birkmeyer JD Stukel T, Siewers A et al. Surgeon Volume and Operative Mortality in the United States, The New England Journal of Medicine. 349 (2003) 2117–2125 10.1056/NEJMsa035205 [DOI] [PubMed] [Google Scholar]
- [6].Jacobson G, Shaber R, Armstrong MA, Hung Y. Hysterectomy rates for benign indications, Obstetrics and gynecology. 107 (2006) 1278–1283 10.1097/01.AOG.0000210640.86628.ff [DOI] [PubMed] [Google Scholar]
- [7].Doo DW, Powell M, Novetsky A, Sheeder J, Guntupalli SR, Preparedness of OB/Gyn residents for fellowship training in gynecologic oncology. Gynecologic Oncology Reports. 12 (2015) 55–60. doi: 10.1016/J.gore.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shah NR, Ward KK, Plaxe SC, Saenz CC, McHale MT, Urinary diversions: A time to enrich surgical training, Gynecologic Oncology. (2015) 1–4. doi: 10.1016/J.ygyno.2015.11.012. [DOI] [PubMed] [Google Scholar]
- [9].Chan JK, Cheung MK, Huh WK, Osann K, Husain A, Teng NN, et al. , Therapeutic role of lymph node resection in endometrioid corpus cancer, Cancer. 107 (2006) 1823–1830. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- [10].Cragun JM, Retrospective Analysis of Selective Lymphadenectomy in Apparent Early-Stage Endometrial Cancer, Journal of Clinical Oncology. 23 (2005) 3668–3675. doi: 10.1200/JC0.2005.04.144. [DOI] [PubMed] [Google Scholar]
- [11].Mariani A, Webb MJ, Galli L, Podratz K. Potential Therapeutic Role of para-aortic lymphadenectomy in Node-Positive Endometrial Cancer. Gynecologic Oncology. 76 (2000): 348–356 [DOI] [PubMed] [Google Scholar]
- [12].The writing committee on behalf of ASTEC, Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study, The Lancet. 373 (2009) 125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Panici PB, Basile S, Maneschi F, Lissoni AA, Signorelli M, et al. , Systematic Pelvic Lymphadenectomy vs No Lymphadenectomy in Early-Stage Endometrial Carcinoma: Randomized Clinical Trial, JNCI Journal of the National Cancer Institute. 100 (2008) 1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- [14].Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC, Predictors of Lymphatic Failure in Endometrial Cancer, Gynecologic Oncology. 84 (2002) 437–442. doi: 10.1006/gyno.2001.6550. [DOI] [PubMed] [Google Scholar]
- [15].Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. , Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging, Gynecologic Oncology. 109 (2008) 11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mariani A, Webb MJ, Rao SK, Lesnick TG, Podratz KC, Significance of Pathologic Patterns of Pelvic Lymph Node Metastases in Endometrial Cancer, Gynecologic Oncology. 80 (2001) 113–120. doi: 10.1006/gyno.2000.6050. [DOI] [PubMed] [Google Scholar]
- [17].Dowdy SC, Borah BJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Haas LR, et al. , Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer, Gynecologic Oncology. 127 (2012) 5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- [18].Abu-Rustum NR, Khoury-Collado F, Gemignani ML, Techniques of sentinel lymph node identification for early-stage cervical and uterine cancer, Gynecologic Oncology. 111 (2008) S44–S50. doi: 10.1016/j.ygyno.2008.07.027. [DOI] [PubMed] [Google Scholar]
- [19].Niikura H, Okamura C, Utsunomiya H, Yoshinaga K, Akahira J, Ito K, et al. , Sentinel lymph node detection in patients with endometrial cancer, Gynecologic Oncology. 92 (2004) 669–674. doi: 10.1016/j.ygyno.2003.10.039. [DOI] [PubMed] [Google Scholar]
- [20].Khoury-Collado F, Murray MP, Hensley ML, Sonoda Y, Alektiar KM, Levine DA, et al. , Sentinel lymph node mapping for endometrial cancer improves detection of metastatic disease to regional lymph nodes. Gynecologic Oncology. 122 (2011) 251–254. doi: 10.1016/j.ygyno.2011.04.030. [DOI] [PubMed] [Google Scholar]
- [21].Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, Soslow RA, Dao F, Sonoda Y, et al. , Sentinel lymph node mapping for grade 1 endometrial cancer: Is it the answer to the surgical staging dilemma, Gynecologic Oncology. 113 (2009) 163–169. doi: 10.1016/j.ygyno.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uterine Neoplasms, NCCN guidelines (2016) 1–95. [Google Scholar]
- [23].SGO clinical practice statement: The role of Sentinel lymph node mapping in endometrial cancer. Society of Gynecologic Oncology, November 2015. [Google Scholar]
- [24].Leitao MM Jr, Khoury-Collado F, Gardner G, Sonoda Y, Brown CL, Alektiar KM, et al. , Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of Stage IIIC endometrial cancer, Gynecologic Oncology. 129 (2013) 38–41. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [25].Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL, et al. , Lymphatic Mapping and Sentinel Lymph Node Biopsy in Women With Squamous Cell Carcinoma of the Vulva: A Gynecologic Oncology Group Study, Journal of Clinical Oncology. 30 (2012) 3786–3791. doi: 10.1200/JCO.2011.41.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].te Grootenhuis NC, van der Zee AGJ, van Doom HC, van der Velden J, Vergote I, Zanagnolo V, et al. , Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I, Gynecologic Oncology. 140 (2016) 8–14. doi: 10.1016/J.ygyno.2015.09.077. [DOI] [PubMed] [Google Scholar]
