Abstract
Foraging by mammalian herbivores has profound impacts on natural and modified landscapes, yet we know little about how they find food, limiting our ability to predict and manage their influence. Mathematical models show that foragers exploiting odour cues outperform a random walk strategy. However, discovering how free-ranging foragers exploit odours in real, complex landscapes has proven elusive because of technological constraints. We took a novel approach, using a sophisticated purpose-built thermal camera system to record fine-scale foraging by a generalist mammalian herbivore, the swamp wallaby (Wallabia bicolor). We tested the hypothesis that odour cues shape forager movement and behaviour in vegetation patches. To do this, we compared wallaby foraging in two odour landscapes: Control (natural vegetation with food and non-food plants interspersed) and +Apple (the same natural vegetation plus a single, highly palatable food source with novel odour (apple)). The +Apple treatment led to strongly directed foraging by wallabies: earlier visits to vegetation patches, straighter movement paths, more hopping and fewer stops than in the Control treatment. Our results provide clear empirical evidence that odour cues are harnessed for efficient, directed search even at this fine scale. We conclude that random walk models miss a key feature shaping foraging within patches.
Keywords: odour cues, fine-scale movements, information-guided search, thermal camera, generalist herbivore
1. Introduction
Mammalian herbivores are dominant players in ecosystems globally, particularly since the disappearance of top predators from many of these ecosystems. Foraging herbivores can also consume threatened plant species, hinder vegetation restoration and hamper both forestry and agricultural crop production [1–4]. To predict and manage these impacts, it is crucial to understand herbivore foraging ecology. Although we know much about how plant characteristics and predation risk influence what herbivores eat and where they forage (e.g. [5]), little is known about how herbivores find food in the first place. Finding food is the initial and most important step in foraging, because if it fails, animals starve.
Two main strategies have been proposed to explain how foragers find food: random walk (undirected) and information-guided search (directed) [6–9]. Random walk may be effective in homogeneous environments, but in more complex scenarios, mathematical models predict that information-guided search using cues reduces movement needed for finding food [10], by concentrating foraging in areas with abundant resources resulting in area-restricted search [11,12].
Information-guided search depends on information cues from prey (i.e. food), which for many foragers is odour [13–15]. Odours provide long-lasting information that can extend over great distances [16]. For foragers, particularly those feeding on stationary prey, such as mammalian herbivores, odour cues can offer relatively reliable information on food and its location, enabling efficient non-random, selective foraging [17–20]. Odour cues should speed up the process of finding food, but how they shape forager movement patterns is poorly understood.
The study of movement patterns is essential to understand the strategies used by foraging animals and quantify movement decisions in time and space. For example, African elephants change movement patterns in different environments, using more tortuous paths in favourable habitats to increase foraging in those areas [21].
Most studies on movement of free-ranging animals use global positioning system (GPS) or very high frequency (VHF) collars to track animal movement over several minutes to (usually) hours, days or weeks with spatial intervals of tens of metres to kilometres. These techniques advanced our understanding of migration [22,23], home-ranges [24,25] and patch selection [26,27]. Although movements that bring animals to a patch are important, foraging is not complete until food is found. Odour cues can guide animal movements from afar [28] and are likely essential in finding food at fine scales (centimetres to metres) too. Food plants for generalist herbivores often occur within metres of each other, and their odour cues could be harnessed for efficient, directed search even at this fine scale. Accuracy and precision limits of VHF and GPS collars hamper the spatial and temporal resolution needed to determine movements at fine scales. Thus, discovering whether odour cues guide herbivore movement at small scales (i.e. within metres) in natural conditions has proven elusive—until now.
To study fine-scale movements, we took a new approach using a high-tech purpose-built thermal camera system to record animal behaviour and location within food patches. This system records objects irrespective of light conditions and unlike other cameras (e.g. motion-triggered camera traps), continuously and from afar.
Our aim was to test predictions from models that incorporate either information-guided or random walk strategies. To do this, we quantified and compared foraging of a generalist herbivore within food patches in two odour landscapes that differed in the spatial dispersion of olfactory information (figure 1a). We studied the elemental units of movement (and associated behaviours) in an experiment manipulating the information in the environment, to investigate the drivers of movement patterns [29].
