Abstract
During the electron transfer through the cytochrome bc1 complex (ubiquinol-cytochrome c oxidoreductase or complex III), protons are translocated across the membrane, and production of superoxide anion radicals is observed. The bc1 complex is purified from broken mitochondrial preparation prepared from frozen heart muscles by repeated detergent solubilization and salt fractionation. The electron transfer of the purified complex is determined spectrophotometrically. The activity depends on the choice of detergent, protein concentration, and ubiquinol derivatives used. The proton translocation activity of 2H+/e− is determined in the reconstituted bc1-PL vesicles. The production by bc1 is determined by measuring the chemiluminescence of the 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazol[1,2-1]pyrazin-3-one hydro-chloride (MCLA)- adduct during a single turnover of bc1 complex, with the Applied Photophysics stopped-flow reaction analyzer SX.18MV, by leaving the excitation light source off and registering the light emission. Production of by bc1 is in an inverse relationship to its electron transfer activity. Inactivation of the bc1 complex by incubating at elevated temperature (37°C) or by treatment with proteinase K results in an increase in -generating activity to the same level as that of the antimycin A–inhibited complex. These results suggest that the structural integrity of protein subunits is not required for -generating activity in the bc1 complex.
1. INTRODUCTION
The mitochondrial electron transport chain is a major intracellular source of superoxide anion radical () production (Chance et al., 1979). The cytochrome bc1 complex (ubiquinol: cytochrome c oxidoreductase, or complex III) has been identified as one of the major production sites in the mitochondrial respiratory chain (Mclennan and Esposti, 2000; Turrens and Boeris, 1980; Turrens et al., 1985). The cytochrome bc1 complex (bc1) is a multisubunit integral membrane protein complex that catalyzes electron transfer from ubiquinol to cyt c with concomitant translocation of protons across the membrane to generate a membrane potential and proton gradient for ATP synthesis (Trumpower and Gennis, 1994). This complex has been purified and its 3-D structure determined (Iwata et al., 1998; Xia et al., 1997, 2007).
The purified bovine bc1 complex is in a dimeric and oxidized form. Each monomer contains a full complement of all 11 protein subunits (3 redox subunits and 8 supernumerary subunits) with a slight excess of cyt c1. Three redox subunits are essential and found in the bc1 complex from different species: the cyt b subunit housing hemes bL and bH (low and high potential hemes), the cyt c1 subunit containing a heme c1, and the iron sulfur protein (ISP) housing a high potential 2Fe-2S cluster. All additional subunits, referred to as supernumerary subunits, are believed to contribute to the increased stability of these complexes (Ljungdahl et al., 1987; Yu et al., 1999). The absorption ratio of Soret over UV is approximately 0.95 in the purified preparation compared with that in crystalline form of 0.88 (Yue et al., 1991). The purified complex catalyzes electron transfer from ubiquinol to cyt c with a specific activity of 24 μmol cyt c reduced per nmol cyt b at room temperature, pH 7.4, with 2,3-dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol (Q0C10BrH2) as the substrate. When the complex is embedded in phospholipid vesicles, it translocates 2 H+ per electron transferred. This preparation is very stable; it lasts for days at 0° or months at −80 °C without activity loss. Thus, it is suitable for crystallization in the presence or absence of inhibitors. The crystals grown from this preparation diffracted X-rays up to 2.1 Å resolution at best (Esser et al., 2006).
Production of during electron transfer through the bc1 complex is thought to result from leakage of electrons from their normal pathways to react with molecular oxygen. Under normal catalytic conditions, only a very small number of electrons leak from the complex to form (Sun and Trumpower 2003; Zhang et al., 1998). This -generating activity increases when the electron transfer is blocked by antimycin or when the electron transport chain becomes overreduced (Zhang et al., 1998). The electron leakage (or superoxide production) site has been speculated at ubisemiquinone of the Qp site (Dröse and Brandt, 2008; Muller et al., 2003) or reduced cytochrome bL (Nohl and Jordan, 1986; Yang et al., 2008), depending on the mechanism by which bifurcation of ubiquinol proceeds in the Q-cycle model (Brandt and Trumpower, 1994; Crofts, 2004; Mitchell, 1976; Yu et al., 2008). If bifurcation of quinol at the Qp site proceeds by the sequential mechanism, semiquinone formed at the Qp site (Cape et al., 2007; De Vries et al., 1981) and reduced heme bL would both be the electron leakage sites during bc1 catalysis. If bifurcation of ubiquinol at the Qp site proceeds by the concerted mechanism (Snyder et al., 2000; Zhu et al., 2007), reduced heme bL would be the only electron leakage site.
This chapter describes methods for measuring the electron transfer, proton translocation, and production activities in the cytochrome bc1 complex. In addition, a large-scale preparation of the cytochrome bc1 complex from frozen heart muscles is described. A reversed relationship between electron transfer activity and production activity in the bc1 complex is established.
