Abstract
Cichlid fishes are exceptionally species-rich, speciated at explosive rates and, hence, are a model system in speciation research. Yet, their reproductive isolating barriers have, so far, not been comprehensively studied. Here, we review current knowledge on pre- and postzygotic mechanisms in cichlids. While premating isolation is the norm in cichlids, its strength varies across lineages and with the geographical setting. Moreover, manipulations of ambient conditions tended to reduce assortative mating among closely related species, suggesting that premating isolation in cichlids is often fragile and context dependent. The observed lack of complete reproductive isolation is supported by past and present hybridization events that have contributed to diversity by creating novel allelic combinations. On the other hand, our meta-analysis highlights that intrinsic postzygotic isolation might accumulate faster than assumed. Mild forms of genetic incompatibilities, such as sex ratio distortion, can already be observed among closely related species. Therefore, cessation of gene flow by strong reproductive isolation in cichlids requires a combination of premating prezygotic isolation supplemented with intrinsic and extrinsic postzygotic barriers. Further, we suggest crucial next steps to improve our knowledge about reproductive barriers in cichlids to understand the evolutionary dynamics of pre- and postzygotic isolation mechanisms during adaptive radiations.
This article is part of the theme issue ‘Towards the completion of speciation: the evolution of reproductive isolation beyond the first barriers'.
Keywords: reproductive isolation, prezygotic reproductive isolation, postzygotic reproductive isolation, cichlids, sex ratio distortion
1. Introduction
Cichlid fishes are one of the most species-rich and phenotypically diverse families of vertebrates (e.g. reviewed in [1]). Approximately 10% of all fish species are cichlids and astonishing inter- and intraspecific variation can be observed in almost every trait including morphology and behaviour, but particularly in coloration, which is often sexually dimorphic [2–5]. Cichlids are not only exceptionally species-rich but also speciated at explosive rates. For example, around 250 species evolved in Lake Tanganyika in 10–12 Myr, more than 800 species are endemic to Lake Malawi originating in less than 4 Myr and around 500 species evolved in Lake Victoria in only 15 000–100 000 years [4,6,7]. In the crater lakes of Nicaragua, several species originated even in only a few hundred generations and are less than 2000 years old [8]. Considering these extremely rapid rates at which some radiations evolved in allopatry, and most remarkably speciation also occurred in sympatry, as mainly demonstrated for smaller lake systems (e.g. [8–10]), it is clear why cichlid fishes constitute a model system for the study of speciation and formation of adaptive radiations in different geographical settings and temporal scales.
Hybridization in cichlids has been observed under laboratory conditions and inferred in nature [8,11–13]. The ease with which often fertile hybrids can be generated is one of the reasons why cichlids are increasingly used to study the genomics of adaptation through forward genetic approaches such as quantitative trait loci-analyses (e.g. [14–16]). More relevant, hybrids between nominal cichlid species are also found in nature [8,17–20], suggesting that reproductive isolation barriers can be ‘leaky’. Evidence has accumulated pointing to an important role of hybridization in catalysing cichlid diversification in some adaptive radiations [8,11,21–27]. However, incomplete reproductive isolation and ongoing hybridization in cichlids raise the question of what prevents divergent genetic clusters from collapsing and thereby disrupting or reversing the process of speciation in this lineage?
Strong reproductive isolation is not necessarily the result of one specific barrier completely ceasing gene flow between divergent genetic clusters, but potentially the combination of multiple imperfect isolation barriers [28]. To identify the individual contributions of these barriers to the speciation of cichlid fishes, we review the literature on reproductive isolation and address the following questions: (i) is strong premating isolation the norm in cichlids, does its strength vary between species complexes belonging to different radiations, what is the role of the geographical setting of speciation and what are the cues affecting it? (ii) what is the evidence that prezygotic postmating barriers contribute to reproductive isolation in cichlids? and (iii) given the evolutionary youth of many cichlids, particularly in crater lake radiations, but also in young East African Rift Valley lakes, what is the contribution of intrinsic and extrinsic postzygotic isolation mechanisms to speciation?
2. The importance of geographical settings for the evolution of reproductive isolation
The geographical setting of speciation (i.e. sympatry or allopatry) has important implications for the establishment of reproductive isolation as it can contribute to pre- and postzygotic isolation in different ways [29]. Temporary spatial isolation, as expected under the model of traditional allopatric speciation, was suggested repeatedly to contribute to the astonishing species richness in African cichlids [30–32]. Periods of allopatry have been common in all African Great Lakes owing to fluctuations in the water level, resulting in habitat fragmentation and limiting gene flow [33,34]. Most researches agree on the importance of such isolation events and/or limited dispersal, owing to strong philopatry that cichlids seem to exhibit, for building up genetic divergence between allopatric populations. Evidence for this is provided by phylogeographic studies that tend to find strong isolation by distance within radiations of African cichlids (e.g. [35–38], but see [39]). Consequently, as a by-product of evolutionary divergence, incompatibilities between species accumulate (i.e. Dobzhansky–Muller incompatibilities, DMIs), that can be manifested in pre- and postzygotic isolation barriers [40]. However, most commonly DMIs have been implicated in postzygotic intrinsic isolation owing to the potentially detrimental effects of incompatible alleles being brought together in interspecific hybrids [40]. Theoretically, assuming that mate recognition systems are not under stabilizing selection, allopatric divergence can also favour the evolution of prezygotic isolation [41]. On the other hand, in sympatrically evolving species or in allopatric species coming into secondary contact, reinforcement through natural selection against hybrids with decreased fitness can be a powerful mechanism strengthening prezygotic barriers to gene flow [41], while the evolution of intrinsic postzygotic barriers has been suggested to be rather unlikely under conditions of continuous gene flow ([42], but see [43–45]). As cichlid fishes represent a study system comprising closely related species evolving in allopatry and sympatry, investigating reproductive isolation in cichlids allows us to consider the importance of biogeographic settings on speciation mechanisms, to determine their influences on the relative prevalence and strength of different isolation barriers.
3. Prezygotic isolation
(a). Premating isolation
Premating isolation encompasses all reproductive barriers that diminish heterospecific interactions between sexes to avoid hybridization [46]. In phylogenetically recent lineages, such as cichlids, premating barriers would be expected to be of particular importance for reproductive isolation [41,46]. Disruptive sexual selection based on intraspecific variation is argued to be one of the main contributors to the rapid rate of speciation seen in some cichlid lineages and to play an important role in maintaining cichlid species diversity in sympatry [6,47–49].
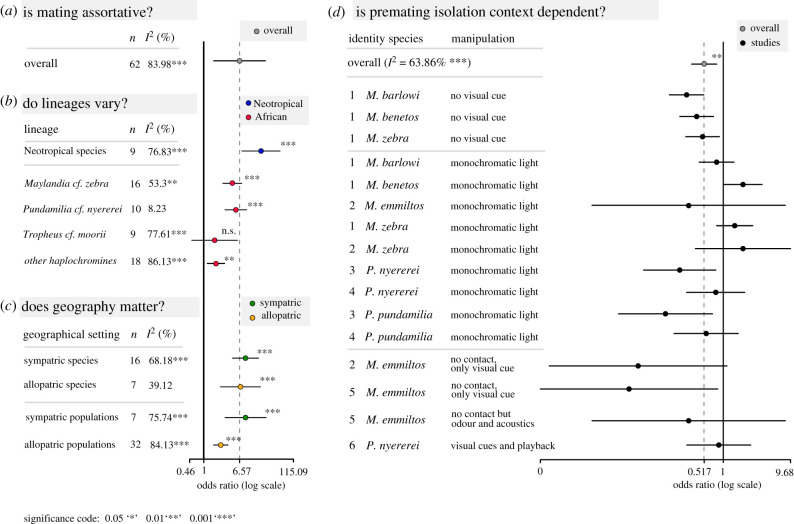
Here, we review the literature asking how commonly premating isolation is observed in cichlids, if it varies across lineages and among sympatric and allopatric species, and what influences its relative strength. A search in ‘Web of Science’ using keywords related to premating isolation in cichlids returned a total of 802 studies (electronic supplementary material, text and table S1). After removing theoretical papers, review articles and studies only citing cichlid literature but using focal species outside of the family Cichlidae, 497 relevant studies remained (electronic supplementary material, text and table S1). Additionally, we added 21 studies that investigated assortative mating in cichlids but were not included in the results of any of these searches. We filtered those studies for direct tests of assortative mating by species or populations. This reduced the number of relevant studies to 39 (electronic supplementary material, table S2), which we subsequently used to conduct a meta-analysis using OpenMEE [50] to investigate premating isolation (for more details, see the electronic supplementary material). Most studies (29 out of 39) reported count data on actual mating events (e.g. spawning events, egg laying, paternity analysis) which we used to conduct a phylogenetically controlled meta-analysis assuming a Brownian motion model (lambda fitted with λ = 1; figure 1). Overall, strong premating isolation seems to be the norm in cichlids (figure 1a). However, heterogeneity (I2, percentage of variation owing to study heterogeneity, [57]) across the investigated studies was high (I2 = 83.98, p < 0.001). There was significant phylogenetic variance (τ2λ = 1.3712) and including phylogenetic correlations as random effects significantly improved the model, as it reduced the amount of unexplained between study variance (τ2 = 0.796 versus τ2 = 0.982 when not controlling for phylogeny). Next, we explored the drivers of this variation. While the strength of premating barriers differed among species complexes, evidence at the meta-analysis level for assortative mate choice was observed in Neotropical lineages (Amphilophus cf. citrinellus and Apistogramma spp.), as well as in the African lineages Maylandia cf. zebra and Pundamilia cf. nyererei, with Tropheus cf. moorii from Lake Tanganyika being the only exception, where mating did not differ from random (figure 1b). All included studies that investigated mate choice in Tropheus cf. moorii were conducted in allopatric populations within species. As it is expected that premating isolation increases with genetic divergence and is favoured in sympatric settings through reinforcement, low levels thereof might potentially explain the absence of assortative mating in the Tropheus species complex. Interestingly, even upon human-induced secondary contact of multiple Tropheus populations differing in colour morphs, there was no evidence of reinforcement but rather signs of introgressive hybridization [58]. Accordingly, we determined if the geographical setting of speciation generally predicts the degree of premating isolation and if the strength of assortative mating differs between nominal species and populations within species (figure 1c). Sympatric and allopatric species differed only slightly in the degree of assortative mating. However, species that occur in allopatry showed more variation in the probability of mating with con- versus heterospecific individuals compared to sympatric ones. By contrast, while sympatric populations (i.e. polymorphisms among populations within species in traits thought to affect mate choice, such as coloration) mated as assortatively as sympatric nominal species, the strength of assortative mating was the weakest among allopatric populations. This might indicate that reinforcement could be especially important for the establishment of premating isolation in populations that have not yet evolved substantial genetic divergence [59]. Some studies reported preference scores as a behavioural proxy for mate choice, which we evaluated in a separate meta-analysis (electronic supplementary material, figure S1). Preference scores usually consider differences in female responsiveness to con- versus heterospecific male behaviour (e.g. quivers, lateral displays). Overall, meta-analyses based on preference scores and count data provided the same qualitative trend: females preferred conspecific over heterospecific males, but there was more variation across studies when considering preference scores. The only lineage differing in the pattern of premating isolation between the analyses was Tropheus cf. moorii. While there was no evidence of assortative mating when considering count data in the Tropheus species complex (figure 1b), the results of the meta-analysis using preference scores were more variable and, in some populations, females did show a preference to interact with conspecific males (electronic supplementary material, figure S1). These differences between count data and preference scores in Tropheus cf. moorii might reflect real differences between populations. However, the large variation across studies might also indicate that comparisons of preference scores across studies can be difficult owing to the differences in measuring female preference.