Figure 1.
Conceptual figure of (a) different odour landscapes and (b) our predictions for animal movement in the Control (green) and +Apple (pink) scenario. ‘X' represents when the animal stops along its movement path (dashed line). Dots represent odour cues emitted by plants; grey from a non-food item, light and dark green from food items A and B, respectively, pink from strongly smelling contrasting food.
Animals using a random walk strategy to find food should show no differences in movements and behaviours between the two odour landscapes. By contrast, if search is information guided (figure 1b), movements and behaviours should differ. Specifically, in natural vegetation (Control) where food plants are within meters of each other and interspersed with non-food plants, foraging animals will travel short distances and use tortuous paths. In contrast, adding a single source of highly palatable novel food with conspicuous odour (+Apple) to this landscape will result in animals moving faster and with a straighter path to find the target. Given unaltered visual cues (apple was not visible from afar), any behavioural changes must be driven by modification of the odour landscape.
We used a mixed feeder/browser, the swamp wallaby (Wallabia bicolor) [30–32]. This Australian macropod (13–17 kg) is ecologically equivalent to mid-size mammalian herbivores in ecosystems around the world: deer in Asia, Europe and America and antelope in Africa. Like these species, swamp wallabies shape vegetation communities in many forests and woodlands, causing significant ecological changes and economic losses [33–35].
2. Material and methods
We filmed free-ranging wallabies in eucalypt woodland dominated by scribbly gum Eucalyptus haemastoma and red bloodwood Corymbia gummifera (Ku-ring-gai Chase National Park, NSW, Australia) between September 2018 and May 2019. We designed and built a thermal camera system that recorded up to 20 h of continuous video and reliably detected animals up to 20 m away in this vegetation. Our system included an Optris 320 thermal camera connected to a Gigabyte mini-PC recording to an external disk and powered by a Drypower 12 V 50 Ah Gel type SLA Cyclic battery. The system saved files every 20 min (facilitating video analysis) and then reset to optimize quality (as sensors lose sensitivity with time).
To quantify within-patch movements and behaviours, we considered an area of approximately 80 m2 to define a food patch. We chose this area to encompass associative effects of neighbouring plants on herbivore foraging decisions, based on [36]. We recorded wallabies foraging in food patches within three sites separated by more than 600 m (to ensure visits of different animals, based on home-range size of this species [37,38]). The sites had low (<25%) shrub (0.5–2 m above ground) cover for suitable visibility by the thermal camera and 25–50% ground vegetation (<0.5 m) cover. The thermal camera was set 2 m above ground, tilted 15–20° downwards and recorded continuously from 16.00 of one day (‘start' of test day) until 08.00 of the following day (‘end' test day).
At each site, we tested two treatments (figure 1a):
-
1.
Control: natural vegetation with food plants interspersed with non-food plants. As foraging wallabies use odour cues [17,18], we considered this landscape to contain dispersed odour cues from food plants.
-
2.
+Apple: the same natural vegetation with an added highly palatable, novel food with conspicuous odour (apple). This target food was localized at one source, emitting a focused odour cue but no visual cue (visually hidden among ground vegetation from approximately 1 m away).
The experiment was run over a period of nine months (see electronic supplementary material). For the Control treatment, we filmed at each site until at least two (up to seven) independent visits (on different days or >30 min apart on the same day) by wallabies were detected. For the +Apple treatment, we then filmed the same sites, but with sliced apple pieces added at the start of the test day in a single location (an area of 50 × 50 cm in the centre of the field of view, 10 m away from the camera). At each site, we ran the Control treatment first to avoid any influence of memory—associated with the highly palatable +Apple treatment—on wallaby movement, which may have arisen if the +Apple treatment preceded the Control treatment (see the electronic supplementary material) as some foragers remember the location of profitable patches, modifying how they visit different patches [39,40].
