Abstract
Because coronavirus disease 2019 (COVID-19) is relatively new, health-care organizations and researchers have been publishing guidelines and recommendations to help health-care providers proceed safely with various aspects of disease management and investigation. Most of the published papers have addressed clinical presentation, diagnostic tests, mitigation measures, and hospital preparedness. Pathological and laboratory issues, including autopsy procedures and the handling of dead bodies, have not yet been well characterized. We reviewed the recent literature for guidelines and reports related to COVID-19 and anatomic pathology, specifically laboratory services, the handling of dead bodies, the conduct of autopsies, and postmortem pathological investigations, to synthesize relevant knowledge to ensure that clinicians are aware of the most recent recommendations for precautions and safety measures, and to support the development of standards in health-care facilities.
Keywords: Autopsy, burial dead body, coronavirus, diffuse alveolar damage, personal protective equipment, severe acute respiratory syndrome CoV-2
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is wreaking havoc around the world.[1] Although the virus belongs to a previously known class of human coronavirus that has appeared sporadically in the past, the current strain remains a subject of inquiry for scientists around the world. Previous outbreaks of Coronaviridae – including HCoV-OC43 (OC43), HCoV-229E (229E), HCoV-NL63 (NL63) and HCoV-HKU1 (HKU1) – have been reported to cause infections of the upper and lower respiratory tract.[2] Over the past 15 years, two new human coronavirus strains, SARS-CoV,[3] and Middle East Respiratory Syndrome CoV (MERS-CoV),[4] have emerged, causing substantial disease and mortality, and in 2019, the SARS-CoV-2 strain emerged. With origins in Wuhan, China, the virus spread worldwide within 2 months, severely decapitating health-care systems around the world.
The virus has been identified to have four major structural proteins, including the spike surface glycoprotein, envelope, matrix, and nucleocapsid protein.[5] The clinical manifestations of the viral infection present as common symptoms such as dry cough, sore throat and fever, and can proceed to muscle pain, chest pain, nausea, vomiting, diarrhea, and respiratory issues.[6] In severe cases, especially in the elderly and those with comorbid conditions, it may lead to respiratory failure and death.
Because this infectious disease is relatively new, healthcare organizations, and researchers have been publishing guidelines and recommendations to help health care providers proceed safely with various aspects of disease management and investigation. Most of the published papers have addressed clinical presentation, diagnostic tests, mitigation measures, and hospital preparedness.[7] Pathological and laboratory issues, including autopsy procedures and the handling of dead bodies, have not yet been well characterized in the published papers. For this reason, we reviewed the recent literature for guidelines and reports related to COVID-19 and anatomic pathology – specifically laboratory services, the handling of dead bodies, the conduct of autopsies, and postmortem pathological investigations. The aim was to synthesize relevant knowledge to ensure that clinicians are aware of the most recent recommendations for precautions and safety measures, and to support the development of standards in health care facilities.
Laboratory Services
The COVID-19 crisis demands the intelligent use of available resources to maximize output and efficiency while adhering to the issues of resilience and social distancing. To continue support to the clinical services, many prominent organizations have issued guidelines for laboratories to prioritize work and to manage staff, equipment, facilities, and reagents to cope with the testing overload. The Royal College of Pathologists has issued the following recommendations for managing laboratory services efficiently.[8] First, testing only for SARS-CoV-2 and ceasing testing for influenza and respiratory viruses and ambulatory testing. Repeat testing is recommended only if a deep lower respiratory tract sample can be obtained postintubation. Private laboratory testing should be conducted in line with national guidance and only when clinically indicated. Second, on screening of multiple body sites, methicillin-resistant Staphylococcus aureus screening should be reduced and refined, including limiting screening to high-risk areas using nasal swabs only. Pseudomonas screening outside of neonatal units is of low value and could be ceased until there is a particular local risk. Third, swab specimens, including genital swabs, possess low clinical value, and can be rejected. In contrast, superficial wound swabs require minimal or no processing. With respect to fecal specimens, the routine culture of nonbloody samples and Salmonella enrichment can be stopped. Testing for Clostridium difficile should continue with scopes to reduce fecal microscopy. Nonpurulent sputum samples should be treated for routine culture. Allergy testing, mycology testing, and Helicobacter pylori antigen, and antibody testing can be avoided. Forth, blood science testing can be reduced, although newborn and antenatal screening can be continued. Cancer screening for symptomatic cases can be prioritized. Routine infertility testing of semen can be deferred for 3–6 months; complex tests can be consolidated on fewer sites. Fifth, laboratories can be connected to avoid repetitive entry and transcription of data. Retest intervals should also be minimized.
