Abstract
Study Design
Literature review.
Objective
To evaluate the association between recombinant human bone morphogenetic protein-2 (rhBMP-2) and malignancy.
Summary of Background Data
The use of rhBMP-2 in spine surgery has been the topic of much debate as studies assessing the association between rhBMP-2 and malignancy have come to conflicting conclusions.
Methods
A systematic review of the literature was performed using the PubMed-National Library of Medicine/National Institute of Health databases. Only non-clinical studies directly addressing BMP-2 and cancer were included. Articles were categorized by study type (animal, in vitro cell line/human/animal), primary malignancy, cancer attributes, and whether BMP-2 was pro-malignancy or not.
Results
A total of 4,131 articles were reviewed. Of those, 515 articles made reference to both BMP-2 and cancer, 99 of which were found to directly examine the role of BMP-2 in cancer. Seventy-five studies were in vitro and 24 were animal studies. Forty-three studies concluded that BMP-2 enhanced cancer function, whereas 18 studies found that BMP-2 suppressed malignancy. Thirty-six studies did not examine whether BMP-2 enhanced or suppressed cancer function. Fifteen studies demonstrated BMP-2 dose dependence (9 enhancement, 6 suppression) and one study demonstrated no dose dependence. Nine studies demonstrated BMP-2 time dependence (6 enhancement, 3 suppression). However, no study demonstrated that BMP-2 caused cancer de novo.
Conclusion
Currently, conflicting data exist with regard to the effect of exogenous BMP-2 on cancer. The majority of studies addressed the role of BMP-2 in prostate (17%), breast (17%), and lung (15%) cancers. Most were in vitro studies (75%) and examined cancer invasiveness and metastatic potential (37%). Of 99 studies, there was no demonstration of BMP-2 causing cancer de novo. However, 43% of studies suggested that BMP-2 enhances tumor function, motivating more definitive research on the topic that also includes clinically meaningful dose- and time-dependence.
Keywords: bone morphogenetic protein, carcinogenicity, fusion, InFuse®, malignancy, rhBMP-2, Spine surgery
Bone morphogenetic proteins (BMPs) are pluripotent factors belonging to the transforming growth factor-beta (TGF-β) superfamily involved in the regulation of embryonic development and postnatal homeostasis of various tissues and organs through their potent regulation of cellular differentiation, proliferation, survival and apoptosis. 1 Since their discovery by Marshall Urist in 1965, over 20 different BMPs have been described in humans.1–6 However, only BMP-2, −4, −6, −7, and −9 have been shown to have significant osteogenic properties through facilitation of intramembranous and endochondral bone formation as well as cartilage formation.3,7,8
BMP-2 appears to be the most oseteogenic BMP discovered and multiple studies have suggested that it could cause bone induction in various animal models.9,10 In 2002, following 13 industry sponsored recombinant human BMP-2 (rhBMP-2) publications regarding safety and efficacy, rhBMP-2 (InFuse; Medtronic Sofamor Danek, Memphis, TN) was Food and Drug Administration (FDA) approved as a bone graft substitute for human use.11 Although it was only approved for single-level anterior lumbar fusion surgery using a tapered titanium cage, 85% of rhBMP-2 has been reported to have been used off-label.12 The original industry-sponsored rhBMP-2 publications regarding safety and efficacy, including reports and analyses of 780 patients receiving rhBMP-2 within prospective controlled study protocols reported a 0% rhBMP-2 – associated adverse events.10 Recently, however, the use of rhBMP-2 in spine surgery has been the topic of much debate as rhBMP-2 has been reported to be associated with a higher incidence of developing new malignancy compared to iliac crest bone graft (Appendix Table 1).
Based on the identified increase cancer risk in patients who received rhBMP-2 and its widespread use in spine surgery, determining the answer to whether BMP-2 is associated with malignancy is of utmost importance. The goal of this study is to evaluate the association between BMP-2 and malignancy by systematically reviewing and analyzing all published literature for all studies examining the role of BMP-2 in malignancy.
MATERIALS AND METHODS
A systematic review and analysis of the published literature in the English language was performed using the PubMed-National Library of Medicine/National Institute of Health databases. Only studies that directly addressed BMP-2 and cancer were included. The terms “bone morphogenetic protein”, “BMP”, “neoplasm”, “tumor”, “malignancy”, “cancer”, “tumorigenesis”, and “metastasis” were used as search terms. Articles were categorized by the study type (animal, in vitro cell line, in vitro human, in vitro animal), primary malignancy, cancer attributes, and whether BMP-2 was pro-malignancy or not. Only articles in English journals or published with English translations were included. Level of evidence (I-V) was assessed for each included article according to published criteria.13
RESULTS
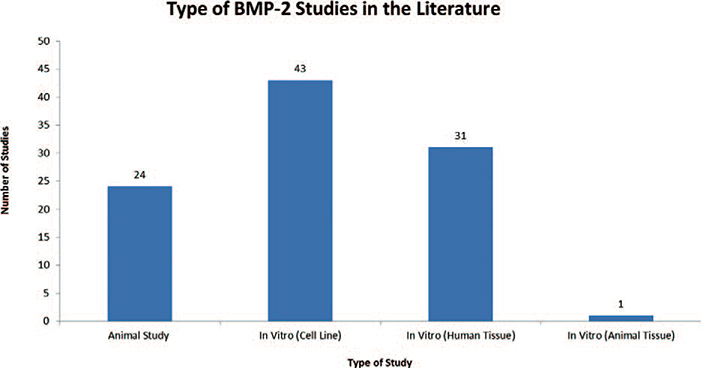
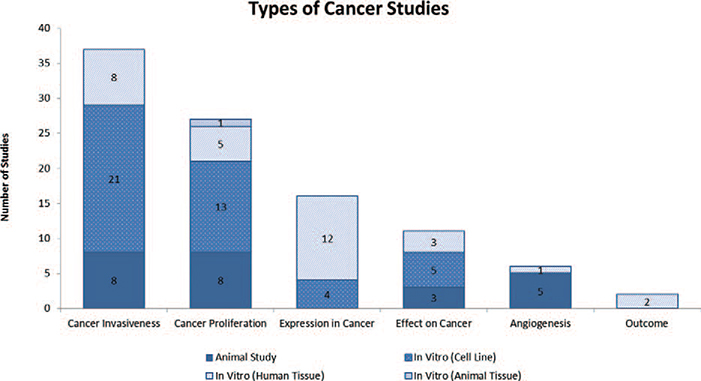
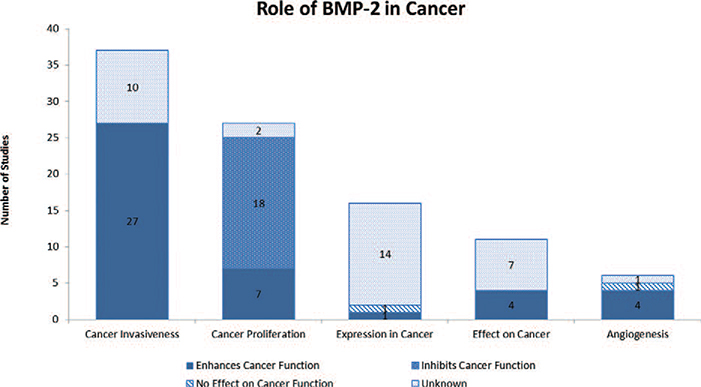
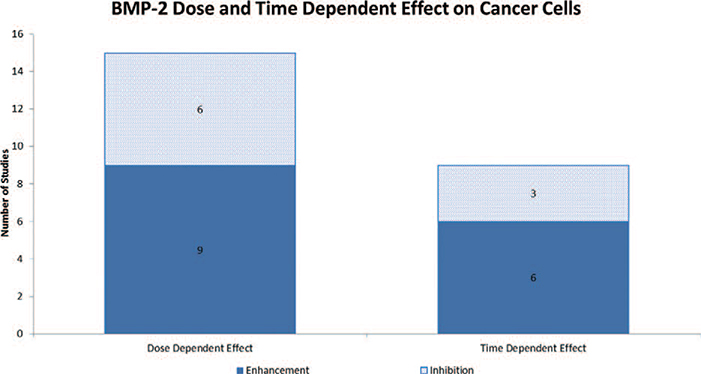