For the Control treatments, we only analysed visits where the animal was browsing (defined as an animal stopping and putting its head down into ground vegetation or manipulating shrubs). We excluded (i) four visits where an animal travelled through the patch without stopping (given our aim was to quantify animals foraging), (ii) repeat visits in the same night if the individual appeared to be the same and (iii) videos with obscured animals. We included two videos where more than one adult individual was present as they were not obviously interacting, using only the first individual coming into the patch. For each visit, we quantified readily identifiable behaviours (e.g. hopping or lowering head) and movements in space and time using BORIS [41] and MTrackJ plugin in FIJI-ImageJ [42,43]. We used known distances to the camera (measured when setting up the camera) at each site, to transform distances measured in FIJI-ImageJ (in pixels) into real distances in metres (see electronic supplementary material). Each visit started when the animal was clearly visible and trackable (no matter the ‘entry point' to the patch) and, for the +Apple treatment, finished when the animal was closest to the apple. For the Control treatment, the same position (without apple) was used as the reference (pointX, electronic supplementary material, figure S1).
We quantified three movement variables:
—Path straightness: difference between maximum and minimum distance to pointX, divided by total distance travelled (straightness index, see [44]); indicates directness of movement and area covered.
—Mean speed: total distance travelled divided by total visit duration; indicates how rapidly the animal moves.
—Stops per metre: total number of stops (no change in location for ≥10 s) divided by total distance travelled; indicates frequency of stops to eat, sniff or look around.
We quantified three mutually exclusive behavioural variables associated with foraging, expressed as percentage of the visit duration (given variation in visit duration):
—Hopping: fast movement using only hind legs to hop (front limbs up), indicates travelling.
—Head down: head lowered below body line, included eating or sniffing at ground level.
—Head up: head raised above body line, included sniffing or looking around.
We also analysed ‘minimum distance reached' (i.e. proximity to pointX) and ‘probability of visiting early' (before midnight). We used midnight to split the total recorded time in half with equal light versus dark hours, representing the two main peaks of wallaby activity, 17.00–21.00 and 04.00–08.00.
(a). Statistical analysis
There were no correlations among the movement or behavioural variables (r < 0.7, p > 0.05). We used generalized linear mixed models in R (‘lme4' package [45]) to test the effect of odour treatments (Control, +Apple) for each behavioural and movement variable individually (dependent variables). We used a data distribution (Gaussian, binomial) appropriate for the form of each dependent variable. The unit of replication was each valid visit. To take into account any effect of visit number within a night, we included ‘first visit' (yes' or ‘no) in our full models as a covariate. For the full model, we tested ‘treatment’ and ‘first visit' as fixed factors and ‘site' as random factor. The effect of ‘first visit' was removed (never significant) and ‘site' was removed when not significant (all variables but Head down). Data were plotted using R (‘graphics' package [46]).
3. Results
We had 21 valid visits across all sites, 11 +Apple replicates and 10 Control replicates. In total, at least 11 different wallabies were observed (distinguished by marks or body size). Wallabies travelled a mean distance of 5.78 m (s.e. = 0.44) per visit, with a mean visit duration of 3.45 min (s.e. = 0.66). In all +Apple replicates, the wallabies found the apple.
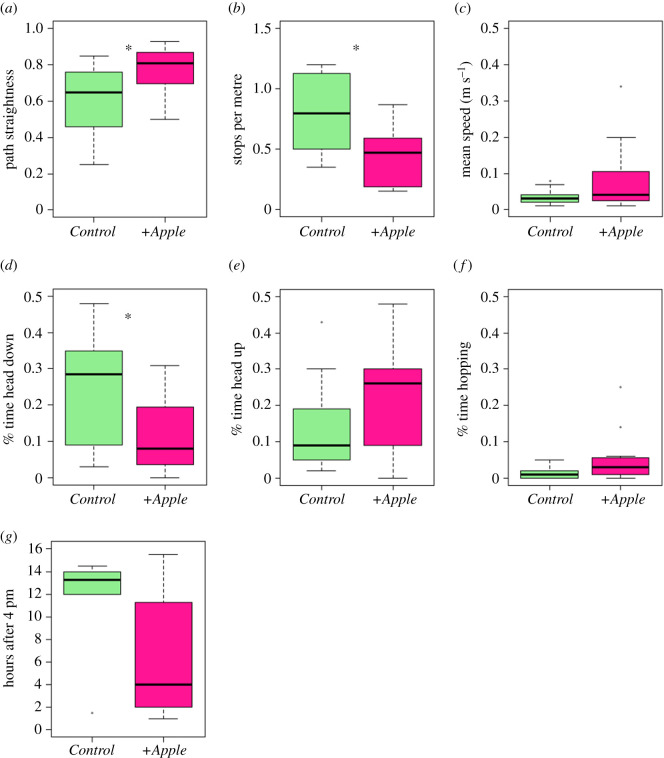
Swamp wallabies visiting Control and +Apple treatments differed in their Path straightness (analysis of deviance likelihood ratio LR , p = 0.028), Stops per metre (LR , p = 0.003), Head down (LR , p = 0.012) and Minimum distance reached (LR , p < 0.001). Mean speed, Hopping and Head up did not differ significantly between treatments (p ≥ 0.1). In terms of movement, in the +Apple treatment paths of wallabies were on average 27% straighter, with half the number of stops and more than twice the speed relative to the Control treatment (figure 2a–c). In terms of behaviour, in the +Apple treatment, wallabies spent on average half the time with their head down, 50% more time with the head up and 420% more time hopping relative to the Control treatment (figure 2d–f).
