Abstract
The cognitive-buffer hypothesis proposes that more harsh and unpredictable environments favour animals with larger brains and resulting greater cognitive skills. Comparisons across taxa have supported the hypothesis, but it has rarely been tested within a species. We measured brain size, as inferred from head dimensions, for 1141 cliff swallow specimens collected in western Nebraska, 1982–2018. Cliff swallows starving to death during unusual late-spring cold snaps had significantly smaller brains than those dying from other causes, suggesting that brain size in this species can affect foraging success and that greater cognitive ability may confer advantages when conditions exceed normal environmental extremes. Brain size declined significantly with the size of the breeding colony from which a specimen came. Larger brains may be favoured in smaller colonies that represent more unpredictable and more challenging social environments where there is less public information on food sources and less collective vigilance against predators, even in relatively normal conditions. Our results provide intraspecific support for the cognitive-buffer hypothesis and emphasize the potential evolutionary impact of rare climatic events.
Keywords: brain size, cognition, coloniality, environmental variability, Petrochelidon pyrrhonota, severe weather
1. Introduction
The evolution of brain size in animals has attracted considerable attention, and evidence now suggests that unpredictable environments select for larger brains [1–5]. When environmental variation presents organisms with novel challenges, such as where to find food, a greater cognitive ability may aid in overcoming these problems by facilitating the adoption of new behaviour [2,6,7]. This has led to the cognitive-buffer hypothesis that larger brains should be associated with more complex socioecological environments [8,9]. Interspecific studies have supported the association between brain size and extent of environmental variability [1–5,8,10], while other studies have shown that higher levels of cognition-driven problem-solving occur in harsher, more unpredictable environments [11,12].
Empirical tests of the cognitive-buffer hypothesis have mostly involved cross-species comparisons, yet the same selective pressures on brain size should also apply within species whenever individuals are exposed to extreme conditions. One component of environmental variability is unusually severe weather, which can lead to intense selection on morphological traits such as body size or behavioural traits such as spring arrival time [13–19], but occurs so rarely that often only long-term studies can detect its effects. Consequently, little is known about how unusually harsh conditions might affect selection on cognitive abilities as reflected in brain size within a species.
Here, we examine brain size in the cliff swallow (Petrochelidon pyrrhonota) using a specimen collection spanning 37 years to investigate whether brain size varies among individuals in a manner predicted by the cognitive-buffer hypothesis. We examined whether brain size differed among birds that died during severe weather when food was scarce, relative to the population at large. This allowed us to infer the degree to which foraging-related cognitive abilities [6,7,12] may have affected survival during these rare events. Because cliff swallows breed in colonies of different sizes that present their own socioecological challenges [20,21], we also measured the effects of colony size (the social environment) that might have independently influenced brain size [22–24].
2. Methods
(a). Study animal and study site
Cliff swallows build gourd-shaped nests out of mud and position their nests underneath overhanging horizontal ledges on the sides of cliffs, bridges, buildings and highway culverts [25]. The birds live in colonies that can vary in size in our study area from 2 to 6000 nests (mean ± s.e. = 404 ± 11 nests, n = 3277 colonies), with some birds nesting solitarily. Cliff swallows feed exclusively on swarms of aerial insects that can be difficult to locate, and the birds often use one another to find food [20,26]. Our study site was in southwestern Nebraska near the University of Nebraska's Cedar Point Biological Station (41.2097° N, 101.6480° W), encompassing parts of Keith, Garden, Lincoln and Morrill counties, where we studied cliff swallows nesting mostly on highway bridges and culverts underneath roads or railroad tracks [20,27].
(b). Specimen collection and weather events
Cliff swallows were collected opportunistically in 1982–2018 whenever salvageable specimens were found in the course of our research and preserved as skins. These included birds dying in mist-netting accidents, on roads owing to collisions with vehicles, during severe weather events, and owing to other miscellaneous causes (e.g. drowning during fights, nest falls, killed by predators). The colony at which a dead bird was found was used to designate the colony size for each specimen. Colony size refers to the number of active nests at a site that year and was determined from active nest counts or estimation from the number of birds present [20,27]. For each colony, where we had more than 50 specimens in a year (all in 1996 as a result of severe weather; see below), we randomly selected 50 from each site to measure.