A total of 4,131 articles were reviewed. Of those, 515 made reference to both BMP-2 and cancer. The 515 studies were further analyzed and a total of 99 studies were found to directly examine the role of BMP-2 in cancer.14–112 Seventeen articles examined the role of BMP-2 in prostate cancer, 17 in breast cancer, 15 in lung cancer, 9 in osteosarcoma, 6 in gastric cancer, 5 in oral cancer, 4 in ovarian cancer, 3 in bladder cancer, 3 in chondrosarcoma, 3 in colon cancer, 3 in melanoma, 3 in pancreatic cancer, 3 in salivary cancer, 1 in adrenal carcinoma, 1 in giant cell tumor, 1 in glioma, 1 in leukemia, 1 in liver cancer, 1 in renal cell carcinoma, 1 in squamous cell carcinoma and 1 in thyroid carcinoma [Table 1]. Twenty-four studies were animal studies and 75 studies were in vitro studies. In vitro studies were further separated based on whether the study used a cultured cell line versus human or animal tissue. Of the 75 in vitro studies, 43 used cell lines, 31 used human tissue and 1 study used animal tissue [Figure 1]. Thirty-seven studies evaluated cancer invasiveness (8 animal, 21 in vitro cell, 8 in vitro human), 27 cancer proliferation (8 animal, 21 in vitro cell, 5 in vitro human, 1 in vitro animal), 16 BMP-2 expression in cancer (4 in vitro cell, 12 in vitro human), 11 effect on cancer (3 animal, 5 in vitro cell, 3 in vitro human), 6 angiogenesis (5 animal, 1 in vitro human), and 2 outcome (2 in vitro human) [Figure 2]. Lastly, the pro-malignancy versus tumor suppression by BMP-2 was assessed. Forty-three studies concluded that BMP-2 enhanced cancer function (43.4%, 13 animal, 25 in vitro cell, 5 in vitro human), 18 studies found that BMP-2 inhibited cancer function (18.2%, 5 animal, 9 in vitro cell, 3 in vitro human, 1 in vitro animal), 2 studies revealed that BMP-2 had no effect on cancer function (2.0%, 1 animal, 1 in vitro human) and 36 studies did not evaluate the role of BMP-2 on cancer function (36.3%, 5 animal, 9 in vitro cell, 22 in vitro human) [Figure 3]. Fifteen studies demonstrated BMP-2 dose dependence (9 enhancement, 6 suppression) and 1 study demonstrated no dose dependence. Nine studies revealed BMP-2 time dependence (6 enhancement, 3 suppression) [Figure 4]. No studies showed that BMP-2 caused cancer de novo, i.e. transformation of normal cells into malignant cells.
TABLE 1.
BMP-2 and Type of Cancer Studied
| Type of Cancer | Number of Studies |
|---|---|
| Prostate | 18 |
| Breast | 17 |
| Lung | 15 |
| Osteosarcoma | 9 |
| Gastric | 6 |
| Oral | 5 |
| Ovarian | 4 |
| Bladder | 3 |
| Chondrosarcoma | 3 |
| Colon | 3 |
| Melanoma | 3 |
| Pancreas | 3 |
| Salivary | 3 |
| Adrenal | 1 |
| Giant Cell Tumor | 1 |
| Glioma | 1 |
| Leukemia | 1 |
| Liver | 1 |
| Renal Cell | 1 |
| Squamous Cell | 1 |
| Thyroid | 1 |
Figure 1.
Table depicting the type and number of BMP-2 studies published in the literature.
Figure 2.
Table depicting the number and type of cancer studies evaluating the role of BMP-2 in various cancer properties.
Figure 3.
Table depicting the role of BMP-2 on cancer function by evaluating its effects on various cancer properties.
Figure 4.
Table depicting the dose- and time-dependent effects of BMP-2 on cancer cells.
DISCUSSION
The widespread off-label use of BMP-2 in fusion surgery and the subsequent concerns of increased risk of malignancy related to its use have led to a whirlwind of controversy in spine surgery. The latest independent review of all published and unpublished data on safety and effectiveness of rhBMP-2 by the Yale University Open Data Access Project (YODA) concluded that at 24 months, cancer risk was increased (RR, 3.45 [95% CI, 1.98–6.00] with rhBMP-2.113,114 However, a recent retrospective review of 467,916 Medicare patients undergoing spinal arthrodesis with and without the use of rhBMP-2 found that BMP-2was not associated with a detectable increase in the risk of cancer within a mean of 2.9-year time window.115 The conflicting findings from studies investigating the use of BMP-2 in spinal fusion surgery and its relationship to cancer continue to be a topic of intense research and debate.
BMPs are a family of proteins secreted to the extracellular environment as an intracellular communication mechanism through their role as ligands of specific receptors found on target cells.116 They form part of a much larger pathway, the TGF-β signaling pathway, responsible for the maintenance of tissue homeostasis and prevention of tumor progression to malignancy by regulation of cellular proliferation, differentiation, survival and adhesion, as well as the cellular microenviroment.117 In order for tumor cells to become malignant, they must elude the tumor-suppressive effects of the regulatory cytokine TGF-β. Malignant cells can do so by bypassing the suppressive effects of TGF-β either through inactivation of core components of the pathway (TGF-β receptors), or by downstream alterations that disable the tumor-suppressive arm of the pathway.117 If cancer cells succeed to circumvent the downstream arm of the pathway, they have at their disposal the remaining regulatory effects of the TGF-β regulatory functions acquiring invasion capabilities, producing autocrine mitogens, or releasing prometastatic cytokines.117
BMP-2 mediates signaling by binding serine/threonine kinase type IA and IB together with a type II receptor with subsequent phosphorylation of Smad 1/5/8 and activation of downstream targets.118 The current theory of stem cell differentiation argues that cell fate decisions are determined by the actions of intracellular signaling networks in response to combinations of extracellular ligands and that crosstalk between different signaling pathways via different transcription factors ultimately results in the expression of patterns of target genes that specify cell fate.119 The effect of BMPs on target cells would thus depend on the context in which the BMP signals are received by those cells. Cells in different organs at different stages of development may express specific combinations of BMP effectors that influence responses to BMP.
