Abstract
One response to the coral reef crisis has been human intervention to enhance selection on the fittest corals through cultivation. This requires genotypes to be identified for intervention, with a primary basis for this choice being growth: corals that quickly grow on contemporary reefs might be future winners. To test for temporal stability of growth as a predictor of future performance, genotypes of the coral Porites spp. were grown in common gardens in Mo'orea, French Polynesia. Growth was measured every two to four months throughout 2018, and each period was used as a predictor of growth over the subsequent period. Area-normalized growth explained less than 29% of the variance in subsequent growth, but for biomass-normalized growth this increased to 45–60%, and was highest when summer growth was used to predict autumn growth. The capacity of initial growth to predict future performance is dependent on the units of measurement and the time of year in which it is measured. The final choice of traits to quantify performance must be informed through consideration of the species and the normalization that best capture the information inherent in the biological processes mediating variation in traits values.
Keywords: Scleractinia, fitness, restoration, genotype
1. Introduction
Human influence on the biosphere defines the Anthropocene [1] through perturbation of biological resources [2]. Faced with the subsequent ecological crises [3], attention is focusing on the taxa that might persist and the traits promoting success [4–6]. Identifying ‘winners' [4,7] has become a priority [4,6], but without an historic analogue of biological responses to future conditions with which such determinations can be informed [8], the task is daunting.
Marine communities provide many examples of changes resulting from anthropogenic effects [9]. Most are undesirable [10], because they impair the capacity of communities to deliver the services with which they have been associated [11,12]. Agriculture provides examples of strategies of human intervention that have alleviated such effects [13], offering hope that similar approaches can be applied in natural ecosystems. Coral reefs provide a compelling example in which human intervention could be considered [14,15], because corals face acute challenges [16,17] and impending extinction [18].
Interest in human-assisted solutions to the coral reef crisis has risen [14,15] as coral mortality has accelerated [19]. These solutions rely on the ability to identify corals suitable for intervention, with the expectation that propagation of their genetic diversity will delay or prevent extinction [14]. The field of evolutionary biology describes how this goal can be achieved [20], but transferring this knowledge is difficult because it is challenging to quantify coral fitness by enumerating offspring, or breeding corals in captivity [21]. Coral fitness therefore is frequently measured through proxies (sensu [22]) such as growth [23], and propagation is often accomplished asexually [24].
The common use of growth to evaluate coral fitness [23] is based on the rationale that it leads to increased fecundity [25–27] and is tractable for measurement. Using growth for this purpose is complicated by inconsistent terminology and methodology [28], so that ‘growth' can mean different things, particularly with respect to fitness. These problems are highlighted through surveys of small corals for which growth has been shown to be a poor predictor of performance [29], possibly because growth has been depressed over decades [30,31]. To screen coral genotypes for candidates suitable for intervention, the mechanism of screening requires careful consideration.
This study explored the utility of coral growth in predicting future growth throughout a year by tracking corals in common gardens to reveal intrinsic phenotypic variation [32,33]. Three hypotheses were tested using Porites spp.: (i) initial and subsequent growth are positively associated, (ii) the association between initial and subsequent growth is temporally stable and (iii) the goodness of fit between initial and subsequent growth is independent of growth normalization.
