Abstract
Regulation of the genome is viewed through the prism of gene expression, DNA replication and DNA repair as controlled through transcription, chromatin compartmentalisation and recruitment of repair factors by enzymes such as DNA polymerases, ligases, acetylases, methylases and cyclin-dependent kinases. However, recent advances in the field of muscle cell physiology have also shown a compelling role for ‘outside-in’ biophysical control of genomic material through mechanotransduction. The crucial hub that transduces these biophysical signals is called the Linker of Nucleoskeleton and Cytoskeleton (LINC). This complex is embedded across the nuclear envelope, which separates the nucleus from the cytoplasm. How the LINC complex operates to mechanically regulate the many functions of DNA is becoming increasingly clear, and recent advances have provided exciting insight into how this occurs in cells from mechanically activated tissues such as skeletal and cardiac muscle. Nevertheless, there are still some notable shortcomings in our understanding of these processes and resolving these will likely help us understand how muscle diseases manifest at the level of the genome.
Keywords: nuclear envelope, mechanotransduction, muscle, DNA replication, genome integrity, LINC complex
1. Introduction
The Linker of Nucleoskeleton and Cytoskeleton (LINC) is a complex of proteins spanning the nuclear envelope (NE), which consists of three core members: lamins, sad1 and UNC84 (SUN) proteins and nuclear envelope spectrin repeat proteins (nesprins). The primary role of the LINC complex is to provide structure for and to tether intranuclear structures such as heterochromatin to structural domains in the cytoplasm such as the actin cytoskeleton and microtubules (MTs). The existence of this complex allows the rapid transmission of signals via mechanical stimulation from peripheral domains of the cell such as the plasma membrane and cytoskeleton directly to the nucleus to enable rapid gene expression responses, as well as facilitate the structural integrity of the nucleus [1]. The LINC complex spans the nuclear membrane via SUN and nesprin family proteins [2]. SUN1 and SUN2 bind directly to lamin A at the inner nuclear membrane and are essential in anchoring the LINC complex. Lamin B1, on the other hand, weakly interacts with SUN1 and SUN2 [3]. SUN1 is dependent on lamin A for nuclear anchoring [4] and SUN domains also span the lipid bilayer of the nuclear membrane, where they bind nesprin via Klarsicht, ANC-1, Syne Homology (KASH) domains on the inner surface of the outer membrane [5]. Nesprins are transmembrane proteins that anchor to SUN in the NE and project into the cytosol, where they bind directly to actin, MTs, intermediate filaments such as desmin, and centrosomal proteins (figure 1) [6,7]. Non-covalent interactions and disulfide bonding between nesprin and SUN resist mechanical force from the cytoskeleton and serve to maintain nuclear shape under stress [5]. Other NE membrane components of importance include LAP2, emerin and MAN1 (the so-called LEM domain proteins), which interact with the core LINC proteins at the interface of the inner nuclear membrane and peripheral heterochromatin [8]. The importance of the LINC complex is highlighted by the spectrum and severity of diseases in which mutation to one of these core LINC members, most commonly lamin A/C, is causative [9–11]. Mutations commonly result in diseases of striated muscle, i.e. muscular dystrophy and cardiomyopathy, and many studies of mechanobiology have identified cell and molecular mechanisms; however, the impact on the regulation of the genome is still relatively poorly understood on a mechanistic level. Some recent findings, however, have expanded our understanding in this field. Herein, we discuss some of the existing models of mechanobiology regarding the LINC complex and known links to genome regulation, and we reveal exciting new areas that require focus.
Figure 1.
The LINC complex in muscle cells. The LINC is a complex of proteins that interact to physically connect the nucleus to the cytoplasm. The nuclear lamina (NL) consists of A-type and C-type lamins localized on the inner nuclear membrane. The lamina is directly connected to SUN1, SUN2 and emerin on the inner nuclear membrane. SUN proteins penetrate through the intermembrane space and bind nesprins on the outer nuclear membrane via the nesprin KASH domain. There are multiple nesprin isoforms, including nesprin 1/2, nesprin 1α and nesprin-3. Nesprin 1/2 contain multiple spectrin repeats that connect the KASH domain to a calponin homology domain in the cytoplasm, which in turn binds actin. Nesprin 1α interacts with MTs in the cytoplasm via kinesin and other MT organizing proteins, i.e. A-kinase anchoring proteins (AKAPs). Nesprin 3 binds cytoskeletal intermediate filament protein desmin via its plectin-interacting domain.
2. The mechanics of LINC complex misregulation in muscle disease
The family of lamin proteins consists of lamins A/C (A-type) and lamins B1 and B2 (B-type). B-type lamins arise from the genes LMNB1 and LMNB2, respectively, whereas lamin A and lamin C arise from the LMNA gene and occur by alternative splicing [12]. Lamin A arises via proteolytic processing of its precursor, prelamin A, by the zinc metalloproteinase ZMPSTE24 [13]. Accumulation of unprocessed prelamin A has been evidenced in genetically mediated laminopathies such as Hutchinson–Gilford Progeria Syndrome (HGPS), Emery–Dreifuss muscular dystrophy (EDMD) and dilated cardiomyopathy (DCM), while also accumulating in response to pharmacological inhibition of ZMPSTE24 [14–16].