The World Health Organization (WHO) has issued interim guidance for laboratory operations during COVID-19.[9] Screening protocols should be adopted according to local conditions for COVID-19 cases, and decisions to test can be based on clinical and epidemiological factors that determine the likelihood of infection. Suspected cases should be tested using nucleic acid amplification tests such as reverse transcription polymerase chain reaction. Patients can be tested for other respiratory pathogens as per local management guidelines for community-acquired pneumonia, but they should be tested for COVID-19 as well. Specimen collection should proceed as per standard operating procedures, labeling specimens as potentially infectious. Specimens need to be stored at 2–8C for up to 72 h, and at −70C if a delay in testing is expected. Workers collecting samples should adhere to infection prevention and control guidelines.
For the testing of clinical samples reaching the laboratory, the WHO has also issued biosafety guidelines.[10] First, all procedures should be performed based on risk assessment, only by personnel with demonstrated capabilities. As well, laboratory personnel handling specimens should wear personal protective equipment (PPE). Second, a validated biology safety cabinet or primary containment device should be used for initial processing. Third, procedures equivalent to Biosafety Level 2 should be adopted for nonpropagative diagnostic work when conducting procedures with a high likelihood of aerosol or droplet generation. Forth, appropriate disinfectants such as hypochlorite, hydrogen peroxide and phenolic compounds must be used against enveloped viruses. Fifth, special precautions must be taken during transport of specimens. For specimen handling and processing, good microbiological practices and procedures should be followed. Sixth, all technical procedures should be conducted in a way to ensure minimized aerosol and droplet generation.
Laboratory personnel engaged in SARS-CoV-2 testing[11] should undergo appropriate testing themselves, as per the required biosafety level and the nature of work. A baseline serum sample should be obtained from laboratory workers and stored for reference. Workers should report any fever or lower respiratory symptoms and be evaluated for exposures. If workers are believed to be exposed, they should be evaluated, counseled, and monitored for symptoms of infection. Local, regional, or public health departments should be informed of any such exposures or illness.
Handling of Dead Bodies
As infections become severe, patients – particularly those with comorbid diseases – may succumb to the disease. The corpses of those who have died due to COVID-19 infection need to be handled with utmost care to avoid transmission of the virus. Evidence is currently lacking about whether individuals can acquire COVID-19 due to exposure to the corpse of an infected individual, but for the safety of those tending to bodies, following safety guidelines and taking the highest precautions should remain a priority.
The WHO has provided interim guidelines for the handling of dead bodies during the COVID-19 pandemic.[12] First, personnel interacting with the body need to practice standard precautions and use PPE[13] as per the level of interaction with the body. The minimum required personal PPE includes gloves and long-sleeved water-resistant gowns. Any fluids leaking from orifices must be contained. Movement and handling of the body must be kept to a minimum. Second, health care workers and mortuary staff should also wear suitable PPE and impermeable disposable gowns. Adults aged 60 years or older and immunocompromised people should not be allowed direct interaction with the body. Embalming is not recommended. Third, because SARS-CoV-2 can stay on surfaces up to 9 days and has been detected even after 72 h under experimental conditions, cleaning of the mortuary environment is recommended. Surfaces where the body is prepared should be adequately cleaned with soap and water or commercial detergent solution. After being cleaned, surfaces should be disinfected using 0.1% sodium hypochlorite or 70% ethanol. Clinical waste needs to be handled carefully and disposed of as per local requirements. Forth, for burial or cremation of a corpse, national and local requirements must be followed for the handling and disposition of remains. Those placing the body in a grave or on a pyre should wear gloves and disinfect hands after the burial. It is advisable to use a checklist to make sure that all necessary steps are followed throughout the process.