2. MATERIALS
Fresh beef hearts were obtained from Wellington Quality Meat Company at Wellington, Kansas; sodium cholate, deoxycholic acid, cytochrome c (horse heart, type III), superoxide dismutase, antimycin A, valinomycin, and asolectin were from Sigma. Proteinase K was from Invitrogen. MCLA was from Molecular Probes Inc. n-Dodecyl-β-D-maltopyranoside (DM) was from Antrace. 2,3-Dimethoxy-5-methyl-6-geranyl-1,4-benzoquinol (Q2H2), 2,3-dimethoxy-5-methyl-6-isoprenyl-1,4-benzoquinol (Q1H2), 2,3-dimethoxy-5-methyl-6-heptyl-1,4-benzoquinol (Q0C7H2), 2,3-dimethoxy-5-methyl-6-decyl-1,4-benzoquinol (Q0C10H2), and 2,3-dimethoxy-5-methyl-6-(10-bromodecyl)-1,4-benzoquinol (Q0C10BrH2) were prepared as previously reported (Yu and Yu, 1982). The His6-tagged four-subunit wild-type complex (Tian et al., 1998), the three-subunit core complex (the complex lacking subunit IV) (Tso et al., 2000), and the [ISP (H131C, H152C)] mutant complex (the complex lacking the iron-sulfur cluster) (Gurung et al., 2005) from Rhodobacter sphaeroides were prepared according to methods previously reported. All other chemicals were of the highest purity commercially available.
3. PURIFICATION OF THE CYTOCHROME bc1 COMPLEX FROM BOVINE HEART SUBMITOCHONDRIAL PARTICLES
3.1. Preparation of submitochondrial particles (SMP) from frozen bovine heart muscles
Bovine hearts removed from carcasses of the slaughtered animals were immediately immersed in an ice water bath and transported to the laboratory. The fat tissues and vesicular tubes were trimmed off; chunks of neat muscles approximately 1 to 2 inches in size were put into plastic bags, 10 pounds each, and placed in a −20° freezer. A bag of frozen meat was taken out from the freezer and thawed by placing it at room temperature for 2 h and then in the cold room overnight. The thawed meat was passed through a meat grinder in a cold room. The ground meat was divided into portions of 600 g each. Each portion was mixed with 1.8 L of 10 mM K2HPO4 in a Warren blender and homogenized at high speed for two 30-sec periods. A 6 to 10 ml amount of 6 N NaOH was added during blending to maintain the pH at 6.8 to 7.0. The blended mixtures were combined and centrifuged at 1600g for 15 min and the supernatant was poured through an eight-layer cheesecloth. The pellets were resuspended in half of the volume of 20 mM K/Na-phosphate buffer, pH 7.4, and centrifuged at 1600g for 15 min. The supernatants were again collected in the same manner as the first centrifugation step, and all supernatants were combined and divided into two portions. The supernatants were acidified to pH 5.5 with 2 N acetic acid and centrifuged immediately at 3300g for 20 min. The precipitates were immediately washed with a half volume of 20 mM K/Na-phosphate buffer and centrifuged at 3300g for 25 min. The precipitates, referred to as the submitochondrial particles (SMP) or broken mitochondria, were homogenized in 0.1 M phosphate-borate buffer, pH 7.8, to a cyt b concentration of approximately 11 μM (4 to 5 L) and stored in a cold room 3 to 4 days before proceeding to the preparation of succinate: cytochrome c oxidoreductase.
3.2. Preparation of succinate: cytochrome c oxidoreductase from SMP
SMP, aged for 3 to 4 days, were stirred in a cold room for 20 h before 45 ml of a 20% sodium cholate solution was slowly added for every liter of SMP. Pulverized ammonium sulfate (AS), 21.2 g/100 ml of solubilized SMP (37.5% saturation), was slowly added under constant stirring and, after the addition, the stirring continued for another 20 min. The mixture was then centrifuged at 14,000g for 90 min. While the precipitates were saved and stored in a deep freezer (−80°C) for the preparation of cyt c oxidase (Yu et al., 1975), the supernatant solutions were combined, and ammonium sulfate (8.9 g/100 ml) was slowly added under constant stirring. To maintain the pH of the mixture, 5 μl of concentrated ammonium hydroxide was added concurrently for every gram of ammonium sulfate used. The stirring was continued for 20 min before the mixture was centrifuged at 14,000g for 20 min. The resulting precipitates containing crude succinate-cytochrome c oxidoreductase (complexes II and III) were dissolved in PES (50 mM phosphate buffer, pH 7.4, containing 1 mM EDTA and 0.25 M sucrose) buffer (~1/6 vol of the SMP used) to a protein concentration of 25 to 30 mg/ml and stored at 0° for 8 h followed by another centrifugation at 96,000g for 40 min. The precipitates, containing mostly cyt c oxidase, were discarded. The supernatants were combined and dialyzed overnight with one change of buffer against 50 mM Na/K phosphate buffer, pH 7.4, containing 1 mM EDTA. The crude succinate: cyt c oxidoreductase appearing as precipitates in the dialysates were recovered by centrifuging at 28,000g for 30 min and homogenized in 50 mM Tris-HCl buffer, pH 7.2, to a protein concentration of 20 mg/ml. Protein concentration was estimated by optical absorption at 278 nm in 1% SDS using a converting factor of 1OD280 = 0.75 mg/ml. This crude succinate cyt c oxidoreductase can be stored at −80°C for future use or subjected to further purification.