Many disease-causing mutations have been described in lamins A/C, but there is very little genotype–phenotype correlation and effects of single mutations are often pleiotropic, making mechanistic study challenging [17]. One explanation may lie in the role of the nuclear lamina (NL) in setting tissue stiffness. Central to the role of the NL is the ability to ‘set’ nuclear stiffness based on the external mechanical environment (cytoskeleton, extracellular matrix) and to modulate this in response to perturbations in mechanical loading. This was demonstrated elegantly by Swift et al. [18] who showed that lamin A acts like a viscous fluid to impede nuclear movement under stress, whereas lamin B acts to return the nucleus to its original shape. Nuclei from a range of cell types were aspirated in micropipettes to imitate tissue stiffness and real-time imaging was used to determine nuclear shape and lamin density. The authors describe the nuclei reacting in a viscoelastic manner much like a balloon, wherein viscosity describes the ability of the nucleus to impede deformation and elasticity describes its ability to return to its normal shape. The viscosity was increased compared to the elasticity in a lamin A : lamin B-dependent manner. Fluorescence correlation spectroscopy showed that lamin A is the mobile component of the NL, while lamin B is the immobile component. Additionally, mRNA analysis showed that the ratio of lamin A versus lamin B in cells was determined by the mechanical environment where ‘stiff’ tissues, i.e. with an increased Young's modulus, result in cells with higher abundance of lamin A [19]. Additionally, a recent study has more detail on the biochemical and biophysical properties of lamin A and revealed that under compression, the coiled coils in the rod domains of lamin A polymers are able to slide onto each other to contract the length of the rod, behaving as a compression spring able to absorb pressure and supporting the idea that A-type lamins can serve as a ‘pressure valve’ [20]. Disease-causing mutations likely affect such properties. In one study, the LMNA-S143P mutant, which causes familial DCM, led to lamin A proteins being more mobile and less tightly bound to the lamin network than wild-type (WT) proteins [21]. Lamin staining and confocal microscopy of DCM patient tissue showed that in the S143P mutant, lamins were mislocalized to the nucleoplasm. The lack of lamin A incorporation into the NL resulted in the aggregate formation that was unequally distributed around the nucleus and detrimental to the structural stability of the nucleus. Moreover, DCM mutations L85R and N195 K and EDMD mutation L530P are known to diminish emerin–lamin interactions [22]. In another study, the ultrastructure of the NL with LMNA mutations E161 K and R190 W exhibited lower cross-link density and increased network bundling. Functionally, reduced elasticity was observed, making it more difficult for the cell to adapt to local stress [23]. The induction of mechanical stress has also been shown to result in nuclear strain changes and nuclear deformation in lamin A knockout (KO) fibroblasts [24] as well as in cells harbouring muscular dystrophy-causing LMNA mutations E358 K and ΔK32 [25]. In summary, mutations in LINC proteins deplete the ability of the overall complex to maintain nuclear shape in the face of external stress.
Nesprins are encoded by SYNE1 and SYNE2 genes and occur in many isoforms [26]. Isoforms abundant in loaded tissue such as skeletal and cardiac muscle include nesprins 1α, 2α and 3. Nesprins are localized at the outer nuclear membrane, where nesprin 1α and 2α bind MTs in the cytoskeleton via kinesin [27] and nesprin 3 interacts with actin via plectin and desmin domains [26,28]. Investigations linking disease-causing mutations in SYNE1 to mechanical defects in the striated muscle are limited compared with lamin A/C, but there is evidence that some are associated with EDMD and DCM [29]. Overexpression of the mutations R8272Q, S8381C and N8406 K in human osteosarcoma cells was associated with abnormal nuclear morphology owing to disrupted LINC complex interactions. Nesprin 1α2 anchors the nucleus to the MT motor protein kinesin during myotube formation, and this interaction was disrupted and the myonuclear position disturbed in the mutated cells. Despite the rarity of these mutations in the DCM population, it is important to note their merit in describing the few clinical manifestations of laminopathies that are not associated with lamins.
Mutations in SUN1 and SUN2 are much more subtly associated with disease than lamins and nesprins, are rare and may not be independently causal. Screening of SUN1/2 in EDMD patients revealed that SUN1/2 sequence changes were correlated with increased severity of the disease [30]. However, when compared with sequenced genome databases, these mutations were rare, which further complicates our understanding of their involvement. LMNA R453 W EDMD is generally not associated with severe cardiac disease, however, in conjunction with SUN1 W337C mutation, resulted in early heart failure. This same study also showed that G68D, G338S and W377C SUN1 mutations, and A56P and R620C SUN2 mutations impaired rearward positioning of the nucleus owing to defective centrosome orientation, with the interaction between SUN1 and emerin being impaired. Moreover, altered regulation of SUN1/2 has also been ascribed to the pathological progression of EDMD and HGPS, since SUN1 expression at the NE in cells from HGPS patients with the G608G and T623S mutations in LMNA was highly variable [31]. Supportive of this role in mechanotransduction via the LINC complex, SUN proteins have recently been identified as a modifier of mechanical stimulation within the ‘outside-in’ mechanotransduction pathway [32].
3. The role of the LINC complex in genome regulation
(a). Gene expression responses to mechanical stimulation
In this section, we outline the variety of evidence in multiple models pointing towards the idea that LINC protein expression and disruption serves to (dys)regulate the gene expression response to mechanical stress. The importance of the lamin network, in particular in potentially regulating gene expression, was evident in the early years of NE biology as disruption of this network was shown to cause chromatin leak into the nucleoplasm and chromatin loss in EDMD cells [33].
Other LINC components have subsequently been implicated in regulating gene expression in experiments showing that epitope-tagged SUN1-expressing cells grown on stiff substrate had enriched calcium ion (Ca2+) transport, response to wound healing, cell adhesion, cell motility and extracellular matrix organization [34]. This study used the DAVID (Database for Annotation, Visualization and Integrated Discovery) assay to group the upregulated genes into functional classes. It was concluded that the LINC complex—via SUN1—was directly related to mRNA expression of multiple genes for cytoskeletal, focal adhesion and NE elements.
Moreover, the LINC complex has been shown to regulate the genome in response to direct mechanical stimulation [35]. This study of mechanotransduction used magnetic tweezers with anti-nesprin 1 antibody to apply force on nesprin 1 of isolated nuclei. This triggered nuclear stiffening and the displacement of the magnetic beads was calculated to show that maintenance of nuclear shape was dependent upon an intact NL and emerin. It is well established that emerin interacts with the LINC complex via the lamina and phosphorylation of emerin at Y74 and Y95 by Src kinase facilitates interactions with lamin A/C. This interaction mediates a functional response to tension, presumed to be as a result of efficient gene expression programmes [36]. Indeed, lamin A-deficient mouse embryonic fibroblasts with EDMD and DCM mutations exhibit more mobile and mislocalized emerin alongside actin polymerization defects that in turn impeded the nuclear localization of megakaryoblastic leukaemia 1, an important cardiac transcription factor [37].