The switch of BMP from a differentiation to a self-renewal signal is highly dependent on the activity of other signaling pathways activated at different times in different tissues.119 The fine balance between self-renewal and differentiation must be maintained in order to maintain normal growth and homeostasis.120 Disruption of this balance can lead to a loss of cellular control in terms of cell growth and proliferation.121 Stem cells have the characteristics of immortality and the loss of contact inhibition, both of which are exhibited by cancer cells.
BMP-2 regulates many essential cellular processes in development including cell proliferation, apoptosis, differentiation, cell-fate determination, and morphogenesis. Given the demonstrated impact of BMP-2 in various stem cell populations and their broad modes of signaling, it is easy to see how BMP-2 may contribute to or foster changes leading to cancer and its spread.122
In this study, the authors aimed to evaluate the effect BMP-2 had on various cancer attributes such as cancer proliferation, invasiveness, and angiogenesis. The goal of this study was to improve our understanding of the effects of exogenous BMP-2 on malignancy, in hopes of further elucidating the intricate relationship between BMP-2 and cancer in the clinical setting.
Of the 99 studies that directly examined the role of BMP-2 in malignancy, 49 were found to examine the role of BMP-2 in prostate (17), breast (17) and lung (15) cancers, the three most common types of cancer as reported by the American Cancer Society.123 Of these three, lung cancer was the only type of cancer where BMP-2 was found to have a role in tumorigenesis through the regulation of the PI3K/mTOR pathway, known to have an important role in regulating growth of human carcinomas.35 BMP-2 is highly expressed in approximately 98% of human lung carcinomas with little to no expression in normal lung tissue or benign lung tumors.35,43 Bieniasz et al.79 revealed that expression of BMP-2 mRNAs was 25.7-fold higher in lung cancer samples compared to normal adjacent lung tissue. Feeley et al.57 found that Noggin, an inhibitor of BMP-2, inhibited mixed metastatic lung lesions in bone and the tumor growth in vivo.
Sixty-three studies assessed whether BMP-2 enhanced or inhibited malignancy. Of those, 43 (68%) studies found BMP-2 to be pro-oncogenic, while 18 (29%) found BMP-2 to be inhibitory and 2 (3%) studies did not find BMP-2 to affect cancer. The specific pro-oncogenic and inhibitory effects of BMP-2 on different tumor types can be found in Tables 2 and 3, respectively. The inhibitory effects of BMP-2 on cancer were only found in studies assessing cancer proliferation. In studies evaluating cancer invasiveness, 73% found that BMP-2 enhanced cancer invasiveness and metastatic potential. Studies evaluating the effects of BMP-2 on angiogenesis found BMP-2 to be enhancing in 67% of the studies. From this early understanding of the effects of BMP-2 on cancer it appears that under specific circumstances, BMP-2 may have the ability to inhibit cancer formation by inhibiting early cellular changes that result in cellular atypia and dysplasia. However, once cells lose further control and become more carcinogenic, BMP-2 may enhance their ability to grow by facilitating neovascularization, and their ability to metastasize and spread to distant organs by enhancing their invasive properties.122,124
TABLE 2.
Breakdown of Studies That Found BMP-2 to be Pro-oncogenic
| Cancer Type | Author | Conclusion of BMP-2 Effect |
|---|---|---|
| Breast | Steinert, S72 | BMP-2 affected apoptosis-related genes which showed pronounced alteration |
| Raida, M68 | BMP-2 expression is activated in invasive breast cancers | |
| Clement, JH36 | Enhances the tumorigenic properties of breast carcinoma cells and drive the cells towards a more aggressive phenotype | |
| Katsuno, Y90 | BMP-2 promotes invasion and bone metastasis of breast cancer | |
| Neman, J92 | Metastatic cells create a permissive niche by steering differentiation through paracrine BMP-2 signaling | |
| Pouliot, F67 | BMPs interacting with type II BMP receptors contribute to the proliferation and/or survival of human breast cancer cells | |
| Lung | Langenfeld, E96,29,43 | Promotes growth, transformation and survival of cancer cells |
| Enhances angiogenic response by stimulating endothelial cells | ||
| Highly overexpressed an growth promoting in cancer cells | ||
| Hsu, Y50 | Induced lung cancer migration, invasion, and epithelial-to-mesenchymal transition | |
| Lee, KB71 | Sequestration of BMP-2 reduced tumor growth | |
| Feeley, BT57 | Mixed metastatic lung lesions in bone are inhibited by noggin overexpression which is a direct inhibitor of BMP-2 | |
| Prostate | Yang, S32 | Induces tube formation in angiogenesis, modulates the biological behavior of prostate tumor cells in diverse and cell type-specific manner |
| Feeley, BT49 | Critical in the formation of the osteoblastic lesions associated with prostate cancer metastases, influence the formation of the osteolytic prostate cancer metastases | |
| Kwon, H52 | Promotes migration of cancer cells in a 3-dimensional gel system | |
| Graham, TR60 | Enhances the invasiveness of C4–2B prostate cancer cell line | |
| Lai, TH42 | Contributes to the migration of prostate cancer cells | |
| Gastric | Kang, MH63 | BMP-2 strongly increases motility and invasiveness, BMP-2 signaling pathways strongly enhance tumor metastasis |
| Park, Y73 | Seems to have a role in progression to metastatic disease, especially in the late stage of tumorigenesis, including invasion and metastasis | |
| Bladder | Hung, TT87 | BMP-2 expression was significantly associated with in vivo tumorigenicity of the cell lines |
| Yang, ZJ95 | Responsible for the mechanism involved in triggering bone metastasis in bladder cancer | |
| Oral | Jin,Y51 | BMP-2 might be involved in the metastasis of oral carcinoma cells |
| Kokorina, NA92 | rhBMP-2 treatment of tumor cells makes them more locally aggressive with worse survival and has adverse effects on invasiveness in human cell lines in vitro | |
| Pancreatic | Chen, X56 | BMP-2 accelerates invasion of panc-1 cells via the PI3K/AKT pathway |
| Gordon, KJ59 | BMP signaling, through Smad1 induction and up-regulation of MMP-2, is an important mediator of pancreatic cancer invasiveness | |
| Chondrosarcoma | Hou, CH39 | Enhances invasiveness of chondrosarcoma cells |
| Fong, YC38 | Contributes to the migration of chondrosarcoma cells through PI3K/AKT pathway | |
| Osteosarcoma | Rubio, R99 | Enhances development of osteoid areas |
| Sotobori, T46 | Enhances cell migration by modulating fibronectin-integrin beta-1 signaling | |
| Melanoma | Rothhammer, T31 | Induces tube formation and migration efficiency of microvascular endothelial cells, play an important role in dissemination of tumor cells from the primary tumor |
| Ovarian | LePage, C85 | Contributes to a modification of tumor cell behavior through a change in motility and adherence |
| Glioma | Guo, M98 | BMP-2 treatment causes marked stimulation of glioma cell growth, migration and invasion |
| Leukemia | Lapperouzas, B88 | Deregulation of intracellular BMP signaling in primary CP-CML samples corrupts and amplifies their response to exogenous BMP2 |
| Chondroblastoma | Yang, X90 | BMP-2 induced protumorigenic effects on chonroblastoma cells |
| Colon | Kang, MH20 | BMP-2 causes resistance and invasion of cancer cells |
TABLE 3.
