Abstract
Current noninvasive treatments for tinnitus have shown mixed results. There have been encouraging developments in using invasive brain or vagal nerve stimulation to modulate neural populations driving the tinnitus percept. However, these invasive treatments can only be used in a small patient population with severe conditions. In this preliminary study, we present a new treatment option we call Multimodal Synchronization Therapy (MST), which attempts to achieve synchronized and localized brain activation without invasive neural stimulation. MST combines multiple sensory, motor, limbic, and cognitive inputs to elicit activation of multimodal neurons to potentially modulate specific neurons driving the tinnitus percept. We present preliminary data in a guinea pig model showing activation of somatosensory and auditory pathways to alter neural activity within the inferior colliculus, a multimodal integration region that has shown pathological changes in animals and patients with tinnitus. Electrical stimulation of different body locations induced excitatory responses in the inferior colliculus, eliciting responses in up to 41% of all recording sites for a given somatic site. Paired somatic and acoustic stimulation resulted in enhanced or suppressed acoustic-driven neural activity in the inferior colliculus that varied depending on stimulation and recording location. Similar modulation effects were observed in the auditory cortex, which may relate to changes in auditory perception. Further studies need to incorporate multiple multimodal pathways and must also confirm that MST can suppress the abnormal neural patterns that directly drive the tinnitus percept.
I. Introduction
Tinnitus, a neurological disorder resulting in a phantom sound generated within the brain in the absence of an external sound source, affects about 250 million people worldwide and is debilitating for about 1% of the world population [1, 2]. Tinnitus is caused by abnormal neural plasticity (changes in the properties of neurons) that occurs throughout the auditory system, and has been linked to tonotopic reorganization, hyperactivity, hyper-synchrony across neurons, and altered spiking patterns [2–5]. Currently, noninvasive treatments show mixed results across patients [1, 2, 6–9]. There have been recent developments in using invasive cortical, deep brain, and vagal nerve stimulation to directly modulate the neurons driving the tinnitus percept [7, 10–12]. However, these treatments are available only to a limited subset of patients due to the surgical risks and costs associated with device implantation.
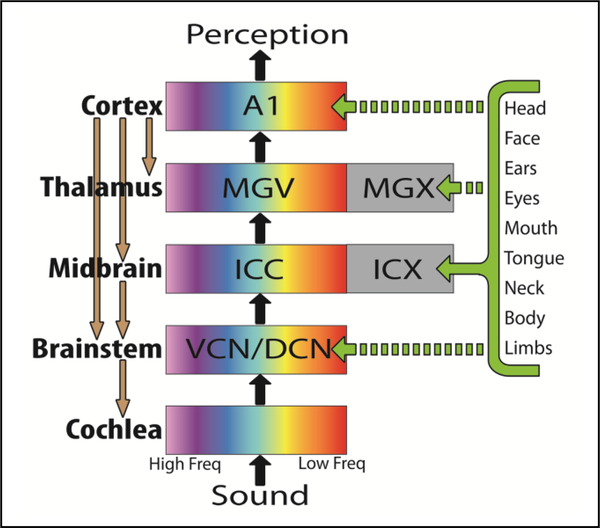
We propose a new tinnitus treatment using noninvasive brain activation that targets specific neural populations in the auditory system through multimodal integration. Previous studies have reported that tinnitus can be modulated through manipulations of the eyes, head, neck, jaw, and shoulders [2, 13, 14], which is consistent with the existence of multimodal integration across motor and sensory pathways [15, 16]. Other studies have identified somatosensory, visual, motor, limbic, and cognitive inputs into the auditory system, including the inferior colliculus (IC) [17–24]. For example, Fig. 1 shows a simplified schematic of somatosensory projections to the external region of the inferior colliculus (ICX) that interact with neurons in the central nucleus of the inferior colliculus (ICC), part of the core auditory pathway. We hypothesize that artificially activating these different multimodal pathways with the appropriate timing will elicit synchronized activation and neural plasticity within specific neural populations in the auditory system. Auditory plasticity has been achieved when co-activating auditory and somatosensory pathways with specific timing [24, 25].