In whole animal models, disruption to the LINC complex caused by lamin A depletion has been shown to disturb the cardiac hypertrophy response to a chronic pressure overload model and implicates impaired mechanotransduction signalling [38]. In this study, Lmna+/− mice were subjected to left ventricular pressure overload via transverse aortic constriction (TAC). Early growth response factor (Egr-1), a mechanosensitive gene, was downregulated in Lmna+/− cells compared to control resulting in accelerated heart failure, apparently bypassing the compensatory hypertrophy response. Post-TAC, the Lmna+/− cells displayed impaired nuclear mechanics and increased sensitivity to mechanical stress. As Egr-1 is also rapidly and transiently induced at the endothelial wound edge following acute aortic surgery, it is clear that Egr-1 is essential in cardiovascular tissue repair and its expression is dependent on the LINC complex [39]. The discrete mechanisms of how LINC complex components directly interact with DNA and chromatin to control gene expression have remained elusive. The NL is closely associated with chromatin and therefore its interaction is important in histone modifications such as acetylation and methylation—key components of transcriptional regulation [40]. Genomic interactions with the lamina occur at specific lamin-associated domains (LADs) and are normally repressive in nature, but cause hyperacetylation in lamin A mutants [41]. Recently, compelling evidence has emerged regarding an epigenetic mechanism for altered cardiomyocyte contractility in induced pluripotent stem cell (iPSC) cardiomyocytes harbouring a K219T mutation in LMNA [42]. The basis of this mechanism was that mutant lamin binding to the promoter region of the SCN5A gene, encoding the sodium channel Nav1.5, resulted in suppression of gene transcription via persistent co-binding of the polycomb repressive complex 2 and trimethylated histone 3 lysine 27. CRISPR–Cas9 correction of this mutation relieved this repressive complex, restored SCN5A gene transcription and Nav1.5 protein abundance and sodium current density. This result supports the idea that the NE can regulate excitation in cardiomyocytes. Whether there is a mechano-regulatory element to this mechanism remains an unresolved question.
In summary, these results indicate a mechanism for mechanically stimulated changes in gene expression in the nuclear adaptation response to mechanical load. The interaction between various LINC components is essential in these responses, and precise mechanisms are starting to become more clear.
(b). The impact of muscle mechanobiology upon genome integrity
Where regulation of the genome is concerned, the best-characterized outcome of NE dysfunction in many cell types is genome instability whereby unrepaired DNA lesions accumulate [43]. This often results in the stalling of cell cycle and senescence in cells that are meant to undergo division. DNA damage and DNA repair are in fact a regular function within the realms of cellular homeostasis. Problems arise when the repair mechanisms become defunct and rates of repair are unable to match rates of damage, leading to the accumulation of DNA lesions. Excess DNA damage results in the expression of cell-cycle inhibitors such as p16, p21 and p53, which cause cell-cycle arrest and senescence [44]. Though cardiac myocytes are post-mitotic, they may still succumb to senescence by reducing protein turnover and cell repair. In fact, emerging evidence suggests that genome instability, brought about by the loss of integrity to the NE, may play an important role in the muscle cell function as these myocytes develop a number of signs of senescence. We recently investigated the role of cardiomyocyte-specific prelamin A accumulation in mice. We found that mice developed rapid-onset cardiomyopathy, which was underpinned by DNA damage accumulation, elevated senescence markers and a profound inflammatory response [14]. Western blot analysis of LINC complex proteins found increases in an abundance of a number of LINC components such as nesprin 2 and SUN2, as well as the intermediate filament (IF) cytoskeletal protein desmin, which is known to link directly to the NE via the nesprin isoform nesprin 3. Indeed, another recent study has provided strong proof of the impact that IF and MT mechanics has on the integrity of the NE itself to reveal that nuclear shape is maintained and fine-tuned by a double-act of MTs and IFs [45]. Furthermore, this study confirmed the role of mechanobiology in the maintenance of the genome as DNA damage was elevated when the MT/IF ratio was increased. The authors were able to show gene expression changes involved in ion handling, contractility and mitochondrial function and metabolism occurred in cardiomyocytes with desmin knockdown. Finally, desmin depletion led to a considerable reduction in co-immunoprecipitation of chromatin LADs and lamin B1 compared to control myocytes indicating loss of heterochromatin regulation in these cells.
More recent evidence that molecular muscle mechanics regulates genome integrity has been provided by the Discher Lab who used the heart muscle of chick embryos to study the role of mechanics in NE and genome integrity [46,47]. They showed that mechanically ‘stiff’ environments caused by increased extracellular matrix synthesis and high actomyosin contractility could lead to NE rupture in cardiomyocytes, especially when lamin A abundance was low via lamin A knockdown. This led to a loss of DNA repair factors from the nucleus, which in turn resulted in an elevation in DNA damage. Phosphorylation of lamin A is known to induce its degradation to facilitate NE breakdown for mitosis in cycling cells and accordingly baseline lamin A phosphorylation is low in muscle cells. Interestingly, this study also showed that inhibition of lamin phosphorylation by cyclin-dependent kinases (CDK) prevented the mechanically regulated loss of lamin abundance. Functionally, DNA damage resulted in rhythmic changes to heart beating. Clinically, these data imply there is potential for CDK inhibitors to protect heart cell function in hearts that have undergone fibrotic disease remodelling. There is also recent evidence from experiments performed in skeletal muscle that disruption to the lamina by disease-causing lamin A/C mutations reduces the ability of nuclei to resist deformation caused by microfluid micropipetting and micro-harpooning experiments. This resulted in nuclear rupture, chromatin protrusions and persistent increases in DNA damage, which could be confirmed in muscle biopsies of patients exhibiting these mutations [48]. In non-muscle cell types, nuclear rupture has been shown to be a characteristic feature of lamin A/C deficiency and has been shown to result in the uncoordinated exchange of nuclear and cytoskeletal components when fluorescently labelled in mouse and human fibroblasts [49]. It was observed that severe rupture events were coupled with resulting focal chromatin condensation likely affecting transcriptional regulation. Evidence suggests that nuclear rupture can cause dysfunctional gene expression as well as DNA damage responses. More work is required to determine whether these are linked or occur independently of each other.