Further guidance on the handling of corpses comes from the U.S. Centers for Disease Control and Prevention and WHO.[14,15] The handling and movement of corpses should always be kept to a minimum.[15] All the key personnel involved in the handling of the dead bodies outside of the healthcare system should be identified to make sure that they are aware of official recommendations and the availability of suitable PPE. It is also advised that funeral directors and embalmers to treat all human remains as infectious.[16] Thus, using standard precautions to minimize possible exposures, and risk of disease transmission remains essential. For this purpose, any puncture holes or wounds should be disinfected using 1% hypochlorite, followed by dressing with an impermeable material. Oral and nasal orifices should be plugged to prevent leakage of bodily fluids. Precaution must be taken while handling intravenous catheters or any other sharp devices. The core elements of standard precautions include hand washing, avoiding smoking or eating, using appropriate protective clothing, and using specific protective clothing if embalming is conducted. As well, all workers and staff handling dead bodies should be trained in infection prevention and control practices. The body should be placed in a leak-proof plastic body bag that can be decontaminated externally using 1% hypochlorite.[17] The body can also be placed in any cloth or tissue for transfer and should be moved from the mortuary area as soon as possible. Environmental cleaning should be practiced in the mortuary using a 1% hypochlorite solution. The body can be viewed by relatives by following standard precautions and PPE to avoid direct contact and possible transmission.[15]
There are three categories of PPE recommended by the Department of Health, Kowloon, for the handling of dead bodies[18] Category 1: gloves, water-repellent gown, and a surgical mask. Use goggles or face shield to protect eyes, if there may be splashes. Category 2: gloves, water-resistant gown/plastic apron over water-repellent gown, and a surgical mask. Use goggles or face shields to protect eyes if there may be splashes. Category 3: water-resistant gown, surgical mask, eye protection (goggles or face shield), double gloves, shoe covers/boots.
The U.S. Centers for Disease Control has also provided a list of recommended PPE when handling corpses, including:[17] double surgical gloves AND cut-proof synthetic mesh gloves, fluid-resistant or impermeable gown, waterproof apron, goggles or face shield, National Institute for Occupational Safety and Health – certified disposable −95 respirator or higher, and powered air-purifying respirators with high efficiency particulate air (HEPA) filters.
Autopsy Procedures
The benefits of clinical autopsy are myriad. However, particularly during a pandemic, the morphological changes in every organ should be meticulously examined and documented at the gross, microscopic and electromicroscopic levels. Immunohistochemistry must also be performed, and frozen tissue must be stored for further investigation. In our experience in Canada during the SARS pandemic, clinical and forensic autopsies are performed according to Centers for Disease Control and College of American Pathologists guidelines. However in Saudi Arabia there is no system for clinical autopsies, although preliminary acceptance of this service is pending at our college (College of Medicine King Saud University), following the Centers for Disease Control and College of American Pathologists guidelines.