Figure 1.
Meta-analysis investigating premating isolation in cichlids. Premating isolation in cichlids (a) seems to be the norm as overall mating is assortative, (b) varies among lineages (all lineages comprise both, allopatric and sympatric comparisons except Tropheus cf. moori, which only includes allopatric comparisons) and (c) its strength is influenced by the geographical setting and genetic divergence. (d) Manipulative studies show that mate choice is context dependent. Log odds ratios depicted with 95% confidence intervals (CI) indicate the likelihood of premating isolation. (a–c) Ratios of 1 indicate mating with con- versus heterospecifics is equally likely, values higher than 1 express the fold increase in the likelihood of mating assortatively and values lower than 1 express the fold increase in mating disassortatively. (d) Ratios of 1 indicate mating with con- versus heterospecifics is equally likely as without manipulation, values higher than 1 express the fold increase in the likelihood of mating assortatively after manipulation and values lower than 1 express the fold increase in mating disassortatively after manipulation; n, number of contrasts (this can deviate from the number of studies included, as studies can include more than one contrast); I2, percentage of variability that is owing to heterogeneity across studies rather than sampling variance, identities correspond to studies from which we extracted corresponding data: 1, [51]; 2, [52]; 3, [53]; 4, [54]; 5, [55]; 6, [56]. For more detailed representations of meta-analyses depicted in (b,c), see the electronic supplementary material, figures S2 and S3, respectively.
Given the substantial diversity of the cichlid clade, large variation in the overall degree of premating isolation (figure 1a) is not surprising. Accordingly, it is unlikely that factors contributing to premating isolation can be generalized across all radiations. Below, we discuss in more detail the different cues contributing to the establishment of premating isolation and variation in their importance for assortative mating across lineages.
(b). Mate choice: the importance of different cues
(i). Visual cues: coloration and pattern
Cichlids are renowned for their astonishing diversity in body coloration, with impressive variation in hue and pattern, and it has been repeatedly determined that visual cues are pivotal for their mating behaviour [51,60–63]. Illustrating this diversity, nuptial coloration is positively associated with species richness at the phylogenetic level [64], often strongly varies between closely related species [65], and sometimes even considerable intraspecific variation among different populations can be observed [49,66,67]. Moreover, striking nuptial coloration in dimorphic species conveys information about the reproductive state, quality and status of the male and, therefore, plays a fundamental role in mate choice and sexual selection [68–70]. Therefore, it is not surprising that attention in cichlid fish research has been focused predominantly on the role of visual cues in mate choice [65,67,71].
Intraspecific sexual selection based on male coloration has been clearly established for some species that are polymorphic for coloration [62,67,72]. Several studies have shown that this extends to interspecific mate choice, with females tending to prefer males exhibiting the coloration of their conspecific males [49,72,73], as indicated by our meta-analysis (figure 1a). This is further supported as females prefer heterospecific males of the most similar species in the absence of conspecific males [65], or when exposed to hybrids segregating for nuptial coloration, females choose males that resemble their conspecifics [74,75]. Moreover, species or populations divergent in coloration have repeatedly been found to have high degrees of assortative mating (e.g. [75–79]). However, these results may not apply universally (figure 1b). Sympatric and allopatric populations of several African cichlid species that differ in their body coloration show reduced degrees of assortative mating (e.g. [52,80,81]).
Taken together, these studies highlight the importance of nuptial coloration but also indicate that their contribution to assortative mating might vary across different cichlid lineages. Given that the majority of studies investigating the effect of coloration on premating isolation have been conducted in only a small subset of species (M. cf. zebra from Lake Malawi, P. cf. nyererei from Lake Victoria, T. cf. moorii from Lake Tanganyika and Amphilophus. cf. citrinellus from the Nicaraguan crater lakes, figure 1b; electronic supplementary material, table S2), it is not clear to which degree those results can be generalized to other lineages.
The importance of visual cues during mate choice and the magnificent variation in nuptial coloration suggest that visual perception might be important for reproductive isolation ([5]; figure 1d). Indeed, there is an impressive amount of diversity in visual sensitivities across cichlid fishes [82]. One of the best studied system in this regard is the Pundamilia complex, where light environment, nuptial coloration and visual sensitivity are strongly associated [83,84]. These associations have been interpreted in the context of sensory drive speciation, where variation in light conditions selected for different alleles of the lws gene that encodes the protein component of the red sensitive photoreceptors of the retina. In turn, this affects female preference for different male nuptial coloration. While female preference is correlated with the genotype at the lws locus [84], no association between opsin gene expression and female preference for male nuptial coloration has been found [85]. Moreover, introgression at this locus following hybridization events has been linked to the divergence among Lake Victoria cichlids [86,87]. Whether these associations hold in other cichlid lineages remains unclear. While in many radiations, variation in visual sensitivity is based on differences in opsin expression patterns [88], a general link between opsin expression and nuptial coloration has not been observed [89] and in some lineages, ecological factors rather than nuptial coloration seem to drive opsin expression [21,90].
(ii). Olfactory cues
Compared to the well-investigated nature of visual cues, our understanding of olfactory signals in cichlids is far less complete, partially because compounds relevant to species isolating mate choice have not yet been identified [91]. Nonetheless, it has been argued that the olfactory system plays an important role in various cichlid behaviours including mate choice [91]. The composition of chemical cues in cichlids is complex and only anecdotal evidence suggests differences that could confer species identity (e.g. major histocompatibility complex, [55,92–94]). Males and females use variation in their chemical compounds to communicate physical condition, reproductive state and initiate courtship behaviour [95–97]. Additionally, the composition of odorants provides a unique signature that is important for individual recognition [98,99] and during sexual imprinting [78,100].
Although chemical cues alone appear not to be sufficient to drive assortative mating in cichlids [51,61,101], it has been suggested that visual signals often have to be supplemented by other cues, such as chemical ones, to guide mate choice ([52,55]; figure 1d). In fact, conspecific odour in the presence of random visual stimuli (i.e. heterospecific individuals) can trigger courtship. Male Astatotilapia burtoni will even court distantly related juvenile Tilapia mariae when their tank is supplemented with water conditioned by a gravid A. burtoni female, but will ignore them in the absence of such chemical cues [102]. Even in cases where visual signals are sufficient to initiate mating, olfactory cues may in some species be required to trigger behavioural and hormonal responses crucial for reproduction [95,96]. However, the role of odour in premating isolation rests on the assumption that cichlids can distinguish between conspecific and heterospecific cues, which to our knowledge has not been tested, at least not systematically. Experiments using a set-up as reported by Giaquinto et al. [97] could help to address this question and provide crucial information needed to further evaluate the importance of olfactory cues for premating isolation in cichlids.
(iii). Acoustic cues
Cichlids are capable of producing a variety of different sounds involving the pharyngeal jaw, stridulation or body movements [103–105]. Variation in acoustic parameters is observed across species, among different populations within species and sometimes even high levels of inter- and intraindividual variation are reported [105–108]. Several studies show that cichlids are sensitive to the range of frequencies of the sounds they produce, but compared to goldfish (Carassius auratus), they appear to have poor sensitivity [109–111]. However, some cichlid species possess specialized connections between the swim bladder and the inner ear that improve their auditory abilities [112]. The role of auditory cues in mate choice is implied by the fact that male vocalization is especially prominent during courtship and female sensitivity to low-frequency sounds, as emitted by males during displays, is increased when they are ready to spawn [113,114].
While cichlids are known to show pronounced interspecific differences in their acoustic parameters, most studies assume, but do not demonstrate, that females are actually capable of discriminating between con- or heterospecifics acoustic signals [105,106,114]. Playback experiments show that females perceive acoustic cues and preferably engage in courtship behaviour with males that are associated with sound playback over mere visual stimuli ([115]; figure 1d). Further, both sexes can discriminate between conspecific sounds and bursts of white noise [56]. However, if the specificity of sound perception allows conspecific to be distinguished from heterospecific vocalizations is yet unknown. Thus, predictions about the relative importance of acoustic cues, compared to visual and olfactory ones, in mating are difficult to make and might differ between species. As with chemical signals, sounds alone appear not to be sufficient for mate attraction and only constitute effective signals in combination with visual/olfactory cues [56,107]. Further evaluation of the contribution of acoustic cues to premating isolation will depend on future research investigating sound discrimination capability of cichlids. Playback experiments, as those conducted by Verzijden et al. [115], but testing con- versus heterospecific sounds, will be helpful to elucidate this issue.
(iv). Multimodality: the interplay of different cues
Integrating the knowledge on which cues govern premating isolation in cichlids makes it evident that species assortative mating is rarely based on a single cue, but rather relies on the combination of multiple cues ([52,55], but see [54]). However, depending on the focal species, the importance of these cues might differ and a hierarchical structure is likely [116]. Studies that manipulated various conditions allow us to disentangle the contribution of the different cues for premating isolation (figure 1d). A large percentage of the tests we evaluated, which manipulated or removed specific cues, resulted in reduced premating isolation. However, the effect varied across cues and species. For example, the complete impairment of visual cues led to the reduction in assortative mating without exception in all tested species. On the other hand, effects of manipulations masking colour differences between species by using monochromatic light strongly depended on the tested lineage. While females of P. cf. nyererei could not distinguish between conspecific and heterospecific males any longer ([54]; figure 1d, [117]), it had no effect on assortative mating in M. cf. zebra ([51,52]; figure 1d). This indicates that the importance of cues might differ, as in some cases not coloration per se but rather colour pattern might be important ([118]; figure 1d). By contrast, the effects of impeding olfactory and auditory cues were more variable and less pronounced compared to visual ones, suggestive of an overall smaller relevance of non-visual cues.