(c). LINC complex involvement in mechanically regulated DNA replication
Tissue dynamics, which one might define as the role of cells and organelles in active tissues regulating their core functions (i.e. contraction in muscle) as well as growth and repair, are critically dependent on molecular events that serve to facilitate them. Whether by the synthesis of new proteins or generation of new cells by cell division in response to injury or stress, replication of DNA is likely important. Whether it can be regulated by direct mechanical modulation is a new question.
Mechanotransduction has been implicated in cell-cycle progression in the epicardial ‘wavefront’ observed in a model of post-ischaemic heart injury in transgenic zebrafish [50]. In this study, epicardial tissue was partially ablated and cellular morphology was examined by immunostaining of ZO1—a tight junction marker. A ‘cellular wavefront’ was observed that was characterized by large multinucleate ‘leader cells’ preceding small, mononucleate ‘follower cells’. Live imaging concluded that defining features of the leader cells were increased stress fibres and endoreplication compared to the follower cells, which underwent cell division. The role of the leader cells was to migrate and maintain cell–cell adhesions while undergoing repair, for which endoreplication is necessary. Once repair was complete, the leader cells underwent apoptosis and were replaced by follower cells. Since actin-dependent migration was the source of leader cell stress, it was concluded that mechanotransduction played a role in leader cell-cycle regulation. These findings are further supported by a recent study showing that lamin B2 is essential for progression to M phase, and may hold some control over the regenerative potential of cardiomyocytes [51]. In this study, augmentation of lamin B2 expression, typically low in adult multinucleate polyploid cardiomyocytes, was shown to facilitate a reduction in ploidy, implicating reduced DNA replication in these cells. This facilitated NE breakdown and metaphase transition, which in turn increased myocardial regeneration in mice and cell division in human cardiomyocytes from induced pluripotent stem cells. Moreover, the authors showed that zebrafish cardiomyocytes—typically regenerative—exhibited naturally high levels of lamin B2.
Though direct evidence for mechano-regulation is lacking in the aforementioned study on lamin B2, there is compelling evidence from Drosophila melanogaster (fruit fly) muscle flank showing that the LINC complex plays a role in mechanically mediated DNA replication [52,53]. Confocal microscopy showed that systematic disruption of LINC complex proteins klarsicht and karoid (D. melanogaster homologues of nesprin and SUN, respectively) resulted in a small myonuclear size as the myonucleus did not grow synchronously with the myofibres themselves. However, analysis of Hoescht intensity revealed substantially increased DNA content indicative of polyploidy. The mutant cells underwent unchecked endoreplication during G1 and S phase of the cell cycle without progression to G2 and M phase. This was coupled with myonuclear positioning defects and highly varied nuclear size compared to myofibre size. Additionally, while control myofibres with an intact LINC slowed their DNA replication towards the end of development, mutant myonuclei persisted with endoreplication. Barrier to autointegration factor (BAF) is a key link between the NL and chromatin and was shown to be downregulated upon genetically mediated disruption of klarsicht and karoid. Subsequent repression of BAF alone resulted in a similar phenotype, suggesting BAF was the key link between the NL and proper cell-cycle progression. Overall, these results indicate that an intact LINC complex supports cells in high-stress environments to maintain physiologic homeostasis, i.e. growth and repair via regulation of DNA replication either directly or indirectly. Arguably, more importantly, the intact LINC complex is essential in repressing endoreplication via BAF once growth and repair are resolved.
Overall, the data discussed in this section broadly support the idea that the NE is a mechanosensitive regulator of DNA replication. One question that remains to be answered unequivocally is whether the mechano-regulation of DNA replication actively contributes to DNA replication or passively regulates it through a scaffolding or structural role.
4. Roles for the LINC complex in mediating Ca2+ handling in myocytes
It has been recently emphasized that the integration of multiple signalling modalities (mechanical, soluble, reactive oxygen species, ionic) is the ‘true physiologic condition’ and needs to be considered in approaches moving forward [54]. With this in mind, Ca2+ signalling in the LINC complex field is desperately understudied. In this section, we will propose the role of LINC in the functions of Ca2+ for cardiac conduction, contraction and genome regulation.
The principal role of Ca2+ in excitation–contraction coupling in the heart is well defined [55], and there is some evidence to support a role for LINC complex proteins in regulating this. For example, HGPS patients display conduction defects in the form of repolarization abnormalities [56]. Electrocardiograms (ECG) of these patients revealed abnormal electrical activity of the heart indicative of dysregulated Ca2+ handling between reticular stores and the cytoplasm known to negatively affect contraction and relaxation of cardiomyocytes. Supporting this, Zmpste24−/− mouse cardiomyocytes exhibited conduction defects owing to reduced ability to maintain stable Ca2+ reuptake because important Ca2+ reuptake proteins sarco/endoplasmic reticulum Ca2+ATPase (SERCA) 2 and calsequestrin 1/2 were reduced. Contrary to this, Lmna−/− hearts showed no dysregulation in Ca2+ handling [57], while recently, it has been shown that there are subtle changes to Ca2+ kinetics in pre-symptomatic cardiomyocytes from mice exhibiting the LMNA H222P mutation known to cause EDMD and DCM [58].