Figure 1.
A simplified schematic of the ascending and descending auditory system. Only some regions and projections relevant for this paper are included. The VCN (ventral cochlear nucleus), DCN (dorsal cochlear nucleus), ICC (central nucleus of inferior colliculus), and MGV (ventral division of medial geniculate nucleus) are ascending tonotopic nuclei leading to A1 (primary auditory cortex). ICX and MGX are external regions involved with multi-modal processing. MST will take advantage of the somatosensory pathways that project to the auditory midbrain and other regions of the auditory system.
With this approach, which we call Multimodal Synchronization Therapy (MST), we will attempt to suppress abnormal firing patterns by targeting specific auditory neural populations that are driving the tinnitus percept. This paper presents our initial work in animals investigating the multimodal interactions between the somatosensory and auditory pathways. In particular, we investigated the effects of electrical stimulation of different locations across the body on auditory responses within the IC and primary auditory cortex (A1).
II. Methods
Experiments were performed on three young female Hartley guinea pigs (295–410 g; Elm Hill Breeding Labs, Chelmsford, MA) according to the policies of the University of Minnesota Institutional Animal Care and Use Committee. Basic surgical and analysis procedures have been described in detail in previous work [26, 27] and are only briefly described in this paper.
Animals were anesthetized with intramuscular injections of a mixture of ketamine (40 mg/kg) and xylazine (10 mg/kg) with supplements to maintain a non-reflexive state. The animals were fixed in a stereotaxic frame (David Kopf Instruments, CA) and a craniotomy was performed to access the right visual and auditory cortices. A silicon-substrate 32-site electrode array (NeuroNexus Technologies, MI) was inserted into either the right ICC or right ICX of the animal using a hydraulic micro-manipulator (David Kopf Instruments, CA). Electrode placements were determined to be in either the ICC or ICX based on frequency response maps (FRMs) derived from responses to acoustic stimulation. ICC placements were characterized by FRM responses with sharp tuning and a clear tonotopic gradient from the shallow, low frequency sites to deeper, high frequency sites. ICX placements were characterized by FRM responses with broad tuning and a reversed or absent tonotopic representation. An additional 32-site array was sometimes inserted into the right primary auditory cortex (A1) depending on the experiment. All recording electrode placements were posthumously verified through histological examination of electrode tracts and three-dimensional computer reconstructions [26]. Subcutaneous needle electrodes were inserted into specific somatic stimulation sites including the neck, shoulders, back, and rear thighs of the animal. An additional needle electrode was placed on the surface of the tongue and a ball electrode was inserted into the animal’s genital area. To ensure consistent stimulation locations across animals, we used defined relations to anatomical landmarks when placing them under the skin (e.g. immediately posterior to shoulder joints).
Experiments were performed inside a sound attenuating, electrically-shielded booth. All acoustic stimulation was presented to the animal's left ear canal using a speaker coupled to a custom-made hollow ear bar calibrated with a 0.25 in condenser microphone (ACO Pacific, CA). Using custom software coded in Matlab (Mathworks, MA) and TDT hardware (Tucker-Davis Technology, FL), multi-unit neural activity was recorded and sampled at a rate of 25 kHz, passed through analog DC-blocking and anti-aliasing filters from 1.6 to 7.5 kHz, and digitally filtered between 0.3 and 3 kHz for spike analysis. Spikes were identified as voltages exceeding 3.5 times the standard deviation of the noise floor. Acoustic stimulation included either pure tones or broadband noise. Pure tones (1–40 kHz at 8 steps per octave; 0–70 dB SPL in 10 dB steps; 50 ms duration with 5 ms rise/fall ramp time) were used to obtain FRMs. Electrical stimulation of the somatic sites consisted of single biphasic, charge-balanced pulses (205 μs/phase, cathodic-leading) at 560 μA, which was the maximum level that elicited activation within the ICC and ICX without causing any muscle twitching across animals. Each of eight somatic sites were stimulated in a randomized pattern for 50 trials at a rate of 2/s. Post-stimulus time histograms (PSTHs) for each of the ICC and ICX recording sites in response to somatic stimulation were plotted for further analysis. Additionally, paired stimulation consisting of somatic stimulation of one body region (same parameters as above) and acoustic broadband noise (50 dB SPL; 50 ms duration with 0.5 ms rise/fall ramp time) was also performed. The ICC, ICX, and A1 responses to paired stimulation were compared to responses to acoustic-only stimulation (100 trials at 2/s for each) to assess the effects of somatic activation. For all cases, an unequal variance two-tailed ranked t-test (p<0.05) was used to confirm a significant change in activity between paired and acoustic-only stimulation based on total spike rate [28].