Breakdown of Studies That Found BMP-2 to be Inhibitory to Cancer
| Cancer Type | Author | Conclusion of BMP-2 Effect |
|---|---|---|
| Breast | Ye, S94 | Inhibited the proliferation of MDA-MB-231 breast cancer cells in vitro |
| Chen, A96 | Significantly inhibited the proliferation of MDA-MB-231 and MCF-7 breast cancer cells | |
| Buijs, JT99 | BMP-2 and -7 strongly inhibited the activity of the breast cancer phenotype | |
| Gosh, N57 | Inhibits estradiol-induced proliferation of human breast cancer cells | |
| Arnold, SF55 | rhBMP-2 decreases proliferation of various breast cancer cell lines | |
| Osteosarcoma | Wang, L25 | Suppresses tumor growth by reducing the gene expression of tumorigenic factors |
| Rici, RE98 | Inhibits the proliferation capacity of osteosarcoma cells by mechanisms of apoptosis and tumor suppression mediated by p53 | |
| Murphy, MG28 | BMP-2 has an inhibitory effect at high doses | |
| Huang, W19 | Increased BMP-2 proliferation caused decreased cellular proliferation and increased osteoblastic differentiation | |
| Prostate | Brubaker, KD16 | BMPs have growth inhibitory effects on prostate cancer cell lines |
| Tomari, K54 | Inhibits dihydrotestosterone-induced growth of prostate cancer cell line LNCaP | |
| Kumagai, T41 | BMP-2 identified in several inhibitory pathways | |
| Gastric | Zhang, J97 | Exerted inhibitory effect on the growth of all types of cells and the inhibition become more evident with the increase of BMP-2 dose |
| Shirai, YT22 | BMP-2 functions as potent tumor suppressors in diffuse-type gastric carcinoma | |
| Adrenal | Johnsen, IK15 | BMP inhibits cell proliferation/viability in a dose and time dependent fashion |
| Colon | Beck, SE18 | RAS/ERK activation prohibits growth suppressive effects of BMP signaling |
| Renal Cell | Wang, L24 | BMP-2 inhibits growth of RCC and causes induction of osseous bone formation |
| Multiple Cancers | Soda, H23 | Significant inhibition was seen in 16 of 65 specimens (24.6%) |
Fifteen studies evaluated the effect of BMP-2 dose on malignancy; 60% of which found a BMP-2 dose-dependent enhancement of cancer cells. Fourteen of the studies used BMP-2 doses ranging from 0–500 ng/mL while one study evaluating tumor growth of human renal cell carcinoma in a rat model used a dosage of 30 μg/mL, three orders of magnitude greater. The current FDA-approved dosage of rh-BMP2 in InFUSE® is 1.5 mg/mL, a value based on nonhuman primate data and adopted to human use.125 A more concentrated preparation of rhBMP-2, Amplify® (2.0 mg/mL) was discontinued from use after a review of the original FDA SSED studies showed increased cancer rates in patients who received Amplify® versus controls (3.8% vs. 0.89%).126 There is currently no available data on the optimal dose of rhBMP-2 for use in humans.
Pharmacokinetic studies using rat and nonhuman primate models have shown that rhBMP-2 is rapidly eliminated from the systemic circulation after intravenous administration with half-lives (t1/2) of 16 minutes in rats and 6.7 minutes in nonhuman primates.127 These half-life values are significantly shorter compared to those of certain established human carcinogens such as cyclosporine (t1/2 in rat, 6–10 hours), 4-aminobiphenyl (t1/2 in rat, 17 hours) and busulfan (t1/2 in rat, 3–8 hours).128–131 Implantation of rhBMP-2 in conjunction with an absorbable collagen sponge resulted in the retention of rhBMP-2 at the site of application (mean resident time (MRT), 4–8 days in the rat) and low levels of detectable rhBMP-2 in the systemic circulation.127 The slow release of rhBMP-2 from an implant site coupled with rapid clearance from the systemic circulation is thought to result in low systemic exposure.132
Only nine studies exist evaluating the time-dependent effect of BMP-2 on cancer cells. Of the currently available data on time dependent effects of BMP-2 on cancer, 67% of the studies revealed a positive association. It is currently unknown whether rhBMP-2, when implanted in the human fusion bed, remains at that location locally or whether it enters the intravascular space and spreads to distant organs. If it does, the concentration and duration of the effect of exogenous rhBMP-2 on distant end organs in humans are unknown.
Two studies assessed the correlation of BMP-2 levels in human serum with patient outcomes. Fei et al.100 showed that BMP-2 up-regulation was an indicator of poor survival in advanced non-small cell lung cancer. The authors concluded that serum BMP-2 level is positively correlated with clinical stage and metastatic burden and may serve as an independent negative predictor for prognosis. Sand et al.97 showed that head and neck squamous cell carcinomas with high baseline BMP-2 protein levels were associated with higher rates of local recurrence, however, overall survival, regional failure, and distant failure were not affected by level of BMP-2 expression.
There are several limitations to this study. It is important to note that 75 of the 99 studies evaluating BMP-2 and malignancy in the literature were cell culture experiments. Although these were validated studies, cell culture experiments may not reveal the full biological effects of BMP-2 since its activity is highly variable depending on the local environment in which it is encountered. A concern with the use of cell lines is that they are engineered for use in proliferation and growth studies and their response to growth factors may not reflect that of normal cells. Another limitation to this study is that only non-clinical (in-vitro and animal) studies were evaluated. Non-clinical studies may not be comparable to human clinical data giving rise to the limitation of inference. A third limitation to this study is the fact that wide ranges of cell and cancer types were evaluated.