In 2007, Zima et al. [59] showed that the NE was a Ca2+ store and that the Ca2+ channels ryanodine receptors (RyRs) and inositol triphospate receptors (IP3Rs) were abundantly expressed in the NE membranes. It is well known that Ca2+, in concert with CamKII-Calmodulin, regulates functions of the genome in cardiomyocytes [60]. Therefore, one might hypothesize that LINC complex proteins may play a role in regulating the function and activity of these channels either indirectly by general NE disruption and spatial disorganization or directly by disruption to binding of complexes crucial to channel function. Indeed, immunofluorescence microscopy and co-immunoprecipitation have shown that the latter of these may be true since the muscle-specific isoform nesprin 1α was shown to co-localize with muscle A-kinase anchoring protein (mAKAP) at the NE in a model of neonatal rat cardiomyocytes [61]. This association was essential for the perinuclear localization of mAKAP and importantly, the RyR—a Ca2+ channel regulating efflux from perinuclear Ca2+ stores into the cytoplasm—was also precipitated with this protein complex. This result provides compelling evidence for the importance of the LINC complex in NE-mediated Ca2+ signalling. These conclusions are, however, complicated by the finding that nesprin 1α has been shown to localize to the Z-disk of the sarcomere, meaning they may play a scaffolding role in regulating sarcoplasmic reticulum (SR) channels and/or myofilament activation in distal locations of the cell rather than at the NE [62,63].
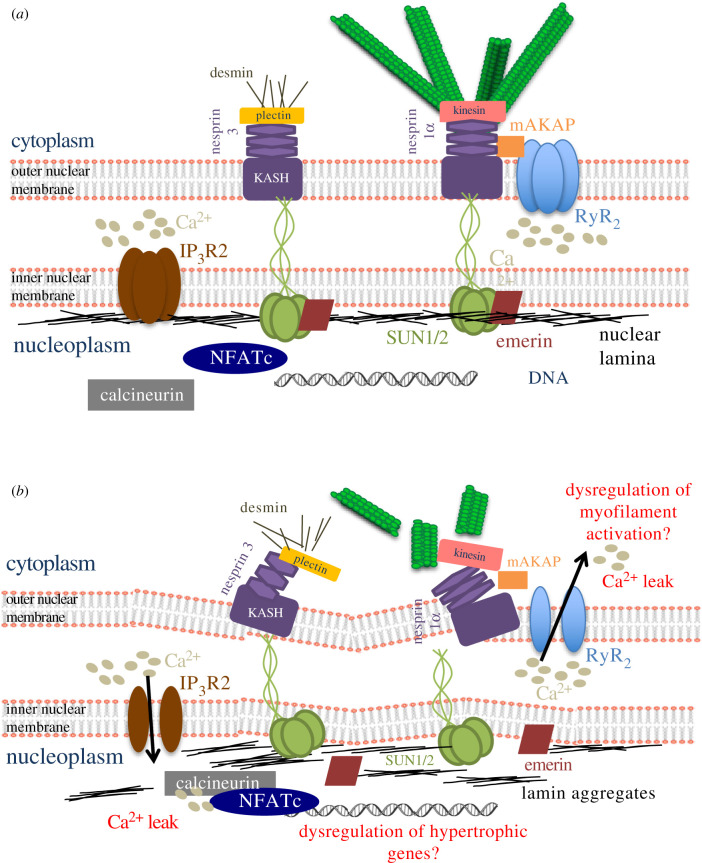
From the perspective of gene regulatory effects via Ca2+ handling, IP3Rs are perhaps of greater interest since inhibiting RyRs in adult cardiomyocytes did not affect perinuclear Ca2+ spark frequency [59]. Moreover, IP3Rs are mostly present within the inner nuclear membrane and regulate ion flow into the nucleoplasm. In ventricular cardiomyocytes, IP3Rs are known to generate a nuclear Ca2+ signal responsible for transcriptional changes that regulate cardiac hypertrophy [64]. Subsequently, western blot analysis of human DCM samples showed increased IP3R2 expression, suggesting that hyperactive transcription via nuclear Ca2+ signalling may be augmented in heart disease. Additionally, ventricular myocytes treated with hypertrophy agonist endothelin-1 were shown to exhibit increased Itpr2 expression, the gene encoding IP3R2. Subsequently, fluorescent labelling indicated that NFATc, a hypertropy regulator, directly bound to the Itpr2 promoter. Since calcineurin-NFATc signalling is a known pathway in cardiac hypertrophy, these results indicate that persistent nuclear Ca2+ sparks via increased IP3R2 expression is a marked feature of pathologic heart remodelling [65]. Given the location of IP3Rs in the NE and their role in gene transcription, it is reasonable to speculate that one of the LINC complex proteins is involved in regulating their function at the inner nuclear membrane (figure 2a).
Figure 2.
Hypothetical model of Ca2+ leakage from the transluminal space of the NE in LINC complex dysfunction. (a) The space between the outer and inner nuclear membrane acts as a Ca2+ store. The IP3R2 channel is located in the inner nuclear membrane and regulates Ca2+ movement into the nucleoplasm, while RyR2 lies in the outer nuclear membrane and controls diffusion into the cytoplasm. (b) A defective LINC complex may cause disruption of the IP3R2 channel. Mishandling of Ca2+ transport into the nucleoplasm such as Ca2+ leak could lead to unregulated activation of the calcineurin-NFATc transcription factor complex. This complex directly binds DNA and promotes hypertrophic gene transcription. Disruption of the connection between the LINC complex and RyR2 occurs via mAKAP and nesprin 1α, leading to Ca2+ leak into the cytoplasm and potentially contributing to perinuclear myofilament activation.
Importantly, there is also a precedent for mechanical regulation of Ca2+ in cardiomyocytes because physical transmission of stress from the sarcolemma to the SR via MTs has been shown to induce Ca2+ leak via the RyR2 [66]. In this study, carbon fibres attached to cell ends were used to induce stretch in rat ventricular myocytes and the Ca2+ spark rate was measured. Axial stretch caused an acute increase in Ca2+ only in the stretched part of the cell, implying a mechanical mechanism of Ca2+ release from the SR. In support of this finding, diastolic stretch in single mouse mdx myocytes generated transient increases in intracellular Ca2+ that propagated in waves, resulting in SR overload and sensitization of RyR2 channels [67]. Moreover, there is also evidence to suggest that the embryonic heartbeat is initiated by direct mechanical modulation of Ca2+ channels of the SR [68]. It is plausible that these mechanical mechanisms operating on the SR could do so in a similar manner at the NE and influence nuclear Ca2+ signalling and gene expression, which is likely to be defective in the face of LINC complex disruption. Based on the evidence presented, disruptions in various components that associate directly with MTs such as nesprin or indirectly such as SUN and lamin A are likely to play important roles in the pathological progression of cardiac disease associated with the nuclear Ca2+ transient (figure 2b).