III. Results & Discussion
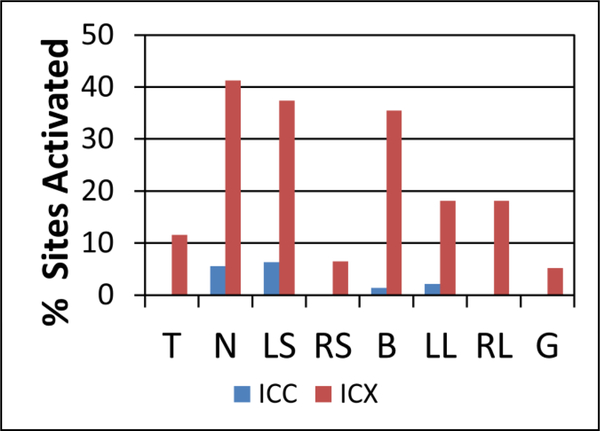
Somatic stimulation alone elicited responses predominately in the ICX along with a few locations in the ICC, as shown in Fig. 2. This is consistent with previous studies [20, 29]. Each recording site was determined to be in ICC or ICX based on FRMs, and sites that were on the ICC/ICX border or difficult to classify were not included. Some somatic stimulation sites yielded responses in a large fraction of the ICX recording locations, with neck stimulation having the highest percentage at 41%. Differences in activation thresholds and patterns were found depending on the somatic site being stimulated. Some regions in the ICX were activated by more than one somatic site at a level of 560 μA (red bars in Fig. 2 sum to over 100%). Also, single recording sites in the IC had different current thresholds depending on which somatic site was stimulated (results not shown).
Figure 2.
Activation of the ICC and ICX in response to somatic stimulation alone. The percentages of ICC (n=143) and ICX (n=155) recording sites that showed excitatory activity in response to each stimulated somatic site at 560 μA are presented. For all stimulation sites, a higher percentage of ICX recording sites showed excitatory responses than ICC recording sites. All percentages represent the number of sites in a given region that showed activity divided by the total number of recording sites in that region. Abbreviations: T-tongue, N-neck, LS-left shoulder, RS-right shoulder, B-back, LL-left hind leg, RL-right hind leg, G-genital area.
The results in Fig. 2 illustrate that somatic stimulation can modulate many neurons in the auditory midbrain, including the ICC within the core auditory pathway, which is encouraging for MST implementation. It is possible that somatosensory activation of the ICC occurs through polysynaptic pathways via the ICX or through other auditory regions that then project to the ICC (Fig. 1). It is also possible that somatosensory activation of the ICC occurs through non-auditory cortical regions that project to the auditory cortex and down to the ICC [21, 30, 31]. By investigating additional current levels closer to the activation threshold for each recording site, we can further assess if it is possible to locally activate regions within the ICC and ICX, which could enable MST to target specific neural populations driving the tinnitus percept.
