Bridging the gap between now and then
Coronavirus disease 2019 (COVID-19) is a viral respiratory disease that mysteriously emerged in late December 2019 in Wuhan City, China [1, 2]. The virus quickly spread worldwide and was announced a global pandemic by the World Health Organization (WHO) in March 2020. Shortly after, a novel coronavirus was identified as the etiologic agent and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the seventh coronavirus to infect humans and the third to cause an outbreak [2–4]. SARS-CoV-2 continues to spread around the world, as of late July, 2020, over 17 million people have been infected, causing over 665,000 deaths (https://coronavirus.jhu.edu/) and these numbers continue to trend upwards. Despite concerted global efforts, only a few targeted therapeutics, such as remdesivir, are available to help prevent or treat this disease [5], therefore, convalescent plasma therapy, a century-old medical remedy is being revisited as a viable and immediate option for mitigating the impact of this disease [6]. Convalescent plasma therapy is a type of passive antibody therapy whereby blood plasma with neutralizing antibodies against a specific virus is recovered from people who have recuperated from an infection, and administered to patients with the infection in order to improve clinical outcome [6]. Although the potential clinical benefit of convalescent plasma therapy in COVID-19 is still uncertain, administering antibody-containing plasma from recovered patients is a near-term option that can be implemented relatively quickly. In fact, because of the high number of patients with severe COVID-19 and the mainstay of current clinical treatment consisting of symptomatic management and mechanical ventilation, administering convalescent plasma for treatment purposes is currently being deployed [7–12]. Although it is still early to tell whether this therapeutic approach is effective against this disease, evidence so far has shown promise in critically ill patients [7–10]. As new targeted therapies against COVID-19 take considerable time to develop, test and deploy, convalescent plasma therapy could buy time needed to develop more sophisticated targeted treatments.
Historical precedent for the use of antibody therapy
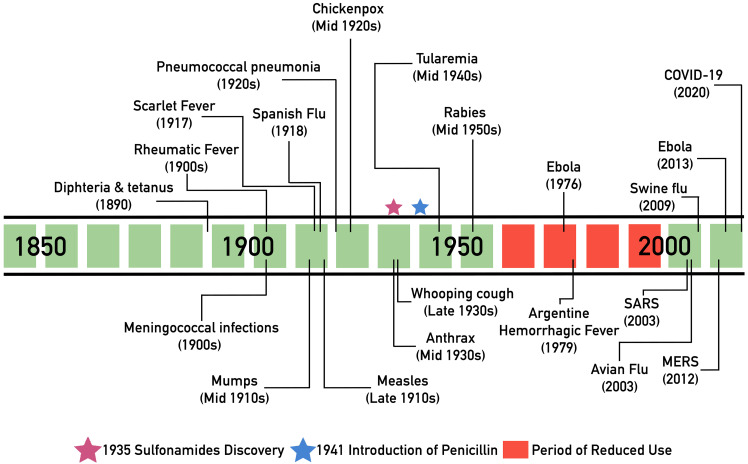
Prior to the antibiotic era, serum (plasma minus clotting factors) therapy was widely used to treat a range of infectious diseases such as scarlet fever and pneumococcal pneumonia. In 1890, the physiologists von Behring and Kitasato used blood serum from immunized animals to treat diphtheria and tetanus [13]; subsequently, serum from recovered animals was identified as a possible source of specific antibodies [14, 15]. The use of convalescent serum gained global recognition and revolutionized the way infectious diseases were treated, and in 1901, Emil von Behring was awarded the Nobel Prize for Medicine for his work, which served as a basis for treatment of multiple diseases in the 1900s as well as the development of vaccines [15]. In fact, there are numerous examples throughout history in which convalescent serum was used with some degree of success to treat an array of diseases, including rheumatic fever [16], scarlet fever [17], mumps [18], measles [18, 19], chickenpox [18], and pneumococcal and meningococcal infections [20] (Fig 1). Most notable use was during the Spanish Flu pandemic (1918 to 1920), where meta-analysis studies showed a significantly reduced mortality risk in patients treated with convalescent serum [8, 12]. However, with the advent of antimicrobials, by the middle of the 20th century, the use of serum therapy had declined. Nevertheless, the interest in passive antibody therapy has been renewed periodically when new epidemics or pandemics have emerged. One example is during the Ebola virus (EBOV) outbreak in 1976 in the Democratic Republic of Congo, where an infected laboratory worker recovered after transfusion with convalescent plasma containing anti-EBOV antibodies. Similarly, in 1979, patients with Argentine hemorrhagic fever virus treated with convalescent plasma had a lower mortality rate compared with subjects treated with normal plasma, and similar results were reported for subsequent epidemics of the disease [21]. Over the following decades, convalescent plasma therapy was successfully employed during the H1N1 swine influenza pandemic (2009), the H5N1 avian flu epidemic (2003), as well as during the EBOV outbreak in West Africa in 2013. Most relevant and encouraging is the use of convalescent plasma during 2 previous coronavirus epidemics: severe acute respiratory syndrome (SARS) in 2003, and Middle East respiratory syndrome (MERS) in 2012 [21]. The high degree of success in achieving favorable clinical outcomes during these coronaviruses outbreaks establishes a strong precedent and supports the notion that convalescent plasma could be a viable option for treatment of COVID-19 patients, particularly upon early administration [6, 9, 12, 21–23].