It has been recommended that autopsy procedures be conducted 12 h after death and storing the corpse at 4–8C.[19] The Royal College of Pathologists recommends conducting a staged autopsy;[19] a detailed examination should be conducted only if necessary. Further, and minimally invasive, a limited autopsy can be performed to collect tissue and fluid samples. Such an examination can further be improved using postmortem computed tomography imaging, which can help identify pulmonary indications associated with SARS-CoV-2 infection.[20]
Autopsy procedures for patients who have died of COVID-19 infection pose an additional challenge because of the risk of inhalation of live viruses present in the lungs and other organs. Additional protective measures, such as using an N95 or other respirator, are important when performing aerosol-generating procedures (AGPs). The use of filtering face piece–3 masks has been recommended by the Royal College of Pathologists.[19] Furthermore, measures to protect against the typical risks of infection from possible splashes during conventional autopsy procedures also need to be followed. When an autopsy of a patient who has died of COVID-19 is conducted, a minimal number of staff should be engaged. The autopsy needs to be performed in an adequately ventilated room while wearing suitable PPE, including a scrub suit, an N95 or surgical mask, long-sleeved fluid-resistant gowns, a face shield, double surgical gloves interposed with mesh gloves, a waterproof apron, goggles, and boots.[14]
AGPs should be minimized in the autopsy room. The use of power saws should be avoided if possible. In addition, while removing, handling, or washing organs such as lung or intestines, splashing should be avoided. Aerosols should be contained using exhaust ventilation, and the volume can be reduced by releasing aerosols in an ambient air environment. Further, AGPs can be reduced by using containment devices such as biosafety cabinets for the examination and handling of small specimens. Intestines can be opened underwater to avoid high-pressure water sprays. Vacuum shrouds should be used with oscillating saws.[18] Hand shears can be used as an alternative to an oscillating bone saw. Other preferable surgical equipment includes round-ended scissors and postmortem–40 or other heavy-duty blades with blunt points. Needles should not be re-sheathed after sampling fluids.[19]
Because morgues are an integral part of combating epidemics, the mortuary and the autopsy room should be built specially and equipped with all required equipment. The autopsy suite or room should have negative pressure ventilation, with at least six times the air change hourly for old buildings, and 12 times for new buildings. Exhaust systems should help direct aerosols and air away from healthcare workers. Autopsies should always be performed in an airborne infection isolation room, and the air should be directed out through a HEPA filter. If airborne infection isolation rooms or HEPA units are not available, procedures should be performed in a protective environment, with air always exhausted outdoor away from human space and not returned to the building.
The autopsy area could also be divided into three zones – the contaminated area; buffer zones 1 and 2; and the clean area – using the building layout.[21] The dissection area is the contaminated area, and the areas designated for personal cleaning and disinfection for laboratory personnel are potentially/semi-contaminated areas. Buffer zone 1 lies between the two and is equipped with washing and disinfection areas and possibly a self-sterilizing air shower room. Buffer zone 2 includes the passage of remains and specimens, which are contaminated items. All areas should be isolated from each other as much as possible.[21] To move across areas, a single-channel closed-loop approach can be adopted, ensuring that the examiner/workers enter from the clean area using one channel and leave the contaminated area from another channel.[22]
Prior to the commencement of the autopsy, examiners should don PPE in the clean area and organize instruments for autopsy in the semi-contaminated area. The autopsy is performed in the contaminated area. Reusable PPE must first be sterilized in the contaminated area, followed by a second round of sterilization in the semi-contaminated area.[22]
The autopsy sequence should follow the abdominal cavity, the pelvic cavity, the neck then the thoracic cavity, and then the cranial cavity. To minimize contamination, the autopsy could be conducted in a leak-proof transparent autopsy bag. Alternatively, a waterproof back sheet could be spread on the dissection table to absorb all liquid. Reusable items could be kept moist between use and decontamination.[21] Only a limited number of personnel should be allowed in the autopsy room and work on the body. When handling needles or other sharps, personnel should exercise caution, and sharps should be disposed of in puncture-proof, closable, labeled sharps containers. If gloves are torn during the procedure because of needles, syringes, or the ends of fractured bones, they should be replaced immediately. In cases of splashing of bodily fluids on examiners' clothing or the autopsy table, strict disinfection should be undertaken. If any of the fluids contaminate the examiner's skin, the area should be treated with 0.5% iodine for more than 3 min, and contaminated mucous membranes need to be rinsed with a large amount of 0.05% iodine or normal saline.[22]
Specimens collected during the autopsy include swab specimens for other respiratory pathogens, indicated microbiological and infectious diseases, and tissues from the lungs, upper airway, and other major organs fixed in formalin [Table 1]. Specimen samples can be taken directly after opening the body cavity to minimize the risk of infection. First, samples for microscopic examination should be extracted, followed by samples for genetic testing. Unfixed organs should be set firmly on the table and sliced using a sponge.[19] Cryogenically frozen specimens may also be obtained and fixed in 3% glutaraldehyde liquid. Organs and tissue can be maintained in a 3.7% formaldehyde buffer fixative. To keep fresh tissue samples, thick needle aspiration may be used immediately after death.[21] For fixing, tissues requiring freezing can be cut into blocks, followed by cutting of tissues in blocks for electron microscopy. Finally, the conventional embedding of diseased organs, tissues, or tissue blocks in paraffin can be performed, using a 4% paraformaldehyde solution for 48–72 h.[22]
Table 1.
