Abstract
Intrinsic postzygotic barriers can play an important and multifaceted role in speciation, but their contribution is often thought to be reserved to the final stages of the speciation process. Here, we review how intrinsic postzygotic barriers can contribute to speciation, and how this role may change through time. We outline three major contributions of intrinsic postzygotic barriers to speciation. (i) reduction of gene flow: intrinsic postzygotic barriers can effectively reduce gene exchange between sympatric species pairs. We discuss the factors that influence how effective incompatibilities are in limiting gene flow. (ii) early onset of species boundaries via rapid evolution: intrinsic postzygotic barriers can evolve between recently diverged populations or incipient species, thereby influencing speciation relatively early in the process. We discuss why the early origination of incompatibilities is expected under some biological models, and detail how other (and often less obvious) incompatibilities may also serve as important barriers early on in speciation. (iii) reinforcement: intrinsic postzygotic barriers can promote the evolution of subsequent reproductive isolation through processes such as reinforcement, even between relatively recently diverged species pairs. We incorporate classic and recent empirical and theoretical work to explore these three facets of intrinsic postzygotic barriers, and provide our thoughts on recent challenges and areas in the field in which progress can be made.
This article is part of the theme issue ‘Towards the completion of speciation: the evolution of reproductive isolation beyond the first barriers’.
Keywords: genetic incompatibilities, introgression, rapid evolution, reinforcement
1. Introduction
Reproductive barriers are the currency of speciation. These barriers can occur before or after a hybrid zygote is formed (i.e. pre- and postzygotic), and selection against hybrids may or may not be mediated by the environment (i.e. extrinsic versus intrinsic). While intrinsic postzygotic barriers were an initial focus of speciation research [1–4], recent work has highlighted the importance of ecology in reproductive isolation, namely prezygotic and extrinsic postzygotic barriers [5–8]. This wave of ecology-focused speciation work, paired with the observation that prezygotic barriers tend to reach completion before intrinsic postzygotic barriers (e.g. [9,10]), has led to the prevalent opinion that prezygotic barriers play a more important role in speciation than intrinsic postzygotic barriers, particularly early on [11–15]. Yet, the fact that intrinsic postzygotic barriers are common across all kingdoms of life, and are a hallmark of most ‘good’ species [16] suggests that their role in speciation may also be essential.
Here, we highlight the contribution of intrinsic postzygotic barriers to speciation and explore how this contribution may change as speciation proceeds. While speciation is a continuous process, we refer to early and late stages based on the degree of reproductive isolation between species, while acknowledging that reproductive isolation is not unidirectional and can be accumulated or lost (figure 1). While these stages roughly correspond to divergence times; wherein recently diverged populations or incipient species may represent earlier stages, and more divergent species pairs may represent later stages, it is not a perfect proxy for reproductive isolation. For example, speciation can be instantaneous (e.g. polyploid speciation [16,32] or ‘single-gene speciation’ [33], but see [34–36]). Alternatively, relatively divergent species pairs may collapse owing to hybridization [37–39].
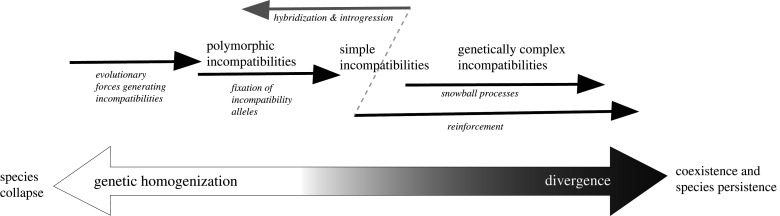
Figure 1.