Multiple-day periods of cold and rainy weather in late-spring (when insects are not active) lead to cliff swallow mortality owing to starvation that varies in severity depending on how long the cold weather lasts [14,19]. We documented that cold weather caused mortality in 1988, 1992, 1996, 2004 and 2017; that of 1996 was the most severe, with at least 53% of the population perishing over a 6-day period [14]. We visited colonies immediately after the bad weather ended and salvaged all dead birds on the ground underneath nests.
(c). Measuring head (brain) size, endocranial volume and body size
Following Møller [28], we recorded head size on each specimen using calipers to measure (i) head length from the cere to the back of the skull; (ii) head width at the widest point behind each ear and (iii) head depth from the base of the jaw to the top of the head. Measurements were taken by one person only (GSW). Brain size was inferred from the head volume, which was calculated using the formula for the volume (v) of an ellipsoid, v = 4/3πlwd (l = head length, w = head width, d = head depth). To examine whether brain size might vary with measures of body size, right wing length was taken on each specimen with a stoppered wing ruler, and bill length from the cere to the tip of the bill was measured with calipers. We measured 1141 specimens, although some were missing information on colony size, cause of death or sex, so sample sizes differ slightly between analyses. Only adult birds ≥ 1 year old (known by their breeding plumage) were used in this study.
Endocranial volume correlates directly with brain size in multiple bird species [29] and head size as measured here strongly predicts brain size in barn swallows (Hirundo rustica; [28]). We assessed this relationship for cliff swallows using 10 randomly selected birds for which head size was measured as described above, the specimen was skinned, the interior of the skull cleared of brain matter and the skull filled with #10 lead shot through the foramen [29]. The mass (m) of the lead shot and the density (d) of lead were used to calculate relative endocranial volume (v) using the equation, v = m/d. Repeatability in measures of brain size was determined by randomly selecting 50 specimens and re-measuring them 3 months later while blind to the previous measures. All specimens were from the collection at the University of Tulsa, except for 9 and 8 specimens from 1984 and 1985, respectively, that were from the American Museum of Natural History and the Peabody Museum of Natural History, respectively.
(d). Statistical analyses
We used mixed models to determine predictors of brain size, beginning with a model including sex, colony size, cause of death, year, wing length and bill length; colony site was treated as a random effect. Variables not significant at p ≤ 0.157 [30] were removed from the final model, as were all interaction terms because none was significant. A repeatability analysis of brain size used the intraclass correlation coefficient [31]. All statistical tests were performed with SAS.
3. Results
Head measurements (from which we inferred brain size) were significantly associated with endocranial volume in cliff swallows (rs = 0.70, p = 0.025, n = 10). Repeatability of head size measurements was highly significant (rI = 0.439, F1,49 = 2.62, p = 0.0005). Brain size varied from 10.33 to 25.09 cm3, representing a range of about 6 standard deviation units.
Brain size in cliff swallows was predicted by sex (F1,829 = 12.24, p = 0.0005; figure 1), cause of death (F3,829 = 20.52, p < 0.0001; figure 1) and colony size (F1,829 = 68.63, p < 0.0001; β ± s.e. = −0.00162 ± 0.000196; figure 2), but there was no significant effect of year (F1,828 = 0.85, p = 0.36; β ± s.e. = 0.0133 ± 0.0145). Wing length had no significant association with brain size (F1,827 = 0.49, p = 0.49; β ± s.e. = 0.0317 ± 0.0455), but there was a weak inverse relationship between bill length and brain size (F1,829 = 4.08, p = 0.044; β ± s.e. = -6.497 ± 3.215). Males (n = 590) averaged (± s.e.) 16.18 (± 0.097) cm3 in brain size and females (n = 524) 15.85 (± 0.11) cm3, a difference of 0.134 standard deviation units. The sex difference applied to birds regardless of cause of death (figure 1).