Figure 2.
Boxplots of the (a–c) movement variables, (d–f) the behavioural variables and (g) the number of hours after 16.00 (time at which apple was placed in +Apple treatment) for swamp wallaby visits to Control (n = 10 visits) and +Apple (n = 11 visits) odour landscapes. Each boxplot shows median, 1st and 3rd quartile, and maximum and minimum values (within 1.5 × IQR). Empty circles are outliers. Asterisks indicate significant difference.
Treatment affected ‘probability of visiting early' (LR , p = 0.039). In natural vegetation (Control), wallabies usually visited patches between 04.00 and 08.00 following day, 12–16 h after the start of the test replicate. When apple was added (+Apple), most of the visits occurred between 17.00–21.00 same day, 1–5 h after the start (figure 2g).
4. Discussion
Our results show that odour cues directed wallaby movement and behaviour at fine temporal and fine spatial scales (within metres). In the Control landscape, when food plants were dispersed in the patch, wallabies used a tortuous path with frequent stops and much time with their head down. These behaviours reflected animals moving in small step-lengths, sniffing or eating vegetation at the ground level and redirecting movements to the next food item, consistent with their use of odour to forage [17,18]. This tortuous path and behaviours including sniffing could result in an area-restricted search (concentrated search effort in high reward areas) as used in mathematical foraging simulations that incorporate information-guided search using odour cues [9,10].
In the +Apple landscape, wallaby movements and behaviours were changed distinctly by a single source, highly palatable and conspicuous food odour (apple). In contrast with the Control landscape, wallabies search within the patch became highly directed (less tortuous path) and efficient (always found the apple), detecting the cue from at least 6 m (maximum distance at which we observed a directed path). They moved faster, by stopping less and spending more time hopping. The increased time with raised head appeared to be associated with wallabies attempting to capture the odour cues and localize the source (‘air scenting', [47]).
For foragers that can detect and find food within metres, travelling far to find one food item is unlikely to be efficient. However, this may change for conspicuous and highly palatable and rewarding food resources. Our results showing that patches with apple were visited earlier (close to dusk same day) than those with natural vegetation only (dawn next day) suggest that apple odour cues were detected at distances beyond the patch.
Our evidence that foragers change search strategies in different odour landscapes demonstrates unambiguously the use of odour information in foraging decisions. This clearly contradicts the random walk hypothesis and supports the contention [8] that animal movement studies must consider the ability of foragers to sense food for information-guided search before invoking any random walk model.
Finally, the short duration of patch visits and small distances travelled by wallabies emphasize why the use of GPS and VHF are unsuitable to reveal fine-scale foraging patterns. In future, combining large-scale studies (by GPS) and small-scale studies (by suitable thermal cameras, as here) will help us better understand the connection between food-searching movements and foraging strategies at different spatio-temporal scales.