The crude preparation, under constant stirring, was slowly mixed with deoxycholate solution (10%) to 0.35 mg/mg protein. The pH of the mixture was maintained at 7.3 to 7.4 with 1 N NaOH or HCl. The mixture was then subject to ammonium acetate fractionation with a 50% saturated solution. The mixture was brought up to 8.3, 12, 15, and 33% saturation, in steps, by slowly adding saturated ammonium acetate solution. Each fractionation step was followed by stirring for 10 min before the solution was centrifuged for 15 min at 28,000g to remove unwanted precipitates. Purified succinate: cyt c oxidoreductase was recovered in the precipitates of the final fractionation step by centrifuging at 96,000g for 30 min with 40% yield. The purified succinate: cyt c oxidoreductase was dissolved in 50 mM Na/K phosphate buffer, pH 7.4, containing 0.25 M sucrose and 1 mM EDTA to a protein concentration of approximately 20 mg/ml. The preparation was then dialyzed against the same buffer extensively over 2 days with two changes of buffer and stored at −80°C.
3.3. Preparation of cytochrome bc1 particles from succinate: cytochrome c oxidoreductase
The extensively dialyzed, frozen succinate: cyt c oxidoreductase was thawed and diluted with an equal volume of 50 mM Na/K phosphate buffer, pH 7.4, and centrifuged at 158,000g for 60 min. The precipitates were collected and homogenized under an argon atmosphere in 50 mM borate-phosphate buffer, pH 7.8, containing 20 mM succinate. The pH of the suspension was adjusted to 10 with 1 N NaOH and stirred for further 10 min under argon before it was centrifuged at 96,000g for 45 min. The supernatant solution was saved for the preparation of succinate dehydrogenase (Yu and Yu, 1980), and the precipitates were homogenized in borate-phosphate buffer, and the preceding pH 10 treatment was repeated. The precipitates (cyt bc1 particles, yield >90%) were collected and homogenized in 50 mM Tris-HCl buffer, pH 8.0, to a protein concentration of approximately 20 mg/ml.
3.4. Preparation of cyt bc1 complex from cyt bc1 particles
The bc1 particles as prepared previously were partially reduced. To purify a fully oxidized bc1 complex, the homogenate was treated with active cyt c oxidase (1% w/w, Yu et al., 1975) and cyt c (2 μM) at 4°C overnight (or until all cyt c/c1 was oxidized). Potassium deoxycholate (10% solution) was slowly added to the mixture under constant stirring to a ratio of 0.35 mg/mg protein. The solution was centrifuged at 28,000g for 20 min to remove any formed precipitate. The supernatant was then subject to a 10-step ammonium acetate fractionation with 50% saturated solution. The volumes of ammonium acetate solution used for each step were 10, 10, 5, 4, 3.5, 3, 2, 1, 1, and 12% (v/v) of the protein solution, respectively. After each addition, the mixture was stirred continuously at 4°C for 30 min before it was centrifuged to remove any precipitates. The solutions of the first two fractionation steps were centrifuged at 96,000g for 30 min to remove both floating, unwanted fat and precipitates. For steps 3 through 9, the mixtures were centrifuged at 28,000g for 20 min, and for the final step it was centrifuged at 96,000g for 30 min. The purified bc1 complex was recovered in the precipitates with a yield of approximately 40%. The precipitates were dissolved in 50 mM Tris-HCl buffer, pH 8.0, containing 0.66 M sucrose and stored at −80°C until use. This preparation is suitable for protein crystallization. The crystals obtained diffract X-rays to 2.9 Å resolution in the absence of inhibitor (Xia et al., 1997) and improved to 2.1 Å in the presence of inhibitor (Esser et al., 2006).
4. ELECTRON TRANSFER ACTIVITY IN THE PURIFIED bc1 COMPLEX
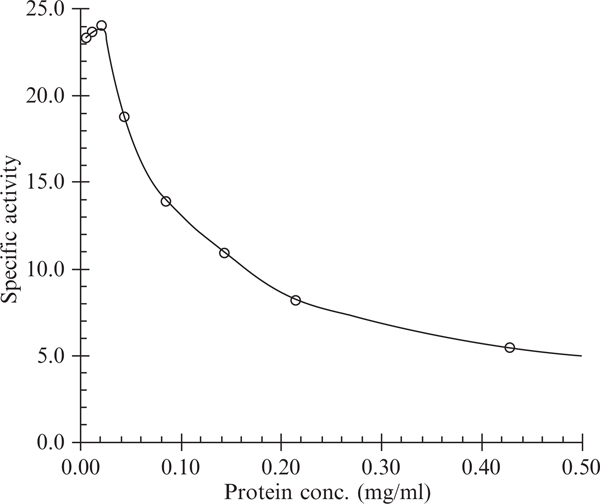
The ubiquinol oxidation activity in purified bc1 is determined by following the reduction of cyt c spectrophotometrically. The assay mixture contains 50 mM Na/K-phosphate buffer, pH 7.4, 100 μM cyt c, 1 mM EDTA, and 25 μM ubiquinol. Diluted cyt bc1 complex (3 to 5 μl) containing 0.1 μM cyt b in the presence of 0.01% DM is added to start the reaction after an initial scan of the mixture for 10 sec at 550 nm for the nonenzymatic reduction of cyt c by the substrate. A difference millimolar extinction coefficient of 18.5 is used for the calculation of cyt c reduction. The substrate, ubiquinol, is prepared by hydrogenation of ubiquinone with Pt-C as catalyst. The ubiquinol stock solution (5 mM) is made in 95% ethanol containing 1 mM HCl. The diluted HCl is used to slow down the autooxidation of substrate. The determination of cyt bc1 activity depends heavily on the physical state of the complex. Proper dilution of the complex in the presence of the right amount of detergent is the key to obtaining the best activity. Fig. 25.1 shows the activity determined under different protein concentrations. As indicated in Table 25.1, ubiquinol and its derivatives give rise to different activities; of all the derivatives tested; Q0C10BrH2 shows the best activity.
