Abstract
In a rural medical center in Upstate New York, we observed an increase in pulmonary blastomycosis cases. Herein, we highlight the increased prevalence of blastomycosis in our region, and our experience with the diagnostic dilemma resulting in delayed diagnosis. This delay may have resulted in an increased mortality. A high index of suspicion may help hasten the diagnosis in an otherwise nonendemic area. A single-center retrospective case series of all patients diagnosed with culture-proven blastomycosis is reported at the Bassett Medical Center from 2007 to 2019. Eight cases of confirmed pulmonary blastomycosis were identified. All patients resided in a rural area along the Susquehanna River Basin. Only one case had a travel history to an endemic state. Males accounted for 100% of cases. There was a 50% mortality rate from acute respiratory distress syndrome. Bronchoalveolar lavage (BAL) reliably made the diagnosis. About 40% of patients had a false-negative blastomycosis serology. There was an average delay of 2.5 months from presentation to correct diagnosis due to a lack of consideration for blastomycosis. BAL resulted in a correct diagnosis, while serology was not reliable to exclude the diagnosis. Physicians should include blastomycosis in the diagnostic differential cases of nonresolving pneumonia in Upstate New York, an area not previously considered as endemic. Bronchoalveolar remains the evaluation method of choice if blastomycosis is suspected.
Keywords: Blastomyces dermatitidis, Otsego County, pulmonary blastomycosis, pulmonary infections, Upstate New York
A dimorphic fungus termed Blastomyces dermatitidis causes blastomycosis. A primary route of infection is respiring the spores, resulting in pneumonia. Infection can also involve the skin, bones, and central nervous system.[1] Clinical manifestations of pulmonary infection can vary from asymptomatic to respiratory failure, and if left untreated, can prove fatal. B. dermatiditis is prevalent along the Great Lakes of North America and the Mississippi and Ohio River basins.[1] Part of this region includes the strip of New York State along the St. Lawrence River,[2] which is not the location of our case series.
This article follows a previous case series done at Bassett Medical Center in 2013[2] and highlights the increasing prevalence of blastomycosis in our Susquehanna River Basin area. We describe eight cases of culture-proven blastomycosis reported at Bassett Medical Center between 2007 and 2019.
Methods
We retrospectively collected and reviewed all the microbiology and pathology reports of all patients diagnosed with culture -proven blastomycosis at Bassett Medical Center in Cooperstown, New York, from 2007 to 2019. We chose not to include the cases of cutaneous blastomycosis. Using our electronic medical records, we reviewed and extracted the data about all the identified patients. Institutional review board approval was not required.
Patients
Case 1 (2007)
A 44-year-old male, farmer and active smoker with no significant past medical history (PMH), was admitted through the emergency department (ED) with hypoxic respiratory failure requiring mechanical ventilation. He reported 3 months of dyspnea, fever (102°F–103°F), nonproductive cough, and 60 lb weight loss. Chest X-ray (CXR) and computed tomography (CT) chest were significant for irregular airspace disease in the right upper lobe (RUL) medially with smaller focus of airway disease at the left base. Blood cultures grew Methicillin-resistant Staphylococcus aureus, and bronchoalveolar lavage (BAL) cultures grew B. dermatiditis. The patient was treated with amphotericin B and made a full recovery.
Case 2 (2012)
A 39-year-old male, construction worker with PMH significant for acute promyelocytic leukemia and diabetes mellitus (DM), who presented to the ED with complaints of nonproductive cough, chills, night sweats, low-grade fevers (100.8°F), 20 lb weight loss, and headache unresponsive to multiple courses of antibiotics (amoxicillin/clavulanic acid, levofloxacin, and sulfamethoxazole/trimethoprim). CXR was significant for new RUL consolidation. CT chest revealed RUL infiltrate with a small area of necrosis/cavitation. BAL cultures grew B. dermatiditis. Serology was negative for Blastomyces antibody. The patient was treated with itraconazole for 6 months and made a full recovery.
Case 3 (2012)
A 63-year-old male, retired marine and former smoker, with no significant PMH presented to the ED with nonproductive cough, dyspnea, pleuritic chest pain, intermittent fevers, fatigue, and decreased appetite. He was admitted to intensive care unit (ICU) for acute hypoxic respiratory failure. CXR was significant for interstitial densities suspicious for vascular congestion. CT chest showed diffuse ground glass and slightly nodular opacities throughout both lungs, more pronounced in the upper lobes. Initially, BAL cultures showed no growth. After further decline, a second BAL was repeated, cultures grew B. dermatiditis. Serology was positive for Blastomyces antibody. The patient was treated with itraconazole for 6 months and a steroid taper. He made a full recovery.