Intrinsic postzygotic barriers play a dynamic role throughout speciation. Incompatibilities can allow for the build up of divergence, while introgression can reverse incompatibilities by replacing incompatible allelic combinations with compatible ones (bottom arrow; lighter colour indicates increasing genetic homogenization, while darker colouring indicates increasing divergence). Three stages of incompatibilities are outlined: polymorphic (involving segregating alleles within species or between incipient species), simple (involving few interacting alleles), and genetically complex (involving many interacting incompatibility alleles with potential for genetic redundancy). The evolutionary processes that connect these stages are listed below the arrows in italics. Early in the speciation process, various evolutionary forces may generate polymorphic incompatibilities (outlined in [17–20]). These in turn may become species-wide, genetically simple incompatibilities if incompatibility alleles fix. Simple incompatibilities can become genetically complex if incompatibility alleles continue to accumulate in a snowball like fashion, in turn creating genetic redundancy [18,21–30]. Although these are drawn as discrete stages, we note that the path of speciation can be substantially more complex (for example, polymorphic incompatibility may be genetically complex). If there is sufficient selection against hybrids, and sufficient production of unfit hybrids, processes such as reinforcement can complete speciation ([31]; note the dotted line between ‘hybridization & introgression’ and ‘reinforcement’). We highlight that divergence is reversible at almost any point along this continuum (although it becomes increasingly more difficult as incompatibilities become more complex). Also, all of these processes can occur relatively rapidly, and may not reflect divergence times between incipient species (e.g. incompatibilities may remain polymorphic for long periods of time if they are maintained by local selection, or reinforcement may happen relatively rapidly if there is strong selection against the production of unfit hybrids).
2. What do we mean by intrinsic postzygotic barriers?
Intrinsic postzygotic barriers manifest when hybrids exhibit lower fitness than either parent, regardless of the environment. Although many examples of intrinsic postzygotic barriers can easily be classified as such, this delimitation is not always clear. For example, barriers that originate prezygotically can also manifest again in hybrids (e.g. [40]). In addition, extrinsic factors can influence the severity of expression of intrinsic postzygotic barriers (e.g. [41,42]). While most research of intrinsic postzygotic reproductive barriers focuses on its most severe forms—hybrid sterility or inviability—these barriers may also manifest as reduced cognitive [43–45] or physiological ability [46,47], or subtler declines in fertility or viability (e.g. [48,49]).
3. The role of intrinsic postzygotic barriers may change throughout the speciation process
Intrinsic postzygotic barriers may be crucial for speciation, but their role is probably dynamic through time. Whether intrinsic postzygotic barriers contribute to the onset of speciation is unresolved. Incompatibilities commonly segregate within species [50–52] and between recently diverged incipient species [53–64], which may serve as an initial barrier. However, polymorphic incompatibility alleles can also be transient, in which case, their contribution to speciation is unclear. For example, incompatibility alleles may be lost by drift or selection before reaching fixation, or polymorphic incompatibility alleles may be polymorphic, because introgression has replaced incompatible allelic combinations with compatibles ones (e.g. [62,64–68]; figure 1). Determining whether polymorphic incompatibility alleles are transient is challenging (and in some cases impossible), and efforts to assess the fate of polymorphic incompatibility alleles requires knowledge of the specific alleles that contribute to reproductive isolation, large population genomic datasets to infer patterns of introgression and selection, and/or the ability to perform crosses. Still, some polymorphic incompatibilities will ultimately reach fixation (figure 1; box 1) and the observation that some barrier alleles predate speciation (e.g. [60,110]) suggests that intrinsic postzygotic barriers may be present at the onset of speciation.
Box 1. The genetic basis of intrinsic postzygotic barriers.
The evolution of intrinsic postzygotic barriers baffled early biologists, as natural selection should never favour the production of unfit hybrids [69]. We now know that these barriers need not evolve in the face of selection, but often evolve as a byproducts of divergence between populations at two or more loci (i.e. DMIs; the Dobzhansky-Muller model of genetic incompatibilities [70–72]). DMIs are common across biological kingdoms [19,20,73], and can underlie both hybrid inviability and sterility [15,17,74], though there are other causes of intrinsic postzygotic barriers (i.e. changes in ploidy, structural genomic changes, differences in endosymbionts [16], meiosis defects owing to substantial sequence divergence [73], or global patterns of inappropriate gene expression as a result of gene regulatory divergence [75]). DMIs can involve interactions between nuclear, or nuclear and organellar genes (reviewed in [16]). They may involve substitutions in two diverging lineages or multiple derived substitutions in one lineage and a preserved ancestral allele in another (derived–derived versus derived–ancestral incompatibilities; [76,77]). Many studies have mapped the genetic location of incompatibility alleles, but few incompatibilities have been resolved to the level of genes [74]. Still, some general patterns have emerged from these mapping efforts while many questions remain.
