Abstract
Vocalizations are an important means to facilitate social interactions, but vocal communication may be affected by infections. While such effects have been shown for mate-attraction calls, other vocalizations that facilitate social contact have received less attention. When isolated, vampire bats produce contact calls that attract highly associated groupmates. Here, we test the effect of an immune challenge on contact calling rates of individually isolated vampire bats. Sickness behaviour did not appear to change call structure, but it decreased the number of contact calls produced. This effect could decrease contact with groupmates and augment other established mechanisms by which sickness reduces social encounters (e.g. mortality, lethargy and social withdrawal or disinterest).
Keywords: sickness behaviour, pathogen transmission, infection, social behaviour, lipopolysaccharide
1. Introduction
Infections can reduce contact between individuals by inducing ‘sickness behavior'. For instance, sickness can decrease physical social encounters through reduced movement [1] or decrease directed social interactions like grooming [2,3]. Reductions in social contact can also occur if infected individuals vocalize less. For example, an immune challenge reduces male mate-attraction vocalizations or some of their components in several species [4–8]. If sick males attract fewer females and if avoiding sick males decreases the likelihood of females acquiring parasites [9–11], then transmission of parasites between the sexes will decrease [12].
Besides courtship vocalizations, a broader range of vocal interactions could be influenced by sickness, but these other call types have received less attention. In many group-living animals that live in conditions of low visibility, or that must maintain cohesion while on the move, individuals produce contact calls to maintain contact with groupmates or particular affiliated individuals [13–21]. If contact calls facilitate physical contact, then sickness behaviour that reduces the rate of contact calling should decrease contact with groupmates. However, if contact calling is used by an individual in need to gain benefits from others, then sick individuals might instead make a greater number of contact calls. For example, when parents can acquire enough food to feed all their offspring, hungry nestlings in worse condition are expected to call more often, not less [22,23]. The expected effect of sickness behaviour on contact calling by distressed individuals is therefore less clear.
Isolated common vampire bats (Desmodus rotundus) produce multi-harmonic contact calls that vary in spectral structure and duration, and that facilitate individual contact and recognition ([15,17], figure 1b,c). Contact calls appear to be important for maintaining co-roosting associations with bonded partners and for finding or recruiting those partners for help; for example, trapped and hungry individuals appear to use contact calls to recruit both kin and non-kin food donors to feed them by regurgitation [17,24].
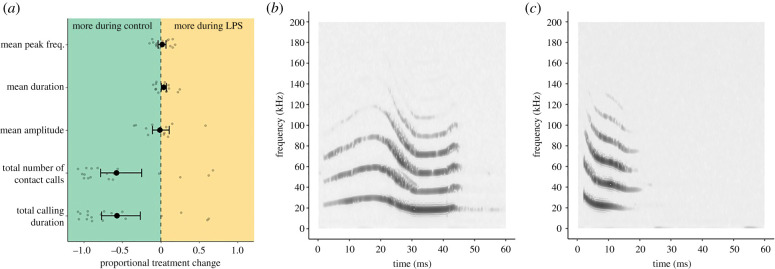
Figure 1.
Effect of lipopolysaccharide (LPS) on vampire bat contact calls. (a) Shows mean standardized LPS effect ± bootstrapped 95% confidence intervals (see electronic supplementary material, table S1) and data points for the mean peak frequency, mean duration, mean amplitude, the total number of contact calls produced and the total calling duration. Example spectrograms of a longer (b) and shorter (c) vampire bat contact call that also vary in peak frequency (darker regions show frequencies of greater relative amplitude).
Here, we mimicked a bacterial infection in vampire bats using lipopolysaccharide (LPS) to trigger transient physiological symptoms and sickness behaviours [2,3,25], and then we tested for the effect of LPS on contact calling behaviour. LPS-injected vampire bats are groomed by fewer bats and have lower social connectedness in the wild, an effect that could be driven in part by a reduction in contact calling [3,25]. We show that LPS-induced sickness behaviour decreases the number of contact calls produced by isolated vampire bats. This effect is relevant for pathogen transmission in social animals that rely on vocalizations to maintain contact because it might further reduce the probability of physical contact between individuals beyond the effects of reduced movement.