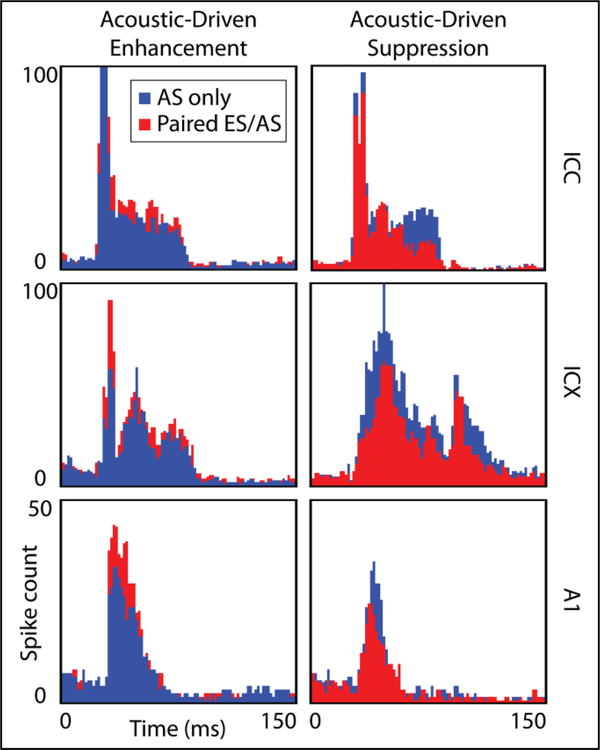
Paired stimulation resulted in a wide range of response patterns across recording locations and somatic stimulation sites. PSTH examples of both paired and acoustic-only stimulation for two different locations in the ICX, ICC, and A1 are presented in Fig. 3. Somatic stimulation induced suppression and enhancement of acoustic-driven activity at various sites in both the ICC and ICX. Summarization of the different patterns and their dependence on recording and somatic stimulation location is currently in progress. Similar to the effects in the midbrain, we observed enhanced and suppressed activity in A1 depending on the recording and stimulation locations. These results demonstrate that somatic stimulation not only elicits excitatory responses in the IC, but can also modulate acoustic-driven activity in A1 either via the ascending auditory pathway or through other non-auditory pathways. The suppression results in Fig. 3 are encouraging for MST implementation considering that tinnitus has been linked to hyperactivity across the auditory midbrain. Somatic stimulation combined with acoustic stimulation could potentially suppress this hyperactivity and reduce or eliminate the tinnitus percept.
Figure 3.
Comparison of paired stimulation and acoustic-only stimulation responses for six PSTHs in ICC, ICX, and A1. Unequal variance two-tailed ranked t-tests were used to test significance. Paired stimulation can result in enhancement (left) and suppression (right) of acoustic-driven activity. A bin width of 1 ms was used. The ICC examples show a 16% increase in total spike rate for enhancement (p=1.2 × 10−5) and a 27% decrease for suppression of acoustic-driven activity (p=2.9 × 10−15). The ICX and A1 examples show differences of 25% (p=9.4 × 10−4) and 31% (p=9.4 × 10−4) for enhancement and 33% (p=3.9 × 10−28) and 24% (p=1.4 × 10−2) for suppression, respectively. AS: acoustic stimulation, ES: electrical stimulation.
There are still several questions that need to be investigated to assess if MST can in fact suppress pathological neural activity related to the tinnitus percept. The preliminary results presented in this paper provide initial neurophysiological evidence that somatic stimulation across the body can modulate and suppress activity within the auditory system. However, tinnitus can be associated with different types of abnormal patterns (e.g., varying temporal/bursting patterns and hypersynchrony) that only occur in a subset of neurons [2–5]. The ability to appropriately modulate specific neurons in the IC and A1 would be required of MST in order to target particular neural populations with abnormal firing patterns driving the tinnitus percept. We are currently investigating if a somatotopic map exists within the IC that could enable systematic activation of specific auditory neurons. In particular, we would attempt to stimulate multiple body locations with appropriate timing among sites to cause synchronized activation of a subset of multimodal neurons within this somatotopic map. An investigation of latencies for different somatic sites and for different IC recording areas would reveal appropriate stimulation sites and timing parameters to use for MST. We will also investigate visual, motor, limbic, and cognitive inputs as additional multimodal integration sources. Additionally, MST must be able to induce long-term changes such that continuous treatment would not be required to suppress tinnitus. Particularly, we need to demonstrate that spontaneous (not just acoustic-driven) spiking activity of neurons can be altered and neural networks can be desynchronized using MST since those features have been linked to tinnitus. Following these investigations, we must demonstrate that this alteration of neural activity directly corresponds to the elimination of the tinnitus percept. This demonstration can be achieved through MST experiments in tinnitus animal models, utilizing behavioral testing for changes in tinnitus perception [25, 32]. Since MST is noninvasive and can use electrical and acoustic stimulators already approved for human use, clinical trials can also be conducted with tinnitus patients. These questions are currently being considered and investigated by our research group as we continue to develop MST as a treatment option for tinnitus. We will also consider MST as a potential treatment for other neurological disorders that consist of abnormal but reversible brain patterns (e.g., phantom limb pain and motor tremors).