Importantly, some of these studies exemplify that premating barriers are context dependent. Under the conditions in which the respective cues evolved, they seem to be a powerful driver for premating isolation. However, drastic environmental changes that constrain the perception of some cues may exacerbate mate choice, increasing interspecific gene flow. Eutrophication and associated turbidity in some regions of Lake Victoria, for example, can result in hybridization between the sympatric species pair P. cf. pundamilia and P. cf. nyererei, while these species mate strongly assortatively in clear water where visual signals are not affected by turbidity [18].
Determining if the importance of different cues for premating isolation varies among cichlid radiations will be highly interesting and might be important to study the genetic basis of premating isolation. However, comprehensive analyses elucidating this issue will depend on further manipulation studies, disentangling the relative importance of different sensory modalities as conducted by Blais et al. [52] and Selz et al. [54] and including more species complexes.
(c). Postmating prezygotic isolation
Postmating prezygotic isolation (or gametic isolation) comprises all reproductive barriers acting between spawning and fertilization and is comparatively less studied in most organisms than premating isolation mechanisms [46], with cichlids being no exception. This is particularly unfortunate because the rapid evolution of reproductive proteins, mediating different processes after copulation including fertilization [119], has been attributed an important role in speciation in the last decades [46,120,121]. Gametic proteins evolve as by-product of sexual selection and an arms race between male and female proteins could result in particularly fast establishment of gametic isolation [46]. One particular good study, investigating interspecific fertilization rates in 26 heterospecific pairs of African cichlids, showed that fertilization failure linearly increases with divergence time, with complete fertilization failure observed after around 4 Myr (divergence time estimate depends on molecular clock used and can have wide confidence intervals; here, divergence time was calculated from a linear, internally calibrated clock using only recent biogeographic events, [12]). One of the few other examples testing specifically for postmating isolation between the closely related Lake Malawi species M. zebra and M. benetos, however, found no differences between interspecific fertilization rates and conspecific controls [122]. This indicates that gametic isolation in cichlids might not be as common and accumulating not as rapidly as proposed for other taxa [121,123,124].
4. Postzygotic isolation
(a). Intrinsic postzygotic isolation
Intrinsic postzygotic isolation constitutes one of the most effective reproductive barriers owing to its irreversibility [125]. It can affect various life stages and is marked by flawed hybrid development that, at worst, results in hybrid inviability or physiological or behavioural hybrid sterility [46]. While the relative contribution of intrinsic barriers to reproductive isolation differs among species, there is agreement upon the fact that such barriers accumulate exponentially with genetic distance [12,46,126,127]. It has been implied that intrinsic incompatibilities are unlikely to evolve in the face of gene flow [42], however, this view has been challenged recently (e.g. [43–45]). In cichlids, intrinsic barriers have been suggested to be neglectable among closely related species (e.g. [128,129]) and only cause complete hybrid inviability after around 4 Myr of divergence time (divergence time estimate depends on molecular clock used and can have wide confidence intervals; here, divergence time was calculated from a linear, internally calibrated clock using only recent biogeographic events; [12]). Considering that many cichlid radiations are much younger [21], it does not come as a surprise that only a minor role in conferring reproductive isolation in cichlids has been attributed to intrinsic barriers and some studies even consider their contribution to maintain species boundaries unlikely [49,128,130].
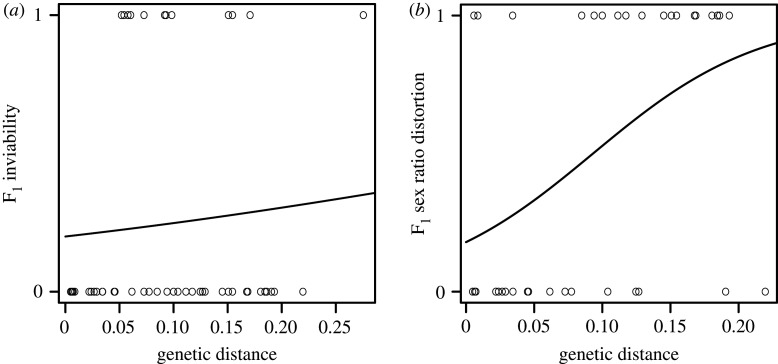
We reviewed the literature to evaluate signatures of intrinsic incompatibilities in the form of inviability and sex ratio distortion among F1 hybrids of interspecific cichlid crosses (electronic supplementary material, table S3) with respect to genetic distance. D-loop sequences obtained from NCBI GenBank were used to compute pairwise genetic distances for interspecific crosses. The D-loop region is commonly sequenced and was, therefore, available for the majority of included species (for species without available D-loop sequences in GenBank, we used sequences of closely related species, electronic supplementary material, table S3). While we acknowledge that D-loop sequences might underestimate the actual level of genetic differentiation compared to more accurate estimates provided by larger datasets [131,132], those were not readily available for all the species used in this analysis. Intrinsic hybrid incompatibilities were analysed using a generalized linear model with binomial error distribution in R considering F1 viability or sex ratio distortion as response variable and genetic distance as explanatory variable (for more details on the methods, see the electronic supplementary material). While Stelkens et al. [12] provide a quantitative measure of hybrid inviability, the vast majority of studies only scored hybrids as viable or inviable. The same was done in studies reporting on sex ratio distortion. Therefore, we only consider binary data. While we acknowledge that this might not quantify the absolute strength of intrinsic incompatibilities, it provides comprehensive insights into the relative timing of the accumulation of hybrid inviability and hybrid sex ratio distortion, contributing to overall intrinsic postzygotic isolation.
Based on our analysis, hybrid inviability is typically not observed among closely related species (figure 2a). These results are in line with previous studies [12], suggesting that complete hybrid inviability is reached only among more divergent cichlid species. The non-significant effect of genetic distance for F1 viability (estimate = 2.801, z = 0.581, p = 0.561) can probably be accounted for by the small number of studies reporting inviable hybrid crosses (electronic supplementary material, table S3). However, hybrid inviability appears not to be a necessary consequence of divergence in cichlids, because viable F1 offspring could be observed even in crosses with substantial genetic distance (figure 2a).
Figure 2.
Intrinsic incompatibilities in cichlids have been evaluated using (a) F1 hybrid inviability (n = 52 interspecific comparisons) and (b) F1 hybrid sex ratio distortion (n = 39 interspecific comparisons) as a function of pairwise genetic distances computed using D-loop sequences. Individual data points represent single interspecific crosses. Inviability of crosses and reports of sex ratio distortion were scored 1, while viable crosses and no reported skew in sex ratio were scored 0. Logistic regressions are depicted as black lines for F1 hybrid inviability (a; estimate = 2.801, z = 0.581, p = 0.561) and F1 sex ratio distortion (b; estimate = 16.330, z = 2.764, p = 0.006).
Sex ratio distortion was also frequently detected in interspecific cichlid crosses (figure 2b; electronic supplementary material, table S3). Although its frequency increases significantly with genetic distance (estimate = 16.330, z = 2.764, p = 0.006), it can also be observed among closely related species (figure 2b), indicating that less severe intrinsic incompatibilities are manifested earlier than assumed. Skewed sex distributions are intriguing as they potentially point towards Haldane's rule, which states ‘when in offspring of two animal races one sex is absent, rare or sterile, that sex is the heterozygous sex’ [133, p. 1]. As the incompatibility described by this rule depicts an early stage in the evolution of postzygotic reproductive isolation, it is especially meaningful for young lineages where signs of intrinsic isolation seem to follow Haldane's rule in many species [46]. Because sex determination in cichlids is variable and complex [134], adherence to Haldane's rule as an intrinsic barrier remains only speculative [128,135]. Owing to the scant information on hybrid viability and other factors like hybrid sex ratio, our conclusions are only tentative. Yet, they might encourage future studies to report fitness consequences for hybrid crosses like growth rate, sex ratio and mortality, as this information will be required to improve our understanding of the importance of intrinsic isolation in cichlids.
Whereas intrinsic incompatibilities between the parental genomes may be shielded in F1 hybrids, incompatible alleles causing severe developmental problems are often unmasked in the second generation by hybrid breakdown [136–138]. Only few studies in cichlids have focused on the fitness consequences of these postzygotic barriers, i.e. DMIs [139,140]. Second-generation hybrids of seven interspecific crosses of African haplochromine cichlids (mostly comprising crosses among sympatric species but also some among allopatric ones, for details, see [139]) showed significantly lower fitness compared to the F1 hybrids and the pure grandparental lineages, and F2 inviability increased with divergence time [139]. Interestingly, segregation distortion in F2 hybrids between closely related sympatric species of Nicaraguan Midas cichlids indicate that hybrid incompatibilities in some cases might emerge earlier than assumed [140].
Extreme cases of intrinsic incompatibilities are posed by major genomic alterations, such as changes in karyotypes or chromosomal rearrangements. Such forms of intrinsic incompatibilities have been reported in multiple different cichlid lineages (reviewed in [141]) and probably played a role in allopatric (e.g. Apistogramma spp., [142]), as well as in sympatric settings (e.g. Laetacara cf. dorsigera, [143]). While this form of intrinsic incompatibilities can generally cause strong and irreversible reproductive isolation, it is not clear if these incompatibilities were instrumental in the speciation process of the respective lineages or whether they only evolved after strong reproductive isolation was already established. Clearly, more comparative studies investigating such major genomic rearrangements (e.g. [144]) are needed to determine the generality of those findings and their contribution to reproductive isolation in different cichlid radiations. Taken together, all these studies stress that even though intrinsic barriers require divergence time to confer strong reproductive isolation, they could still play a role in young lineages, such as most cichlid radiations, and should not be excluded based solely on evolutionary youth.