2. Material and methods
We recorded contact calls by physically isolating an adult female vampire bat (n = 18) in a soft mesh cage at a distance of 10–30 cm from a CM16 ultrasound condenser microphone (frequency range 1–200 KHz, Avisoft Bioacoustics, Berlin, Germany). The mesh cage was inside a 68 l plastic bin lined with acoustic dampening foam and within hearing range of conspecifics of a captive colony. To selectively record contact calls, we used a digitizer (116 Hn UltrasoundGate, Avisoft Bioacoustics, Berlin, Germany, sampling frequency of 250 or 500 kHz) to save a .wav file whenever a 10–50 kHz sound was detected at greater than 5% amplitude. We used Avisoft SASLabPro (Avisoft Bioacoustics, Berlin, Germany) to measure the onset, duration, peak amplitude and peak frequency of all calls. We excluded echolocation calls and other noise by deleting sounds that were longer than 60 ms and shorter than 10 ms.
For each bat, we recorded two trial types. In LPS trials, we induced sickness behaviour in subjects by injecting them subcutaneously with LPS (L2630 Sigma-Aldrich, USA, dose: 5 mg kg−1 body mass of bat) in phosphate-buffered saline (PBS) before the recording period. We chose this dose based initially on observed effects in another bat species [26], and on later studies in vampire bats, which showed that this dose increases white blood cell count and neutrophil to lymphocyte ratio [2] and decreases physical activity, social encounters and social grooming [2,3,25]. In control trials, the same bats were injected with an equivalent volume of only PBS as a control treatment.
Treatments were given in random order, and eight bats received the control treatment first. We recorded bats for 4–6 h immediately after the injection, because we previously detected symptoms for at least 6 h post-injection [2]. Different bats were recorded for different times after injection, but the paired LPS and control trials were always the same duration and time of the night. Since bats often sleep in the recording chamber, we excluded hours when the bat did not call during either the treatment night or control night, but our results do not change if we include these hours in our analysis. The inter-trial period was at least 5 days to ensure recovery of the bats [2,25]. To calculate a standardized effect (proportional change) of LPS on vocalizations for each bat, we used (YLPS − YC)/(YLPS + YC), where YLPS and YC are the measures of vocal activity during the bat's LPS and control trial, respectively.
To test for an effect of LPS on contact calling, we randomly swapped the control and LPS trial data within each bat to calculate a distribution of t-statistics under the null hypothesis of no difference between the LPS and control trial, then compared the observed t-statistic to this distribution to obtain a two-sided p-value (i.e. a nonparametric permuted paired t-test). To estimate 95% confidence intervals for LPS effect sizes, we used nonparametric bootstrapping with accelerated bias-corrected percentile limits [27]. We used 5000 permutations for both methods. We calculated the mean and bootstrapped 95% CI for the LPS effect on five measures of contact calling behaviour: the total number of contact calls produced, the sum of call durations, the mean call duration, the mean amplitude and the mean peak frequency (the frequency at the point of the maximum amplitude of the entire element). Data and R script to repeat our analysis are available on figshare [28].
3. Results
LPS injections led to fewer contact calls. The average contact calling rates per bat during the control and LPS trials were, respectively, 66 and 16 contact calls per hour (see electronic supplementary material, figure S1 for details on each bat and electronic supplementary material, figure S2 for average call production of bats after LPS and control over time). On average, LPS injections caused female vampire bats to produce 30% fewer contact calls, with 15 of 18 bats producing fewer contact calls during the LPS trial compared to the control trial (p = 0.0006, figure 1a and table 1, electronic supplementary material, figure S1). Fewer calls led to an average decrease of 32% in total calling duration (p = 0.0012, figure 1a and table 1). We did not detect an effect of LPS on mean call amplitude (figure 1a and table 1). Although vampire bats produce contact calls that vary in call structure (figure 1b,c), we did not detect an effect of LPS injections on mean call duration (figure 1a and table 1) or mean peak frequency (figure 1a and table 1).
Table 1.