In terms of nuclear Ca2+ events during disease processes, studies have shown that, upon hypertrophic stimuli, Ca2+ is diffused from the nuclear membrane into the nucleoplasm and invagination is required for Ca2+ to be pumped back into the nucleoplasm [69]. Accumulation of perinuclear RyRs and SERCA after transthoracic aortic constriction in mouse hearts has also been observed and suggests that enhanced Ca2+ transport may be occurring at the NE and detrimentally affecting nuclear Ca2+ transients. This may suggest dysregulation, potentially augmentation, of Ca2+-dependent NFAT and Ca2+/calmodulin-dependent protein kinase (CaMKII) signalling occurs that mediates hypertrophic gene expression programmes. There is early evidence to both refute and support the notion that the LINC complex could regulate the nuclear Ca2+ transient. First, Muchir and colleagues have very recently published a study where they examined the amplitude of the nuclear Ca2+ transient in cardiomyocytes isolated from mice with a LMNA mutation known to cause EDMD and DCM in humans and found no difference compared to wild-type control mice at an early pre-symptomatic time point, suggesting that the LINC complex may not be all that important in regulating nuclear Ca2+, though it is important to note these experiments were performed in baseline conditions, i.e. under no mechanical stress [58]. Indeed, hypertrophic stimulation of neonatal rat ventricular myocytes with emerin knockdown resulted in increased half-decay time of the nuclear Ca2+ transient, increased nuclear size and decreased nuclear invagination [70]. Emerin re-expression restored nuclear invagination, and increased nucleoplasmic Ca2+ transient. This result implicates emerin as a regulator of the nuclear Ca2+ transient, though it is not clear whether this is by direct association with nuclear Ca2+ channels or through general disruption of the NE. It is interesting that the regulation of nuclear invagination formation and regression was attenuated in this study. On the one hand, the mechanical interplay between the cytoskeleton, NE and heterochromatin could regulate this, as it is now known that heterochromatin is also mechanically compliant and contributes to nucleus stiffness in non-muscle cell types (imagine a tug-of-war type scenario) [71,72]. On the other hand, there may be a regulatory role for the NL in the de novo synthesis of phospholipid membrane to drive NE membrane extension, as recently shown by experiments combining Nanoscale Secondary Ion Mass Spectrometry with Light microscopy that tracked nascent phospholipid formation and observed selective lamin incorporation during synthesis [73]. These processes may even be co-dependent.
Clearly, much work is required to establish a role for the LINC complex and mechano-mediated nuclear Ca2+ handling. Answering some of these questions could begin with investigating the consequences to the nuclear Ca2+ transient of mechanical strain alongside systematic removal of LINC complex components from myocytes. Moreover, defining a potential biophysical and biochemical relationship between LINC complex components and Ca2+ channels such as RYRs and IP3R2 via contact prediction modelling, immunofluorescence colocalization studies and co-immunoprecipitation will be fundamental to making progress in this area.
5. Conclusion
The current state of the LINC complex literature shows that components of the LINC complex act to convey mechanical pressure from the cytoskeleton in order to maintain nuclear shape and genome integrity. Emerging data indicate that the LINC complex is able to help regulate dynamic events such as DNA replication and gene transcription. Disruption of the NE makes the nuclear membrane more susceptible to mechanical stress and leads to misregulation of the genome in multiple models of LINC complex disruption. In-depth mechanisms are still being elucidated, but recent progress has been promising and the field is moving quickly.
The mechanobiology field is still quite new and understanding the relative contributions of mechanical versus chemical and electrical signalling is proving challenging. Indeed, improving our understanding of the position that the LINC complex occupies within integrated models of signalling, especially with regard to nuclear Ca2+ regulation, is required to provide a more complete picture of how mechanical stress elicits a (patho)physiological response. For example, does the LINC complex regulate nuclear Ca2+ transient in skeletal and cardiac muscle cells under stress? If so, is this through direct mechanical modulation of local Ca2+ channels, or is this mostly a structural role, i.e. maintaining an intact NE for stable membrane incorporation of channels? Can any observed effects be isolated from altered electrical or chemical modulation for a full reductionist understanding of these signalling realms?
Acknowledgements
We thank Cathy Shanahan and Elizabeth Halton for their useful comments and feedback on this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors listed made a substantial contribution to the generation and synthesis of ideas and writing of this manuscript, and approved it for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was not directly supported by funding.