5. Conclusion
We have shown that incorporating the use of odour cues is crucial for understanding fine-scale foraging of generalist herbivores. Mathematical models show theoretically that foraging efficiency is improved by incorporating the use of odour cues [10], and we have now provided quantitative information that can be used to pin these models down to the real world. By linking foraging, movement and sensory ecology, we have identified the mechanisms shaping movement patterns at small spatio-temporal resolution [29]. The techniques and equipment developed here can be used to study other animals in different environments, helping answer the crucial question of how they find their food.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
To Dr Catherine Price, who has helped with her comments and advice.
Ethics
This research was conducted with the approval of the University of Sydney Animal Ethics Committee (AEC; permit nos. 2014#717 and #2018/1416), and the New South Wales Government Office of Environment and Heritage (Scientific Licence permit nos. SL100443 and SL102186).
Data accessibility
The datasets supporting this article were uploaded as part of the electronic supplementary material.
Authors' contributions
C.G.O., C.M., P.B. and A.T. designed the experiments. A.T. designed the software and provided the monitoring system (courtesy of the CSIRO). C.G.O. conducted the experiments. C.G.O. and C.M. did the formal analysis. C.G.O., C.M. and P.B. wrote the paper. C.M., P.B. and A.T. reviewed and edited the paper. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests
Funding
This research was supported by the Australian Research Council ARC-DP [DP190101441].
References
- 1.Côté SD, Rooney TP, Tremblay J-P, Dussault C, Waller DM. 2004. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147. ( 10.1146/annurev.ecolsys.35.021103.105725) [DOI] [Google Scholar]
- 2.Bråthen KA, Ims RA, Yoccoz NG, Fauchald P, Tveraa T, Hausner VH. 2007. Induced shift in ecosystem productivity? Extensive scale effects of abundant large herbivores. Ecosystems. 10, 773–789. ( 10.1007/s10021-007-9058-3) [DOI] [Google Scholar]
- 3.Dexter N, Hudson M, James S, MacGregor C, Lindenmayer DB. 2013. Unintended consequences of invasive predator control in an Australian forest: overabundant wallabies and vegetation change. PLoS ONE 8, e69087 ( 10.1371/journal.pone.0069087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripple WJ, Larsen EJ, Renkin RA, Smith DW. 2001. Trophic cascades among wolves, elk and aspen on Yellowstone National Park's northern range. Biol. conserv. 102, 227–234. ( 10.1016/S0006-3207(01)00107-0) [DOI] [Google Scholar]
- 5.McArthur C, Banks PB, Boonstra R, Forbey JS. 2014. The dilemma of foraging herbivores: dealing with food and fear. Oecologia. 176, 677–689. ( 10.1007/s00442-014-3076-6) [DOI] [PubMed] [Google Scholar]
- 6.Bell WJ. 1990. Searching behavior patterns in insects. Annu. Rev. Entomol. 35, 447–467. ( 10.1146/annurev.en.35.010190.002311) [DOI] [Google Scholar]
- 7.Auger-Méthé M, Derocher AE, DeMars CA, Plank MJ, Codling EA, Lewis MA. 2016. Evaluating random search strategies in three mammals from distinct feeding guilds. J. Anim. Ecol. 85, 1411–1421. ( 10.1111/1365-2656.12562) [DOI] [PubMed] [Google Scholar]
- 8.Pyke G. 2009. Animal movements: an optimal foraging approach. In Encyclopedia of animal behavior, pp. 149–156. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 9.Vergassola M, Villermaux E, Shraiman BI. 2007. ‘Infotaxis’ as a strategy for searching without gradients. Nature 445, 406 ( 10.1038/nature05464) [DOI] [PubMed] [Google Scholar]