2. Methods
(a). Overview
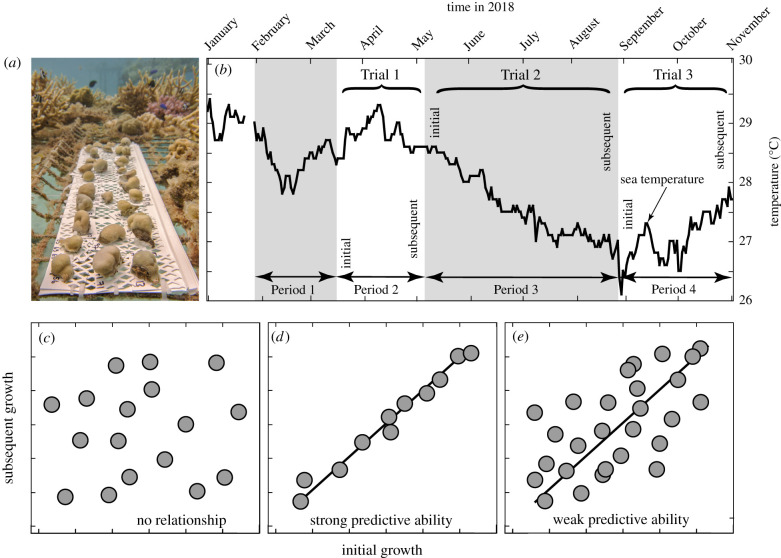
Small colonies (less than or equal to 4 cm diameter) of Porites spp. (P. lobata and P. lutea) were collected in January 2018 and their growth measured by change in mass in common gardens from 28 January to 15 March, 15 March to 5 May, 5 May to 27 August, and 27 August to 1 November 2018 (figure 1). Small colonies increased the likelihood that each was genetically unique, as they originate through recruitment of sexual larvae [34]. Temperature was recorded (Hobo U22, ± 0.2°C) at approximately 2 m depth. Using area- and biomass-normalized growth, associations between initial and subsequent growth were predicted (figure 1).
Figure 1.
Schematic illustrating experimental corals (a), the experimental chronology and seawater temperature (b), and potential outcomes (c–e). The relationships between initial and subsequent growth over each period were analysed over three trials (Trial 1–3), with three outcomes hypothesized: none (c), strong (high r2) (d), or weak (low r2) (e).
(b). Corals and dependent variables
Corals were collected on January 25 and 26, from 2–3 m depth in the back reef (17.475°S, 149.816°W) and transported to the laboratory, where they were glued to bases (Coral Glue, Ecotech, USA). Prepared corals were kept in seawater where their diameters were measured (±1 mm), and their masses were determined by buoyant weighing (±1 mg [35]). Corals were haphazardly assigned on 28 January to common gardens at 5 m or 8 m depth. Depth initially was part of the experiment, but when this effect was absent (see electronic supplementary material), the results were pooled by depth. Light was measured using a meter (LI-1400 fitted with LI-193SA, Li-Cor, Nebraska), and around noon on 1 February 2018, was 443 ± 2 µmol quanta m–2 s−1 at 5 m depth and 324 ± 1 µmol quanta m−2 s−1 at 8 m depth.
The diameter and buoyant weight of the corals were measured on approximately 15 March, 5 May, 27 August and 1 November. Measurements were taken over approximately 3–4 days before the corals were returned to the common gardens, and the measurement of all corals reduced the likelihood of species identity confounding time in modifying growth. Changes in buoyant weight were converted to dry weight using the density of aragonite (2.93 g cm−3) and seawater (1.017–1.023 g cm−3) [35]. Net calcification was standardized to time and mean tissue area over each period (mg cm−2 d−1), with area calculated using previous data and a regression of area on size (electronic supplementary material). In November, biomass was measured by fixation (5% formalin), decalcifying in 10% hydrochloric acid and drying at 60°C; biomass was normalized to the area of coral tissue. Using the relationship between biomass and season (electronic supplementary material), biomass at the other four sampling times was estimated for each coral, and the mean biomass over each period was used to normalize growth (mg mg−1 d−1).
(c). Statistical analysis
Results were analysed using area- and biomass-normalized growth. Hypothesis 1 was tested with Pearson correlations using initial and subsequent growth over three trials (figure 1) and Model I linear regressions [36]. The first initial growth was recorded over Period 1 with the subsequent growth over Period 2 (supporting Trial 1); growth over Period 2 then was the initial growth supporting Trial 2, and so on. Hypothesis 2 was tested using repeated measures (RM) ANCOVA in which coral was the RM factor. Unplanned contrasts of elevations were completed with Tukey's honestly significant test [37]. Residual variation was used to compare the fit of the linear relationships prepared using area- and biomass-normalized growth (Hypothesis 3). Biomass-normalized growth was log-transformed as it was positively skewed. The normality and homoscedasticity assumptions of the statistical procedures were tested through graphical analysis of residuals, and statistical analyses were completed with Systat 13.0 software.