(b). Extrinsic postzygotic isolation
Extrinsic ecological reproductive isolation, often referred to as ecological inviability, is characterized by decreased performance of hybrids in the respective environment of the parental species [46]. Studies investigating hybrid performance in the wild are rare (but see [145–147]). Nonetheless, extrinsic isolation has been attributed an important role in speciation owing to the potential to diminish the number of hybrids, and Coyne & Orr [46, p. 255] even argued that ‘it has become fashionable to suggest that extrinsic, and especially ecological, postzygotic isolation is more common or more important than intrinsic in nature. This might well be true. But at present, such assertations rest more on intuition than data’. This lack of knowledge is partially owing to the difficulty of obtaining large-scale ecological information required to correctly assess the effect of extrinsic postzygotic isolation [46]. Unfortunately, more than 15 years later, this still reflects our rather poor understanding of extrinsic barriers compared to our knowledge of premating barriers in cichlids.
Ecologically relevant phenotypes are frequently found to be intermediate in cichlid hybrids compared to the parental species [15,16]. Hence, it is commonly assumed that hybrids might have poor ecological performance and suffer from reduced fitness. Given that many cichlid species are highly specialized, it is possible that hybrids with intermediate phenotypes are often not as fit in nature as the parental phenotypes and some studies support this assumption [129,148–150]. The importance of extrinsic ecological isolation rests on the strong assumption that there are no free niches and no ecological opportunities for hybrids with intermediate phenotypes in nature. In case those niches do exist, they might be occupied by other species that potentially outcompete hybrids [46,151], as the ecological space inhabited by cichlids tends to be heavily packed [47,152].
Besides ecological inviability, extrinsic isolation also comprises behavioural hybrid sterility, as intermediate states of phenotypes important for mate choice can lower attractiveness and render it difficult for hybrids to find mates [46]. The importance of this barrier has been demonstrated for various taxa (e.g. [153,154]) and it probably also contributes to reproductive isolation in cichlids as hybrids often exhibit intermediate phenotypes in traits relevant for mate choice [23,74,75,148,155]. In most cases, females of the parental species show a clear preference for conspecific males over heterospecifics and hybrids [75,155]. Taken together, these studies suggest reduced mating success of hybrids consistent with behavioural sterility.
5. Hybridization
Traditionally, hybridization has been considered to disrupt the process of speciation [156–158]. Owing to incomplete reproductive isolation, species can hybridize, which potentially causes divergent genetic clusters to collapse, negatively affecting species richness [18]. However, recent studies of East African cichlids have challenged this view by providing evidence that hybridization is not necessarily a destructive force to cichlid species diversity but actually fuelled explosive speciation bursts at early stages of these adaptive radiations [21,25,86]. Hybridization has the potential to drastically increase genetic and phenotypic diversity and it has been shown that entire species flocks are of hybrid origin for Lake Tanganyika [21], Lake Mweru [25] and the entire Lake Victoria region [86]. Ongoing hybridization is still frequently observed among cichlids and can create new phenotypes with extreme trait values [22,159]. Given ecological opportunity, such novelty can give hybrids the potential to outperform parental species outside of their respective niches [149] and if paired with non-random mating preferences, as demonstrated for some interspecific hybrids [75], this might set the stage for potential hybrid speciation. Therefore, strong but leaky reproductive isolation allowing for rare hybridization events in the presence of ecological opportunity has been demonstrated to be an important source of genetic variation and can catalyse radiation.
6. Conclusion
The importance of cichlid fishes as an evolutionary model system can be, among others, attributed to their incredible species richness and explosive diversification rates [1,2,4]. While cichlid diversification has been extensively studied (e.g. [1,3,4]), much less is known about what causes cessation of gene flow between diverging populations. However, in order to understand the origin and maintenance of the magnificent cichlid radiations, we urgently need to improve our knowledge concerning the barriers that confer reproductive isolation.
Based on theory, premating isolation has been predicted to contribute most to overall reproductive isolation, as it acts early in the sequence of reproductive barriers and, therefore limits gene flow substantially [46,160,161]. Accordingly, premating isolation plays an important role in cichlids, as suggested by strong evidence at the meta-analysis level for assortative mating (figure 1a), which is in line with previously published work (e.g. [12,47,48]). However, its strength is variable. While fishes within certain cichlid lineages mate strongly assortatively, this does not necessarily apply for other lineages (figure 1b). Variation in the degree of premating isolation is probably influenced by a combination of genetic divergence and the geographical context, as expected under reinforcement (figure 1c). Studies investigating premating isolation are currently restricted to relatively few species complexes (i.e. Maylandia cf. zebra, Pundamilia cf. nyererei, Tropheus cf. moori, Amphilophus cf. citrinellus and Apistogramma spp.). Considering this lack of phylogenetic coverage and given the great diversity of the cichlid clade (e.g. reviewed in [1]), it is not surprising that strength and mechanisms of premating isolation differ across lineages and no general pattern has emerged, yet. Moreover, premating isolation in cichlids seems to be context dependent (figure 1d). While premating barriers can be very effective in limiting gene flow under the conditions they evolved in, they might not be highly resilient when environments are subject to drastic changes, as it is demonstrated for some lineages where species diversity is reduced after environmental change [18,162]. This indicates that strong reproductive isolation in cichlids most likely depends on the combination of multiple reproductive barriers. Our meta-analysis suggests that mild forms of intrinsic incompatibilities, such as sex ratio distortion, can already be observed among closely related species (figure 2b) and potentially supplement premating barriers. Moreover, a contribution of extrinsic postzygotic isolation is likely [129,148,149]. However, the importance of these different barriers for conferring reproductive isolation can vary along the speciation continuum [163,164].
The speciation continuum has been suggested to consist of different stages based on divergence and strength of reproductive isolation among populations [44,165–167]. Adaptive radiations are very informative to elucidate the signatures of these different stages because lineages within radiations may differ in the strength of reproductive isolation, as observed in stickleback fishes (Gasterosteidae) and Heliconius butterflies (Nymphalidae) [166,167]. Our meta-analyses suggest that members of different radiations of cichlid fishes also seem to vary in their stages along the speciation continuum (figures 1 and 2). Some cichlid lineages, such as Tropheus cf. moori, appear to be still at an early stage, where populations have undergone differentiation in some phenotypic traits (e.g. coloration), but levels of reproductive isolation are still low and genetic divergence might not be sufficient for reinforcement to act upon secondary contact ([58]; figure 1b). By contrast, other lineages, for example Pundamilia cf. nyererei, represent more advanced stages along the speciation continuum, marked by strong reproductive isolation (figure 1b). However, speciation is still incomplete, as changes in environmental conditions can disrupt species assortative mating [18]. As proposed for late stages in sticklebacks [167], completion of the speciation process in cichlids might require the establishment of major intrinsic incompatibilities, such as changes in karyotypes or chromosomal rearrangements, which can already be observed in some cichlid lineages [141–144]. Because of great variation in the strength of reproductive isolation among different lineages, cichlid fishes might provide powerful contrasts to elucidate further characteristics distinguishing different stages along the speciation continuum associated with different biogeographic settings. However, this will require an increase in the number of comparative studies on different aspects of reproductive isolation across a broad taxonomic range of cichlid lineages, especially among radiations that differ in their evolutionary time.
We suggest two lines of research that will substantially further our understanding of the barriers that might be essential to identify stages along the speciation continuum in cichlid fishes. Firstly, it will be crucial to improve the knowledge on linkage of genomic loci important for reproductive isolation. Early stages of differentiation are usually marked by signatures of divergent selection on few genomic loci and their surrounding regions (e.g. divergence hitchhiking, [165,168]). During later stages along the speciation continuum, linkage among multiple such loci under divergent selection might lead to an overall reduction in gene flow throughout the genome that results in genome-wide differentiation (e.g. genome hitchhiking, [44]). Given the increase in genomic resources available for cichlid fishes in the last decade (e.g. [169]), a necessary next step will be to look for linkage among genes known to contribute to reproductive isolation. Interesting candidates could be genes affecting visual sensitivity, female preference and nuptial coloration (see §3b(i), Visual cues: coloration and pattern). Knowledge about the genomic regions controlling visual sensitivity (e.g. [170]) and coloration (e.g. [15]) in cichlids is emerging. However, much less is known about the genetic basis of female preferences for nuptial coloration, although experimental studies suggest that it indeed has a genetic component [75,171]. Progress in determining the genomic regions affecting preference, coloration and visual sensitivity, as well as exploring the link among them will have a great impact on our understanding of premating isolation. Secondly, at the moment literature on reproductive isolation in cichlid fishes is highly skewed towards premating isolation, while the conclusions about the commonality and strength of postzygotic barriers rest on few studies. Therefore, it will be important to increase research directed towards determining the contribution of postzygotic reproductive isolation. It will be especially important to understand the role of divergence time and geographical settings for the evolution of intrinsic postzygotic barriers. The Lake Tanganyika radiations might be key in addressing the question of how genetic distance relates to the evolution of intrinsic incompatibilities. Lake Tanganyika is not only the oldest of the East African Rift Lakes, but its cichlid species flock is also the most diverse in ecological, morphological and genetic terms [172]. Different Tanganyikan lineages, like Ectodini, Lamprologini and Tropheini, have radiated to different degrees and vary significantly in their divergence time [21]. Thus, Lake Tanganyika offers the possibility for comparisons among radiations within the same lake, that might be essential to identify DMIs. Further, it also allows for meaningful contrasts to determine the strength of reproductive isolation during different stages along the speciation continuum. Following these aforementioned lines of research will not only improve our knowledge of the barriers that triggered and maintain the current cichlid diversity, but can also contribute to the understanding of the evolutionary dynamics that characterize different stages along the speciation continuum.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Andreas F. Kautt for fruitful discussion and valuable comments.
Data accessibility
All the data used in this Review are included in the electronic supplementary material
Authors' contributions
S.J.R., J.T.-D. and A.M. conceptualized the project and S.J.R. and J.T.-D. collected and analysed the data. S.J.R. wrote the manuscript with revisions from all authors.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the University of Konstanz. S.J.R. was funded by a fellowship from the Hector Fellow Academy and J.T.-D. was supported by grants of the DFG (grant no. TO914/3-1) and (grant no. ME1725/22-1) and the Zukunftskolleg of the University of Konstanz.