Figure 25.1.
Effect of protein concentration on the electron transfer activity measurement of cyt bc1 complex. Cytochrome bc1 complex preparation was dissolved in 50 mM Tris-HCl buffer, pH 8.0, containing 0.66 M sucrose and 0.01% DM at indicated protein concentrations. Three- to five-microliter aliquots were used for activity determination at room temperature. Specific activity is expressed as μmoles cytochrome c reduced/nmol cytochrome b/min.
Table 25.1.
Relative effectiveness of ubiquinol derivatives in the electron transfer reaction of cyt bc1 complex
| Substrate | Relative activity, % |
|---|---|
| Q2H2 | 100 |
| Q1H2 | 35 |
| Q0C7H2 | 65 |
| Q0C10H2 | 105 |
| Q0C10BrH2 | 120 |
5. PROTON TRANSLOCATION IN THE PURIFIED bc1 COMPLEX
Phospholipid (PL) vesicles embedded with cyt bc1 complex were prepared essentially according to the cholate dialysis method (Gurung et al., 2005; Kagawa and Racker, 1971). Asolectin micellar solution was prepared by sonicating 200 mg of acetone-washed asolectin in 4 ml of 50 mM sodium phosphate buffer, pH 7.4, containing 2% sodium cholate and 100 mM KCl in an ice-water batch under an anaerobic environment maintained by continuously passing argon into the vessel. The cyt bc1 complex (1.25 mg) was mixed with 1 ml of asolectin micellar solution to give an asolectin/protein ratio of 40. The bc1 complex-PL mixtures were incubated at 0° for 30 min before overnight dialysis at 4°C against 100 × volumes of 50 mM sodium phosphate buffer, pH 7.4, containing 100 mM KCl with three changes of buffer. The mixture was then dialyzed against 100 × volume of 150 mM KCl (without buffer) for 3 to 4 h.
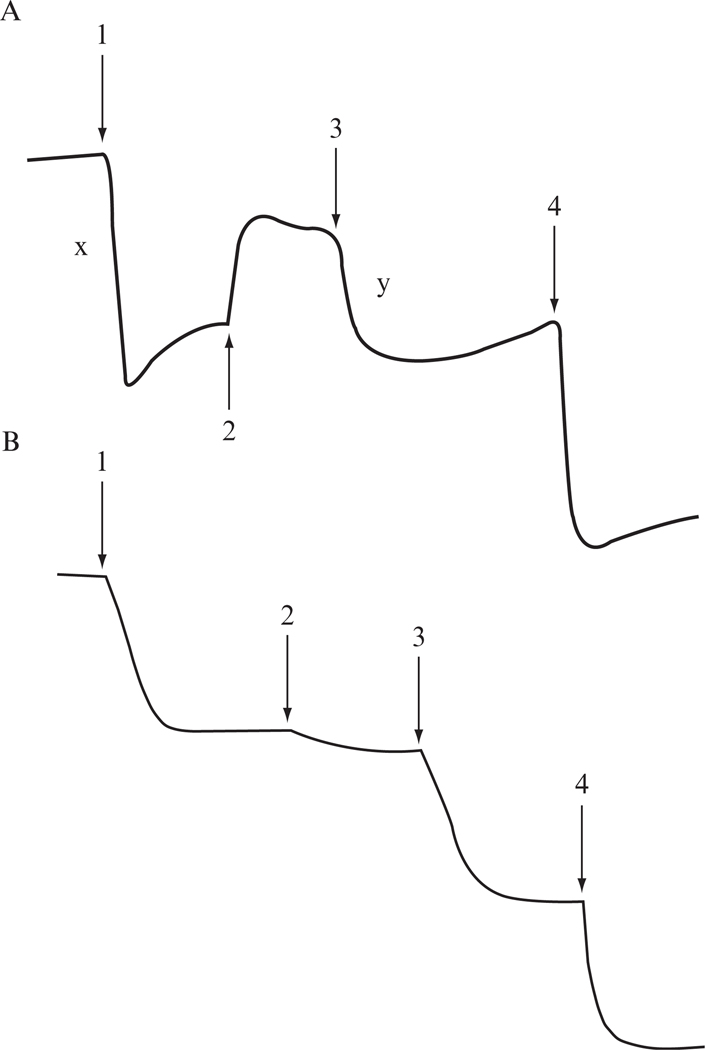
Proton translocation coupled to electron flow through the bc1 complex-PL vesicles is measured at room temperature with an Accumet Model 10 pH meter and a Model 13-620-96 combination pH electrode. Twenty-five nmol of Q0C10BrH2 were added to 1.6 ml reaction mixture containing 150 mM KCl, 4 μM ferricytochrome c, 1 μM valinomycin, and an appropriate amount of bc1-PL vesicles (30 to 50 μl). Electron flow is initiated by the addition of 5 nmol of ferricyanide, which oxidized cyt c, and thus provided an electron acceptor for the complex. Electron flow is also measured in an identical manner except for the presence of the protonophore, m-chloro carbonyl cyanide phenylhydrazone (CCCP), at a concentration of 2 μM, under which the vesicles are permeable to protons and no cross-membrane ΔpH exists. Proton-pumping stoichiometry (H+/e− ) of 2 is calculated as the ratio of the decrease in pH on ferricyanide addition to bc1-PL vesicles before and after treatment with CCCP (Fig. 25.2 A, also Gurung et al., 2005). When proton-pumping activity is determined with PL-vesicles coembedded with bovine bc1 and a R. sphaeroides mutant complex lacking the iron-sulfur cluster [ISP (H131C-H152C)] (Fig. 25.2B), a H+/e− of 1 is obtained, indicating that a proton leakage channel is provided by this mutant complex (Gurung et al., 2005).
Figure 25.2.