Figure 1.

Mean (±1 s.e.) brain size (in cm3) of male and female cliff swallows in relation to cause of death. Numbers above bars indicate number of specimens measured (sample size). ‘Other' category includes birds killed by predators and nest falls, drownings and presumed natural causes. Brain size of weather fatalities was significantly smaller than that of net fatalities, road kills and other causes of death.
Figure 2.
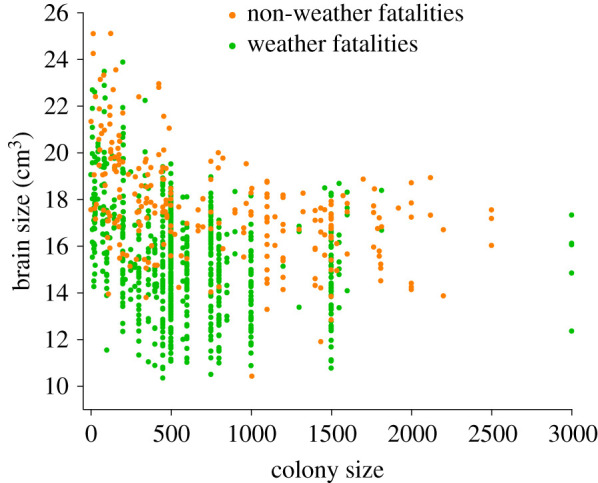
Brain size (in cm3) of cliff swallows in relation to colony size for birds killed by weather versus all other causes. Each dot represents one bird, although points overlap extensively in some cases. Brain size declined significantly with colony size (see §3). Sexes are combined here.
Cliff swallows killed in cold weather had smaller brains than birds dying owing to all other causes (figure 1). Male and female weather fatalities had brain sizes 0.89 and 0.93 standard deviation units, respectively, smaller than the next closest cause of death category. Among the other causes of death, there were no significant differences: for example, net casualties and road kills did not differ in brain sizes for either males (Z = 0.075, p = 0.94, Wilcoxon test) or females (Z = 0.775, p = 0.44).
Brain size declined as colony size increased for both weather fatalities and non-weather fatalities (figure 2). This relationship was the same regardless of cause of death, with no significant interaction between cause of death and colony size (F1,826 = 0.50, p = 0.68).
4. Discussion
Our results provide intraspecific support for the cognitive-buffer hypothesis [8,9], in that smaller brained cliff swallows were more likely to succumb during harsh conditions that exceeded normal environmental extremes. These kinds of unusual events are relatively rare, with only five of them occurring during the 37-year duration of the study, but the more severe episodes can impose strong selection for traits that help individuals avoid starvation [14]. Greater cognitive abilities could allow cliff swallows to innovate, for example, by foraging in different places where insects gather in inclement conditions (e.g. on warmer asphalt road surfaces) or by feeding in different ways (e.g. picking insects off a shoreline from the ground rather than in the air). Birds with larger brains that can better problem-solve in novel situations should be at an advantage in these more extreme conditions [11,12], and the smaller brains of weather fatalities (figure 1) support that inference.
If, as predicted, the frequency of bad weather events in the Nebraska study area increases with global climate change [19,32], more frequent episodic selection for larger brains could lead to permanent microevolutionary change in brain size over time, as we have seen for other traits [16]. However, such a directional shift is so far not evident in our data: year had no significant effect on brain size over the 37 years of our study. This result could be partly because brain size can be constrained by energetic costs [33], especially in species like cliff swallows that are long-distance migrants [5].
Our finding that larger brained cliff swallows tended to settle in smaller colonies also supports the cognitive-buffer hypothesis, because residents of small cliff swallow colonies likely encounter greater ecological challenges than do birds settling in large colonies, even when conditions are not severe. For example, individuals in large colonies frequently use public information from conspecifics on where food can be found, information that is more readily available because of the many birds present; those in smaller groups engage in almost no information transfer and often hunt solitarily [20]. Thus, smaller colonies may select for cognitively superior cliff swallows that have greater foraging ability and select against those smaller brained birds that are less creative foragers. Being prone to feeding innovations could partly compensate for the lack of foraging information from conspecifics in smaller cliff swallow colonies.