References
- 1.Stiassny ML, Meyer A. 1999. Cichlids of the rift lakes. Sci. Am. 280, 64–69. ( 10.1038/scientificamerican0299-64) [DOI] [Google Scholar]
- 2.Henning F, Meyer A. 2014. The evolutionary genomics of cichlid fishes: explosive speciation and adaptation in the postgenomic era. Annu. Rev. Genomics Hum. Genet. 15, 417–441. ( 10.1146/annurev-genom-090413-025412) [DOI] [PubMed] [Google Scholar]
- 3.Salzburger W. 2009. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol. Ecol. 18, 169–185. ( 10.1111/j.1365-294X.2008.03981.x) [DOI] [PubMed] [Google Scholar]
- 4.Kocher TD. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298. ( 10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 5.Maan ME, Sefc KM (eds). 2013. Colour variation in cichlid fish: developmental mechanisms, selective pressures and evolutionary consequences. Semin. Cell Dev. Biol. 24, 516–528. ( 10.1016/j.semcdb.2013.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner CE, Harmon LJ, Seehausen O. 2012. Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366–369. ( 10.1038/nature11144) [DOI] [PubMed] [Google Scholar]
- 7.Cohen AS, Soreghan MJ, Scholz CA. 1993. Estimating the age of formation of lakes: an example from Lake Tanganyika, East African Rift system. Geology 21, 511–514. () [DOI] [Google Scholar]
- 8.Kautt AF, Machado-Schiaffino G, Meyer A. 2016. Multispecies outcomes of sympatric speciation after admixture with the source population in two radiations of Nicaraguan crater lake cichlids. PLoS Genet. 12, e1006157 ( 10.1371/journal.pgen.1006157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A. 2006. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439, 719–723. ( 10.1038/nature04325) [DOI] [PubMed] [Google Scholar]
- 10.Malinsky M, et al. 2015. Genomic islands of speciation separate cichlid ecomorphs in an East African crater lake. Science 350, 1493–1498. ( 10.1126/science.aac9927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzburger W, Baric S, Sturmbauer C. 2002. Speciation via introgressive hybridization in East African cichlids? Mol. Ecol. 11, 619–625. ( 10.1046/j.0962-1083.2001.01438.x) [DOI] [PubMed] [Google Scholar]
- 12.Stelkens RB, Young KA, Seehausen O. 2010. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution 64, 617–633. ( 10.1111/j.1558-5646.2009.00849.x) [DOI] [PubMed] [Google Scholar]
- 13.Olave M, Meyer A. In press. Implementing large genomic single nucleotide polymorphism data sets in phylogenetic network reconstructions: a case study of particularly rapid radiations of cichlid fish. Syst. Biol. [DOI] [PubMed] [Google Scholar]
- 14.Franchini P, Fruciano C, Spreitzer ML, Jones JC, Elmer KR, Henning F, Meyer A. 2014. Genomic architecture of ecologically divergent body shape in a pair of sympatric crater lake cichlid fishes. Mol. Ecol. 23, 1828–1845. ( 10.1111/mec.12590) [DOI] [PubMed] [Google Scholar]
- 15.Kratochwil CF, Liang Y, Gerwin J, Woltering JM, Urban S, Henning F, Machado-Schiaffino G, Hulsey CD, Meyer A. 2018. Agouti-related peptide 2 facilitates convergent evolution of stripe patterns across cichlid fish radiations. Science 362, 457–460. ( 10.1126/science.aao6809) [DOI] [PubMed] [Google Scholar]
- 16.Henning F, Machado-Schiaffino G, Baumgarten L, Meyer A. 2017. Genetic dissection of adaptive form and function in rapidly speciating cichlid fishes. Evolution 71, 1297–1312. ( 10.1111/evo.13206) [DOI] [PubMed] [Google Scholar]
- 17.Elder H, Garrod D, Whitehead P. 1971. Natural hybrids of the African cichlid fishes Tilapia spilurus nigra and T. leucosticta: a case of hybrid introgression. Biol. J. Linn. Soc. 3, 103–146. ( 10.1111/j.1095-8312.1971.tb00173.x) [DOI] [Google Scholar]
- 18.Seehausen O, Van Alphen JJ, Witte F. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. ( 10.1126/science.277.5333.1808) [DOI] [Google Scholar]
- 19.Koblmüller S, Duftner N, Sefc KM, Aibara M, Stipacek M, Blanc M, Egger B, Sturmbauer C. 2007. Reticulate phylogeny of gastropod-shell-breeding cichlids from Lake Tanganyika—the result of repeated introgressive hybridization. BMC Evol. Biol. 7, 7 ( 10.1186/1471-2148-7-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loiselle P. 1971. Hybridization in cichlids. Buntb. Bull. 27, 9–18. [Google Scholar]
- 21.Irisarri I, et al. 2018. Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes. Nat. Commun. 9, 3159 ( 10.1038/s41467-018-05479-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelkens RB, Schmid C, Selz O, Seehausen O. 2009. Phenotypic novelty in experimental hybrids is predicted by the genetic distance between species of cichlid fish. BMC Evol. Biol. 9, 283 ( 10.1186/1471-2148-9-283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PF, Konings A, Kornfield I. 2003. Hybrid origin of a cichlid population in Lake Malawi: implications for genetic variation and species diversity. Mol. Ecol. 12, 2497–2504. ( 10.1046/j.1365-294X.2003.01905.x) [DOI] [PubMed] [Google Scholar]
- 24.Bell MA, Travis MP. 2005. Hybridization, transgressive segregation, genetic covariation, and adaptive radiation. Trends Ecol. Evol. 20, 358–361. ( 10.1016/j.tree.2005.04.021) [DOI] [PubMed] [Google Scholar]
- 25.Meier JI, et al. 2019. The coincidence of ecological opportunity with hybridization explains rapid adaptive radiation in Lake Mweru cichlid fishes. Nat. Commun. 10, 5391 ( 10.1038/s41467-019-13278-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marques DA, Meier JI, Seehausen O. 2019. A combinatorial view on speciation and adaptive radiation. Trends Ecol. Evol. 34, 531–544. ( 10.1016/j.tree.2019.02.008) [DOI] [PubMed] [Google Scholar]
- 27.Rüber L, Meyer A, Sturmbauer C, Verheyen E. 2001. Population structure in two sympatric species of the Lake Tanganyika cichlid tribe Eretmodini: evidence for introgression. Mol. Ecol. 10, 1207–1225. ( 10.1046/j.1365-294X.2001.01259.x) [DOI] [PubMed] [Google Scholar]
- 28.Butlin RK, Smadja CM. 2018. Coupling, reinforcement, and speciation. Am. Nat. 191, 155–172. ( 10.1086/695136) [DOI] [PubMed] [Google Scholar]
- 29.Butlin R, Debelle A, Kerth C, Snook RR, Beukeboom LW, Castillo RC et al. 2012. What do we need to know about speciation? Trends Ecol. Evol. 27, 27–39. ( 10.1016/j.tree.2011.09.002) [DOI] [PubMed] [Google Scholar]
- 30.Sturmbauer C. 1998. Explosive speciation in cichlid fishes of the African Great Lakes: a dynamic model of adaptive radiation. J. Fish Biol. 53, 18–36. ( 10.1111/j.1095-8649.1998.tb01015.x) [DOI] [Google Scholar]
- 31.Sturmbauer C, Meyer A. 1992. Genetic divergence, speciation and morphological stasis in a lineage of African cichlid fishes. Nature 358, 578 ( 10.1038/358578a0) [DOI] [PubMed] [Google Scholar]
- 32.Mayr E. 1984. Evolution of fish species flocks: a commentary. In Evolution of fish species flocks (eds Echelle AA, Kornfield I), pp. 3–12. Orono, ME: University of Maine at Orono Press. [Google Scholar]
- 33.Echelle AA, Kornfield I. 1984. Evolution of fish species flocks Orono, ME: University of Maine at Orono Press. [Google Scholar]
- 34.Verheyen E, Rüber L, Snoeks J, Meyer A. 1996. Mitochondrial phylogeography of rock-dwelling cichlid fishes reveals evolutionary influence of historical lake level fluctuations of Lake Tanganyika, Africa. Phil. Trans. R. Soc. Lond. B 351, 797–805. ( 10.1098/rstb.1996.0074) [DOI] [PubMed] [Google Scholar]
- 35.Taylor MI, Rüber L, Verheyen E. 2001. Microsatellites reveal high levels of population substructuring in the species-poor Eretmodine cichlid lineage from Lake Tanganyika. Proc. R. Soc. Lond. B 268, 803–808. ( 10.1098/rspb.2000.1580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PF, Kornfield I. 2002. Phylogeography of Lake Malawi cichlids of the genus Pseudotropheus: significance of allopatric colour variation. Proc. R. Soc. Lond. B 269, 2495–2502. ( 10.1098/rspb.2002.2188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koblmüller S, Sefc KM, Duftner N, Warum M, Sturmbauer C. 2007. Genetic population structure as indirect measure of dispersal ability in a Lake Tanganyika cichlid. Genetica 130, 121–131. ( 10.1007/s10709-006-0027-0) [DOI] [PubMed] [Google Scholar]
- 38.Raeymaekers JA, et al. 2013. Contrasting parasite communities among allopatric colour morphs of the Lake Tanganyika cichlid Tropheus. BMC Evol. Biol. 13, 41 ( 10.1186/1471-2148-13-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer A, Knowles L, Verheyen E. 1996. Widespread geographical distribution of mitochondrial haplotypes in rock-dwelling cichlid fishes from Lake Tanganyika. Mol. Ecol. 5, 341–350. ( 10.1111/j.1365-294X.1996.tb00325.x) [DOI] [PubMed] [Google Scholar]
- 40.Orr HA, Turelli M. 2001. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution 55, 1085–1094. ( 10.1111/j.0014-3820.2001.tb00628.x) [DOI] [PubMed] [Google Scholar]
- 41.Butlin R. 1987. Speciation by reinforcement. Trends Ecol. Evol. 2, 8–13. ( 10.1016/0169-5347(87)90193-5) [DOI] [PubMed] [Google Scholar]