Proton-pumping of cyt bc1 complexes embedded in PL vesicles. Measurement of pH change as an indicator for the proton-pumping activity of PL vesicles embedded with (A) mitochondria bc1 complex only, (B) with mitochondrial bc1 complex and the 2Fe-2S cluster lacking mutant complex, H131C-H152C, with ubiquinol as the substrate. Arrows indicate the points of addition of 5 nmol of ferricyanide (1), 3 μM CCCP (2), 5 nmol of ferricyanide (3), and 5 nmol HCl (4). Note: proton-pumping ratio (H+/e− ) = x/y.
6. SUPEROXIDE GENERATION BY THE PURIFIED bc1 COMPLEX
Although the rate of production by the cyt bc1 complex can be determined by measuring the decrease in rate of cyt c reduction in the presence of superoxide dismutase under conditions of continuous turnover of the bc1 complex (Muller et al., 2002), the small rate of formation, compared with the normal rate of cyt c reduction, compromises the accuracy of this method. MCLA has a high sensitivity for in the neutral pH range (Nakano, 1990). The MCLA- chemiluminescence method has been widely used to detect (Midorikawa et al., 2001; Uehara et al., 1993; Zhang et al., 1998). However, when the MCLA- chemiluminescence method was adopted to determine the production during continuing turnover of the bc1 complex (in the presence of ubiquinol and cytochrome c) with Lumac/3M Biocounter (model 2110A), a high background of production, resulting from the nonenzymatic oxidation of ubiquinol by cytochrome c, was encountered. This makes it difficult to accurately measure production by the cyt bc1 complex.
This difficulty has finally been overcome by measuring the chemiluminescence of the MCLA- adduct during a single turnover of bc1 complex, with Applied Photophysics stopped-flow reaction analyzer SX.18MV (Leatherhead, England). By leaving the excitation light source off, the chemiluminescence of MCLA- generated when cyt bc1 complex is mixed with ubiquinol and MCLA is registered in light emission (Denicola et al., 1995). Because the system contains no cytochrome c, chemiluminescence of MCLA-, resulting from nonenzymatic oxidation of ubiquinol by cytochrome c, is eliminated. This method enables us to unambiguously compare production by various bc1 complexes.
Experimentally, the reaction is carried out at 25° by mixing solutions A and B in a 1:1 ratio. Solution A contains 100 mM Na+/K+ phosphate buffer, pH 7.4, 1 mM EDTA, 1 mM KCN, 1 mM NaN3, 0.1% bovine serum albumin, 0.01% DM, and an appropriate concentration of bovine cyt bc1 or other systems. Solution B contains 125 μM Q0C10BrH2 and 4 μM MCLA in the same buffer. Once the reaction starts, the produced chemiluminescence, in voltage, is consecutively monitored for 2 sec. generation is expressed in xanthine oxidase (XO) units. One XO unit is defined as chemiluminescence (maximum peak height of light intensity) generated by 1 unit of XO, which equals 2.0 V from an Applied photophysics stopped-flow reaction anlyzer SX.18MV, when solution A containing 100 mM Na+/K+ phosphate buffer, pH 7.4, 100 μM hypoxanthine, 4 μM MCLA, and 1 mM NaN3 is mixed with solution B containing 100 mM Na+/K+ phosphate buffer, pH 7.4, 1 mM NaN3 and 1 unit of XO.