3. Results
(a). Overview
Of the prepared corals, 60 were placed on the common gardens at 5 m depth and 57 at 8 m depth. Sample size declined over time as corals were lost to attrition and sampled for other purposes; this analysis is based on the 60 corals that were weighed on all five occasions (approx. 28 January, approx. 15 March, approx. 5 May, approx. 27 August, approx. 1 November).
(b). Hypothesis 1: subsequent versus initial growth
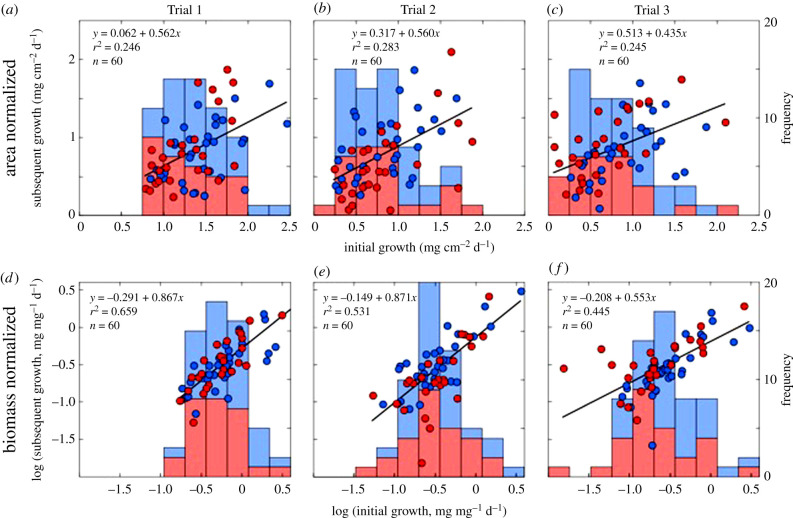
Area-normalized growth was higher in the warmer versus the cooler portion of the year. Mean (± s.e.) growth over the four periods (figure 1b) was 1.4 ± 0.1 mg cm−2 d−1, 0.8 ± 0.1 mg cm−2 d−1, 0.8 ± 0.1 mg cm−2 d−1 and 0.9 ± 0.1 mg cm−2 d−1 (all n = 60). Initial and subsequent growth rates over each of the three trials were significantly and positively associated (r ≥ 0.495, d.f. = 60, p ≤ 0.001) and linear regressions for each trial were significant (F1,58 ≥ 18.811, p ≤ 0.001) (figure 2).
Figure 2.
Growth of Porites spp. at 5 m (red) and 8 m (blue) depth on area- (a–c) and biomass- (d–f) normalized scales for corals that were measured on five occasions. Plots show initial and subsequent growth over each trial, together with Model I regression. Stacked histograms show the frequency distribution of initial growth at 5 m (red) and 8 m (blue) depth.
Previous data from the western Atlantic [38,39] show that coral biomass varies throughout the year, and in November in the southern hemisphere (when biomass was measured in the present study), it is predicted to be at 97.4% of the annual maximum value inferred to occur in October. The relationship between coral biomass and time (electronic supplementary material, figure S2) indicates that biomass was 88.7% of the maximum in January, 83.1% in March, 89.9% in May and 95.4% in August. In November, measured biomass ranged from 0.3 to 7.7 mg cm−2, with a mean (±s.e.) of 3.1 ± 0.2 mg cm−2 (n = 60). Biomass-normalized growth was higher in the first versus the other three periods, and initial and subsequent growth (log-transformed) were positively associated over each trial (r ≥ 0.759, d.f. = 58, p < 0.001), and the regressions of subsequent on initial growth were significant (F1,58 ≥ 46.500, p ≤ 0.001) (figure 2).