Collection of fixed autopsy tissue specimens
| Specimen site |
|---|
| Respiratory sites |
| Trachea (proximal and distal) |
| Central (hilar) lung with segmental bronchi, right and left primary bronchi |
| Representative pulmonary parenchyma from right and left lung |
| Liver, spleen, kidney, heart, gastrointestinal tract |
| Any other tissues showing significant gross pathology |
After the specimens have been obtained, they must be packaged carefully by placing them in a primary container, and then a secondary container. The secondary container can then be placed in a resealable plastic bag, and the plastic bag can be placed in a biological specimen bag with absorbent material. For nasopharyngeal and lung swabs, specimens should be stored at 2–8C for up to 72 h and shipped on ice packs. Package or the container surfaces should be disinfected.[14]
Pathological Findings: Diagnosing Coronavirus Disease 2019 Postmortem
The pathological findings for COVID-19 disease continue to be unraveled as more reports are published. The clinical features recommended by Public Health England form the basis for the patient to be categorized as infected with COVID-19 and are the same for choosing to undertake further examination in the deceased. These criteria include the presence of clinical or radiological evidence of pneumonia or acute respiratory distress syndrome, or influenza-like illness, hoarseness, shortness of breath, sneezing, sore throat, wheezing, or nasal congestion. The decision to undertake autopsy to acquire deeper insights into pathological features depends on the individual case.
In COVID-19, the lung weight may be higher than normal. Purulent inflammation, characteristic of bacterial infection, may result from a secondary infection. As well, the presentation of pneumocyte hyperplasia, focal inflammation, and multinucleated giant cell formation without any hyaline membranes have been reported in patients who initially underwent surgery for lung adenocarcinoma.[23] These patients were identified as having COVID-19 infection, and because they were asymptomatic at the time of surgery, the mentioned changes are indicative of early alterations associated with COVID-19 infection. Further, in a symptomatic man who died of COVID-19 infection, multiple ground-glass opacities or patchy bilateral shadows were observed in chest X-ray. The uncontrolled inflammation, accumulation of fluid, and progressive fibrosis compromise the ability to exchange gas severely,[24] as the surfactant levels decrease due to dysfunction of type-I and type II pneumocytes, the surface tension increases, and the expansion ability of lungs decreases.[2] This further increases the risk of lung collapse.
Microscopic examination has revealed the presence of diffuse alveolar damage with exudate, with lymphocytic inflammation in COVID-19. Multinucleated giant cells were also present. Whole-lung biopsy of those who have died from COVID-19 has revealed another important pathological finding: that the lungs have a diffuse congestive appearance. The gross examination also highlighted partly hemorrhagic necrosis on the outer edge of the lower lung. Hyperplasia of small vessels, thickening of vessels of the wall, lumen stenosis, microthrombosis, and occlusion have also been seen. The pulmonary interstitial space was infiltrated with focal monocytes, plasma cells, and lymphocytes. Such infiltration has also been observed in the blood vessels of the alveolar septum, with the majority being CD4+ T cells, which were congested and edematous.[25] The cells of epithelial and alveolar epithelium showed desquamation and squamous metaplasia, atrophy, and vacuolar degeneration, bronchiolitis, and alveolitis.[26] The alveolar cavity showed congestion with mucus, edema, inflammatory cells, and desquamated cells.[26,27] Several multinucleate giant cells with intracytoplasmic viral inclusion bodies were also seen along with pulmonary interstitial fibrosis.[26] SARS-CoV2 has shown similar findings to SARS-CoV [Figure 1].
Figure 1.