Case 4 (2015)
A 66-year-old male, sawyer, with PMH significant for hypertension, end-stage renal disease (ESRD), DM, deep-vein thrombosis (DVT), methicillin-sensitive S. aureus bacteremia, and osteomyelitis status post debridement. He presented to the ED with complaints of fever (101°F), nonproductive cough, night sweats, and weight loss for 2 months. CXR revealed LUL infiltrate. Despite intravenous (IV) vancomycin, piperacillin-tazobactam, and caspofungin, the patient continued to be febrile with clinical deterioration. CT chest was obtained and showed confluent parenchymal density consolidations, extending more prominently in the upper lobes. BAL cultures grew B.deratiditis. He was treated with amphotericin B and IV steroids. Over 2 weeks, he improved and was discharged to a rehabilitation center on itraconazole. He died 2 months later of unknown cause.
Case 5 (2016)
A 56-year-old male, former mechanic and welder with a PMH of hypertension, DM presented to the ED with 3 weeks of nonproductive cough, chills, generalized aches, and malaise. CXR was significant for LUL consolidation extending to the perihilar and lingular lung fields. The patient refused admission and was discharged on levofloxacin. He returned a few days later with persistent symptoms. He was admitted and treated with azithromycin and ceftriaxone, later discharged on azithromycin and cefpodoxime. He was re-admitted 6 days later for persistent symptoms and worsening consolidation on CXR. He was started on azithromycin, tobramycin, and cefepime for healthcare-associated pneumonia with BAL showing acute inflammatory cells. On his third re-admission, he was noted to have new skin lesions on his left shoulder. Chest CT was significant for extensive pneumonia in the left lung sparing a small portion of the LUL superiorly. Bronchoscopy was repeated and BAL cultures grew B. dermatiditis. Serology was positive for Blastomyces antibody. He was treated with amphotericin B and methylprednisolone. After 7 days of therapy, he was switched to the oral taper of itraconazole and prednisone. Follow-up chest CT 2 months later showed progressive disease despite clinical improvement. He had a peripherally inserted central catheter placed and began IV amphotericin but was subsequently discontinued due to acute kidney injury. Therapy was completed with voriconazole. After 18 months of anti-fungal therapy, he made a full recovery.
Case 6 (2018)
A 55-year-old male, grain inspector, with a history of provoked pulmonary embolus and DVT, was transferred to our ICU for acute hypoxic respiratory disease requiring mechanical ventilation. CXR was significant for diffuse multifocal consolidations. He reported 6 months of nonproductive cough, fever (101.3°F), dyspnea, night sweats, pleuritic chest pain, and symmetric bilateral joint redness. Initial treatment included azithromycin, ceftriaxone, levofloxacin, clindamycin, meropenem, vancomycin, and IV methylprednisolone. CT Chest was significant for extensive diffuse nodular infiltrates and bilateral consolidations, minimal bilateral pleural effusions, and enlarged mediastinal lymphadenopathy. BAL cultures grew B. dermatiditis. He continued to decline and received extracorporeal membrane oxygenation (ECMO) but did not survive.
Case 7 (2019)
A 55-year-old male, retired chef, with a PMH of alcoholic cirrhosis, chronic pancreatitis, DM, chronic obstructive pulmonary disease, and chronic opioid use presented with hypoxic respiratory failure after 1 month of progressive dyspnea during which he was treated for CAP. CXR was significant for patchy opacities in the bilateral lower bases, left more than right. He deteriorated while on vancomycin, meropenem, and azithromycin, so he was transferred to ICU. CT chest was significant for multifocal consolidation in both lungs with prominent and mildly enlarged mediastinal lymph nodes. BAL cultures grew B. dermatiditis. Blastomyces antibody serology was positive. He was started on amphotericin B but developed worsening hypoxemia, hypotension, and tachycardia. He was deemed not a candidate for ECMO and expired.
Case 8 (2019)
A 73-year-old male, retired rabbi very active in the community, with a PMH of coronary artery disease status postcoronary artery bypass surgery, atrial fibrillation, systolic heart failure, pacemaker placement for third-degree atrioventricular (AV) block, ESRD, and DM presented with a few days of low-grade fevers, nonproductive cough, and malaise. Initial CXR was unremarkable. He was empirically started on meropenem, vancomycin, levofloxacin, and amphotericin for pneumonia. Repeat CXR 24 h later was significant for diffuse nodular opacities in both lungs. He developed hypoxic respiratory failure and shock, was intubated and transferred to ICU. CT chest was significant for a cavitary mass in the left lower lobe. BAL was performed but due to continued deterioration, family withdrew care, and he expired. BAL cultures and serum serology came back positive for B. dermatiditis.