References
- 1.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. 2006. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41–53. ( 10.1083/jcb.200509124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosa BA, Rothballer A, Kutay U, Schwartz TU. 2012. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035–1047. ( 10.1016/j.cell.2012.03.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. 2009. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64, 173–187. ( 10.1016/j.neuron.2009.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi YH, Chen CY, Jeang KT. 2012. Reversal of laminopathies: the curious case of SUN1. Nucleus 3, 418–421. ( 10.4161/nucl.21714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W, Worman HJ, Gundersen GG. 2015. Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol. 208, 11–22. ( 10.1083/jcb.201409047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajgor D, Shanahan CM. 2013. Nesprins: from the nuclear envelope and beyond. Expert Rev. Mol. Med. 15, e5 ( 10.1017/erm.2013.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimpel P, et al. 2017. Nesprin-1α-dependent microtubule nucleation from the nuclear envelope via Akap450 is necessary for nuclear positioning in muscle cells. Curr. Biol. 27, 2999–3009. ( 10.1016/j.cub.2017.08.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner N, Krohne G. 2007. LEM-domain proteins: new insights into lamin-interacting proteins. Int. Rev. Cytol. 261, 1–46. ( 10.1016/S0074-7696(07)61001-8) [DOI] [PubMed] [Google Scholar]
- 9.De Sandre-Giovannoli A, et al. 2003. Lamin A truncation in Hutchinson–Gilford progeria. Science 300, 2055 ( 10.1126/science.1084125) [DOI] [PubMed] [Google Scholar]
- 10.Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, Edvardsen T, Haugaa KH. 2018. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur. Heart J. 39, 853–860. ( 10.1093/eurheartj/ehx596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worman HJ, Bonne G. 2007. ‘Laminopathies’: a wide spectrum of human diseases. Exp. Cell Res. 313, 2121–2133. ( 10.1016/j.yexcr.2007.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F, Worman HJ. 1993. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, 16 321–16 326. ( 10.1006/geno.1995.1036) [DOI] [PubMed] [Google Scholar]
- 13.Barrowman J, Hamblet C, Kane MS, Michaelis S. 2012. Requirements for efficient proteolytic cleavage of prelamin A by ZMPSTE24. PLoS ONE 7, e32120 ( 10.1371/journal.pone.0032120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brayson D, et al. 2019. Prelamin A mediates myocardial inflammation in dilated and HIV-associated cardiomyopathies. JCI Insight 4, 126315 ( 10.1172/jci.insight.126315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casasola A, Scalzo D, Nandakumar V, Halow J, Recillas-Targa F, Groudine M, Rincon-Arano H. 2016. Prelamin A processing, accumulation and distribution in normal cells and laminopathy disorders. Nucleus 7, 84–102. ( 10.1080/19491034.2016.1150397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young SG, Meta M, Yang SH, Fong LG. 2006. Prelamin A farnesylation and progeroid syndromes. J. Biol. Chem. 281, 39 741–39 745. ( 10.1074/jbc.R600033200) [DOI] [PubMed] [Google Scholar]
- 17.Scharner J, Gnocchi VF, Ellis JA, Zammit PS. 2010. Genotype–phenotype correlations in laminopathies: how does fate translate? Biochem. Soc. Trans. 38, 257–262. ( 10.1042/BST0380257) [DOI] [PubMed] [Google Scholar]
- 18.Swift J, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 ( 10.1126/science.1240104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swift J, Discher DE. 2014. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 127, 3005–3015. ( 10.1242/jcs.149203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makarov AA, Zou J, Houston DR, Spanos C, Solovyova AS, Cardenal-Peralta C, Rappsilber J, Schirmer EC. 2019. Lamin A molecular compression and sliding as mechanisms behind nucleoskeleton elasticity. Nat. Commun. 10, 3056 ( 10.1038/s41467-019-11063-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West G, et al. 2016. Deleterious assembly of the lamin A/C mutant p.S143P causes ER stress in familial dilated cardiomyopathy. J. Cell Sci. 129, 2732–2743. ( 10.1242/jcs.184150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raharjo WH, Enarson P, Sullivan T, Stewart CL, Burke B. 2001. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 114, 4447–4457. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee A, Rathee V, Krishnaswamy R, Bhattacharjee P, Ray P, Sood AK, Sengupta K. 2013. Viscoelastic behavior of human lamin A proteins in the context of dilated cardiomyopathy. PLoS ONE 8, e83410 ( 10.1371/journal.pone.0083410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. 2004. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370–378. ( 10.1172/JCI19670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, Herrmann H, Wallrath LL, Lammerding J. 2013. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 22, 2335–2349. ( 10.1093/hmg/ddt079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroud MJ, Banerjee I, Veevers J, Chen J. 2014. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ. Res. 114, 538–548. ( 10.1161/CIRCRESAHA.114.301236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Rao L, Shanahan CM, Zhang Q. 2018. Nesprin-1/2: roles in nuclear envelope organisation, myogenesis and muscle disease. Biochem. Soc. Trans. 46, 311–320. ( 10.1042/BST20170149) [DOI] [PubMed] [Google Scholar]
- 28.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. 2005. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 171, 799–810. ( 10.1083/jcb.200506083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C, et al. 2017. Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis. Hum. Mol. Genet. 26, 2258–2276. ( 10.1093/hmg/ddx116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinke P, et al. 2014. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 10, e1004605 ( 10.1371/journal.pgen.1004605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. 2010. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J. Biol. Chem. 285, 3487–3498. ( 10.1074/jbc.M109.071910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert HTJ, Mallikarjun V, Dobre O, Jackson MR, Pedley R, Gilmore AP, Richardson SM, Swift J. 2019. Nuclear decoupling is part of a rapid protein-level cellular response to high-intensity mechanical loading. Nat. Commun. 10, 4149 ( 10.1038/s41467-019-11923-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidzianska A, Hausmanowa-Petrusewicz I. 2003. Architectural abnormalities in muscle nuclei. Ultrastructural differences between X-linked and autosomal dominant forms of EDMD. J. Neurol. Sci. 210, 47–51. ( 10.1016/s0022-510x(03)00012-1) [DOI] [PubMed] [Google Scholar]
- 34.Alam SG, et al. 2016. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 6, 38063 ( 10.1038/srep38063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K.. 2014. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16, 376–381. ( 10.1038/ncb2927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tifft KE, Bradbury KA, Wilson KL. 2009. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J. Cell Sci. 122, 3780–3790. ( 10.1242/jcs.048397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. 2013. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511. ( 10.1038/nature12105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cupesi M, Yoshioka J, Gannon J, Kudinova A, Stewart CL, Lammerding J. 2010. Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J. Mol. Cell. Cardiol. 48, 1290–1297. ( 10.1016/j.yjmcc.2009.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khachigian LM. 2006. Early growth response-1 in cardiovascular pathobiology. Circ. Res. 98, 186–191. ( 10.1161/01.RES.0000200177.53882.c3) [DOI] [PubMed] [Google Scholar]