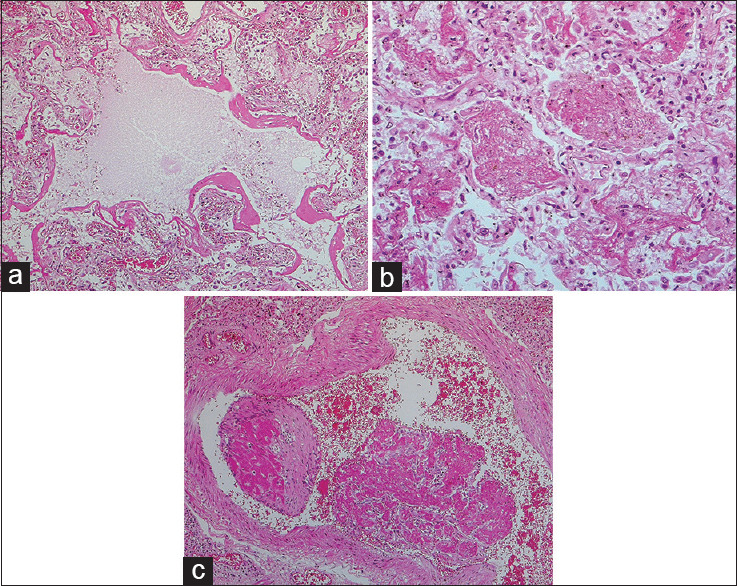
(a) Diffuse alveolar damage in a severe acute respiratory syndrome patient: acute/exudative phase, with classic hyaline membranes (H and E, ×100). (b) Acute fibrinous and organizing pneumonia pattern in a severe acute respiratory syndrome patient: intra-alveolar fibrin “balls” are observed, rather than conventional hyaline membranes (H and E, ×200). (c) Acute and organizing thromboembolus in severe acute respiratory syndrome patient (H and E, ×100). Photomicrographs courtesy of Dr. David Hwang (University of Toronto)
Another study entailing postmortem needle core biopsy of heart, lung, and liver for four patients (3 males and one female) revealed diffuse alveolar damage: Injured alveolar epithelial cells, the formation of the hyaline membrane, and type II pneumocyte hyperplasia.[28] Pulmonary consolidation by fibroblastic proliferation with extracellular matrix was seen, and airspaces presented fibrin-forming clusters. One patient presented with consolidation with abundant neutrophil infiltration in the intra-alveolar region, superimposed with bacterial bronchopneumonia. Pathological changes in the liver and heart were regarded as secondary to the viral infection. Patchy necrosis with mild lobular infiltration was seen in the liver, and mild focal fibrosis with myocardial hypertrophy was seen in the heart. Similar observations of acute bronchopneumonia with diffused alveolar damage were also reported by an autopsy study involving SARS-CoV-2 testing in the United States.[29] Reduced lymphocyte numbers, necrosis, and degeneration of cells in the spleen, while necrotic parenchymal cells, hyaline thrombus formation in small vessels have also been reported.[25]
Cardiac complications may occur in COVID-19, including myocardial injury, myocarditis, acute myocardial infarction, heart failure, dysrhythmias, and venous thromboembolic events.[30]
Such pathological findings remain in alignment with SARS-CoV infection.[31] Diffuse alveolar damage remains the prominent histopathology in SARS-CoV-2 and Middle East respiratory syndrome coronavirus as well MERS-CoV.[32] The gross pathology of lungs includes pleural effusions, mucopurulent material in the tracheobronchial region, lung consolidation, pulmonary edema, pericarditis, and focal hemorrhage.[33] It also presents the formation of hyaline membrane, exudation of fibrin in alveolar space, and alveolar hemorrhage,[34,35] similar to COVID-19 infection. Infection with SARS-CoV has been reported to cause severe pneumonia with massive infiltration of inflammatory cells and elevated pro-inflammatory chemokine/cytokine response, resulting in acute lung injury and acute respiratory distress syndrome.[36] The airways, cells of the alveolar epithelium, vascular endothelium, monocytes, lymphocytes, and macrophages were infected upon staining for viral antigens. The interstitium and alveoli showed extensive cellular infiltrates, including neutrophils and predominantly macrophages on histological examination of the lungs, and lower CD4 and CD8 T cells counts occurred in peripheral blood samples.[37]
Conclusion
COVID-19 is caused by SARS-CoV-2, a highly infectious virus that can be transmitted between humans. Thus, it is important to practice great caution when excising samples from corpses for examination; conducting autopsies for pathological study; or handling, packaging, and storing specimens for laboratory examination. Recommended guidelines from authorities, researchers, and experts provide a foundation for correct practices in this respect. It is essential to conduct examinations in a carefully orchestrated manner to minimize any chances of exposure among laboratory personnel and health care workers. Such exposure could not only endanger the workers but also the people who come into contact with them. As well, the important pathological findings described by researchers could help in the efficient diagnosis of COVID-19 and also in its management. Continued autopsies can help verify findings and develop an improved understanding of the workings of the disease.