Acknowledgments
The authors thank Malgorzata Straka for assistance with experimental protocols and manuscript revisions. They also thank Sarah Offutt for assistance with manuscript revisions and figure generation.
Research was supported by University of Minnesota and Institute for Translational Neuroscience start-up funds, NIH NIDCD 1R03-DC011589, MD5M Hearing Foundation, NSF IGERT DGE-1069104, and the Frieda Martha Kunze Fellowship.
Contributor Information
Cory D. Gloeckner, The authors are with the Department of Biomedical Engineering, University of Minnesota, MN 55455 USA
Benjamin T. Smith, The authors are with the Department of Biomedical Engineering, University of Minnesota, MN 55455 USA
Craig D. Markovitz, The authors are with the Department of Biomedical Engineering, University of Minnesota, MN 55455 USA
Hubert H. Lim, The authors are with the Department of Biomedical Engineering, University of Minnesota, MN 55455 USA; Institute for Translational Neuroscience and the Department of Otolaryngology at the University of Minnesota.
References
- [1].ATA, "American Tinnitus Association," http://www.ata.org/research/resources-researchers.
- [2].Moller AR, Langguth B, De Ridder D, and Kleinjung T, Eds., Textbook of Tinnitus. New York: Springer Science+Business Media, LLC; 2011. [Google Scholar]
- [3].Eggermontand JJ, Roberts LE,"Theneuroscienceof tinnitus," Trends Neurosci, vol. 27, pp. 676–82, November 2004. [DOI] [PubMed] [Google Scholar]
- [4].Bauer CA, Turner JG, Caspary DM, Myers KS, and Brozoski TJ, "Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma," J Neurosci Res, vol. 86, pp. 2564–78, August 15 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lanting CP, de Kleine E, and van Dijk P, "Neural activity underlying tinnitus generation: results from PET and fMRI," Hear Res, vol. 255, pp. 1–13, September 2009. [DOI] [PubMed] [Google Scholar]
- [6].Tass PA, Adamchic I, Freund HJ, von Stackelberg T, and Hauptmann C, "Counteracting tinnitus by acoustic coordinated reset neuromodulation," Restor Neurol Neurosci, vol. 30, pp. 137–59, 2012. [DOI] [PubMed] [Google Scholar]
- [7].Vanneste S and De Ridder D, "Noninvasiveandinvasive neuromodulation for the treatment of tinnitus: an overview," Neuromodulation, vol. 15, pp. 350–60, July 2012. [DOI] [PubMed] [Google Scholar]
- [8].Vanneste S, van Dongen M, De Vree B, Hiseni S, van der Velden E, Strydis C, et al. , "Does enriched acoustic environment in humans abolish chronic tinnitus clinically and electrophysiologically? A double blind placebo controlled study," Hear Res, vol. 296, pp. 141–8, February 2013. [DOI] [PubMed] [Google Scholar]
- [9].Hobson J, Chisholm E, and El Refaie A, "Sound therapy (masking) in the management of tinnitus in adults," Cochrane Database Syst Rev, vol. 11, p. CD006371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Ridder D, Vanneste S, Kovacs S, Sunaert S, Menovsky T, van de Heyning P, et al. , "Transcranial magnetic stimulation and extradural electrodes implanted on secondary auditory cortex for tinnitus suppression," J Neurosurg, vol. 114, pp. 903–11, April 2011. [DOI] [PubMed] [Google Scholar]
- [11].Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. , "Reversing pathological neural activity using targeted plasticity," Nature, vol. 470, pp. 101–4, February 3 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheungand SW, Larson PS, "Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC)," Neuroscience, vol. 169, pp. 1768–78, September 15 2010. [DOI] [PubMed] [Google Scholar]