- 42.Bank C, Bürger R, Hermisson J. 2012. The limits to parapatric speciation: Dobzhansky–Muller incompatibilities in a continent–island model. Genetics 191, 845–863. ( 10.1534/genetics.111.137513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulmuni J, Westram A. 2017. Intrinsic incompatibilities evolving as a by-product of divergent ecological selection: considering them in empirical studies on divergence with gene flow. Mol. Ecol. 26, 3093–3103. ( 10.1111/mec.14147) [DOI] [PubMed] [Google Scholar]
- 44.Feder JL, Nosil P, Wacholder AC, Egan SP, Berlocher SH, Flaxman SM. 2014. Genome-wide congealing and rapid transitions across the speciation continuum during speciation with gene flow. J. Hered. 105, 810–820. ( 10.1093/jhered/esu038) [DOI] [PubMed] [Google Scholar]
- 45.Agrawal AF, Feder JL, Nosil P. 2011. Ecological divergence and the origins of intrinsic postmating isolation with gene flow. Int. J. Ecol. 2011, 1–15. ( 10.1155/2011/435357) [DOI] [Google Scholar]
- 46.Coyne J, Orr H. 2004. Speciation. Sunderland, MA: Sinauer. [Google Scholar]
- 47.Seehausen O. 2000. Explosive speciation rates and unusual species richness in haplochromine cichlid fishes: effects of sexual selection. Adv. Ecol. Res. 31, 237–274. ( 10.1016/S0065-2504(00)31015-7) [DOI] [Google Scholar]
- 48.Wilson AB, Noack-Kunnmann K, Meyer A. 2000. Incipient speciation in sympatric Nicaraguan crater lake cichlid fishes: sexual selection versus ecological diversification. Proc. R. Soc. Lond. B 267, 2133–2141. ( 10.1098/rspb.2000.1260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maan ME, Seehausen O, Söderberg L, Johnson L, Ripmeester EA, Mrosso HD, Taylor MI, Van Dooren TJ, Van Alphen JJ. 2004. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. Lond. B 271, 2445–2452. ( 10.1098/rspb.2004.2911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace BC, Lajeunesse MJ, Dietz G, Dahabreh IJ, Trikalinos TA, Schmid CH, Gurevitch J. 2017. Open MEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 8, 941–947. ( 10.1111/2041-210X.12708) [DOI] [Google Scholar]
- 51.Jordan R, Kellogg K, Juanes F, Stauffer J Jr. 2003. Evaluation of female mate choice cues in a group of Lake Malawi mbuna (Cichlidae). Copeia 2003, 181–186. ( 10.1643/0045-8511(2003)003[0181:EOFMCC]2.0.CO;2) [DOI] [Google Scholar]
- 52.Blais J, Plenderleith M, Rico C, Taylor MI, Seehausen O, van Oosterhout C, Turner GF. 2009. Assortative mating among Lake Malawi cichlid fish populations is not simply predictable from male nuptial colour. BMC Evol. Biol. 9, 53 ( 10.1186/1471-2148-9-53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seehausen O, Witte F, Van Alphen J, Bouton N. 1998. Direct mate choice maintains diversity among sympatric cichlids in Lake Victoria. J. Fish Biol. 53, 37–55. ( 10.1111/j.1095-8649.1998.tb01016.x) [DOI] [Google Scholar]
- 54.Selz OM, Pierotti ME, Maan ME, Schmid C, Seehausen O. 2014. Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 25, 612–626. ( 10.1093/beheco/aru024) [DOI] [Google Scholar]
- 55.Plenderleith M, Oosterhout C, Robinson RL, Turner GF. 2005. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 1, 411–414. ( 10.1098/rsbl.2005.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estramil N, Bouton N, Verzijden MN, Hofker K, Riebel K, Slabbekoorn H. 2014. Cichlids respond to conspecific sounds but females exhibit no phonotaxis without the presence of live males. Ecol. Freshw. Fish 23, 305–312. ( 10.1111/eff.12081) [DOI] [Google Scholar]
- 57.Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egger B, Sefc KM, Makasa L, Sturmbauer C, Salzburger W. 2012. Introgressive hybridization between color morphs in a population of cichlid fishes twelve years after human-induced secondary admixis. J. Hered. 103, 515–522. ( 10.1093/jhered/ess013) [DOI] [PubMed] [Google Scholar]
- 59.Pfennig KS. 2016. Reinforcement as an initiator of population divergence and speciation. Cur. Zool. 62, 145–154. ( 10.1093/cz/zow033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight M, Turner G. 1999. Reproductive isolation among closely related Lake Malawi cichlids: can males recognize conspecific females by visual cues? Anim. Behav. 58, 761–768. ( 10.1006/anbe.1999.1206) [DOI] [PubMed] [Google Scholar]
- 61.Venesky MD, Andraso GM, Ropski SJ. 2005. Behavior of male Kenyi cichlids, Pseudotropheus lombardoi, in response to visual and olfactory cues from females. Bios 76, 77–84. ( 10.1893/0005-3155(2005)076[0077:RABOMK]2.0.CO;2) [DOI] [Google Scholar]
- 62.Barlow GW, Rogers W, Cappeto RV. 1977. Incompatibility and assortative mating in the Midas cichlid. Behav. Ecol. Sociobiol. 2, 49–59. ( 10.1007/BF00299288) [DOI] [Google Scholar]
- 63.Baerends GP, Baerends-van Roon J. 1950. An introduction to the study of the ethology of the cichlid fishes. Behaviour 1, 1–243. [Google Scholar]
- 64.Seehausen O, van Alphen JJ, Witte F. 1999. Can ancient colour polymorphisms explain why some cichlid lineages speciate rapidly under disruptive sexual selection? Belg. J. Zool. 129, 43–60. [Google Scholar]
- 65.Couldridge VC, Alexander GJ. 2002. Color patterns and species recognition in four closely related species of Lake Malawi cichlid. Behav. Ecol. 13, 59–64. ( 10.1093/beheco/13.1.59) [DOI] [Google Scholar]
- 66.Alphen V. 1999. Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity in Lake Victoria? Ecol. Lett. 2, 262–271. ( 10.1046/j.1461-0248.1999.00082.x) [DOI] [Google Scholar]
- 67.Elmer KR, Lehtonen TK, Meyer A. 2009. Color assortative mating contributes to sympatric divergence of neotropical cichlid fish. Evolution 63, 2750–2757. ( 10.1111/j.1558-5646.2009.00736.x) [DOI] [PubMed] [Google Scholar]
- 68.Allender CJ, Seehausen O, Knight ME, Turner GF, Maclean N. 2003. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proc. Natl Acad. Sci. USA 100, 14 074–14 079. ( 10.1073/pnas.2332665100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson C, Wong S, Fuller A, Zigelsky K, Earley R. 2015. Carotenoid-based coloration is associated with predation risk, competition, and breeding status in female convict cichlids (Amatitlania siquia) under field conditions. Environ. Biol. Fishes. 98, 1005–1013. ( 10.1007/s10641-014-0333-9) [DOI] [Google Scholar]
- 70.Dijkstra PD, Hekman R, Schulz RW, Groothuis TG. 2007. Social stimulation, nuptial colouration, androgens and immunocompetence in a sexual dimorphic cichlid fish. Behav. Ecol. Sociobiol. 61, 599–609. ( 10.1007/s00265-006-0289-7) [DOI] [Google Scholar]
- 71.Seehausen O. 1997. Distribution of and reproductive isolation among color morphs of a rock-dwelling Lake Victoria cichlid (Haplochromis nyererei). Ecol. Freshw. Fish 6, 59–66. ( 10.1111/j.1600-0633.1997.tb00145.x) [DOI] [Google Scholar]
- 72.Maan ME, Seehausen O, Van Alphen JJ. 2010. Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biol. J. Linn. Soc. 99, 398–406. ( 10.1111/j.1095-8312.2009.01368.x) [DOI] [Google Scholar]
- 73.Selz O, Thommen R, Pierotti M, Anaya-Rojas JM, Seehausen O. 2016. Differences in male coloration are predicted by divergent sexual selection between populations of a cichlid fish. Proc. R. Soc. B 283, 20160172 ( 10.1098/rspb.2016.0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stelkens RB, Pierotti ME, Joyce DA, Smith AM, van der Sluijs I, Seehausen O. 2008. Disruptive sexual selection on male nuptial coloration in an experimental hybrid population of cichlid fish. Phil. Trans. R. Soc. B 363, 2861–2870. ( 10.1098/rstb.2008.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selz O, Thommen R, Maan M, Seehausen O. 2014. Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish. J. Evol. Biol. 27, 275–289. ( 10.1111/jeb.12287) [DOI] [PubMed] [Google Scholar]
- 76.Siepen G, Caprona M. 1986. The influence of parental color morph on mate choice in the cichlid fish Cichlasoma nigrofasciatum. Ethology 71, 187–200. ( 10.1111/j.1439-0310.1986.tb00583.x) [DOI] [Google Scholar]
- 77.Salzburger W, Niederstätter H, Brandstätter A, Berger B, Parson W, Snoeks J, Sturmbauer C. 2006. Color-assortative mating in the cichlid species Tropheus moorii from Lake Tanganyika, East Africa. Proc. R. Soc. B 273, 257–266. ( 10.1098/rspb.2005.3321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verzijden MN, ten Cate C. 2007. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 3, 134–136. ( 10.1098/rsbl.2006.0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zoppoth P, Koblmüller S, Sefc K. 2013. Male courtship preferences demonstrate discrimination against allopatric colour morphs in a cichlid fish. J. Evol. Biol. 26, 577–586. ( 10.1111/jeb.12074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sefc KM, et al. 2015. Asymmetric dominance and asymmetric mate choice oppose premating isolation after allopatric divergence. Ecol. Evol. 5, 1549–1562. ( 10.1002/ece3.1372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stelkens RB, Seehausen O. 2009. Phenotypic divergence but not genetic distance predicts assortative mating among species of a cichlid fish radiation. J. Evol. Biol. 22, 1679–1694. ( 10.1111/j.1420-9101.2009.01777.x) [DOI] [PubMed] [Google Scholar]