(c). Hypothesis 2: stability of initial–subsequent growth association
For area-normalized growth, the slopes of the regressions were homogeneous among trials (F2,115 = 0.917, p = 0.403), and overall growth rate (i.e., the elevation of the regressions) differed among trials (F2,117 = 5.320, p = 0.006) (figure 2b,c). Overall growth rates were higher in Trial 1 versus 2 and 3 (p ≤ 0.050), but similar in Trial 2 and 3 (p = 0.537). For biomass-normalized growth, the slopes of the regressions were homogeneous among trials (F2,115 = 0.857, p = 0.427) and elevations differed among trials (F2,117 = 11.362, p < 0.001). Overall growth rates were higher in Trial 1 versus 2 and 3 (p ≤ 0.001), but similar between Trials 2 and 3 (p = 0.902) (figure 2).
(d). Hypothesis 3: goodness of fit comparing growth normalizations
For area-normalized data, the relationships explained 25–28% of the variation in subsequent growth. For biomass-normalized growth, the relationships explained 45–66% of the variation in subsequent growth (figure 2).
4. Discussion
The extent to which organism performance is consistent across space and time has profound implications for population stability. In heterogeneous habitats, non-plastic reaction norms can lead to reduced genetic diversity [40] and impaired genetic capacity to respond to changing conditions, while genotype-by-environment interactions facilitate plasticity, which can allow phenotypic performance to vary across space and time [41]. Within a human-disturbed biosphere, predicting organism performance from present-day phenotypes necessitates decisions about selecting for plastic or non-plastic reaction norms for the trait(s) of interest, which is critical to understanding the function of future communities [42–44]. Accurate prediction of organism performance is a pre-requisite for human intervention to facilitate desirable outcomes to the changes affecting community structure [44]. While these principles have broad application in the Anthropocene, they are particularly relevant to coral reefs, which are at the forefront of systems at a tipping point with respect to human disturbances [14,45]. These anthropogenic forces have initiated rapid ecological changes, ensuring that future reef communities will be different from those of the past [46] and pushing the foundation taxon towards extinction [18]. For corals, the need to predict future performance is acute, and the time for an intense focus on the science for rigorous decision making for human intervention is now [14,45].
For massive Porites spp. colonies that we infer are genetically distinct, the present study shows that colony growth predicts future growth performance over two to four months (Hypothesis 1), but prediction accuracy varies over time (Hypothesis 2) and is higher for biomass-normalized (r2 > 0.44) versus area-normalized (r2 ≤ 0.28) growth (Hypothesis 3). In terms of the pressing need to identify ‘winning' corals [4,7] that might populate future reefs, our results provide an objective evaluation of the limited potential to accurately predict the future performance of corals from present-day responses. Using growth rate as an indicator of one such trait that might be used for this purpose, our results show that corals with fast, area-normalized growth are unlikely to sustain rapid growth over at least a year and, therefore, are poor candidates for human intervention [sensu 14]; area-normalized growth is a weak proxy for performance. Biomass-normalized growth was a better predictor of future performance, and corals growing fast on this scale likely continue to grow fast throughout the year.
Intraspecific phenotypic variation is common in corals [47,48], but it has re-emerged as a research topic in the study of genetic variation in the response of corals to asexual propagation [49–51] and environmental stressors [51,52]. The limitations of growth in corals as a predictive tool are beginning to be described, including the value of mass deposition versus linear extension [53], and evidence of weak capacity to predict field growth from cultured growth [50], and over time [49]. Nevertheless, growth remains a common means to evaluate coral performance based on the inference that it is relatively stable, and is one of several traits recommended to assay genotypes for future performance [45]. As high growth in corals is likely to be traded against other traits determining performance [49,54], measurement of single traits to predict future performance will have limitations [45]. Yet, our analyses reveal the circumstances under which short-term growth has a strong predictive capacity for future growth, and therefore, how it can best inform a search for coral ‘winners' [4,7]. The accuracy of biomass-normalized growth for this purpose highlights the need to better understand the physiological mechanisms of variation in growth in order to sharpen the capacity to identify winning genotypes. By extension, it will be important to begin comprehensive measurements of coral biomass, for example, through non-destructive approaches [55] or through tissue biopsies sampled with precision tools. Finally, it is notable that the accuracy of predicting future performance was greatest around the Austral summer when growth was maximized. This trend suggests that long-term declines in coral growth [30,31] may erode the capacity to predict their future performance [29] at a time when this capacity is urgently needed.