- 40.Prokocimer M, Barkan R, Gruenbaum Y. 2013. Hutchinson–Gilford progeria syndrome through the lens of transcription. Aging Cell 12, 533–543. ( 10.1111/acel.12070) [DOI] [PubMed] [Google Scholar]
- 41.Guelen L, et al. 2008. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951. ( 10.1038/nature06947) [DOI] [PubMed] [Google Scholar]
- 42.Salvarani N, et al. 2019. The K219T-Lamin mutation induces conduction defects through epigenetic inhibition of SCN5A in human cardiac laminopathy. Nat. Commun. 10, 2267 ( 10.1038/s41467-019-09929-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misteli T, Scaffidi P. 2005. Genome instability in progeria: when repair gets old. Nat. Med. 11, 718–719. ( 10.1038/nm0705-718) [DOI] [PubMed] [Google Scholar]
- 44.Shaltiel IA, Krenning L, Bruinsma W, Medema RH. 2015. The same, only different—DNA damage checkpoints and their reversal throughout the cell cycle. J. Cell Sci. 128, 607–620. ( 10.1242/jcs.163766) [DOI] [PubMed] [Google Scholar]
- 45.Heffler J, et al. 2019. A balance between intermediate filaments and microtubules maintains nuclear architecture in the cardiomyocyte. Circ. Res. 126, e10–e26. ( 10.1161/CIRCRESAHA.119.315582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho S, et al. 2019. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev. Cell 49, 920–935. ( 10.1016/j.devcel.2019.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xanthis I, Iskratsch T. 2019. Lamin-A mechano-protects the heart. Dev. Cell 49, 821–822. ( 10.1016/j.devcel.2019.05.041) [DOI] [PubMed] [Google Scholar]
- 48.Earle AJ, et al. 2020. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 19, 464–473. ( 10.1038/s41563-019-0563-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robijns J, Molenberghs F, Sieprath T, Corne TD, Verschuuren M, De Vos WH.. 2016. In silico synchronization reveals regulators of nuclear ruptures in lamin A/C deficient model cells. Sci. Rep. 6, 30325 ( 10.1038/srep30325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao J, et al. 2017. Tension creates an endoreplication wavefront that leads regeneration of epicardial tissue. Dev. Cell 42, 600–615. ( 10.1016/j.devcel.2017.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han L, et al. 2020. Lamin B2 levels regulate polyploidization of cardiomyocyte nuclei and myocardial regeneration. Dev. Cell 53, 42–59. ( 10.1016/j.devcel.2020.01.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Stoops E, Cp U, Markus B, Reuveny A, Ordan E, Volk T. 2018. Mechanotransduction via the LINC complex regulates DNA replication in myonuclei. J. Cell Biol. 217, 2005–2018. ( 10.1083/jcb.201708137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brayson D, Ho CY, Shanahan CM. 2018. Muscle tensions merge to cause a DNA replication crisis. J. Cell Biol. 217, 1891–1893. ( 10.1083/jcb.201804041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izu LT, Kohl P, Boyden PA, Miura M, Banyasz T, Chiamvimonvat N, Trayanova N, Bers DM, Chen-Izu Y. 2019. Mechano-electric and mechano-chemo-transduction in cardiomyocytes. J. Physiol. 598, 1285–1305. ( 10.1113/JP276494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bers DM. 2002. Cardiac excitation–contraction coupling. Nature 415, 198–205. ( 10.1038/415198a) [DOI] [PubMed] [Google Scholar]
- 56.Rivera-Torres J, et al. 2016. Cardiac electrical defects in progeroid mice and Hutchinson–Gilford progeria syndrome patients with nuclear lamina alterations. Proc. Natl Acad. Sci. USA 113, E7250–E7259. ( 10.1073/pnas.1603754113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikolova V, et al. 2004. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113, 357–369. ( 10.1172/JCI19448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez BM, et al. 2020. Activation of sarcolipin expression and altered calcium cycling in LMNA cardiomyopathy. Biochem. Biophys. Rep. 22, 100767 ( 10.1016/j.bbrep.2020.100767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zima AV, Bare DJ, Mignery GA, Blatter LA. 2007. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J. Physiol. 584, 601–611. ( 10.1113/jphysiol.2007.140731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saucerman JJ, Bers DM. 2008. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys. J. 95, 4597–4612. ( 10.1529/biophysj.108.128728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. 2005. Nesprin-1α contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp. Cell Res. 303, 388–399. ( 10.1016/j.yexcr.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q, et al. 2007. Nesprin-1 and -2 are involved in the pathogenesis of Emery–Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, 2816–2833. ( 10.1093/hmg/ddm238) [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. 2005. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J. Cell Sci. 118, 673–687. ( 10.1242/jcs.01642) [DOI] [PubMed] [Google Scholar]
- 64.Sankar N, deTombe PP, Mignery GA. 2014. Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J. Biol. Chem. 289, 6188–6198. ( 10.1074/jbc.M113.495242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higazi DR, et al. 2009. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol. Cell 33, 472–482. ( 10.1016/j.molcel.2009.02.005) [DOI] [PubMed] [Google Scholar]
- 66.Iribe G, et al. 2009. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ. Res. 104, 787–795. ( 10.1161/CIRCRESAHA.108.193334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prosser BL, Ward CW, Lederer WJ. 2011. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445. ( 10.1126/science.1202768) [DOI] [PubMed] [Google Scholar]
- 68.Chiou KK, et al. 2016. Mechanical signaling coordinates the embryonic heartbeat. Proc. Natl Acad. Sci. USA 113, 8939–8944. ( 10.1073/pnas.1520428113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ljubojevic S, et al. 2014. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130, 244–255. ( 10.1161/CIRCULATIONAHA.114.008927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimojima M, et al. 2017. Emerin plays a crucial role in nuclear invagination and in the nuclear calcium transient. Sci. Rep. 7, 44312 ( 10.1038/srep44312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hobson CM, Kern M, O'Brien ET, Stephens AD, Falvo MR, Superfine R. 2020. Correlating nuclear morphology and external force with combined atomic force microscopy and light sheet imaging separates roles of chromatin and lamin A/C in nuclear mechanics. Mol. Biol. Cell mbcE20010073 ( 10.1091/mbc.E20-01-0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nava MM, et al. 2020. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800–817. ( 10.1016/j.cell.2020.03.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drozdz MM, Jiang H, Pytowski L, Grovenor C, Vaux DJ. 2017. Formation of a nucleoplasmic reticulum requires de novo assembly of nascent phospholipids and shows preferential incorporation of nascent lamins. Sci. Rep. 7, 7454 ( 10.1038/s41598-017-07614-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.