- 82.Carleton KL, Dalton BE, Escobar-Camacho D, Nandamuri SP. 2016. Proximate and ultimate causes of variable visual sensitivities: insights from cichlid fish radiations. Genesis 54, 299–325. ( 10.1002/dvg.22940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carleton KL, Parry JW, Bowmaker JK, Hunt DM, Seehausen O. 2005. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol. Ecol. 14, 4341–4353. ( 10.1111/j.1365-294X.2005.02735.x) [DOI] [PubMed] [Google Scholar]
- 84.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 85.Wright DS, Demandt N, Alkema JT, Seehausen O, Groothuis TG, Maan ME. 2017. Developmental effects of visual environment on species-assortative mating preferences in Lake Victoria cichlid fish. J. Evol. Biol. 30, 289–299. ( 10.1111/jeb.13001) [DOI] [PubMed] [Google Scholar]
- 86.Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8, 14363 ( 10.1038/ncomms14363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meier JI, Marques DA, Wagner CE, Excoffier L, Seehausen O. 2018. Genomics of parallel ecological speciation in Lake Victoria cichlids. Mol. Biol. Evol. 35, 1489–1506. ( 10.1093/molbev/msy051) [DOI] [PubMed] [Google Scholar]
- 88.O'Quin KE, Hofmann CM, Hofmann HA, Carleton KL. 2010. Parallel evolution of opsin gene expression in African cichlid fishes. Mol. Biol. Evol. 27, 2839–2854. ( 10.1093/molbev/msq171) [DOI] [PubMed] [Google Scholar]
- 89.Schneider RF, Rometsch SJ, Torres-Dowdall J, Meyer A. 2020. Habitat light sets the boundaries for the rapid evolution of cichlid fish vision, while sexual selection can tune it within those limits. Mol. Ecol. 29, 1476–1493. ( 10.1111/mec.15416) [DOI] [PubMed] [Google Scholar]
- 90.Torres-Dowdall J, Pierotti ME, Härer A, Karagic N, Woltering JM, Henning F, Elmer KR, Meyer A. 2017. Rapid and parallel adaptive evolution of the visual system of Neotropical Midas cichlid fishes. Mol. Biol. Evol. 34, 2469–2485. ( 10.1093/molbev/msx143) [DOI] [PubMed] [Google Scholar]
- 91.Keller-Costa T, Canário AV, Hubbard PC. 2015. Chemical communication in cichlids: a mini-review. Gen. Comp. Endocrinol. 221, 64–74. ( 10.1016/j.ygcen.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 92.Blais J, Rico C, Van Oosterhout C, Cable J, Turner GF, Bernatchez L. 2007. MHC adaptive divergence between closely related and sympatric African cichlids. PLoS ONE 2, e734 ( 10.1371/journal.pone.0000734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Milinski M, Griffiths S, Wegner KM, Reusch TB, Haas-Assenbaum A, Boehm T. 2005. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA 102, 4414–4418. ( 10.1073/pnas.0408264102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hofmann MJ, Bracamonte SE, Eizaguirre C, Barluenga M. 2017. Molecular characterization of MHC class IIB genes of sympatric Neotropical cichlids. BMC Genet. 18, 15 ( 10.1186/s12863-017-0474-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almeida O, Miranda A, Frade P, Hubbard P, Barata E, Canário A. 2005. Urine as a social signal in the Mozambique tilapia (Oreochromis mossambicus). Chem. Senses. 30(Suppl. 1), i309–i310. ( 10.1093/chemse/bjh238) [DOI] [PubMed] [Google Scholar]
- 96.Crapon de Caprona M. 1974. The effect of chemical stimuli from conspecifics on the behavior of Haplochromis burtoni (Cichlidae, Pisces). Experientia 30, 1394–1395. [DOI] [PubMed] [Google Scholar]
- 97.Giaquinto PC, da Silva Berbert CM, Delicio HC. 2010. Female preferences based on male nutritional chemical traits. Behav. Ecol. Sociobiol. 64, 1029–1035. ( 10.1007/s00265-010-0918-z) [DOI] [Google Scholar]
- 98.Barnett C. 1977. Chemical recognition of the mother by the young of the cichlid fish, Cichlasoma citrinellum. J. Chem. Ecol. 3, 461–466. ( 10.1007/BF00988188) [DOI] [Google Scholar]
- 99.Barnett C. 1981. The role of urine in parent-offspring communication in a cichlid fish. Z. Tierpsychol. 55, 173–182. ( 10.1111/j.1439-0310.1981.tb01267.x) [DOI] [Google Scholar]
- 100.Thünken T, Waltschyk N, Bakker TC, Kullmann H. 2009. Olfactory self-recognition in a cichlid fish. Anim. Cogn. 12, 717–724. ( 10.1007/s10071-009-0231-2) [DOI] [PubMed] [Google Scholar]
- 101.Barlow GW. 1992. Is mating different in monogamous species? The Midas cichlid fish as a case study. Am. Zool. 32, 91–99. ( 10.1093/icb/32.1.91) [DOI] [Google Scholar]
- 102.Crapon de Caprona MD. 1980. Olfactory communication in a cichlid fish, Haplochromis burtoni. Z. Tierpsychol. 52, 113–134. [DOI] [PubMed] [Google Scholar]
- 103.Lobel PS. 2001. Acoustic behavior of cichlid fishes. J. Aquaricult. Aquat. Sci. 9, 167–186. [Google Scholar]
- 104.Amorim MCP. 2006. Diversity of sound production in fish. Commun. Fishes 1, 71–104. [Google Scholar]
- 105.Danley PD, Husemann M, Chetta J. 2012. Acoustic diversity in Lake Malawi's rock-dwelling cichlids. Environ. Biol. Fishes 93, 23–30. ( 10.1007/s10641-011-9886-z) [DOI] [Google Scholar]
- 106.Amorim MCP, Knight ME, Stratoudakis Y, Turner GF. 2004. Differences in sounds made by courting males of three closely related Lake Malawi cichlid species. J. Fish Biol. 65, 1358–1371. ( 10.1111/j.0022-1112.2004.00535.x) [DOI] [Google Scholar]
- 107.Amorim MCP, Simões JM, Fonseca PJ, Turner GF. 2008. Species differences in courtship acoustic signals among five Lake Malawi cichlid species (Pseudotropheus spp.). J. Fish Biol. 72, 1355–1368. ( 10.1111/j.1095-8649.2008.01802.x) [DOI] [Google Scholar]
- 108.Van Staaden MJ, Smith AR. 2011. Cutting the Gordian knot: complex signaling in African cichlids is more than multimodal. Cur. Zool. 57, 237–252. ( 10.1093/czoolo/57.2.237) [DOI] [Google Scholar]
- 109.Smith ME, Kane AS, Popper AN. 2004. Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water? J. Exp. Biol. 207, 3591–3602. ( 10.1242/jeb.01188) [DOI] [PubMed] [Google Scholar]
- 110.Ladich F, Wysocki LE. 2003. How does tripus extirpation affect auditory sensitivity in goldfish? Hear. Res. 182, 119–129. ( 10.1016/S0378-5955(03)00188-6) [DOI] [PubMed] [Google Scholar]
- 111.Kenyon TN, Ladich F, Yan HY. 1998. A comparative study of hearing ability in fishes: the auditory brainstem response approach. J. Comp. Physiol. A 182, 307–318. ( 10.1007/s003590050181) [DOI] [PubMed] [Google Scholar]
- 112.Schulz-Mirbach T, Heß M, Metscher BD, Ladich F. 2013. A unique swim bladder-inner ear connection in a teleost fish revealed by a combined high-resolution microtomographic and three-dimensional histological study. BMC Biol. 11, 75 ( 10.1186/1741-7007-11-75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lobel PS. 1998. Possible species specific courtship sounds by two sympatric cichlid fishes in Lake Malawi, Africa. Environ. Biol. Fishes 52, 443–452. ( 10.1023/A:1007467818465) [DOI] [Google Scholar]
- 114.Maruska KP, Ung US, Fernald RD. 2012. The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS ONE 7, e37612 ( 10.1371/journal.pone.0037612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verzijden MN, Van Heusden J, Bouton N, Witte F, ten Cate C, Slabbekoorn H. 2010. Sounds of male Lake Victoria cichlids vary within and between species and affect female mate preferences. Behav. Ecol. 21, 548–555. ( 10.1093/beheco/arq018) [DOI] [Google Scholar]
- 116.Escobar-Camacho D, Carleton KL. 2015. Sensory modalities in cichlid fish behavior. Curr. Opin. Behav. Sci. 6, 115–124. ( 10.1016/j.cobeha.2015.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Seehausen O, van Alphen JJ. 1998. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav. Ecol. Sociobiol. 42, 1–8. ( 10.1007/s002650050405) [DOI] [Google Scholar]
- 118.Genner MJ, Nichols P, Carvalho GR, Robinson RL, Shaw PW, Turner GF. 2007. Reproductive isolation among deep-water cichlid fishes of Lake Malawi differing in monochromatic male breeding dress. Mol. Ecol. 16, 651–662. ( 10.1111/j.1365-294X.2006.03173.x) [DOI] [PubMed] [Google Scholar]
- 119.Swanson WJ, Vacquier VD. 2002. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3, 137 ( 10.1038/nrg733) [DOI] [PubMed] [Google Scholar]
- 120.Matute DR. 2010. Reinforcement of gametic isolation in Drosophila. PLoS Biol. 8, e1000341 ( 10.1371/journal.pbio.1000341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albrecht T, Opletalová K, Reif J, Janoušek V, Piálek L, Cramer ER, Johnsen A, Reifová R. 2019. Sperm divergence in a passerine contact zone: indication of reinforcement at the gametic level. Evolution 73, 202–213. ( 10.1111/evo.13677) [DOI] [PubMed] [Google Scholar]
- 122.Brashears C.2016. Post-zygotic isolation and Haldane's rule in two closely related Lake Malawi cichlid species. PhD thesis, Baylor University, Waco, TX, USA.