Supplementary Material
Supplementary Material
Acknowledgements
This study was permitted by the French Polynesian Government (Délégation à la Recherche) (Protocole d'Accueil 2018), and is CSUN contribution number 310.
Ethics
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Data accessibility
Data included as electronic supplementary material to be archived in association with the paper at Biology Letters. Data are accessible at: https://portal.edirepository.org/nis/mapbrowse?scope=knb-lter-mcr&identifier=5040&revision=10. This links to the data doi:10.6073/pasta/643be961dc6ba5791023a0526b6ceef4.
Authors' contributions
The study was designed, executed, analysed and written by P.J.E. and H.M.P.; both authors approve the final version and agree to be held accountable for the content.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the National Science Foundation (grant nos. 16-37396 and 14-39173).
References
- 1.Waters CN, et al. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, aad2622 ( 10.1126/science.aad2622) [DOI] [PubMed] [Google Scholar]
- 2.Lewis SL, Maslin MA. 2015. Defining the Anthropocene. Nature 519, 171–180. ( 10.1038/nature14258) [DOI] [PubMed] [Google Scholar]
- 3.Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. 2005. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29. ( 10.1111/j.1461-0248.2004.00686.x) [DOI] [Google Scholar]
- 4.Webster MS, Colton MA, Darling ES, Armstrong J, Pinsky ML, Knowlton N, Schindler DE. 2017. Who should pick the winners of climate change? Trends Ecol. Evol. 32, 167–173. ( 10.1016/j.tree.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 5.Merow C, Bois ST, Allen JM, Xie Y, Silander JA Jr. 2017. Climate change both facilitates and inhibits invasive plant ranges in New England. Proc. Natl Acad. Sci. USA. 114, E3276–E3284. ( 10.1073/pnas.1609633114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson GJ. 2018. ‘Winners’ and ‘losers’ in the Anthropocene: understanding adaptation through phenotypic plasticity. Funct. Ecol. 32, 1906–1907. ( 10.1111/1365-2435.13108) [DOI] [Google Scholar]
- 7.Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R. 2001. Coral bleaching: winners and losers. Ecol. Lett. 4, 122–131. ( 10.1046/j.1461-0248.2001.00203.x) [DOI] [Google Scholar]
- 8.Calosi P, Putnam HM, Twitchett RJ, Vrmandele F. 2018. Marine metazoan modern mass extinction: improving predictions by integrating fossil, modern, and physiological data. Annu. Rev. Mar. Sci. 11, 20.1–20.22. ( 10.1146/annurev-marine-010318-095106) [DOI] [PubMed] [Google Scholar]
- 9.Lotze HK, et al. 2019. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl Acad. Sci. USA 116, 12 907–12 912. ( 10.1073/pnas.1900194116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 11.Pratchett MS, Hoey AS, Wilson SK. 2014. Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 7, 37–43. ( 10.1016/j.cosust.2013.11.022) [DOI] [Google Scholar]
- 12.Pedrono M, Locatelli B, Ezzine-de-Blas D, Pesche D, Morand S, Binot A. 2015. Impact of climate change on ecosystem services. In Climate change and agriculture worldwide (ed. Torquebiau E.), pp. 251–261. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 13.Tyczewska A, Woźniak E, Gracz J, Kuczyński J, Twardowski T. 2018. Towards food security: current state and future prospects of agrobiotechnology. Trends Bioechnol. 36, 1219–1229. ( 10.1016/j.tibtech.2018.07.008) [DOI] [PubMed] [Google Scholar]
- 14.van Oppen MJH, Oliver JK, Putnam HM, Gates RD. 2015. Building coral reef resilience through assisted evolution. Proc. Natl Acad. Sci. USA 112, 2307–2313. ( 10.1073/pnas.1422301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony K, et al. 2017. New interventions are needed to save coral reefs. Nature Eco. Evo. 1, 1420–1422. ( 10.1038/s41559-017-0313-5) [DOI] [PubMed] [Google Scholar]