Fig 1. Notable historic uses of antibody therapy against infectious diseases.
COVID-19, coronavirus disease 2019; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
Buying time with the help of the convalescent
The convalescent plasma therapeutic approach is based on the principle of passive antibody therapy, a short-term strategy whereby antibodies from the blood of someone who recovered from an infection can be administered to protect or treat another person [6, 21]. Effectively, the end goal is the same as vaccines, making antibodies against a specific infectious agent readily available. For instance, a vaccine relies on the host immune cells (B lymphocytes specifically) to produce antibodies after antigen recognition and signal amplification by the immune system, a process that may take weeks [24]; on the other hand, in the case of passive antibody therapy, the process is expedited by providing a patient with immediate immunity when the premade antibodies are given. Therefore, for COVID-19 patients, the expedited approach could prove lifesaving. Nevertheless, this advantage does not come without caveats, as immunization with passive antibody therapy is typically of shorter-term protection, in part because of the half-life of antibodies in circulation [25] and lack of new production by B lymphocytes. Today, passive antibody therapy relies primarily on pooled immunoglobulin preparations that contain high concentrations of antibodies. In contrast, plasma has been used emergently in epidemics in which there is insufficient time or resources to generate immunoglobulin preparations [21].
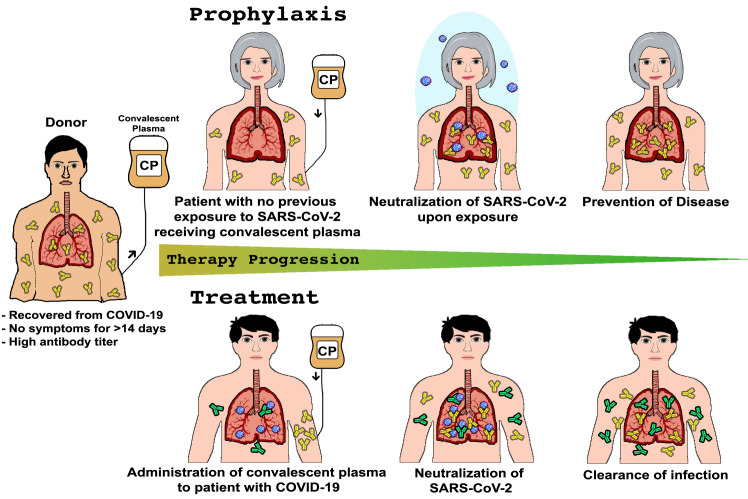
Despite the high rate of SARS-CoV-2 infection, the relatively low mortality rate provides a rich pool of donors [26]. However, potential COVID-19 donors must meet several eligibility criteria that ensure the donor has antibodies against SARS-CoV-2 and lacks the presence of other types of infections [21, 27]. Additionally, only plasma with high anti-SARS-CoV-2 titers of immunoglobulins G and M (IgG and IgM) are used. Once collected, plasma can be tested and administered within hours, following conventional donor–patient blood compatibility typing [27]. In addition to rapid mobilization, this therapeutic approach is also versatile in applicability as it can be used for prophylaxis or treatment, as illustrated in Fig 2. In the case of prophylaxis, a subject considered at high risk for infection (because of age or underlying medical conditions or who is likely to be in contact with people with COVID-19) could be administered convalescent plasma or neutralizing antibodies for protection against infection. Alternatively, plasma can be administered to treat subjects who have contracted the infection but have not made sufficient antibodies against the virus yet in order to augment their immune response, improve disease course, and enhance recovery [21]. However, it is important to note that passive antibody therapy is most effective when administered prophylactically or implemented early after the onset of symptoms [21].
Fig 2. Overview of the use and applications of CP therapy.