Financial support and sponsorship
This work was supported by the College of Medicine Research Center, King Saud University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Dr. David Hwang (University of Toronto) for providing photomicrographs of histologic findings in SARS.
References
- 1.Somily AM, BaHammam AS. Coronavirus disease-19 (severe acute respiratory syndrome-coronavirus-2) is not just simple influenza: What have we learned so far? J Nat Sci Med. 2020;3:79–82. [Google Scholar]
- 2.Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235:185–95. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Revised Interim Case Definition for Reporting to WHO – Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Emergency Preparedness, Response 2013. [Last accessed on 2020 Apr 20]. Available from: https://www.who.int/csr/disease/coron avirus_infections/case_definit ion_03_7_2014/en/p .
- 5.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–8. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry M, Ghonem L, Alsharidi A, Alanazi A, Alotaibi NH, AlShahrani FS, et al. Coronavirus disease2019 pandemic in the Kingdom of Saudi Arabia: Mitigation Measures and Hospital Preparedness. J Nat Sci Med. 2020:3. [doi: 10.4103/JNSM.JNSM_29_20] [Google Scholar]
- 8.The Royal College of Pathologists. Prioritisation/Deferral of Pathology Laboratory Work in Light of SARSCoV-2 (COVID19) epidemic. Recommendations from RC Path and Professional Bodies (IBMS, ACP and ACB) 2020. [Last accessed on 2020 Apr 20]. pp. 1–19. Available from: https://www.rcpath.org/uploads/assets/f5123842-950f-49c5-bf69ed866a7ca3da/Prioritisation-deferral-of-pathology-laboratory-work.pdf pp.
- 9.World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. Institutional Repository for Information Sharing. 2020. [Last accessed on 2020 Apr 20]. Available from: https://apps.who.int/iris/handle/10665/331329p .
- 10.World Health Organization. Laboratory Biosafety Guidance Related to Coronavirus Disease 2019 (COVID-19): Interim Guidance. Institutional Repository for Information Sharing. 2020. [Last accessed on 2020 Apr 20]. Available from: https://apps.who.int/iris/handle/10665/331138p .
- 11.Center for Disease Control. Supplement F: Laboratory guidance. Severe Acute Respiratory Syndrome. 2004. [Last accessed on 2020 Apr 20]. pp. 1–32. Available from: https://www.cdc.gov/sars/guidance/f-lab/downloads/F-lab-full-pdfpp .
- 12.World Health Organization. Infection Prevention and Control for the Safe Management of a Dead Body in the Context of COVID-19: Interim Guidance. 2020. [Last accessed on 2020 Apr 20]. pp. 1–6. Available from: https://apps.who.int/iris/bits tream/handle/10665/331538/WHO-COVID-19-lPC_DBMgmt-2020-1-en g.pdf?sequence=1 &isAllowed=y .
- 13.World Health Organization. How to Conduct Safe and Dignified Burial of a Patient who has Died from Suspected or Confirmed Ebola or Marburg Virus Disease. 2017. [Last accessed on 2020 Apr 20]. pp. 1–17. Available from: https://apps.who.int/iris/bitstrea m/handle/10665/137379/WHO_EVD_GUIDANCE_Burials_14.2_eng.pdf?sequence=1 .