- 16.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 17.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 18.Carpenter KE, et al. 2008. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. ( 10.1126/science.1159196) [DOI] [PubMed] [Google Scholar]
- 19.Hughes TP, et al. 2018. Global warming transforms coral reef assemblages. Nature 556, 492–496. ( 10.1038/s41586-018-0041-2) [DOI] [PubMed] [Google Scholar]
- 20.Carroll SP, Jørgensen PS, Kinnison MT, Bergstrom CT, Denison RF, Gluckman P, Smith TB, Strauss SY, Tabashnik BE. 2014. Applying evolutionary biology to address global challenges. Science 346, 1245993 ( 10.1126/science.1245993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leal MC, Ferrier-Pagès C, Petersen D, Osinga R. 2016. Coral aquaculture: applying scientific knowledge to ex situ production. Rev. Aquaculture 8, 136–153. ( 10.1111/raq.12087) [DOI] [Google Scholar]
- 22.Calow P. 1983. Evolutionary principles. Tertiary level biology. Boston, MA: Springer. [Google Scholar]
- 23.Kenkel CD, Setta SP, Matz MV. 2015. Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity 115, 509–516. ( 10.1038/hdy.2015.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton JA, Willis BL, Hutson KS. 2015. Coral propagation: a review of techniques for ornamental trade and reef restoration. Rev. Aquaculture 9, 238–256. ( 10.1111/raq.12135) [DOI] [Google Scholar]
- 25.Connell JH. 1973. Population ecology of reef-building corals. In Biology and geology of coral reefs, vol. II (eds Jones OA, Endean R), pp. 205–245. New York, NY: Academic Press. [Google Scholar]
- 26.Hughes TP. 1989. Community structure and diversity of coral reefs: the role of history. Ecology 70, 275–279. ( 10.2307/1938434) [DOI] [Google Scholar]
- 27.Hughes TP, Ayre D, Connell JH. 1992. The evolutionary ecology of corals. Trends Ecol. Evol. 7, 292–295. [DOI] [PubMed] [Google Scholar]
- 28.Edmunds PJ, Gates RD. 2002. Normalizing physiological data for scleractinian corals. Coral Reefs 21, 193–197. ( 10.1007/s00338-002-0214-0) [DOI] [Google Scholar]
- 29.Edmunds PJ. 2017. Intraspecific variation in growth rate is a poor predictor of fitness for reef corals. Ecology 98, 2191–2200. ( 10.1002/ecy.1912) [DOI] [PubMed] [Google Scholar]
- 30.Edmunds PJ. 2007. Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Mar. Ecol. Prog. Ser. 341, 1–13. ( 10.3354/meps341001) [DOI] [Google Scholar]
- 31.De'ath G, Lough JM, Fabricius KE. 2009. Declining coral calcification on the Great Barrier Reef. Science 323, 116–119. ( 10.1126/science.1165283) [DOI] [PubMed] [Google Scholar]
- 32.Linhart YB, Grant MC. 1996. Evolutionary significance of local genetic differentiation in plants. Annu. Rev. Ecol. Syst. 27, 237–277. ( 10.1146/annurev.ecolsys.27.1.237) [DOI] [Google Scholar]
- 33.de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I. 2016. Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 116, 249–254. ( 10.1038/hdy.2015.93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richmond RH, Hunter CL. 1990. Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 60, 185–203. [Google Scholar]
- 35.Davies SP. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395. ( 10.1007/BF00428135) [DOI] [Google Scholar]
- 36.Sokal RR, Rohlf JF. 2010. Biometry, 4th edn New York, NY: W.H. Freeman & Company. [Google Scholar]
- 37.Zar JH. 1999. Biostatistical analysis, 4th edn Upper Saddle River, NJ: Prentice Hall, Inc. [Google Scholar]
- 38.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. 2000. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685. ( 10.4319/lo.2000.45.3.0677) [DOI] [Google Scholar]