7. COMPARISON OF PRODUCTION BY THE bc1 COMPLEXES WITH VARYING ELECTRON TRANSFER ACTIVITIES
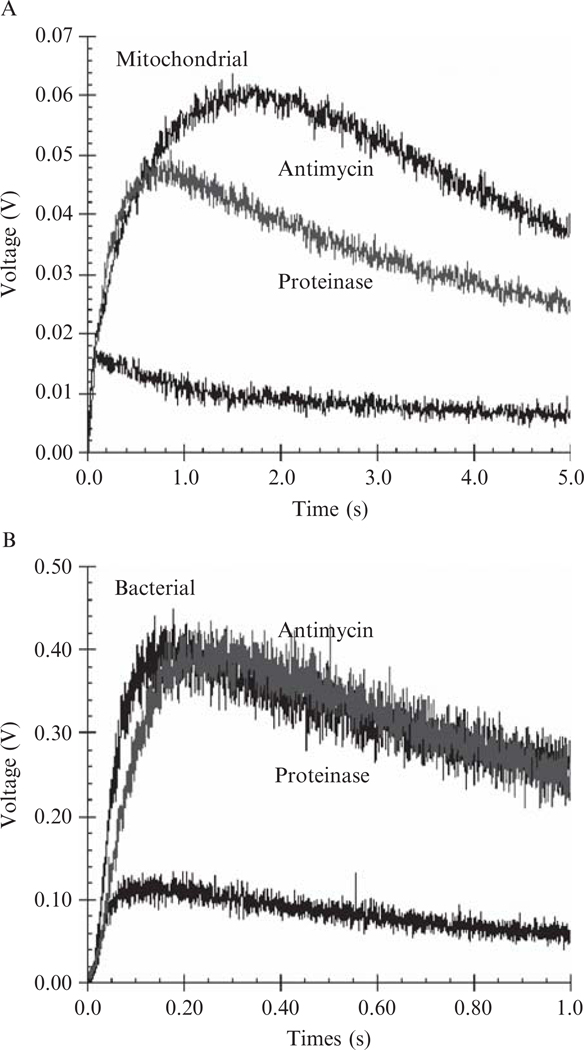
Under normal catalytic conditions only a very small number of electrons leak from the bovine complex to form (Fig. 25.3A). The production significantly increases (fivefold) when the electron transfer reaction is blocked by antimycin A (Fig. 25.3A). This inhibitor effect is also observed in the bacterial complex (Fig. 25.3B). Because no ubisemiquinone can be detected in antimycin A–inhibited complexes in the presence of ubiquinol and such systems feature an increase in the reduction level of cyt bL, the reduced cyt bL is most probably the electron source for the superoxide generation.
Figure 25.3.
Generation of in intact, antimycin A–inhibited and proteinase K-treated cytochrome bc1 complexes. (A) Data for the mitochondrial bc1 complex and (B) for R. sphaeroides four-subunit, wild-type bc1 complex. The concentrations of bovine and R. sphaeroides bc1 complexes in solution A were 5 μM. Detailed experimental conditions are given in Methods for determining superoxide generation. To digest subunits of the cyt bc1 complex, the bc1 solution was diluted with 50 mM Tris-HCl buffer, pH 7.4, containing 0.01% DM, to a protein concentration of 20 mg/ml and incubated with 0.4 mg/ml of proteinase K at room temperature. The electron transfer activity and superoxide generation activity were followed during the course of incubation. When electron transfer activity was completely lost, the incubated mixture was subjected to SDS-PAGE to confirm the protein digestion and to determine production.
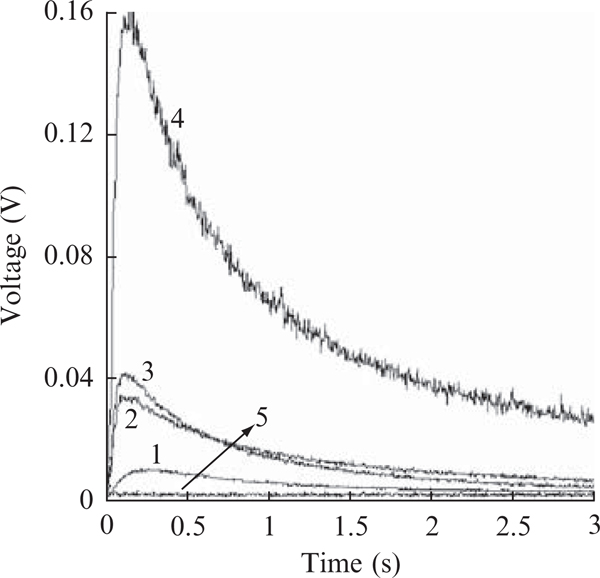
When productions in cyt bc1 complexes with varying electron transfer activities, a reversed relationship between electron transfer activity and production is revealed (Fig. 25.4). The specific activities, μmol cyt c reduced/min/nmol b, for the bovine complex, the four subunit, wild-type, reconstituted, and the three-subunit core complexes of R. sphaeroides are 24 , 2.2, 2.0, and 0.6., respectively, whereas the production, XO unit/nmol b, by these four complexes are 0.03, 0.063, 0.067, and 0.25, respectively (calculated from Fig. 25.4). Inactivation of the bc1 complex by incubating at elevated temperature (37°C) or treatment with proteinase k results in an increase in superoxide production to the same level as that of the antimycin A–inhibited complex (Fig. 25.3). These results suggest that the structural integrity of protein subunits is not required for superoxide generating activity.