Results
Males accounted for 100% of the cases, with a median age of 56.4 years. All patients were residents of a rural county and resided along the Susquehanna River Valley [Figure 1]. Only one patient had traveled to a known Blastomyces endemic area. We found a common theme of no suspicion for blastomycosis with empiric treatment of bacterial pneumonia that failed to respond to antibiotics. Subsequently, respiratory failure developed, resulting in admission, and evaluation with BAL. There was a delay in diagnosis from the presentation for approximately 6 months to a year, average of 2.2 months [Table 1]. All cases in our series had significant occupational and/or recreational exposures [Table 1].
Figure 1.
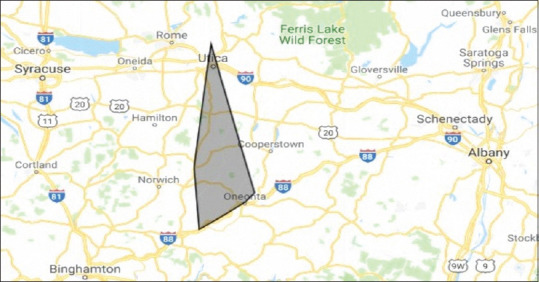
Geographical location of blastomycosis in Upstate New York
Table 1.
Case characteristics
| Cases | New York county | Occupation | Serology | Delay in diagnosis | Deceased |
|---|---|---|---|---|---|
| 1 | Oneida | Farmer | Not applicable | 1 month | Yes |
| 2 | Otsego | Construction | Negative | 6 months | No |
| 3 | Chenango | Marine | Positive | 1 weeks | No |
| 4 | Chenango | Sawyer | Not applicable | 2 months | Yes |
| 5 | Otsego | Mechanic and welder | Positive | 1 month | No |
| 6 | Otsego | Grain inspector | Not applicable | 6 months | Yes |
| 7 | Otsego | Chef | Positive | 1 month | Yes |
| 8 | Otsego | Rabbi | Negative | 1 week | Yes |
Nonproductive cough, dyspnea, and fever were the most common chief complaints. There were no specific clinical or radiologic findings associated with the cases.[1] CXR/CT chest findings ranged from the upper lobe consolidations or diffuse ground opacities [Figures 2 and 3]. Initial negative blastomycosis serologies lead to premature exclusion of the diagnosis in some cases. Definitive diagnosis required growth of the organism in culture material obtained by BAL. Notably, two patients required repeat BAL before the diagnosis was made.
Figure 2.
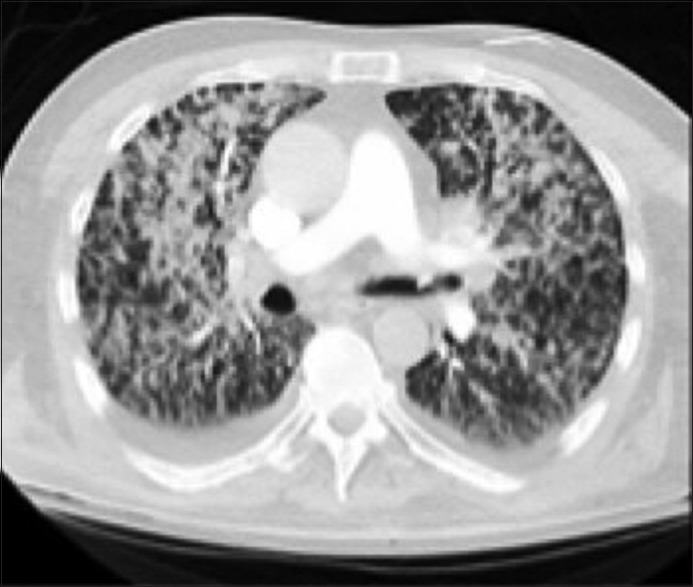
Computed tomography chest with confluent parenchymal density consolidations extending more prominently in the upper lobes
Figure 3.