- 14.Centers for Disease Control and Prevention. Collection and Submission of Postmortem Specimens from Deceased Persons with Known or Suspected COVID-19: CDC. 2020. [Last updated on 2020 Mar 19]. Available from: https://www.cdc.gov/coronavir us/2019-ncov/hcp/guidance-postmortem-specimens.html .
- 15.World Health Organization and Pan American Health Organization. Dead body Management in the Context of the Novel Coronavirus (COVID-19) 2020. [Last updated on 2020 Mar 19]. pp. 1–5. Available from: https://www.paho.org/en/docum ents/dead-body-management-context-novel-coronavirus-covid-19 pp .
- 16.Health Protection Surveillance Centre (HPSC). Guidance note for Funeral Directors/Embalmers Handling Potentially Infectious Human Remains. Management of Deceased Individuals Harbouring Infectious Diseases. 2014. [Last accessed on 2020 Apr 20]. pp. 1–3. Available from: https://www.hpsc.ie/a-z/lifest ages/modi/File, 14332, en.pdfpp .
- 17.Ministry of Health and Family Welfare Directorate General of Health Services. COVID19: Guidelines on Dead Body Management. 2020. [Last accessed on 2020 Apr 20]. pp. 1–7. Available from: https://www.mohfw.gov.in/pdf/1584423700568_COVID19GuidelinesonDeadbodymanagement.pdf pp .
- 18.Food and Environmental Hygiene Department. Precautions for Handling and Disposal of Dead Bodies. 2020. [Last accessed on 2020 Apr 20]. pp. 1–24. Available from: https://www.chp.gov.hk/files/pdf/grp.guideline-hp-ic-precautions_for_handli ng_and_disposal_of_dead_bodie s_en.pdf pp .
- 19.Osborn M, Lucas S, Stewart R, Swift B, Youd E. Briefing on COVID-19 autopsy practice relating to possible cases of COVID-19 (2019-nCov, novel coronavirus from China 2019/2020). The pulisher is Registered charity in England and Wales. The Royal College of Pathologists. 2020. [Last accessed on 2020 Apr 20]. pp. 1–14. Available from: https://www.rcpath.org/uploads/assets/d5e28baf-5789-4b0f-acecfe370eee 6223/447e37d0-29dd-4994-a11fe27b93de0905/Briefing-on-COVID-19-autopsy-Feb-2020.pdfpp .
- 20.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–42. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 21.Pathologist Branch of Chinese Medical Doctor Association PBoCMA. Operation guide for coronavirus disease-19 dead cases autopsy examination (Trial) Chin J Pathol. 2020;49:3–10. [Google Scholar]
- 22.Mao DM, Zhou N, Zheng D, Yue JC, Zhao QH, Luo B, et al. Guide to the forensic pathology practice on death cases related to corona virus disease 2019 (COVID-19) trial draft. Fa Yi Xue Za Zhi. 2020;36:6–5. doi: 10.12116/j.issn.1004-5619.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–4. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi Y, Lagniton PN, Ye S, Li E, Xu RH. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–66. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 26.Luo W, Yu H, Gou J, Li X, Sun Y, Li J, et al. Linical Pathology of critical patient with novel coronavirus pneumonia (COVID-19) Preprints. 2020. [Last accessed on 2020 Apr 20]. p. 2020020407. Available from: https://www.preprints.org/manu script/202002.0407/v4 .
- 27.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med. 2020;172:629–32. doi: 10.7326/M20-0533. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;14:1–8. doi: 10.1038/s41379-020-0536-x. doi: 10.1038/s41379-020-0536-x. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–33. doi: 10.1093/ajcp/aqaa062. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. N Engl J Med. 2020;382:1653–9. doi: 10.1016/j.ajem.2020.04.048. doi: 10.1056/NEJMsr2005760. Epub 2020 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–8. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–24. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Brand JM, Haagmans BL, van Riel D, Osterhaus AD, Kuiken T. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J Comp Pathol. 2014;151:83–112. doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136–47. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–8. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