In conclusion, this study revealed the existence of conflicting data with regards to the effect of exogenous BMP-2 on cancer. We establish the fact that BMP-2 is a ubiquitous growth factor integral to growth, development and homeostasis with a wide range of effects on both normal and malignant cells. Of the 99 studies we examined, there was no demonstration of BMP-2 causing cancer de novo. However, 43% of studies suggested that BMP-2 enhances tumor function, motivating more definitive research on this topic that also includes clinically meaningful dose- and time-dependence.
Key Points.
BMP-2 is an ubiquitous growth factor integral to growth, development and homeostasis of both normal and malignant cells.
The use of rhBMP-2 in spine surgery has been the topic of much debate as rhBMP-2 has been reported to be associated with a higher incidence of developing new malignancy compared to iliac crest bone graft.
Of the 99 non-clinical studies examined, sixty-three assessed whether BMP-2 enhanced or inhibited malignancy. Of those, 43 (68%) studies found BMP-2 to be pro-oncogenic, while 18 (29%) found BMP-2 to be inhibitory and 2 (3%) studies did not find BMP-2 to affect cancer.
There was no demonstration of BMP-2 causing cancer de novo, however, 43% of studies suggested that BMP-2 enhances tumor function, motivating more definitive research on this topic that also includes clinically meaningful dose- and time-dependence.
This study revealed the existence of conflicting data with regards to the effect of exogenous BMP-2 on cancer.
Acknowledgments
No funds were received in support of this work.
Relevant financial activities outside the submitted work: board membership, consultancy, employment, grants, payment for lectures, royalties, stocks.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
References
- 1.Ye L, Mason MD, Jiang WG. Bone morphogenetic protein and bone metastasis, implication and therapeutic potential. Front Biosci 2011;16:865–97. [DOI] [PubMed] [Google Scholar]
- 2.Urist MR. Bone: formation by autoinduction. Science 1965;150 (3698):893–9. [DOI] [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science 1988;242 (4885):1528–34. [DOI] [PubMed] [Google Scholar]
- 4.Celeste AJ, Iannazzi JA, Taylor RC, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA 1990;87 (24):9843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ. Identification of a novel member (GDF-1) of the transforming growth factor-beta superfamily. Mol Endocrinol 1990;4 (7):1034–40. [DOI] [PubMed] [Google Scholar]
- 6.Ozkaynak E, Rueger DC, Drier EA, et al. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J 1990;9 (7):2085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel DI, Nove J, Kerns KM, et al. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors 1992;7 (2):139–50. [DOI] [PubMed] [Google Scholar]
- 8.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res (346):1998;26–37. [PubMed] [Google Scholar]
- 9.Even J, Eskander M, Kang J. Bone morphogenetic protein in spine surgery: current and future uses. J Am Acad Orthop Surg 2012;20 (9):547–52. [DOI] [PubMed] [Google Scholar]
- 10.Carragee EJ, Hurwitz EL, Weiner BK. Acritical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11 (6):471–91. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083432.htm Accessed 3/18/2013.
- 12.Ong KL, Villarraga ML, Lau E, et al. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine 2010;35 (19):1794–800. [DOI] [PubMed] [Google Scholar]
- 13.Wright JG, Swiontowski MF, Heckman JD. Introducing levels of evidence to the Journal. J Bone Joint Surg Am 2003;85:1–3. [PubMed] [Google Scholar]
- 14.Reinholz MM, Iturria SJ, Ingle JN, et al. Differential gene expression of TGF-beta family members and osteopontin in breast tumor tissue: analysis by real-time quantitative PCR. Breast Cancer Res Treat 2002;74 (3):255–69. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen IK, Kappler R, Auernhammer CJ, et al. Bone morphogenetic proteins 2 and 5 are down-regulated in adrenocortical carcinoma and modulate adrenal cell proliferation and steroidogenesis. Cancer Res 2009;69 (14):5784–92. [DOI] [PubMed] [Google Scholar]
- 16.Brubaker KD, Corey E, Brown LG, et al. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem 2004;91 (1):151–60. [DOI] [PubMed] [Google Scholar]
- 17.Spanjol J, Djordjevic G, Markic D, et al. Role of bone morphogenetic proteins in human prostate cancer pathogenesis and development of bone metastases: immunohistochemical study. Coll Antropol 2010;34 (Suppl 2):119–25. [PubMed] [Google Scholar]
- 18.Beck SE, Jung BH, Del Rosario E, et al. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal 2007;19 (7):1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Rudkin GH, Carlsen B, et al. Overexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cells. Exp Cell Res 2002;274 (2):226–34. [DOI] [PubMed] [Google Scholar]
- 20.Kang MH, Kang HN, Kim JL, et al. Inhibition of PI3 kinase/Akt pathway is required for BMP2-inducedEMT and invasion. Oncol Rep 2009;22 (3):525–34. [DOI] [PubMed] [Google Scholar]
- 21.Rothhammer T, Poser I, Soncin F, et al. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 2005;65 (2):448–56. [PubMed] [Google Scholar]
- 22.Shirai YT, Ehata S, Yashiro M, et al. Bone morphogenetic protein-2 and −4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol 2011;179 (6):2920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soda H, Raymond E, Sharma S, et al. Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming units. Anticancer Drugs 1998;9 (4): 327–31. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Park P, Zhang H, et al. BMP-2 inhibits tumor growth of human renal cell carcinoma and induces bone formation. Int J Cancer 2012;13198:1941–50. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Park P, Zhang H, et al. BMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99–1 cell line. Cancer Biol Ther 2011;11 (5):457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen XZ, Akiyama Y, Baylin SB, et al. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene 2006;25 (18): 2666–73. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Ma L, Guo Q, et al. Expression of bone morphogenetic protein-2 and its receptors in epithelial ovarian cancer and their influence on the prognosis of ovarian cancer patients. J Exp Clin Cancer Res 2010;29:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MG, Mailhot J, Borke J, et al. The effects of rhBMP-2 on human osteosarcoma cells and human gingival fibroblasts in vitro. J Oral Implantol 2001;27 (1):16–24. [DOI] [PubMed] [Google Scholar]