Virus-neutralizing antibodies in the plasma of a patient who recovered from COVID-19 can be administered prophylactically to prevent infection in vulnerable individuals and those with known exposure to the virus (Prophylaxis). Convalescent plasma can also be administered to infected individuals to improve the clinical outcome (Treatment). CP, convalescent plasma; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Convalescent plasma with neutralizing antibodies is currently being used for investigational purposes in the COVID-19 pandemic, and preliminary results from 2 small studies performed in China are encouraging. A pilot study exploring the feasibility of convalescent plasma transfusion to rescue a group of 10 patients with severe disease showed that 1 dose (200 mL) of convalescent plasma with high neutralizing antibody titers was well tolerated, resulted in disappearance of viremia, and improved clinical symptoms in all patients within days of administration [8]. Similar results were reported from another study with 5 critically ill patients on mechanical ventilation [7]. Although these small, nonrandomized studies had limitations, these findings indicate that convalescent plasma could be a promising rescue option for severe COVID-19 [21].
Limitations and potential risks
Although convalescent plasma therapy is considered a relatively safe therapeutic modality, there are some potential risks [12]. One theoretical complication that may arise is an antibody-mediated proinflammatory disease enhancement known as antibody-dependent enhancement (ADE), whereby antibodies that developed during a prior infection exacerbate severity of the disease [12, 28]. The transfer of these antibodies may aberrantly activate fragment crystallizable (Fc) or complement receptors, increasing recruitment of proinflammatory cytokines and chemokines to the site of infection and causing severe tissue damage [29–31]. Additionally, the presence of non-neutralizing antibodies may exacerbate viral endocytosis or phagocytosis into host cells via Fc receptors, potentializing viral replication [29, 32]. However, although this phenomenon is well known with Dengue and other viral diseases, there have not been any reported ADE cases with the use of convalescent plasma for SARS, MERS, or COVID-19 [12, 29, 32–35]. Nevertheless, it is crucial to have a clear understanding of the role of the recipients' immune response [12]. Transmission of the virus through transfusion is another concern; however, the risk is relatively low because of strict transfusion protocols, and when used for treatment purposes, the recipient is already infected [12, 36].
The success of convalescent plasma therapy hinges on the availability of plasma with high concentrations of antibodies, which may not be a major limitation for COVID-19 because SARS-CoV-2 has been shown to elicit high neutralizing antibody titers in recently convalesced individuals [7–9]. Apheresis is an automated technology that allows for selective collection of a blood fraction while other components can be transfused back to the donor. Therefore, for donation of convalescent plasma, plasmapheresis is recommended because it is highly efficient and approximately 400–800 mL of plasma can be collected in a single donation, providing 2–4 units of convalescent plasma for transfusion [21]. There are some practical and logistical limitations for the implementation of a large-scale convalescent plasma transfusion program such as training of study personnel, recruitment of donors, and transport of plasma to hospitals, as well considerations for plasma shelf-life and half-life of antibodies in the plasma [21, 36].
Although large-scale randomized clinical trials will ultimately confirm the safety and efficacy of convalescent plasma therapy for COVID-19, a recent safety study by Joyner and colleagues [12] provided encouraging data. By analyzing key safety metrics following transfusion of convalescent plasma in 5,000 hospitalized adults with severe or life-threatening COVID-19, the incidence of serious adverse events was found to be <1%, and the 7-day mortality incidence was 14.9%. These early indicators are encouraging and highly supportive of the use of convalescent plasma as a rescue therapy in hospitalized patients, given that the reported fatality rate of COVID-19 among patients admitted to the intensive care units (ICUs) is 57% [37]. In fact, the low risk indicated by the study support expanding the use of convalescent plasma therapy in less ill individuals [6].
Future perspectives
The global reach of the COVID-19 pandemic and the desperate need for effective treatments have provided an impetus to develop convalescent plasma therapy into a viable (albeit short-term) treatment option particularly for the critically ill. Although its efficacy and safety have not yet been fully proven, convalescent plasma therapy for COVID-19 patients is projected to be a safe and potentially effective therapy for prophylaxis and treatment. However, it is critically important to perform rigorous randomized controlled trials to confirm efficacy and safety and to provide evidence for improved meaningful clinical outcomes. Nevertheless, despite the nuanced challenges, the substantial evidence of benefit with use for prior viral infections offers strong precedent for convalescent plasma as a therapeutic approach. Importantly, efforts should be focused not only on evaluation of the feasibility of plasma treatment for infectious diseases but also ensure that use of convalescent plasma therapy takes place according to ethical and controlled conditions.
Acknowledgments
We would like to thank Dr. Arturo Casadevall for critical review of the manuscript.