Effect of LPS on vampire bat contact calls. Means and their bootstrapped 95% confidence intervals for the standardized LPS effects for contact call number, total calling duration, mean amplitude, mean peak frequency and mean call duration (table corresponds to figure 1a).
| measure | N | lower CI | mean | upper CI | p-value |
|---|---|---|---|---|---|
| call number | 18 | −0.78 | −0.58 | −0.27 | 0.0006 |
| calling duration | 18 | −0.77 | −0.57 | −0.26 | 0.0012 |
| mean amplitude | 14a | −0.11 | −0.01 | 0.11 | 0.8754 |
| mean peak frequency | 14a | −0.03 | 0.02 | 0.07 | 0.4656 |
| mean duration | 14a | 0.01 | 0.04 | 0.08 | 0.0510 |
aWe could not calculate the LPS effect on mean amplitude, mean peak frequency and mean duration for four bats because they did not produce any calls when injected with LPS.
4. Discussion
Infection-induced sickness behaviours can affect vocal communication as evident in LPS-injected male house mice that produce fewer call syllables, which likely contributes to reduced associations with females [7]. Similarly, immune-challenged males decrease their song rate in collared flycatchers (Ficedula albicollis), white-browed sparrow weavers (Plocepasser mahali), field crickets (Gryllus campestris) and white-crowned sparrows (Zonotrichia leucophrys gambelii) [5,6,8,29]. In comparison to these mate-attraction calls, contact calls and signals of need are interesting to consider because a state of poor condition could lead to either a higher or lower calling rate. In vampire bats, contact calling can attract food donors and might act as a signal of need [15,17]. Here, we showed that an immune challenge reduces contact calling, which could potentially help to explain why immune-challenged vampire bats encounter fewer individuals [3,25], in addition to the more obvious explanation of reduced movement.
In a previous study using the same dose as we used here, LPS-injected vampire bats were more lethargic, spending less time awake, moving or engaging in hygienic behaviours such as self-grooming [2]. So, our results are most consistent with the simplest explanation that reduced contact calling is also owing to lethargy. Reduced contact calling is unlikely to be explained as a kin-selected mechanism for reducing pathogen transmission [30–32] because contact calls attract both kin and non-kin [17]. We hypothesize that vampire bats reduce contact calling to support the energetic demands of the physiological response. Across several taxa, the metabolic costs of acoustic signalling are estimated to be about eight times that of remaining silent [33] and call rate is sensitive to other ecological constraints like reduced food availability [34]. Experiments with a related frugivorous bat species show that LPS injections reduce body mass and increase resting metabolic rate by 40% [35].
We used a dose of LPS for which we knew the physiological and behavioural effects in vampire bats [2,3,25]. It is important to note, however, that the physiological responses to LPS are dose-dependent and involve both pro-inflammatory and anti-inflammatory responses [36]. To determine what doses are most ecologically relevant for different diseases, future work must compare the relationship between the dose-dependent effects of LPS against the effects of natural bacterial infections in vampire bats and other species.
It is also important to note that infection-induced changes to social vocalizations are pathogen specific. LPS mimics common symptoms of a bacterial infection in vampire bats and other animals [1,2]. Some live pathogens, however, could increase specific social behaviours to favour their transmission [37]. For instance, chytrid-fungus infected Japanese tree frogs (Hyla japonica) increase their mating call effort, which potentially favours the transmission of the fungus [38]. Vampire bats harbour multiple pathogens in their saliva that rely on directed social interactions, like Bartonella [39], hemoplasmas [40] and, most notably, rabies [41]. It would be particularly interesting to look at how rabid vampire bats change their calling behaviour and their response to calls of conspecifics.
Besides call rate, the structure of animal vocalizations might also depend on infection status [4,42]. Common vampire bats produce highly variable contact calls (e.g. figure 1b and c), but we found no evidence that sick bats consistently produced any particular contact call structure more or less. However, for some pathogens, such as rabies, which could affect the vocal tract, there may be clear differences in call structure.