In addition, the heightened awareness of predators in large colonies, owing to greater vigilance because of many eyes [20], provides more protection for birds that do not invest in the cognitive capacity to better detect or predict predator attacks. Given that in some animals' brain size is positively associated with the likelihood of avoiding predators [34,35], differing predation risk among groups might favour smaller brained cliff swallows that settle in the safer, larger colonies. Cognitively superior individuals with bigger brains that are more competent at avoiding predators and/or finding food should prefer smaller colonies where they minimize the inevitable costs of coloniality [20,36].
Our finding smaller brained cliff swallows settling in larger colonies contrasts with results from barn swallows, in which brain size varied directly with colony size [28]. Møller [28] argued that larger barn swallow colonies represented more complex social environments where bigger brains and greater cognitive abilities might be important in tracking social relationships among residents. This ‘social-brain' hypothesis [22–24] has attracted considerable interest, but applies best to species that establish long-term social bonds among group members. Because cliff swallows mostly interact with a relatively small subset of close neighbours within a colony regardless of colony size and do not form any long-term social bonds with specific colony members [20,37], the social-brain hypothesis probably does not apply to cliff swallows.
Unlike most studies that use a measure of relative brain size corrected for body size, we used absolute brain size because in cliff swallows brain size did not increase significantly with body size. Bill length was our measure of body size for cliff swallows, with bill length correlating directly with other skeletal metrics such as tarsus length [16]. Interestingly, larger brained cliff swallows had shorter bills. This indicates that larger brained birds were not favoured simply because they had larger body size. Wing length was not a predictor of brain size in cliff swallows, and thus any selection on brain size occurs independently of selection on wing length. For example, while wing length in cliff swallows is under selection brought about by road-associated mortality [38], we found no differences in brain size between birds killed on roads versus ones dying owing to other non-weather-related causes. This suggests that mortality from vehicles does not select for greater cognitive abilities in cliff swallows and does not support an analysis [39] suggesting that birds in general killed by vehicles have smaller brains than those dying for other reasons. While we found a significant sex difference in brain size, the difference was slight and less than that reported for barn swallows [28]. In cliff swallows the cognitive advantages that may lead to innovative foraging should apply to both sexes, and the bad weather and colony size relationships were the same for the sexes.
Acknowledgements
We thank Lizzie Connor, Stacey Hannebaum, Allison Johnson, Shadi Nadri, Catherine Page and especially Mary Bomberger Brown for preparing the specimens; Jessica Cargill and Maria Pereyra for the endocranial volume measurements; the Cedar Point Biological Station of the University of Nebraska-Lincoln for use of facilities; the American Museum of Natural History and Yale University's Peabody Museum of Natural History for loan of specimens; and three anonymous reviewers for comments on the manuscript.
Ethics
Specimens were collected under salvage permits issued by the U. S. Fish and Wildlife Service and the Nebraska Game and Parks Commission, and under Institutional Animal Care and Use Committee protocols of Yale University, the University of Nebraska and the University of Tulsa.
Data accessibility
Raw data for this paper are deposited in Dryad: https://dx.doi.org/10.5061/dryad.bcc2fqz8g [40].
Authors' contributions
G.S.W. collected data and assisted in the manuscript preparation; C.R.B. conceived project, analysed data and wrote manuscript. Both authors approved the final version and are accountable for the work reported.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the National Science Foundation (grant nos. BSR-8600608, BSR-9015734, DEB-9613638, IBN-9974733, DEB-0075199, DEB-0514824, DEB-1019423, DEB-1453971 and IOS-1556356) and the National Institutes of Health (grant no. R01AI057569).