Funding Statement
The work in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH under award number R01AI130170 (NIAID). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. Epub 2020/02/03. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020. Epub 2020/02/23. 10.1093/ije/dyaa033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–92. Epub 2018/12/12. 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Y, Zhao K, Shi ZL, Zhou P. Bat Coronaviruses in China. Viruses. 2019;11(3). Epub 2019/03/06. 10.3390/v11030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N Engl J Med. 2020. Epub 2020/05/24. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 6.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. Epub 2020/03/14. 10.1172/JCI138003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323(16):1582–1589. Epub 2020/03/29. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. Epub 2020/04/08. 10.1073/pnas.2004168117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Pang R, Xue X, Bao J, Ye S, Dai Y, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging. 2020;12(8):6536–6542. Epub 2020/04/23. 10.18632/aging.103102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;1–12. Epub 2020/04/16. 10.1002/jmv.25577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheridan C. Convalescent serum lines up as first-choice treatment for coronavirus. Nat Biotechnol. 2020;(38):655–658. Epub 2020/05/03. 10.1038/d41587-020-00011-1 . [DOI] [PubMed] [Google Scholar]
- 12.Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K, Klassen S, et al. Early Safety Indicators of COVID-19 Convalescent Plasma in 5,000 Patients. medRxiv 2020May1220099879 [Preprint]. 2020 [cited 2020 May 16]. 10.1101/2020.05.12.20099879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behring Ev. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. 2013. 10.17192/eb2013.0164 [DOI] [PubMed] [Google Scholar]
- 14.Behring Ev. Untersuchungen über das Zustandekommen der Diphtherie-Immunität bei Thiere. Philipps-Universität Marburg; 2013. 10.17192/eb2013.0165 [DOI] [Google Scholar]
- 15.Kaufmann SH. Remembering Emil von Behring: from Tetanus Treatment to Antibody Cooperation with Phagocytes. mBio. 2017;8(1). Epub 2017/03/02. 10.1128/mBio.00117-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore JJ. The Action of Vaccines and of Concentrated Antistreptococcus Serum in Experimental Streptococcal Arthritis. J Infect Dis. 1914;15(1):215–26. [Google Scholar]
- 17.Smith DD. Serum treatment of scarlet fever [doctoral thesis]. Omaha (Nebraska): University of Nebraska; 1934.
- 18.The use of convalescent serum in the treatment of measles, chickenpox, mumps and whooping cough, including the prophylactic value of parental blood: J. M. and Barenberg L. H. N. Y. State J. Med. 33: 2, 1933. Int J Orthod Dent Child. 1933;19(3):328–9. 10.1016/S0097-0522(33)90316-0 PubMed PMID: S0097052233903160. [DOI] [Google Scholar]
- 19.Kohn JL, Klein IF, Schwarz H. Treatment of preeruptive measles with convalescent serum. JAMA. 1938;111(26):2361–4. 10.1001/jama.1938.02790520017004 [DOI] [Google Scholar]
- 20.Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38(8):1695–702. Epub 1994/08/01. 10.1128/aac.38.8.1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020. Epub 2020/04/08. 10.1172/JCI138745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–56. Epub 2011/01/21. 10.1093/cid/ciq106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung IFN, To KKW, Lee CK, Lee KL, Yan WW, Chan K, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464–73. Epub 2013/03/02. 10.1378/chest.12-2907 . [DOI] [PubMed] [Google Scholar]
- 24.Prevention CfDCa. Principles of Vaccination. In: Epidemiology and Prevention of Vaccine-Preventable Diseases [Internet]. Washington, D.C.: Public Health Foundation. 13th. [cited 2020 June 11]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html
- 25.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33. Epub 2009/05/23. 10.1111/j.1476-5381.2009.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924 Epub 2020/02/23. 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang. 2020. Epub 2020/04/23. 10.1111/vox.12939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70. Epub 2020/04/11. 10.1038/s41577-020-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55:102768 Epub 2020/04/29. 10.1016/j.ebiom.2020.102768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz HU. How immune complexes from certain IgG NAbs and any F(ab')(2) can mediate excessive complement activation. Adv Exp Med Biol. 2012;750:186–96. Epub 2012/08/21. 10.1007/978-1-4614-3461-0_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jancar S, Sanchez Crespo M. Immune complex-mediated tissue injury: a multistep paradigm. Trends Immunol. 2005;26(1):48–55. Epub 2005/01/05. 10.1016/j.it.2004.11.007 . [DOI] [PubMed] [Google Scholar]
- 32.Yip MS, Leung HL, Li PH, Cheung CY, Dutry I, Li D, et al. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med J. 2016;22(3 Suppl 4):25–31. Epub 2016/07/09. . [PubMed] [Google Scholar]
- 33.Katzelnick LC, Harris E, Participants in the Summit on Dengue Immune Correlates of P. Immune correlates of protection for dengue: State of the art and research agenda. Vaccine. 2017;35(36):4659–69. Epub 2017/08/02. 10.1016/j.vaccine.2017.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–3. Epub 2020/02/25. 10.1016/j.micinf.2020.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146(1):201–17. Epub 1977/07/01. 10.1084/jem.146.1.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–7. Epub 2015/12/18. 10.2450/2015.0131-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. Epub 2020/02/28. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]