First, the number of loci involved in DMIs varies greatly (from simple incompatibilities involving a single pair of interacting alleles [78]; to complex incompatibilities involving many interacting alleles [79]). The number of loci contributing to DMIs should increase through time, although the rate of increase is debated [18,21–28]. The number of loci involved in DMIs does not reflect the severity of the barrier; many severe inviability or sterility phenotypes are controlled by simple incompatibilities [29]. However, as the complexity of intrinsic postzygotic barriers increase, so does the potential for genetic redundancy (e.g. an increase in the number of incompatibility loci with no substantial increase in reproductive isolation). For example, hybrid inviability between Drosophila melanogaster and each of Drosophila simulans and Drosophila santomea is equally strong, but is controlled by roughly six times more loci in the latter cross [24].
Second, a knowledge of the genes responsible for intrinsic postzygotic barriers has provided insight into the underlying causes for their evolution. Of the incompatibility genes discovered to date, many exhibit genomic signatures of strong positive selection ([53,80–86], reviewed in [19,87,88]), although others do not [89–92]. Yet, the evolutionary drivers of most incompatibilities are unresolved. Several evolutionary explanations have been put forth as the underlying cause of incompatibilities, including local adaptation [93–96], hitchhiking [97,98], systems drift (i.e. stochastic evolution of the genetic basis of a trait without change to the phenotype [99–101]), gene duplication [102–104], intra-genomic conflicts [19,87,88], and host-pathogen conflict [20,50,105]. Although the original proposals [106,107] were met with skepticism [108,109], of the handful of incompatibilities for which the underlying genes have been identified, many seem to have evolved via conflicts. Yet, the relative importance of conflict, other types of selection and other processes, in the evolution of incompatibility alleles remains unknown.
Third, many species exhibit genetic variation for reproductive isolation. This variation can exist as segregating incompatibilities within a species [50–52], or as a genetic polymorphism for the ability of a species to cross to a close relative (e.g. [53–64] reviewed in [18]). The relative importance of polymorphic incompatibilities in speciation has been debated, and their contribution will depend on the allele frequencies of each incompatibility allele [18].
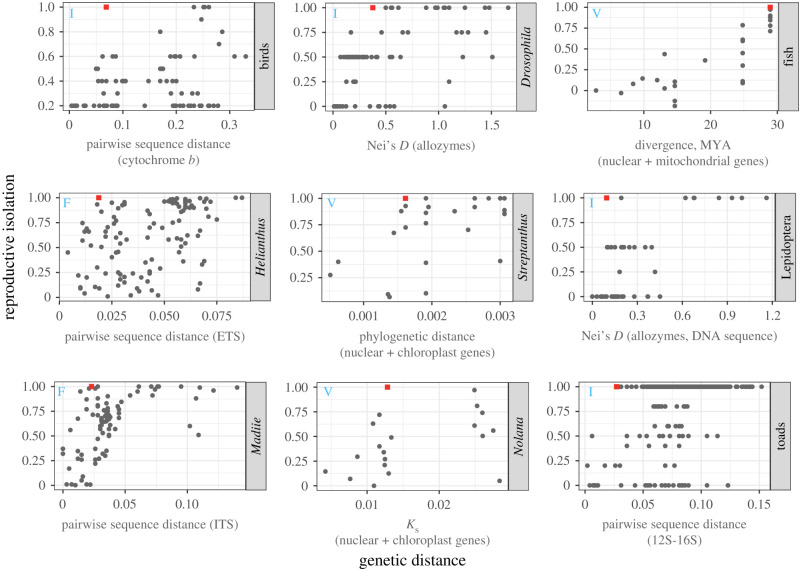
Strong evidence that intrinsic postzygotic barriers play a role at low to intermediate levels of divergence stems from the commonality of these barriers between incipient and recently diverged species pairs. We amassed data from eight previously published comparative studies, comprising nine taxonomic groups, to determine whether significant intrinsic postzygotic isolation can appear early in divergence and whether this is common across taxonomic groups [9,111–117]. In each study, the degree of reproductive isolation was assessed as a function of genetic distance between species. However, these studies differ in how reproductive isolation and genetic distance were measured. Some used categorical indices of intrinsic postzygotic isolation [9,111–113], while others measured individual components of intrinsic postzygotic isolation [114–117]. Studies also varied in both the statistic calculated to infer divergence (e.g. Nei's D [9,111], phylogenetic distance [116], pairwise sequence distance [112,113,115], divergence in millions of years [117] or Ks [114]), as well as the genetic markers used (a small number of nuclear or mitochondrial genes [112–117] or allozymes [9,111]). While these methodological differences prevent us from quantitatively comparing studies, their amalgamation can qualitatively inform us of general patterns in speciation.