References
- 1.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and responses of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sol D, Lefebvre L, Rodríguez-Teijeiro JD. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441. ( 10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sol D, Székely T, Liker A, Lefebvre L. 2007. Big-brained birds survive better in nature. Proc. R. Soc. B 274, 763–769. ( 10.1098/rspb.2006.3765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. 2016. Environmental variation and the evolution of large brains in birds. Nat. Comm. 7, 13971 ( 10.1038/ncomms13971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincze O. 2016. Light enough to travel or wise enough to stay? Brain size evolution and migratory behavior in birds. Evolution 70, 2123–2133. ( 10.1111/evo.13012) [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. 1997. Feeding innovations and forebrain size in birds. Anim. Behav. 53, 549–560. ( 10.1006/anbe.1996.0330) [DOI] [Google Scholar]
- 7.Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim. Behav. 78, 1001–1010. ( 10.1016/j.anbehav.2009.06.033) [DOI] [Google Scholar]
- 8.Allman J, McLaughlin T, Hakeem A. 1993. Brain weight and life-span in primate species. Proc. Natl Acad. Sci USA 90, 118–122. ( 10.1073/pnas.90.1.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sol D. 2009. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130–133. ( 10.1098/rsbl.2008.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuck-Paim C, Alonso WJ, Ottoni EB. 2008. Cognition in an ever-changing world: climatic variability is associated with brain size in neotropical parrots. Brain Behav. Evol. 71, 200–215. ( 10.1159/000119710) [DOI] [PubMed] [Google Scholar]
- 11.Roth TC, LaDage LD, Pravosudov VV. 2010. Learning capabilities enhanced in harsh environments: a common garden approach. Proc. R. Soc. B 277, 3187–3193. ( 10.1098/rspb.2010.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlovsky DY, Branch CL, Pravosudov VV. 2015. Problem-solving ability and response to novelty in mountain chickadees (Poecile gambeli) from different elevations. Behav. Ecol. Sociobiol. 69, 635–643. ( 10.1007/s00265-015-1874-4) [DOI] [Google Scholar]
- 13.Bumpus HC. 1899. The elimination of the unfit as illustrated by the introduced sparrow, Passer domesticus. Biol. Lect. Woods Hole Mar. Biol. Stat. 6, 209–226. [Google Scholar]
- 14.Brown CR, Brown MB. 1998. Intense natural selection on body size and wing and tail asymmetry in cliff swallows during severe weather. Evolution 52, 1461–1475. ( 10.1111/j.1558-5646.1998.tb02027.x) [DOI] [PubMed] [Google Scholar]
- 15.Brown CR, Brown MB. 2000. Weather-mediated natural selection on arrival time in cliff swallows (Petrochelidon pyrrhonota). Behav. Ecol. Sociobiol. 47, 339–345. ( 10.1007/s002650050674) [DOI] [Google Scholar]
- 16.Brown MB, Brown CR. 2011. Intense natural selection on morphology of cliff swallows (Petrochelidon pyrrhonota) a decade later: did the population move between adaptive peaks? Auk 128, 69–77. ( 10.1525/auk.2011.10219) [DOI] [Google Scholar]
- 17.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711. ( 10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 18.Campbell-Staton SC, Cheviron ZA, Rochette N, Catchen J, Losos JB, Edwards SV. 2017. Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science 357, 495–498. ( 10.1126/science.aam5512) [DOI] [PubMed] [Google Scholar]
- 19.Brown CR, Brown MB, Hannebaum SL, Hosak PK, Kucera AJ, Page CE, Strickler SA, Wagnon GS. 2018. Changing patterns of natural selection on morphology of cliff swallows during severe weather. Wilson J. Ornithol. 130, 755–762. ( 10.1676/17-00057.1) [DOI] [Google Scholar]