- [13].Simmons R, Dambra C, Lobarinas E, Stocking C, and Salvi R, "Head, Neck, and Eye Movements That Modulate Tinnitus," Semin Hear, vol. 29, pp. 361–370, November 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levine RA,Nam EC, Oron Y, and Melcher JR, "Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities," Prog Brain Res, vol. 166, pp. 195–207, 2007. [DOI] [PubMed] [Google Scholar]
- [15].Dehmel S, Cui YL, and Shore SE, "Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness," Am J Audiol, vol. 17, pp. S193–209, December 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huffman RF and Henson OW Jr., "The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus," Brain Res Brain Res Rev, vol. 15, pp. 295–323, Sep-Dec 1990. [DOI] [PubMed] [Google Scholar]
- [17].Gruters KG and Groh JM, "Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus," Front Neural Circuits, vol. 6, p. 96, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hurley LM and Sullivan MR, "From behavioral context to receptors: serotonergic modulatory pathways in the IC," Front Neural Circuits, vol. 6, p. 58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schofield BR, Motts SD, and Mellott JG, "Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex," Hear Res, vol. 279, pp. 85–95, September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aitkin LM, Kenyon CE, and Philpott P, "The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus," J Comp Neurol, vol. 196, pp. 25–40, February 10 1981. [DOI] [PubMed] [Google Scholar]
- [21].Winer JA, "Decoding the auditory corticofugal systems," Hear Res, vol. 212, pp. 1–8, February 2006. [DOI] [PubMed] [Google Scholar]
- [22].Marsh RA, Fuzessery ZM, Grose CD, and Wenstrup JJ, "Projection to the inferior colliculus from the basal nucleus of the amygdala," J Neurosci, vol. 22, pp. 10449–60, December 1 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ledoux JE, Ruggiero DA, Forest R, Stornetta R, and Reis DJ, "Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat," J Comp Neurol, vol. 264, pp. 123–46, October 1 1987. [DOI] [PubMed] [Google Scholar]
- [24].Basura GJ, Koehler SD, and Shore SE, "Multi-sensory integration in brainstem and auditory cortex," Brain Res, vol. 1485, pp. 95–107, November 16 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dehmel S, Pradhan S, Koehler S, Bledsoe S, and Shore S, "Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity?," in J Neurosci. vol. 32, ed United States, 2012, pp. 1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Markovitz CD, Tang TT, Edge DP, and Lim HH, "Three-dimensional brain reconstruction of in vivo electrode tracks for neuroscience and neural prosthetic applications," Frontiers in Neural Circuits, vol. 6, 2012-June-27 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Limand HH, Anderson DJ, "Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant," J Neurophysiol, vol. 96, pp. 975–88, September 2006. [DOI] [PubMed] [Google Scholar]
- [28].Ruxton GD,"The unequal variancet-test is an underused alternative to Studenťs t-test and the Mann–Whitney U test," Behavioral Ecology, vol. 17, pp. 688–690, 2006. [Google Scholar]
- [29].Jain R and Shore S, "External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig," Neurosci Lett, vol. 395, pp. 71–5, February 27 2006. [DOI] [PubMed] [Google Scholar]
- [30].Lemus L, Hernandez A, Luna R, Zainos A, and Romo R, "Do sensory cortices process more than one sensory modality during perceptual judgments?," Neuron, vol. 67, pp. 335–48, July 29 2010. [DOI] [PubMed] [Google Scholar]
- [31].King AJ and Walker KM, "Integrating information from different senses in the auditory cortex," Biol Cybern, vol. 106, pp. 617–25, December 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Turner JG,"Behavioral measures of tinnitus in laboratory animals," Prog Brain Res, vol. 166, pp. 147–56, 2007. [DOI] [PubMed] [Google Scholar]