- 29.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2004;2 (3):141–9. [PubMed] [Google Scholar]
- 30.Raida M, Clement JH, Leek RD, et al. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol 2005;131 (11):741–50. [DOI] [PubMed] [Google Scholar]
- 31.Rothhammer T, Bataille F, Spruss T, et al. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 2007;26 (28):4158–70. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Pham LK, Liao CP, et al. A novel bone morphogenetic protein signaling in heterotypic cell interactions in prostate cancer. Cancer Res 2008;68 (1):198–205. [DOI] [PubMed] [Google Scholar]
- 33.Kleeff J, Maruyama H, Ishiwata T, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology 1999;116 (5):1202–16. [DOI] [PubMed] [Google Scholar]
- 34.Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene 2006;25 (5):685–92. [DOI] [PubMed] [Google Scholar]
- 35.Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein-2-induced transformation involves the activation of mammalian target of rapamycin. Mol Cancer Res 2005;3 (12): 679–84. [DOI] [PubMed] [Google Scholar]
- 36.Clement JH, Raida M, Sanger J, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol 2005;27 (2):401–7. [PubMed] [Google Scholar]
- 37.Feeley BT, Krenek L, Liu N, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone 2006;38 (2):154–66. [DOI] [PubMed] [Google Scholar]
- 38.Fong YC, Li TM, Wu CM, et al. BMP-2 increases migration of human chondrosarcoma cells via PI3K/Akt pathway. J Cell Physiol 2008;217 (3):846–55. [DOI] [PubMed] [Google Scholar]
- 39.Hou CH, Hsiao YC, Fong YC, et al. Bone morphogenetic protein-2 enhances the motility of chondrosarcoma cells via activation of matrix metalloproteinase-13. Bone 2009;44 (2):233–42. [DOI] [PubMed] [Google Scholar]
- 40.Kokorina NA, Zakharkin SO, Krebsbach PH, et al. Treatment effects of rhBMP-2 on invasiveness of oral carcinoma cell lines. Laryngoscope 2011;121 (9):1876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumagai T, Tomari K, Shimizu T, et al. Alteration of gene expression in response to bone morphogenetic protein-2 in androgen-dependent human prostate cancer LNCaP cells. Int J Mol Med 2006;17 (2):285–91. [PubMed] [Google Scholar]
- 42.Lai TH, Fong YC, Fu WM, et al. Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate 2008;68 (12):1341–53. [DOI] [PubMed] [Google Scholar]
- 43.Langenfeld EM, Calvano SE, Abou-Nukta F, et al. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 2003;24 (9):1445–54. [DOI] [PubMed] [Google Scholar]
- 44.Le Page C, Puiffe ML, Meunier L, et al. BMP-2 signaling in ovarian cancer and its association with poor prognosis. J Ovarian Res 2009;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nissinen L, Pirila L, Heino J. Bone morphogenetic protein-2 is a regulator of cell adhesion. Exp Cell Res 1997;230 (2): 377–85. [DOI] [PubMed] [Google Scholar]
- 46.Sotobori T, Ueda T, Myouli A, et al. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Exp Cell Res 2006;312 (19):3927–38. [DOI] [PubMed] [Google Scholar]
- 47.Wu JB, Fu HQ, Huang LZ, et al. Effects of siRNA-targeting BMP-2 on the abilities of migration and invasion of human liver cancer SMMC7721 cells and its mechanism. Cancer Gene Ther 2011;18 (1):20–5. [DOI] [PubMed] [Google Scholar]
- 48.Bentley H, Hamdy FC, Hart KA, et al. Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer 1992;66 (6):1159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feeley BT, Gamradt SC, Hsu WK, et al. Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. J Bone Miner Res 2005;20 (12):2189–99. [DOI] [PubMed] [Google Scholar]
- 50.Hsu YL, Huang MS, Yang CJ, et al. Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem 2011;286 (43):37335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin Y, Tipoe GL, Liong EC, et al. Overexpression of BMP-2/4, −5 and BMPR-IA associated with malignancy of oral epithelium. Oral Oncol 2001;37 (3):225–33. [DOI] [PubMed] [Google Scholar]
- 52.Kwon H, Kim HJ, Rice WL, et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J Tissue Eng Regen Med 2010;4 (8):590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothhammer T, Braig S, Bosserhoff AK. Bone morphogenetic proteins induce expression of metalloproteinases in melanoma cells and fibroblasts. Eur J Cancer 2008;44 (16):2526–34. [DOI] [PubMed] [Google Scholar]
- 54.Tomari K, Kumagai T, Shimizu T, et al. Bone morphogenetic protein-2 induces hypophosphorylation of Rb protein and repression of E2F in androgen-treated LNCaP human prostate cancer cells. Int J Mol Med 2005;15 (2):253–8. [PubMed] [Google Scholar]
- 55.Arnold SF, Tims E, Mcgrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine 1999;11 (12):1031–7. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, Liao J, Lu Y, et al. Activation of the PI3K/Akt pathway mediates bone morphogenetic protein 2-induced invasion of pancreatic cancer cells Panc-1. Pathol Oncol Res 2011;17 (2):257–61. [DOI] [PubMed] [Google Scholar]
- 57.Feeley BT, Liu NQ, Conduah AH, et al. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank:Fc administration. J Bone Miner Res 2006;21 (10):1571–80. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh-Choudhury N, Ghosh-Choudhury G, Celeste A, et al. Bone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochim Biophys Acta 2000;1497 (2):186–96. [DOI] [PubMed] [Google Scholar]
- 59.Gordon KJ, Kirkbride KC, How T, et al. Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a Smad1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis 2009;30 (2):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graham TR, Agrawal KC, Abdel-Mageed AB. Independent and cooperative roles of tumor necrosis factor-alpha, nuclear factor-kappaB, and bone morphogenetic protein-2 in regulation of metastasis and osteomimicry of prostate cancer cells and differentiation and mineralization of MC3T3-E1 osteoblast-like cells. Cancer Sci 2010;101 (1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graham TR, Odero-Marah VA, Chung LW, et al. PI3K/Akt-dependent transcriptional regulation and activation of BMP-2-Smad signaling by NF-kappaB in metastatic prostate cancer cells. Prostate 2009;69 (2):168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ide H, Katoh M, Sasaki H, et al. Cloning of human bone morphogenetic protein type IB receptor (BMPR-IB) and its expression in prostate cancer in comparison with other BMPRs. Oncogene 1997;14 (11):1377–82. [DOI] [PubMed] [Google Scholar]
- 63.Kang MH, Kim JS, Seo JE, et al. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res 2010;316 (1):24–37. [DOI] [PubMed] [Google Scholar]