- [27].van der Zee AGJ, Oonk MH, de Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. , Sentinel Node Dissection Is Safe in the Treatment of Early-Stage Vulvar Cancer, Journal of Clinical Oncology. 26 (2008) 884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- [28].Mariani A, Keeney GL, Aletti G, Webb MJ, Haddock MG, Podratz KC, Endometrial carcinoma: paraaortic dissemination, Gynecologic Oncology. 92 (2004) 833–838. doi: 10.1016/J.ygyno.2003.11.032. [DOI] [PubMed] [Google Scholar]
- [29].Lopes L, Micolau S, Baracat F et al. , Sentinel lymph node in endometrial cancer, International Journal of Gynecological Cancer. 17 (2007) 1113–1117. doi: 10.1111/j.l525-1438.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- [30].Khoury-Collado F, Glaser GE, Zivanovic O, Sonoda Y, Levine DA, Chi DS, et al. , Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecologic Oncology. 115 (2009) 453–455. doi: 10.1016/j.ygyno.2009.08.026. [DOI] [PubMed] [Google Scholar]
- [31].Ballester M, Dubernard G, Lecuru F et al. , Detection rate and diagnostic accuracy of sentinel-nodebiopsy in early stage endometrial cancer: a prospectivemulticentre study (SENTI-ENDO), Lancet Oncology. 12 (2011) 469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- [32].Kang S, Yoo HJ, Hwang JH, Lim MC, Seo S-S, Park SY, Sentinel lymph node biopsy in endometrial cancer: meta-analysis of 26 studies, Gynecologic Oncology. 123 (2011) 522–527. doi: 10.1016/j.ygyno.2011.08.034. [DOI] [PubMed] [Google Scholar]
- [33].Rossi EC, Ivanova A, Boggess JF, Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study, Gynecologic Oncology. 124 (2012) 78–82. doi: 10.1016/j.ygyno.2011.09.025. [DOI] [PubMed] [Google Scholar]
- [34].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. , The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes, Gynecologic Oncology. 125 (2012) 531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- [35].How J, Lau S, Press J, Ferenczy A, Pelmus M, Stern J, et al. , Accuracy of sentinel lymph node detection following intra-operative cervical injection for endometrial cancer: A prospective study, Gynecologic Oncology. 127 (2012) 332–337. doi: 10.1016/j.ygyno.2012.08.018. [DOI] [PubMed] [Google Scholar]
- [36].Holloway RW, Bravo RAM, Rakowski JA, James JA, Jeppson CN, Ingersoll SB, et al. , Detection of sentinel lymph nodes in patients with endometrial cancer undergoing robotic-assisted staging: A comparison of colorimetric and fluorescence imaging, Gynecologic Oncology. 126 (2012) 25–29. doi: 10.1016/j.ygyno.2012.04.009. [DOI] [PubMed] [Google Scholar]
- [37].Alkhoury F, Martin JT, Contessa J, Zuckerman R, Nadzam G, The Impact of Laparoscopy on the Volume of Open Cases in General Surgery Training, Journal of Surgical Education. 67 (2015) 316–319. doi: 10.1016/j.jsurg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- [38].Carson JS, Smith L, Are M, Edney J, Azarow K, Mercer DW, et al. , National trends in minimally invasive and open operative experience of graduating general surgery residents: implications for surgical skills curricula development? American Journal of Surgery. 202 (2011) 720–726. doi: 10.1016/j.amjsurg.2011.06.045. [DOI] [PubMed] [Google Scholar]
- [39].Hoekstra AV, Morgan JM, Lurain JR, Buttin BM, Singh DK, Schink JC, et al. , Robotic surgery in gynecologic oncology: Impact on fellowship training, Gynecologic Oncology. 114 (2009) 168–172. doi: 10.1016/j.ygyno.2009.04.022. [DOI] [PubMed] [Google Scholar]
- [40].Eisenkop SM, Spirtos NM, The relative importance of surgical training and laboratory research in a gynecologic oncology fellowship, International Journal of Gynecological Cancer. 14 (2004) 23–34. [DOI] [PubMed] [Google Scholar]
- [41].burkett D, Horwitz J, Kennedy V, Graziano S, Kenton K, Assessing Current Trends in Resident Hysterectomy Training, Female Pelvic Medicine and Reconstructive Surgery. 17 (2011) 1–5. doi: 10.1097/SPV.0b013e3182309a22. [DOI] [PubMed] [Google Scholar]
- [42].Pulliam S, Berkowitz LR, Smaller Pieces of the Hysterectomy Pie, Obstetrics & Gynecology. 113 (2009) 395–398. [DOI] [PubMed] [Google Scholar]
- [43].Lentz GM, Mandel LS, Goff BA, A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models, American Journal of Obstetrics and Gynecology. 193 (2005) 2056–2061. doi: 10.1016/j.ajog.2005.07.064. [DOI] [PubMed] [Google Scholar]
- [44].Schreuder H, Wolswijk R, Zweemer RP, Schijven MP, Verheijen R, Training and learning robotic surgery, time for a more structured approach: a systematic review, BJOG: an Internal Journal of Obs Gyn. 119 (2011) 137–149. doi: 10.1111/j.l471-0528.2011.03139.x. [DOI] [PubMed] [Google Scholar]
- [45].Escobar PF, Kebria M, Falcone T, Evaluation of a novel single-port robotic platform in the cadaver model for the performance of various procedures in gynecologic oncology, Gynecologic Oncology. 120 (2011) 380–384. doi: 10.1016/j.ygyno.2010.11.005. [DOI] [PubMed] [Google Scholar]