- 123.Swanson WJ, Wong A, Wolfner MF, Aquadro CF. 2004. Evolutionary expressed sequence tag analysis of Drosophila female reproductive tracts identifies genes subjected to positive selection. Genetics 168, 1457–1465. ( 10.1534/genetics.104.030478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Devigili A, Fitzpatrick JL, Gasparini C, Ramnarine I, Pilastro A, Evans J. 2018. Possible glimpses into early speciation: the effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. J. Evol. Biol. 31, 66–74. ( 10.1111/jeb.13194) [DOI] [PubMed] [Google Scholar]
- 125.Matute DR, Butler IA, Turissini DA, Coyne JA. 2010. A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science 329, 1518–1521. ( 10.1126/science.1193440) [DOI] [PubMed] [Google Scholar]
- 126.Butlin RK, Tregenza T. 1998. Levels of genetic polymorphism: marker loci versus quantitative traits. Phil. Trans. R. Soc. Lond. B 353, 187–198. ( 10.1098/rstb.1998.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Orr HA. 1995. The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139, 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Der Sluijs I, Van Dooren TJ, Seehausen O, Van Alphen J. 2008. A test of fitness consequences of hybridization in sibling species of Lake Victoria cichlid fish. J. Evol. Biol. 21, 480–491. ( 10.1111/j.1420-9101.2007.01495.x) [DOI] [PubMed] [Google Scholar]
- 129.Rajkov J, Weber AAT, Salzburger W, Egger B. 2018. Immigrant and extrinsic hybrid inviability contribute to reproductive isolation between lake and river cichlid ecotypes. Evolution 72, 2553–2564. ( 10.1111/evo.13612) [DOI] [PubMed] [Google Scholar]
- 130.Baylis JR. 1976. A quantitative study of long-term courtship: I. Ethological isolation between sympatric populations of the Midas cichlid, Cichlasoma citrinellum, and the arrow cichlid, C. zaliosum. Behaviour 59, 59–69. ( 10.1163/156853976X00460) [DOI] [Google Scholar]
- 131.Wagner CE, Keller I, Wittwer S, Selz OM, Mwaiko S, Greuter L, Sivasundar A, Seehausen O. 2013. Genome-wide RAD sequence data provide unprecedented resolution of species boundaries and relationships in the Lake Victoria cichlid adaptive radiation. Mol. Ecol. 22, 787–798. ( 10.1111/mec.12023) [DOI] [PubMed] [Google Scholar]
- 132.Machado-Schiaffino G, Kautt AF, Kusche H, Meyer A. 2015. Parallel evolution in Ugandan crater lakes: repeated evolution of limnetic body shapes in haplochromine cichlid fish. BMC Evol. Biol. 15, 9 ( 10.1186/s12862-015-0287-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haldane J. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 134.Gammerdinger WJ, Kocher TD. 2018. Unusual diversity of sex chromosomes in African cichlid fishes. Genes 9, 480 ( 10.3390/genes9100480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Svensson O, Smith A, García-Alonso J, Van Oosterhout C. 2016. Hybridization generates a hopeful monster: a hermaphroditic selfing cichlid. R. Soc. Open Sci. 3, 150684 ( 10.1098/rsos.150684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dobzhansky T. 1950. Genetics of natural populations. XIX. Origin of heterosis through natural selection in populations of Drosophila pseudoobscura. Genetics 35, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Endler JA. 1977. Geographic variation, speciation, and clines. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 138.Burton RS. 1990. Hybrid breakdown in physiological response: a mechanistic approach. Evolution 44, 1806–1813. ( 10.1111/j.1558-5646.1990.tb05251.x) [DOI] [PubMed] [Google Scholar]
- 139.Stelkens RB, Schmid C, Seehausen O. 2015. Hybrid breakdown in cichlid fish. PLoS ONE 10, e0127207 ( 10.1371/journal.pone.0127207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Recknagel H, Elmer KR, Meyer A. 2013. A hybrid genetic linkage map of two ecologically and morphologically divergent Midas cichlid fishes (Amphilophus spp.) obtained by massively parallel DNA sequencing (ddRADSeq). G3: Genes, Genomes, Genet. 3, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Feldberg E, Porto JIR, Bertollo LAC. 2003. Chromosomal changes and adaptation of cichlid fishes during evolution. Fish Adapt. 285, 308. [Google Scholar]
- 142.Wagner Werneck Félix da Costa G, de Bello Cioffi M, Liehr T, Feldberg E, Antonio Carlos Bertollo L, Franco Molina W. 2019. Extensive chromosomal reorganization in Apistogramma fishes (Cichlidae, Cichlinae) fits the complex evolutionary diversification of the genus. Int. J. Mol. Sci. 20, 4077 ( 10.3390/ijms20174077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martins-Santos, Cristina I, De Brito Portela-Castro AL, Julio HF Jr. 2005. Chromosomal polymorphism and speciation in Laetacara cf. dorsigera (Teleostei, Perciformes, Cichlidae) from the river Parana PR Brazil. Caryologia 58, 95–101. ( 10.1080/00087114.2005.10589439) [DOI] [Google Scholar]
- 144.Hodaňová L, Kalous L, Musilová Z. 2014. Comparative cytogenetics of Neotropical cichlid fishes (Nannacara, Ivanacara and Cleithracara) indicates evolutionary reduction of diploid chromosome numbers . Comp. Cytogenet. 8, 169 ( 10.3897/compcytogen.v8i3.7279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hatfield T, Schluter D. 1999. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53, 866–873. ( 10.1111/j.1558-5646.1999.tb05380.x) [DOI] [PubMed] [Google Scholar]
- 146.Martin CH, Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339, 208–211. ( 10.1126/science.1227710) [DOI] [PubMed] [Google Scholar]
- 147.Arnegard ME, et al. 2014. Genetics of ecological divergence during speciation. Nature 511, 307–311. ( 10.1038/nature13301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Machado-Schiaffino G, Kautt AF, Torres-Dowdall J, Baumgarten L, Henning F, Meyer A. 2017. Incipient speciation driven by hypertrophied lips in Midas cichlid fishes? Mol. Ecol. 26, 2348–2362. ( 10.1111/mec.14029) [DOI] [PubMed] [Google Scholar]
- 149.Selz OM, Seehausen O. 2019. Interspecific hybridization can generate functional novelty in cichlid fish. Proc. R. Soc. B 286, 20191621 ( 10.1098/rspb.2019.1621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baumgarten L, Gonzalo M-S, Frederico H, Axel M. 2015. What big lips are good for: on the adaptive function of repeatedly evolved hypertrophied lips of cichlid fishes. Biol. J. Linn. Soc. 115, 448–455. ( 10.1111/bij.12502) [DOI] [Google Scholar]
- 151.Mallet J. 2007. Hybrid speciation. Nature 446, 279 ( 10.1038/nature05706) [DOI] [PubMed] [Google Scholar]
- 152.Kautt AF, Machado-Schiaffino G, Meyer A. 2018. Lessons from a natural experiment: allopatric morphological divergence and sympatric diversification in the Midas cichlid species complex are largely influenced by ecology in a deterministic way. Evol. Lett. 2, 323–340. ( 10.1002/evl3.64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gottsberger B, Mayer F. 2007. Behavioral sterility of hybrid males in acoustically communicating grasshoppers (Acrididae, Gomphocerinae). J. Comp. Physiol. A 193, 703–714. ( 10.1007/s00359-007-0225-y) [DOI] [PubMed] [Google Scholar]
- 154.Höbel G, Gerhardt HC. 2003. Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57, 894–904. ( 10.1111/j.0014-3820.2003.tb00300.x) [DOI] [PubMed] [Google Scholar]
- 155.Sluijs I VD, Van Dooren TJ, Hofker KD, van Alphen JJ, Stelkens RB, Seehausen O. 2008. Female mating preference functions predict sexual selection against hybrids between sibling species of cichlid fish. Phil. Trans. R. Soc. B. 363, 2871–2877. ( 10.1098/rstb.2008.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rieseberg LH. 1991. Hybridization in rare plants: insights from case studies in Cercocarpus and Helianthus. In Genetics and conservation of rare plants (eds Falk DA, Holsinger KE), pp. 171–181. New York, NY: Oxford University Press. [Google Scholar]
- 157.Cade TJ. 1983. Hybridization and gene exchange among birds in relation to conservation. In Genetics and conservation (eds Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas WL), pp. 288–309. Menlo Park, CA: The Benjamin/Cummings Publishing Company Inc. [Google Scholar]
- 158.Thymer J, Simberloff D. 1996. Extinction by hybridisation. Annu. Rev. Ecol. Syst. 27, 83–109. ( 10.1146/annurev.ecolsys.27.1.83) [DOI] [Google Scholar]
- 159.Albertson RC, Kocher TD. 2005. Genetic architecture sets limits on transgressive segregation in hybrid cichlid fishes. Evolution 59, 686–690. ( 10.1111/j.0014-3820.2005.tb01027.x) [DOI] [PubMed] [Google Scholar]
- 160.Jiggins CD, Linares M, Naisbit RE, Salazar C, Yang ZH, Mallet J. 2001. Sex-linked hybrid sterility in a butterfly. Evolution 55, 1631–1638. ( 10.1111/j.0014-3820.2001.tb00682.x) [DOI] [PubMed] [Google Scholar]
- 161.Kirkpatrick M, Ravigné V. 2002. Speciation by natural and sexual selection: models and experiments. Am. Nat. 159, S22–S35. ( 10.1086/338370) [DOI] [PubMed] [Google Scholar]
- 162.Seehausen O. 2006. Conservation: losing biodiversity by reverse speciation. Curr. Biol. 16, R334–R3R7. ( 10.1016/j.cub.2006.03.080) [DOI] [PubMed] [Google Scholar]
- 163.Nosil P, Harmon LJ, Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 24, 145–156. ( 10.1016/j.tree.2008.10.011) [DOI] [PubMed] [Google Scholar]
- 164.Schemske DW. 2010. Adaptation and the origin of species. Am. Nat. 176, S4–S25. ( 10.1086/657060) [DOI] [PubMed] [Google Scholar]
- 165.Kulmuni J, Butlin RK, Lucek K, Savolainen V, Westram AM. 2020. Towards the completion of speciation: the evolution of further reproductive isolation once the first barriers are in place. Phil. Trans. R. Soc. B 286, 20190528 ( 10.1098/rstb.2019.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Merot C, Salazar C, Merrill RM, Jiggins CD, Joron M. 2017. What shapes the continuum of reproductive isolation? Lessons from Heliconius butterflies. Proc. R. Soc. B 284, 20170335 ( 10.1098/rspb.2017.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hendry AP, Bolnick DI, Berner D, Peichel CL. 2009. Along the speciation continuum in sticklebacks. J. Fish Biol. 75, 2000–2036. ( 10.1111/j.1095-8649.2009.02419.x) [DOI] [PubMed] [Google Scholar]
- 168.Via S, West J. 2008. The genetic mosaic suggests a new role for hitchhiking in ecological speciation. Mol. Ecol. 17, 4334–4345. ( 10.1111/j.1365-294X.2008.03921.x) [DOI] [PubMed] [Google Scholar]
- 169.Brawand D, et al. 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375–381. ( 10.1038/nature13726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nandamuri SP, Conte MA, Carleton KL. 2018. Multiple trans QTL and one cis-regulatory deletion are associated with the differential expression of cone opsins in African cichlids. BMC Genomics 19, 945 ( 10.1186/s12864-018-5328-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haesler MP, Seehausen O. 2005. Inheritance of female mating preference in a sympatric sibling species pair of Lake Victoria cichlids: implications for speciation. Proc. R. Soc. B 272, 237–245. ( 10.1098/rspb.2004.2946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koblmüller S, Sefc KM, Sturmbauer C. 2008. The Lake Tanganyika cichlid species assemblage: recent advances in molecular phylogenetics. Hydrobiologia 615, 5 ( 10.1007/s10750-008-9552-4) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used in this Review are included in the electronic supplementary material