- 64.Kang MH, Oh SC, Lee HJ, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-(B pathway, and MMP-9 expression. Exp Cell Res 2011;317 (12):1746–62. [DOI] [PubMed] [Google Scholar]
- 65.Kudo N, Ogose A, Ariizumi T, et al. Expression of bone morphogenetic proteins in giant cell tumor of bone. Anticancer Res 2009;29 (6):2219–25. [PubMed] [Google Scholar]
- 66.Langenfeld EM, Bojnowski J, Perone J, et al. Expression of bone morphogenetic proteins in human lung carcinomas. Ann Thorac Surg 2005;80 (3):1028–32. [DOI] [PubMed] [Google Scholar]
- 67.Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res 2003;63 (2):277–81. [PubMed] [Google Scholar]
- 68.Raida M, Clement JH, Ameri K, et al. Expression of bone morphogenetic protein 2 in breast cancer cells inhibits hypoxic cell death. Int J Oncol 2005;26 (6):1465–70. [PubMed] [Google Scholar]
- 69.Jiang S, Fritz DT, Rogers MB. A conserved post-transcriptional BMP2 switch in lung cells. J Cell Biochem 2010;110 (2):509–21. [DOI] [PubMed] [Google Scholar]
- 70.Kiyozuka Y, Nakagawa H, Senzaki H, et al. Bone morphogenetic protein-2 and type IV collagen expression in psammoma body forming ovarian cancer. Anticancer Res 2001;21 (3B): 1723–30. [PubMed] [Google Scholar]
- 71.Lee KB, Murray SS, Duarte ME, et al. Effects of the bone morphogenetic protein binding protein spp24 (secreted phosphoprotein 24 kD) on the growth of human lung cancer cells. J Orthop Res 2011;29 (11):1712–8. [DOI] [PubMed] [Google Scholar]
- 72.Steinert S, Kroll TC, Taubert I, et al. Differential expression of cancer-related genes by single and permanent exposure to bone morphogenetic protein 2. J Cancer Res Clin Oncol 2008;134 (11):1237–45. [DOI] [PubMed] [Google Scholar]
- 73.Park Y, Kim JW, Kim DS, et al. The Bone Morphogenesis Protein-2 (BMP-2) is associated with progression to metastatic disease in gastric cancer. Cancer Res Treat 2008;40 (3):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soares AF, Xavier RL, da Costa Miguel MC, et al. Bone morphogenetic protein-2/4 and bone morphogenetic protein receptor type IA expression in metastatic and nonmetastatic oral squamous cell carcinoma. Am J Otolaryngol 2010;31 (4): 266–71. [DOI] [PubMed] [Google Scholar]
- 75.Bobinac D, Maric I, Zoricic S, et al. Expression of bone morphogenetic proteins in human metastatic prostate and breast cancer. Croat Med J 2005;46 (3):389–96. [PubMed] [Google Scholar]
- 76.Komai Y, Morimoto S, Saito K, et al. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol 2006;13 (8):1126–8. [DOI] [PubMed] [Google Scholar]
- 77.Davies SR, Watkins G, Douglas-Jones A, et al. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol 2008;7 (4):327–38. [PubMed] [Google Scholar]
- 78.Imai N, Iwai A, Hatekeyama S, et al. Expression of bone morphogenetic proteins in colon carcinoma with heterotopic ossification. Pathol Int 2001;51 (8):643–8. [DOI] [PubMed] [Google Scholar]
- 79.Bieniasz M, Oszajca K, Eusebio M, et al. The positive correlation between gene expression of the two angiogenic factors: VEGF and BMP-2 in lung cancer patients. Lung Cancer 2009;66 (3):319–26. [DOI] [PubMed] [Google Scholar]
- 80.Zhao M, Takata T, Ogawa I, et al. Immunohistochemical demonstration of bone morphogenetic protein-2 and type II collagen in pleomorphic adenoma of salivary glands. J Oral Pathol Med 1998;27 (7):293–6. [DOI] [PubMed] [Google Scholar]
- 81.Mehdi R, Shimizu T, Yoshimura Y, et al. Expression of bone morphogenetic protein and its receptors in osteosarcoma and malignant fibrous histiocytoma. Jpn J Clin Oncol 2000;30 (6):272–5. [DOI] [PubMed] [Google Scholar]
- 82.Horvath LG, Henshall SM, Kench JG, et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate 2004;59 (3):234–42. [DOI] [PubMed] [Google Scholar]
- 83.Doak SH, Jenkins SA, Hurle RA, et al. Bone morphogenic factor gene dosage abnormalities in prostatic intraepithelial neoplasia and prostate cancer. Cancer Genet Cytogenet 2007;176 (2):161–5. [DOI] [PubMed] [Google Scholar]
- 84.Choi YJ, Kim ST, Park KH, et al. The serum bone morphogenetic protein-2 level in non-small-cell lung cancer patients. Med Oncol 2012;29 (2):582–8. [DOI] [PubMed] [Google Scholar]
- 85.Le Page C, Ouellet V, Madore J, et al. Gene expression profiling of primary cultures of ovarian epithelial cells identifies novel molecular classifiers of ovarian cancer. Br J Cancer 2006;94 (3):436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao Q, Tong W, Luria JS, et al. Effects of bone morphogenetic protein-2 on proliferation and angiogenesis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2010;39 (3):266–71. [DOI] [PubMed] [Google Scholar]
- 87.Hung TT, Wang H, Kingsley EA, et al. Molecular profiling of bladder cancer: involvement of the TGF-beta pathway in bladder cancer progression. Cancer Lett 2008;265 (1):27–38. [DOI] [PubMed] [Google Scholar]
- 88.Hatakeyama S, Ohara-Nemoto Y, Kyakumoto S, et al. Expression of bone morphogenetic protein in human adenocarcinoma cell line. Biochem Biophys Res Commun 1993;190 (3):695–701. [DOI] [PubMed] [Google Scholar]
- 89.Kusafuka K, Yamaguchi A, Kayano T, et al. Expression of bone morphogenetic proteins in salivary pleomorphic adenomas. Virchows Arch 1998;432 (3):247–53. [DOI] [PubMed] [Google Scholar]
- 90.Katsuno Y, Hanyu A, Kanda H, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 2008;27 (49):6322–33. [DOI] [PubMed] [Google Scholar]
- 91.Liu F, Bloch N, Bhushan KR, et al. Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. Mol Imaging 2008;7 (4):175–86. [PMC free article] [PubMed] [Google Scholar]
- 92.Kokorina NA, Lewis JS Jr, Zakharkin SO, et al. rhBMP-2 has adverse effects on human oral carcinoma cell lines in vivo. Laryngoscope 2012;122 (1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreau JE, Anderson K, Mauney JR, et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res 2007;67 (21):10304–8. [DOI] [PubMed] [Google Scholar]
- 94.Lee Y, Schwarz E, Davies M, et al. Differences in the cytokine profiles associated with prostate cancer cell induced osteoblastic and osteolytic lesions in bone. J Orthop Res 2003;21 (1):62–72. [DOI] [PubMed] [Google Scholar]
- 95.Langenfeld E, Deen M, Zachariah E, et al. Small molecule antagonist of the bone morphogenetic protein type I receptors suppresses growth and expression of Id1 and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer 2013;12 (1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Langenfeld E, Hong CC, Lanke G, et al. Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS One 2013;8 (4):e61256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sand JP, Kokorina NA, Zakharkin SO, et al. BMP-2 Expression correlates with local failure in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 2014;150 (2):245–50. [DOI] [PubMed] [Google Scholar]