Figure 25.4.
Time traces of production by various bc1 complexes. Curves 1 to 4 represent the bovine and R. sphaeroides complexes of wild-type, reconstituted, and three-subunit core, respectively. Reconstituted complex refers to the complex reconstituted from the core complex and recombinant wild-type subunit IV. The concentrations of bc1 complexes used in solution Awere 3 μM. Curve 5 is for control experiment when no bc1 complexes nor Q0C10BrH2 is present in the system. A similar curve was obtained when 300 unit/ml superoxide dismutase was added to the complete system.
ACKNOWLEDGMENTS
This research was supported in part by an NIH grant (GM 30721) to C. A. Y. by the Oklahoma Agricultural Experiment Station (Projects #1819 and #2372), Oklahoma State University, and by the Intramural Research Program of National Cancer Institute, Center for Cancer Research, NIH.
REFERENCES
- Brandt U, and Trumpower B. (1994). The proton-motive Q cycle in mitochondria and bacteria. Crit. Rev. Biochem. Mol. Biol 29, 165–197. [DOI] [PubMed] [Google Scholar]
- Cape JL, Bowman MK, and Kramer DM (2007). A semiquinone intermediate generated at the Qo site of the cytochrome bc1 complex: Importance for the Q-cycle and superoxide production. Proc. Natl. Acad. Sci. USA 104, 7887–7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, and Boveris A. (1979). Hydroperoxide metabolism in mammalian organs. Physiol. Rev 59, 527–605. [DOI] [PubMed] [Google Scholar]
- Crofts AR (2004). The cytochrome bc1 complex: Function in the context of structure. Ann. Rev. Physiol 66, 689–733. [DOI] [PubMed] [Google Scholar]
- Denicola A, Souza JM, Gatti RM, Augusto O, and Radi R. (1995). Desferrioxamine inhibition of the hydroxyl radical-like reactivity of peroxynitrite: Role of the hydroxamic groups. Free Radic. Biol. Med 19, 11–19. [DOI] [PubMed] [Google Scholar]
- De Vries S, Albracht SP, Berden JA, and Slater EC (1981). A new species of bound ubisemiquinone anion in QH2: Cytochrome c oxidoreductase. J. Biol. Chem 256, 11996–11998. [PubMed] [Google Scholar]
- Dröse S, and Brandt U. (2008). The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem 283, 21649–21654. [DOI] [PubMed] [Google Scholar]
- Esser L, Gong X, Yang S, Yu L, Yu CA, and Xia D. (2006). Surface-modulated motion switch: Capture and release of iron-sulfur protein in the cytochrome bc1 complex. Proc. Natl. Acad. Sci. USA 103, 13045–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung B, Yu L, Xia D, and Yu CA (2005). The iron-sulfur cluster of the rieske iron-sulfur protein functions as a proton-exiting gate in the cytochrome bc1 complex. J. Biol. Chem 280, 24895–24902. [DOI] [PubMed] [Google Scholar]
- Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, and Jap BK (1998). Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281, 64–71. [DOI] [PubMed] [Google Scholar]
- Kagawa Y, and Racker E. (1971). Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXV. Reconstitution of vesicles catalyzing 32p adenosine triphosphate exchange. J. Biol. Chem 246, 5477–5487. [Google Scholar]
- Ljungdahl PO, Pennoyer JD, Robertson DE, and Trumpower BL (1987). Purification of highly active cytochrome bc1 complexes from phylogenetically diverse species by a single chromatographic procedure. Biochim. Biophys. Acta 891, 227–241. [DOI] [PubMed] [Google Scholar]
- Mclennan HR, and Esposti MD (2000). The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg. Biomembr 32, 152–162. [DOI] [PubMed] [Google Scholar]
- Mitchell P. (1976). Possible molecular mechanisms of the protonmotive function of cytochrome systems. J. Theor.Biol 62, 327–367. [DOI] [PubMed] [Google Scholar]
- Midorikawa J, Maehara K, Yaoita H, Watanabe T, Ohtani H, Ushiroda S, and Maruyama Y. (2001). Continuous observation of superoxide generation in an in-situ ischemia-reperfusion rat lung model. Jpn. Circ. J 65, 207–212. [DOI] [PubMed] [Google Scholar]
- Muller F, Crofts AR, and Kramer DM (2002). Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc1 complex. Biochemistry 41, 7866–7874. [DOI] [PubMed] [Google Scholar]
- Muller F, Roberts AG, Bowman MK, and Kramer DM (2003). Architecture of the Qo site of the cytochrome bc1 complex probed by superoxide production. Biochemistry 42, 6493–6499. [DOI] [PubMed] [Google Scholar]
- Nakano M. (1990). Determination of superoxide radical and singlet oxygen based on chemiluminescence of luciferin analogs. Methods Enzymol. 186, 585–591. [DOI] [PubMed] [Google Scholar]