- 20.Brown CR, Brown MB. 1996. Coloniality in the cliff swallow: the effect of group size on social behavior. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Brown CR, Brown MB. 2000. Heritable basis for choice of group size in a colonial bird. Proc. Natl Acad. Sci. USA 97, 14825–14830. ( 10.1073/pnas.97.26.14825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolly A. 1966. Lemur social behavior and primate intelligence. Science 153, 501–506. ( 10.1126/science.153.3735.501) [DOI] [PubMed] [Google Scholar]
- 23.Humphrey NK. 1976. The social function of intellect. In Growing points in ethology (eds Bateson PPG, Hinde RA), pp. 303–317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. 6, 178–190. () [DOI] [Google Scholar]
- 25.Brown CR, Brown MB, Pyle P, Patten MA. 2017. Cliff swallow (Petrochelidon pyrrhonota). In The birds of North America (ed. Rodewald PG.). Ithaca, NY: Cornell Lab of Ornithology; See https://birdsna.org/Species-Account/bna/species/cliswa [Google Scholar]
- 26.Brown CR, Brown MB, Shaffer ML. 1991. Food-sharing signals among socially foraging cliff swallows. Anim. Behav. 42, 551–564. ( 10.1016/S0003-3472(05)80239-8) [DOI] [Google Scholar]
- 27.Brown CR, Brown MB, Roche EA. 2013. Spatial and temporal unpredictability of colony size in cliff swallows across 30 years. Ecol. Monogr. 83, 511–530. ( 10.1890/12-2001.1) [DOI] [Google Scholar]
- 28.Møller AP. 2010. Brain size, head size and behaviour of a passerine bird. J. Evol. Biol. 23, 625–635. ( 10.1111/j.1420-9101.2009.01928.x) [DOI] [PubMed] [Google Scholar]
- 29.Iwaniuk AN, Nelson JE. 2002. Can endocranial volume be used as an estimate of brain size in birds?. Can. J. Zool. 80, 16–23. ( 10.1139/z01-204) [DOI] [Google Scholar]
- 30.Vergouw D, Heymans MW, Peat GM, Kuijpers T, Croft PR, de Vet HCW, van der Horst HE, van der Windt DAWM. 2010. The search for stable prognostic models in multiple imputed data sets. BMC Med. Res. Method. 10, 81 ( 10.1186/1471-2288-10-81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zar JH. 2001. Biostatistical analysis, 4th edn Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 32.Mitchell JFB, Lowe J, Wood RA, Vellinga M. 2006. Extreme events due to human-induced climate change. Phil. Trans. R. Soc. A 364, 2117–2133. ( 10.1098/rsta.2006.1816) [DOI] [PubMed] [Google Scholar]
- 33.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz S, Dunbar RIM. 2006. Chimpanzee and felid diet composition is influenced by prey brain size. Biol. Lett. 2, 505–508. ( 10.1098/rsbl.2006.0519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotrschal A, Buechel SD, Zala SM, Corral-Lopez A, Penn DJ, Kolm N. 2015. Brain size affects female but not male survival under predation threat. Ecol. Lett. 18, 646–652. ( 10.1111/ele.12441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander RD. 1974. The evolution of social behavior. Ann. Rev. Ecol. Syst. 5, 325–383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 37.Brown CR, Brown MB. 1991. Selection of high-quality host nests by parasitic cliff swallows. Anim. Behav. 41, 457–465. ( 10.1016/S0003-3472(05)80848-6) [DOI] [Google Scholar]
- 38.Brown CR, Brown MB. 2013. Where has all the road kill gone? Curr. Biol. 23, R233–R234. ( 10.1016/j.cub.2013.02.023) [DOI] [PubMed] [Google Scholar]
- 39.Møller AP, Erritzøe J. 2017. Brain size in birds is related to traffic accidents. R. Soc. Open Sci. 4, 161040 ( 10.1098/rsos.161040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagnon GS, Brown CR. 2020. Data from: Smaller brained cliff swallows are more likely to die during harsh weather Dryad Digital Repository. ( 10.5061/dryad.bcc2fqz8g) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wagnon GS, Brown CR. 2020. Data from: Smaller brained cliff swallows are more likely to die during harsh weather Dryad Digital Repository. ( 10.5061/dryad.bcc2fqz8g) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Raw data for this paper are deposited in Dryad: https://dx.doi.org/10.5061/dryad.bcc2fqz8g [40].