- 10.Hein AM, McKinley SA. 2012. Sensing and decision-making in random search. Proc. Natl Acad. Sci. USA 109, 12 070–12 074. ( 10.1073/pnas.1202686109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedix J., Jr 1993. Area-restricted search by the plains pocket gopher (Geomys bursarius) in tallgrass prairie habitat. Behav. Ecol. 4, 318–324. ( 10.1093/beheco/4.4.318) [DOI] [Google Scholar]
- 12.Romanach SS, Seabloom EW, Reichman O. 2007. Costs and benefits of pocket gopher foraging: linking behavior and physiology. Ecology 88, 2047–2057. ( 10.1890/06-1461.1) [DOI] [PubMed] [Google Scholar]
- 13.Hoballah ME, Stuurman J, Turlings TCJ, Guerin PM, Connétable S, Kuhlemeier C. 2005. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta. 222, 141–150. ( 10.1007/s00425-005-1506-8) [DOI] [PubMed] [Google Scholar]
- 14.Hodgkison R, Ayasse M, Kalko EKV, Häberlein C, Schulz S, Mustapha WAW, Zubaid A, Kunz TH. 2007. Chemical ecology of fruit bat foraging behavior in relation to the fruit odors of two species of paleotropical bat-dispersed figs (Ficus hispida and Ficus scortechinii). J. Chem. Ecol. 33, 2097–2110. ( 10.1007/s10886-007-9367-1) [DOI] [PubMed] [Google Scholar]
- 15.Hughes NK, Price CJ, Banks PB. 2010. Predators are attracted to the olfactory signals of prey. PLoS ONE 5, e13114 ( 10.1371/journal.pone.0013114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doty RL. 1986. Odor-guided behavior in mammals. Experientia 42, 257–271. ( 10.1007/BF01942506) [DOI] [PubMed] [Google Scholar]
- 17.Stutz RS, Banks PB, Proschogo N, McArthur C. 2016. Follow your nose: leaf odour as an important foraging cue for mammalian herbivores. Oecologia 182, 643–651. ( 10.1007/s00442-016-3678-2) [DOI] [PubMed] [Google Scholar]
- 18.Finnerty PB, Stutz RS, Price CJ, Banks PB, McArthur C. 2017. Leaf odour cues enable non-random foraging by mammalian herbivores. J. Anim. Ecol. 86, 1317–1328. ( 10.1111/1365-2656.12748) [DOI] [PubMed] [Google Scholar]
- 19.Schmitt MH, Shuttleworth A, Ward D, Shrader AM. 2018. African elephants use plant odours to make foraging decisions across multiple spatial scales. Anim. Behav. 141, 17–27. ( 10.1016/j.anbehav.2018.04.016) [DOI] [Google Scholar]
- 20.McArthur C, Finnerty PB, Schmitt MH, Shuttleworth A, Shrader AM. 2019. Plant volatiles are a salient cue for foraging mammals: elephants target preferred plants despite background plant odour. Anim. Behav. 155, 199–216. ( 10.1016/j.anbehav.2019.07.002) [DOI] [Google Scholar]
- 21.Duffy KJ, Dai X, Shannon G, Slotow R, Page B. 2011. Movement patterns of African elephants (Loxodonta africana) in different habitat types. Afr.J. Wildl. Res. 41, 21–28. ( 10.3957/056.041.0107) [DOI] [Google Scholar]
- 22.Thomas B, Minot E, Holland J. 2010. Fledging behaviour of juvenile northern royal albatrosses (Diomedea sanfordi): a GPS tracking study. Notornis 57, e147. [Google Scholar]
- 23.Thirgood S, et al. (ed) 2004. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. forum 7, 113–120. ( 10.1017/S1367943004001404) [DOI] [Google Scholar]
- 24.Wat KKY, Herath APHM, Rus AI, Banks PB, Mcarthur C. 2019. Space use by animals on the urban fringe: interactive effects of sex and personality. Behav. Ecol. 31, 330–339. [Google Scholar]
- 25.Fryxell JM, Hazell M, Börger L, Dalziel BD, Haydon DT, Morales JM, McIntosh T, Rosatte RC. 2008. Multiple movement modes by large herbivores at multiple spatiotemporal scales. Proc. Natl Acad. Sci. USA 105, 19 114–19 119. ( 10.1073/pnas.0801737105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stefano J, York A, Swan M, Greenfield A, Coulson G. 2009. Habitat selection by the swamp wallaby (Wallabia bicolor) in relation to diel period, food and shelter. Aust. Ecol. 34, 143–155. ( 10.1111/j.1442-9993.2008.01890.x) [DOI] [Google Scholar]