Supplementary Material
Acknowledgements
We thank the Smithsonian Tropical Research Institute for logistical support. Samuel Kaiser, Vanessa Pérez, Jineth Berrio-Martinez, Imran Razik, Bridget Brown, David Girbino, Simon Ripperger and Emma Kline for help in the field and animal caretaking.
Ethics
Our work was approved by the Smithsonian Tropical Research Institute Animal Care and Use Committee (#2016-0728-2019-A2), the Animal Care and Use Committee of the University of Texas at Austin (AUP-2016-00124) and by the Panamanian Ministry of Environment (protocol: SE/A-64-17).
Data accessibility
We have uploaded Data and R code to a figshare repository. The repository is cited in the methods part of the main text: Stockmaier S. 2020 Dataset and R.code from: ‘Sickness behavior reduces contact calling in vampire bats'. Figshare digital repository. https://doi.org/10.6084/m9.figshare.11861877.v4.
Authors' contributions
S.S. and G.G.C. designed the study and carried out the experiments and husbandry. G.G.C. captured the bats and established the captive colony. S.S., G.G.C. and D.J. contributed to the data collection and analysis. G.G.C., D.I.B. and R.A.P. coordinated the study and provided valuable resources and laboratory space. All authors contributed to draft the manuscript, gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Smithsonian Tropical Research Institute.
References
- 1.Lopes PC, Block P, Konig B. 2016. Infection-induced behavioural changes reduce connectivity and the potential for disease spread in wild mice contact networks. Sci. Rep. 6, 31790 ( 10.1038/srep31790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockmaier S, Bolnick DI, Page RA, Carter GG. 2018. An immune challenge reduces social grooming in vampire bats. Anim. Behav. 140, 141–149. ( 10.1016/j.anbehav.2018.04.021) [DOI] [Google Scholar]
- 3.Stockmaier S, Bolnick DI, Page RA, Carter GG. 2020. Sickness effects on social interactions depend on the type of behaviour and relationship. J. Anim. Ecol. 89, 1387–1394. ( 10.1111/1365-2656.13193) [DOI] [PubMed] [Google Scholar]
- 4.Dreiss AN, Navarro C, De Lope F, Møller AP. 2008. Effects of an immune challenge on multiple components of song display in barn swallows Hirundo rustica: implications for sexual selection. Ethology 114, 955–964. ( 10.1111/j.1439-0310.2008.01546.x) [DOI] [Google Scholar]
- 5.Garamszegi LZ, Møller AP, Török J, Michl G, Péczely P, Richard M. 2004. Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav. Ecol. 15, 148–157. ( 10.1093/beheco/arg108) [DOI] [Google Scholar]
- 6.Jacot A, Scheuber H, Brinkhof MWG. 2004. Costs of an induced immune response on sexual display and longevity in field crickets. Evolution 58, 2280–2286. ( 10.1111/j.0014-3820.2004.tb01603.x) [DOI] [PubMed] [Google Scholar]
- 7.Lopes PC, König B. 2016. Choosing a healthy mate: sexually attractive traits as reliable indicators of current disease status in house mice. Anim. Behav. 111, 119–126. ( 10.1016/j.anbehav.2015.10.011) [DOI] [Google Scholar]
- 8.York JE, Radford AN, Groothuis TG, Young AJ. 2016. Dominant male song performance reflects current immune state in a cooperatively breeding songbird. Ecol. Evol. 6, 1008–1015. ( 10.1002/ece3.1938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillgarth N. 1996. Ectoparasite transfer during mating in ring-necked pheasants Phasianus colchicus. J. Avian Biol. 27, 260–262. ( 10.2307/3677232) [DOI] [Google Scholar]
- 10.Luong LT, Platzer EG, Zuk M, Giblin-Davis RM. 2000. Venereal worms: sexually transmitted nematodes in the decorated cricket. J. Parasitol. 86, 471–477. ( 10.1645/0022-3395(2000)086[0471:VWSTNI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Padilla J, Vergara P, Mougeot F, Redpath SM. 2012. Parasitized mates increase infection risk for partners. Am. Nat. 179, 811–820. ( 10.1086/665664) [DOI] [PubMed] [Google Scholar]