- 98.Guo M, Jiang Z, Zhang X, et al. miR-656 inhibits glioma tumorigenesis through repression of BMPR1A. Carcinogenesis 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 99.Rubio R, Abarrategi A, Garcia-Castro J, et al. Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 100.Fei ZH, Yao CY, Yang XL, et al. Serum BMP-2 up-regulation as an indicator of poor survival in advanced non-small cell lung cancer patients. Asian Pac J Cancer Prev 2013;14 (9):5293–9. [DOI] [PubMed] [Google Scholar]
- 101.Laperrousaz B, Jeanpierre S, Sagorny K, et al. Primitive CML cell expansion relies on abnormal levels of BMPs provided by the niche and on BMPRIb overexpression. Blood 2013;122 (23):3767–77. [DOI] [PubMed] [Google Scholar]
- 102.Takeda M, Mikami T, Numata Y, et al. Papillary thyroid carcinoma with heterotopic ossification is a special subtype with extensive progression. Am J Clin Pathol 2013;139 (5):587–98. [DOI] [PubMed] [Google Scholar]
- 103.Yang X, Wang YP, Liu FX, et al. Increased invasiveness of osteosarcoma mesenchymal stem cells induced by bone-morphogenetic protein-2. In Vitro Cell Dev Biol Anim 2013;49 (4): 270–8. [DOI] [PubMed] [Google Scholar]
- 104.Mu X, Isaac C, Schott T, et al. Rapamycin Inhibits ALDH Activity, Resistance to Oxidative Stress, and Metastatic Potential in Murine Osteosarcoma Cells. Sarcoma 2013;2013:480713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neman J, Choy C, Kowolik CM, et al. Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin Exp Metastasis 2013;30 (6):753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye XY, Niu XM, Tang NW, et al. Adenovirus mediated knockdown of bone morphogenetic protein 2 inhibits human lung cancer growth and invasion in vitro and in vivo. Int J Immunopathol Pharmacol 2012;25 (4):967–76. [DOI] [PubMed] [Google Scholar]
- 107.Ye S, Park BH, Song KJ, et al. In vivo inhibition of bone morphogenetic protein-2 on breast cancer cell growth. Spine 2013;38 (3):E143–150. [DOI] [PubMed] [Google Scholar]
- 108.Yang ZJ, Liu FX, Yang YS, et al. Expression of bone-morphogenetic protein 2 and tumor necrosis factor ( correlates with bone metastases in bladder urothelial carcinoma. Ann Diagn Pathol 2013;17 (1):51–3. [DOI] [PubMed] [Google Scholar]
- 109.Chen A, Wang D, Liu X, et al. Inhibitory effect of BMP-2 on the proliferation of breast cancer cells. Mol Med Rep 2012;6 (3):615–20. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, Ge Y, Sun L, et al. Effect of bone morphogenetic protein-2 on proliferation and apoptosis of gastric cancer cells. Int J Med Sci 2012;9 (2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rici RE, Alcantara D, Fratini P, et al. Mesenchymal stem cells with rhBMP-2 inhibits the growth of canine osteosarcoma cells. BMC Vet Res 2012;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buijs JT, van der Horst G, van den Hoogen C, et al. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene 2012;31 (17):2164–74. [DOI] [PubMed] [Google Scholar]
- 113.Simmonds MC, Brown JV, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med 2013;158 (12):877–89. [DOI] [PubMed] [Google Scholar]
- 114.Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med 2013;158 (12):890–902. [DOI] [PubMed] [Google Scholar]
- 115.Kelly MP, Savage JW, Bentzen SM, et al. Cancer risk from bone morphogenetic protein exposure in spinal arthrodesis. J Bone Joint Surg Am 2014;96 (17):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blanco Calvo M, Bolós Fernández V, Medina Villaamil V, et al. Biology of BMP signalling and cancer. Clin Transl Oncol 2009;11 (3):126–37. [DOI] [PubMed] [Google Scholar]
- 117.Massague J TGF( in cancer. Cell 2008;134 (2):215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein-2-induced transformation involves the activation of mammalian target of rapamycin. Mol Cancer Res 2005;3 (12):679–84. [DOI] [PubMed] [Google Scholar]
- 119.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene 2005;24 (37):5713–21. [DOI] [PubMed] [Google Scholar]
- 120.Lin H Stem cell: to be or not to be. Nature 2003;425 (6956):353–5. [DOI] [PubMed] [Google Scholar]
- 121.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001;414 (6859):98–104. [DOI] [PubMed] [Google Scholar]
- 122.Thawani JP, Wang AC, Than KD, et al. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery 2010;66 (2):233–46; discussion 246. [DOI] [PubMed] [Google Scholar]
- 123.American Cancer Society: cancer Facts and Figures 2014. Atlanta, Ga: American Cancer Society, 2014. Available at: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf Accessed April 10, 2014. [Google Scholar]
- 124.Ehata S, Yokoyama Y, Takahashi K, et al. Bi-directional roles of bone morphogenetic proteins in cancer: another molecular Jekyll and Hyde?. Pathol Int 2013;63 (6):287–96. [DOI] [PubMed] [Google Scholar]
- 125.Summary of Safety and Effective Data Premarket Approval Application P000058. Rockville, MD: 2002. FDA. InFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion Device; Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf Accessed April 11, 2014. [Google Scholar]
- 126.Devine JG, Dettori JR, France JC, et al. The use of rhBMP in spine surgery: is there a cancer risk?. Evid Based Spine Care J 2012;3 (2):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 2002;27 (16 Suppl 1): S40–48. [DOI] [PubMed] [Google Scholar]
- 128.Wagner O, Schreier E, Heitz F, et al. Tissue distribution, disposition, and metabolism of cyclosporine in rats. Drug Metab Dispos 1987;15 (3):377–83. [PubMed] [Google Scholar]
- 129.Karreth S, Lenk W. The metabolism of 4-aminobipheyl in rat. I. Reaction of N-hydroxy-4-aminobiphenyl with rat blood in vivo. Xenobiotica 1991;21 (3):417–28. [DOI] [PubMed] [Google Scholar]
- 130.Hassan M, Ehrsson H, Wallin I, et al. Pharmacokinetic and metabolic studies of busulfan in rat plasma and brain. Eur J Drug Metab Pharmacokinet 1988;13 (4):301–5. [DOI] [PubMed] [Google Scholar]
- 131.American Cancer Society. Known and Probable Human Carcinogens. Available at: http://www.cancer.org/cancer/cancercauses/othercarcinogens/generalinformationaboutcarcinogens/knownand-probable-human-carcinogens Accessed April 11, 2015.
- 132.United States Food and Drug Administration. InFUSETM Bone Graft/LT-CAGETM Lumbar Tapered Fusion Device – P000058. Summary of Safety and Effectiveness Data. Accessed March 29, 2015 Available at: http://www.fda.gov/ohrms/dockets/dailys/04/June04/060704/04m-0249-aav0001–03-SSED-vol1.pdf.