- 27.Fehlmann G, O'Riain MJ, Kerr-Smith C, Hailes S, Luckman A, Shepard ELC, King AJ. 2017. Extreme behavioural shifts by baboons exploiting risky, resource-rich, human-modified environments. Sci. Rep. 7, 15057 ( 10.1038/s41598-017-14871-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mella VS, Possell M, Troxell-Smith SM, McArthur C. 2018. Visit, consume and quit: patch quality affects the three stages of foraging. J. Anim. Ecol. 87, 1615–1626. ( 10.1111/1365-2656.12882) [DOI] [PubMed] [Google Scholar]
- 29.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis C, Hollis C, Robertshaw J, Robertshaw J, Harden R, Harden R. 1986. Ecology of the swamp wallaby (Wallabia bicolor) in Northeastern New-South-Wales. 1. Diet. Wildl. Res. 13, 355–365. ( 10.1071/WR9860355) [DOI] [Google Scholar]
- 31.Osawa R. 1990. Feeding Strategies of the swamp wallaby, Wallabia bicolor, on North Stradbroke Island, Queensland. I: Composition of diets. Wildl. Res. 17, 615–621. ( 10.1071/WR9900615) [DOI] [Google Scholar]
- 32.Di Stefano J, Newell GR. 2008. Diet selection by the swamp wallaby (Wallabia bicolor): feeding strategies under conditions of changed food availability. J. Mammal. 89, 1540–1549. ( 10.1644/07-MAMM-A-193.1) [DOI] [Google Scholar]
- 33.Montague T, Pollock D, Wright W (eds) 1990. An examination of the browsing animal problem in Australian eucalypt and pine plantations. In Proceedings Vertebrate Pest Conference (USA). [Google Scholar]
- 34.Di Stefano J. 2004. The importance of ecological research for ecosystem management: the case of browsing by swamp wallabies (Wallabia bicolor) in commercially harvested native forests. Ecol. Manag. Restor. 5, 61–67. ( 10.1111/j.1442-8903.2004.00170.x) [DOI] [Google Scholar]
- 35.Foster C, Barton P, Sato C, Wood J, MacGregor C, Lindenmayer D. 2016. Herbivory and fire interact to affect forest understory habitat, but not its use by small vertebrates. Anim. Conserv. 19, 15–25. ( 10.1111/acv.12210) [DOI] [Google Scholar]
- 36.Stutz RS, Banks PB, Dexter N, McArthur C. 2015. Associational refuge in practice: can existing vegetation facilitate woodland restoration? Oikos. 124, 571–580. ( 10.1111/oik.01782) [DOI] [Google Scholar]
- 37.Di Stefano J, Coulson G, Greenfield A, Swan M. 2011. Resource heterogeneity influences home range area in the swamp wallaby Wallabia bicolor. Ecography 34, 469–479. ( 10.1111/j.1600-0587.2010.06523.x) [DOI] [Google Scholar]
- 38.Troy S, Coulson G. 1993. Home range of the swamp wallaby, Wallabia bicolor. Wildl. Res. 20, 571–575. ( 10.1071/WR9930571) [DOI] [Google Scholar]
- 39.Fagan WF, et al. 2013. Spatial memory and animal movement. Ecol. Lett. 16, 1316–1329. ( 10.1111/ele.12165) [DOI] [PubMed] [Google Scholar]
- 40.Inglis IR, Langton S, Forkman B, Lazarus J. 2001. An information primacy model of exploratory and foraging behaviour. Anim. Behav. 62, 543–557. ( 10.1006/anbe.2001.1780) [DOI] [Google Scholar]
- 41.Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. ( 10.1111/2041-210X.12584) [DOI] [Google Scholar]
- 42.Meijering E, Dzyubachyk O, Smal I. 2012. Methods for cell and particle tracking. In Methods in enzymology, vol. 504, pp. 183–200. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 43.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676 ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benhamou S. 2004. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J. Theor. Biol. 229, 209–220. ( 10.1016/j.jtbi.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 45.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv arXiv:14065823.
- 46.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 47.Gadbois S, Reeve C. 2014. Canine olfaction: scent, sign, and situation. In Domestic dog cognition and behavior: the scientific study of Canis familiaris (ed. Horowitz A.), pp. 3–29. Berlin, Germany: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article were uploaded as part of the electronic supplementary material.