- 12.Able DJ. 1996. The contagion indicator hypothesis for parasite-mediated sexual selection. Proc. Natl Acad. Sci. USA 93, 2229–2233. ( 10.1073/pnas.93.5.2229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold BD, Wilkinson GS. 2011. Individual specific contact calls of pallid bats (Antrozous pallidus) attract conspecifics at roosting sites. Behav. Ecol. Sociobiol. 65, 1581–1593. ( 10.1007/s00265-011-1168-4) [DOI] [Google Scholar]
- 14.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication Sunderland, MA: Sinauer Associates. [Google Scholar]
- 15.Carter GG, Logsdon R, Arnold BD, Menchaca A, Medellin RA. 2012. Adult vampire bats produce contact calls when isolated: acoustic variation by species, population, colony, and individual. PLoS ONE 7, e38791 ( 10.1371/journal.pone.0038791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter GG, Fenton MB, Faure PA. 2009. White-winged vampire bats (Diaemus youngi) exchange contact calls. Can. J. Zool. 87, 604–608. ( 10.1139/Z09-051) [DOI] [Google Scholar]
- 17.Carter GG, Wilkinson GS. 2016. Common vampire bat contact calls attract past food-sharing partners. Anim. Behav. 116, 45–51. ( 10.1016/j.anbehav.2016.03.005) [DOI] [Google Scholar]
- 18.Cortopassi KA, Bradbury JW. 2006. Contact call diversity in wild orange-fronted parakeet pairs, Aratinga canicularis. Anim. Behav. 71, 1141–1154. ( 10.1016/j.anbehav.2005.09.011) [DOI] [Google Scholar]
- 19.Janik VM, Sayigh LS. 2013. Communication in bottlenose dolphins: 50 years of signature whistle research. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 199, 479–489. ( 10.1007/s00359-013-0817-7) [DOI] [PubMed] [Google Scholar]
- 20.Maurello MA, Clarke JA, Ackley RS. 2000. Signature characteristics in contact calls of the white-nosed Coati. J. Mammal. 81, 415–421. () [DOI] [Google Scholar]
- 21.van Oosterom L, Montgomery JC, Jeffs AG, Radford CA. 2016. Evidence for contact calls in fish: conspecific vocalisations and ambient soundscape influence group cohesion in a nocturnal species. Sci. Rep. 6, 19098 ( 10.1038/srep19098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caro SM, Griffin AS, Hinde CA, West SA. 2016. Unpredictable environments lead to the evolution of parental neglect in birds. Nat. Commun. 7, 10985 ( 10.1038/ncomms10985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godfray HCJ. 1991. Signalling of need by offspring to their parents. Nature 352, 328–330. ( 10.1038/352328a0) [DOI] [Google Scholar]
- 24.Carter GG, Wilkinson GS, Page RA. 2017. Food-sharing vampire bats are more nepotistic under conditions of perceived risk. Behav. Ecol. 28, 565–569. ( 10.1093/beheco/arx006) [DOI] [Google Scholar]
- 25.Ripperger SP, Stockmaier S, Carter GG. 2020. Sickness behaviour reduces network centrality in wild vampire bats. bioRxiv 2020.03.30.015545 ( 10.1101/2020.03.30.015545) [DOI]
- 26.Stockmaier S, Dechmann DK, Page RA, O'Mara MT. 2015. No fever and leucocytosis in response to a lipopolysaccharide challenge in an insectivorous bat. Biol. Lett. 11, 20150576 ( 10.1098/rsbl.2015.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puth M-T, Neuhäuser M, Ruxton GD. 2015. On the variety of methods for calculating confidence intervals by bootstrapping. J. Anim. Ecol. 84, 892–897. ( 10.1111/1365-2656.12382) [DOI] [PubMed] [Google Scholar]
- 28.Stockmaier S. 2020. Dataset and R.code from: ‘Sickness behavior reduces contact calling in vampire bats'. Figshare digital repository. ( 10.6084/m9.figshare.11861877.v4) [DOI]