- Nohl H, and Jordan W. (1986). The mitochondrial site of superoxide formation. Biochem. Bioph. Res. Co 138, 533–539. [DOI] [PubMed] [Google Scholar]
- Snyder CH, Gutierrez-Cirlos EB, and Trumpower BL (2000). Evidence for a concerted mechanism of ubiquinol oxidation by the cytochrome bc1 complex. J. Biol. Chem 275, 13535–13541. [DOI] [PubMed] [Google Scholar]
- Sun J, and Trumpower BL (2003). Superoxide anion generation by the cytochrome bc1 complex. Arch. Biochem. Biophys 419, 198–206. [DOI] [PubMed] [Google Scholar]
- Tian H, Yu L, Mather MW, and Yu C-A (1998). Flexibility of the neck region of the rieske iron-sulfur protein is functionally important in the cytochrome bc1 complex. J. Biol. Chem 273, 27953–27959. [DOI] [PubMed] [Google Scholar]
- Tso SC, Shenoy SK, Quinn BN, Yu L, and Yu CA (2000). Subunit IV of cytochrome bc1 complex from Rhodobacter sphaeroides: Location of regions essential for interaction with the three-subunit core complex. J. Biol. Chem 275, 15287–15294. [DOI] [PubMed] [Google Scholar]
- Trumpower BL, and Gennis RB (1994). Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: The enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem 63, 675–716. [DOI] [PubMed] [Google Scholar]
- Turrens JF, and Boveris A. (1980). Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J 191, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, and Lehninger AL (1985). Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys 237, 408–414. [DOI] [PubMed] [Google Scholar]
- Uehara K, Maruyama N, Huang CK, and Nakano M. (1993). The first application of a chemiluminescence probe, 2-methyl-6-[p-methoxyphenyl]-3,7-dihydroimidazo[1,2-1] pyrazin-3-one (MCLA), for detecting O2 production, in vitro, from Kupffer cells stimulated by phorbol myristate acetate. FEBS Lett. 335, 167–170. [DOI] [PubMed] [Google Scholar]
- Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, and Deisenhofer J. (1997). Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science 277, 60–66. [DOI] [PubMed] [Google Scholar]
- Xia D, Esser L, Yu L, and Yu CA (2007). Structural basis for the mechanism of electron bifurcation at the quinol oxidation site of the cytochrome bc1 complex. Photosyn. Res 92, 17–34. [DOI] [PubMed] [Google Scholar]
- Yang S, Ma H-W, Yu L, and Yu CA (2008). On the mechanism of quinol oxidation at Qp site in cytochrome bc1 complex: Studied by mutants without cytochrome bL or bH. J. Biol. Chem 283, 28767–28776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CA, Yu L, and King TE (1975). Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J. Biol. Chem 250, 1383–1392. [PubMed] [Google Scholar]
- Yu CA, and Yu L. (1980). Resolution and reconstitution of succinate-cytochrome c reductase: Preparations and properties of high purity succinate dehydrogenase and ubiquinol-cytochrome c reductase. Biochim. Biophys. Acta 591, 409–420. [DOI] [PubMed] [Google Scholar]
- Yu CA, and Yu L. (1982). Synthesis of biologically active ubiquinone derivatives. Biochemistry 21, 4096–4101. [DOI] [PubMed] [Google Scholar]
- Yu CA, Cen X, Ma H-W, Yin Y, Yu L, Esser L, and Xia D. (2008). Domain conformational switch of the iron-sulfur protein in cytochrome bc1 complex is induced by the electron transfer from cytochrome bL to bH. Biochim. Biophys. Acta 1777, 1038–1043. [DOI] [PubMed] [Google Scholar]
- Yu L, Tso SC, Shenoy SK, Quinn BN, and Xia D. (1999). The role of the supernumerary subunit of Rhodobacter sphaeroides cytochrome bc1 complex. J. Bioenerg. Biomembr 31, 251–257. [DOI] [PubMed] [Google Scholar]
- Yu L, Tso SC, Shenoy SK, Quinn BN, and Xia D. (1999). The role of the supernumerary subunit of Rhodobacter sphaeroides cytochrome bc1 complex. J. Bioenerg. Biomembr 31, 251–258. [DOI] [PubMed] [Google Scholar]
- Yue WH, Zou YP, Yu L, and Yu CA (1991). Crystallization of mitochondria ubiquinol-cytochrome c reductase. Biochemistry 30, 2303–2306. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu L, and Yu CA (1998). Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J. Biol. Chem 273, 33972–33976. [DOI] [PubMed] [Google Scholar]
- Zhu J, Egawa T, Yeh SR, Yu L, and Yu CA (2007). Simultaneous reduction of iron-sulfur protein and cytochrome bL during ubiquinol oxidation in cytochrome bc1 complex. Proc. Natl. Acad. Sci. USA 104, 4864–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]