- 39.Thornhill DJ, et al. 2011. A connection between colony biomass and death in Caribbean reef-building corals. PLoS ONE 6, e29535 ( 10.1371/journal.pone.0029535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sultan SE, Bazzaz FA. 1993. Phenotypic plasticity in Polygonum persicaria. II. Norms of reaction to soil moisture and the maintenance of genetic diversity. Evolution 47, 1032–1049. ( 10.1111/j.1558-5646.1993.tb02133.x) [DOI] [PubMed] [Google Scholar]
- 41.Stearns SC. 1989. The evolutionary significance of phenotypic plasticity. Bioscience 39, 436–445. ( 10.2307/1311135) [DOI] [Google Scholar]
- 42.Kingsolver JG, Buckley LB. 2017. Evolution of plasticity and adaptive responses to climate change along climate gradients. Proc R. Soc. B 284, 20170386 (doi:0.1098/rspb.2017.0386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicotra AB, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends Plant. Sci. 15, 684–692. ( 10.1016/j.tplants.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 44.Ghalambor CK, McKay JK, Carrol SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 45.Baums IB, et al. 2019. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Appl. 29, e01978 ( 10.1002/eap.1978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. ( 10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 47.Bruno JF, Edmunds PJ. 1997. Clonal variation for phenotypic plasticity in the coral Madracis mirabilis. Ecology 78, 2177–2190. ( 10.1890/0012-9658(1997)078[2177:CVFPPI]2.0.CO;2) [DOI] [Google Scholar]
- 48.Todd PA. 2008. Morphological plasticity in scleractinian corals. Biol. Rev 83, 315–337. ( 10.1111/j.1469-185X.2008.00045.x) [DOI] [PubMed] [Google Scholar]
- 49.Ladd MC, Shantz AA, Bartels E, Burkepile DE. 2017. Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Mar. Ecol. Prog. Ser. 572, 129–139. ( 10.3354/meps12169) [DOI] [Google Scholar]
- 50.O'Donnell KE, Lohr KE, Bartels E, Baums IB, Patterson JT. 2018. Acropora cervicornis performance and symbiont identity throughout the restoration process. Coral Reefs 37, 1109–1118. ( 10.1007/s00338-018-01743-y) [DOI] [Google Scholar]
- 51.Drury C, Manzello D, Lirman D. 2017. Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS ONE 12, e0174000 ( 10.1371/journal.pone.0174000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw EC, Carpenter RC, Lantz CA, Edmunds PJ. 2016. Intraspecific variability in the response to ocean warming and acidification in the scleractinian coral Acropora pulchra. Mar. Biol. 163, 210 ( 10.1007/s00227-016-2986-8) [DOI] [Google Scholar]
- 53.Kuffner IB, Barels E, Stathakopoulos A, Enochs IC, Kolodziej G, Toth LT, Manzello DP. 2017. Plasticity in skeletal characteristics of nursery-raised staghorn coral, Acropora cervicornis. Coral Reefs 36, 679–684. ( 10.1007/s00338-017-1560-2) [DOI] [Google Scholar]
- 54.Cunning R, Gillette P, Capo T, Galvez K, Baker AC. 2014. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34, 155–160. ( 10.1007/s00338-014-1216-4) [DOI] [Google Scholar]
- 55.Huffmyer AS, Matsuda SB, Eggers AR, Lemus JD, Gates RD. 2020. Evaluation of laser scanning confocal microscopy as a method for characterizing reef-building coral tissue thickness and Symbiodiniaceae fluorescence. J. Exp. Biol. 223, jeb220335 ( 10.1242/jeb.220335) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included as electronic supplementary material to be archived in association with the paper at Biology Letters. Data are accessible at: https://portal.edirepository.org/nis/mapbrowse?scope=knb-lter-mcr&identifier=5040&revision=10. This links to the data doi:10.6073/pasta/643be961dc6ba5791023a0526b6ceef4.