- 29.Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. 2006. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii). Horm. Behav. 49, 15–29. ( 10.1016/j.yhbeh.2005.04.009) [DOI] [PubMed] [Google Scholar]
- 30.Bos N, Lefevre T, Jensen AB, d'Ettorre P. 2012. Sick ants become unsociable. J. Evol. Biol. 25, 342–351. ( 10.1111/j.1420-9101.2011.02425.x) [DOI] [PubMed] [Google Scholar]
- 31.Heinze J, Walter B. 2010. Moribund ants leave their nests to die in social isolation. Curr. Biol. 20, 249–252. ( 10.1016/j.cub.2009.12.031) [DOI] [PubMed] [Google Scholar]
- 32.Stroeymeyt N, Grasse AV, Crespi A, Mersch DP, Cremer S, Keller L. 2018. Social network plasticity decreases disease transmission in a eusocial insect. Science 362, 941–945. ( 10.1126/science.aat4793) [DOI] [PubMed] [Google Scholar]
- 33.Ophir AG, Schrader SB, Gillooly JF. 2010. Energetic cost of calling: general constraints and species-specific differences. J. Evol. Biol. 23, 1564–1569. ( 10.1111/j.1420-9101.2010.02005.x) [DOI] [PubMed] [Google Scholar]
- 34.Ritschard M, Brumm H. 2012. Zebra finch song reflects current food availability. Evol. Ecol. 26, 801–812. ( 10.1007/s10682-011-9541-3) [DOI] [Google Scholar]
- 35.Guerrero A, Rivera D, Díaz V, Triana C, Nino A. 2018. Metabolic cost of acute phase response in the frugivorous bat, Artibeus lituratus. Mamm. Res. 63, 397–404. ( 10.1007/s13364-018-0375-z) [DOI] [Google Scholar]
- 36.Armour EM, Bruner TL, Hines JK, Butler MW. 2020. Low-dose immune challenges result in detectable levels of oxidative damage. J. Exp. Biol. 223, jeb220095 ( 10.1242/jeb.220095) [DOI] [PubMed] [Google Scholar]
- 37.Klein SL. 2003. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Tribute Paul MacLean Neurobiol. Relev. Soc. Behav. 79, 441–449. ( 10.1016/S0031-9384(03)00163-X) [DOI] [PubMed] [Google Scholar]
- 38.An D, Waldman B. 2016. Enhanced call effort in Japanese tree frogs infected by amphibian chytrid fungus. Biol. Lett. 12, 20160018 ( 10.1098/rsbl.2016.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker DJ, Bergner LM, Bentz AB, Orton RJ, Altizer S, Streicker DG. 2018. Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. in vampire bats. PLoS Negl. Trop. Dis. 12, e0006786 ( 10.1371/journal.pntd.0006786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volokhov DV, Becker DJ, Bergner LM, Camus MS, Orton RJ, Chizhikov VE, Altizer SM, Streicker DG. 2017. Novel hemotropic mycoplasmas are widespread and genetically diverse in vampire bats. Epidemiol. Infect. 145, 3154–3167. ( 10.1017/S095026881700231X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilar-Setien A, Loza-Rubio E, Salas-Rojas M, Brisseau N, Cliquet F, Pastoret PP, Rojas-Dotor S, Tesoro E, Kretschmer R. 2005. Salivary excretion of rabies virus by healthy vampire bats. Epidemiol. Infect. 133, 517–522. ( 10.1017/s0950268805003705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fedorka KM, Mousseau TA. 2006. Immune system activation affects male sexual signal and reproductive potential in crickets. Behav. Ecol. 18, 231–235. ( 10.1093/beheco/arl067) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stockmaier S. 2020. Dataset and R.code from: ‘Sickness behavior reduces contact calling in vampire bats'. Figshare digital repository. ( 10.6084/m9.figshare.11861877.v4) [DOI]
Supplementary Materials
Data Availability Statement
We have uploaded Data and R code to a figshare repository. The repository is cited in the methods part of the main text: Stockmaier S. 2020 Dataset and R.code from: ‘Sickness behavior reduces contact calling in vampire bats'. Figshare digital repository. https://doi.org/10.6084/m9.figshare.11861877.v4.