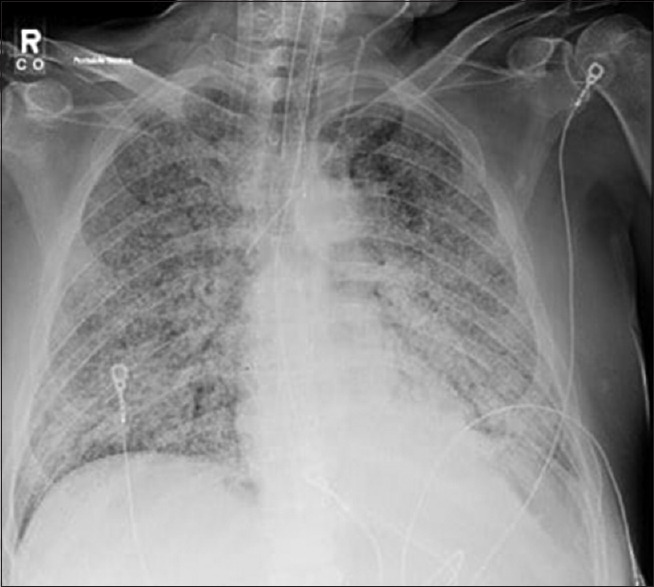
Chest radiograph significant for diffuse multifocal consolidations
There was a 50% mortality rate from acute respiratory distress syndrome (ARDS) in our series. Mortality was proportionate to delay in diagnosis. As would be expected, mortality was highest among those with multiple comorbidities.
Discussion
Blastomycosis is becoming a more prevalent serious infection in Upstate New York. In 2017, it came to the New York Department of Health's attention of an increasing incidence of blastomycosis near the Albany New York area.[3] There was no travel history to an endemic region in the majority of the patients. The annual incidence of blastomycosis has increased from 0.1 cases per 100,000 to 0.2 cases per 100,000 from 2015 to 2016.[3] Our case series demonstrated a similar marked increase in cases at our institution. Admittedly, we have increased our index of suspicion for blastomycosis when patients present with indolent but progressive respiratory symptoms.
There are recognized endemic areas for B.dermatitidis. These areas include states that border the Ohio River and Mississippi River valleys of the United States. The annual incidence has been noted to be higher in residents near waterways of North Central Wisconsin.[2,4] Similarly, our series demonstrated that all of our cases lived along the Susquehanna River Valley. We suspect that the recent flooding in this river basin of Upstate New York[5] could be contributing to the increasing prevalence of blastomycosis. To date, no environmental testing has been conducted.
Interestingly, we observed only males in our series, which in our rural location may predispose work and recreational exposure rather than a sex-related predilection of blastomycosis. Our cohort had significant environmental exposure due to both occupational and recreational hobbies [Table 1]. In the environment, Blastomyces exist as molds, and infection occurs with inhalation of the conidia, which germinate into the yeast phase at body temperature.[2,4] Disruption of the soil at construction sites, excavation, or by other means can release the conidia into the environment. Middle-aged men involved in recreational or occupational activities, for example, hunting, fishing, or activities involved near fresh water, are commonly affected.[1]
Pulmonary blastomycosis presentation in our series varied from an influenza-like illness, clinical pneumonia as well as overt respiratory failure with ARDS. A low index of suspicion and difficulty in diagnosis may have led to a delay in diagnosis and treatment and ultimately increased mortality in our patients. Definite diagnosis requires microscopy and culturing the specific organism. BAL was often required to make the diagnosis. Interestingly, two cases required repeat BAL before the diagnosis was made. Studies have found that sensitivity and specificity for antibody testing are very low, 57% and 30%, respectively.[4] Our series found that serology was not sensitive for diagnosis, with only 42.9% patients having a positive serology.
Conclusions
In summary, this case series highlights the increasing prevalence of blastomycosis in a rural Upstate New York area [Figure 1], not previously considered an endemic region. Blastomycosis should be considered in nonresolving respiratory illness, especially in patients with significant outdoor exposures. Serology is not reliable to exclude blastomycosis,[5] and BAL should continue to be the test of choice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are also grateful to the IT, Microbiology, and Pathology Department at the Bassett Medical Center for their assistance.
References
- 1.McBride JA, Gauthier GM, Klein BS. Clinical manifestations and treatment of blastomycosis. Clin Chest Med. 2017;38:435–49. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Permpalung N, Kaewpoowat Q, Prasidthrathsint K, Chongnarungsin D, Hyman CL. Pulmonary blastomycosis: A new endemic area in New York state. Mycoses. 2013;56:592–5. doi: 10.1111/myc.12073. [DOI] [PubMed] [Google Scholar]
- 3.McDonald R, Dufort E, Jackson BR, Tobin EH, Newman A, Benedict K, et al. Notes from the field: Blastomycosis cases occurring outside of regions with known endemicity - New York, 2007-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1077–8. doi: 10.15585/mmwr.mm6738a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367–81. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susquehanna River Basin Commission. June 2006 Flood: A Summary of the Flood and Performance of the Susquehanna Flood Forecast and Warning System. Susquehanna River Basin Commission. 2007 [Google Scholar]