We find that substantial intrinsic reproductive isolation appears relatively early in divergence for most groups (figure 2). In fact, in eight of nine comparative studies, the first species pair to reach complete intrinsic postzygotic reproductive isolation is within the youngest half of species tested, and the distribution of genetic distances for species pairs with greater than 50% intrinsic postzygotic isolation is largely overlapping with the distribution of genetic distances for species pairs with less than 50% reproductive isolation (figure 2; we note that centrarchid fishes is the exception in both of these observations [117]). In addition, there are many examples of rapid evolution of intrinsic barriers. For example, hybrid sterility evolves rapidly in stalk-eyed flies [118], mountain pine beetles [63,119], and several plant lineages [120–122], sometimes in the absence of significant prezygotic barriers (e.g. [123]). Hybrid inviability has been shown to evolve rapidly in several plant species [60,124–127], as well as mammals [128]. In line with the rapid evolution of reproductive barriers is the observation that diverging populations tend to show a very sharp transition between freely sharing migrants to exhibiting no signs of migration [129–131] (although these patterns do not uniquely support a role of intrinsic postzygotic barriers). Understanding at what level of reproductive isolation this transition will happen is a key aim in speciation biology.
Figure 2.
Intrinsic postzygotic reproductive isolation can evolve rapidly across many types of taxonomic groups. The rate of evolution of different types of intrinsic postzygotic reproductive isolation across nine different taxonomic groups. Scatterplots display the rate of accumulation of either hybrid inviability (V), sterility (F) or an index of both (I) for species pairs of differing divergence times, with the first species pair to achieve complete reproductive isolation (greater than 99.5% reproductive isolation) highlighted in red boxes. Note that the units of the X-axis differ among panels, as genetic distance was measured using different methods in each study (the statistic calculated as well as the markers used are listed as individual panel X-axis labels). References for the data are as follows: Birds [112], Drosophila [9], centrarchid fishes [117], Helianthus and Madiie [115], Streptanthus [116], Lepidoptera [111], Nolana [114], toads [113]. For both Nolana and Streptanthus, multiple components of intrinsic postzygotic reproductive isolation were measured. Here, we display reproductive isolation conferred by the number of viable seeds produced, as it is one of the earliest acting barriers. (Online version in colour.)
Not all species pairs evolve intrinsic postzygotic isolation rapidly. Variation exists within and between taxonomic groups for the rate of evolution of intrinsic postzygotic barriers. For example, complete intrinsic postzygotic isolation is not achieved in centrarchid fishes until approximately 28 Myr [117], while similar levels of isolation can be reached within approximately 3–5 Myr for birds, Drosophila, and Streptanthus (figure 2, [9,112,116]). Similarly, intrinsic barriers tend to evolve faster in mammals than birds or frogs [4,132,133]. Studies comparing species pairs with and without strong reproductive isolation at comparable levels of divergence can shed light on the underlying evolutionary drivers of intrinsic postzygotic barriers. For example, hybrid male sterility accumulates faster in the tropics in Drosophila [134]. In plants, mating system [135] and life history [115] affect the rate of evolution of hybrid inviability and sterility, respectively.
Lastly, intrinsic barriers probably play an important role late in the speciation process. Most divergent species show strong intrinsic reproductive isolation (figure 2). The accumulation of reproductive isolation may simply be a consequence of time (i.e. a deterministic outcome of genomic divergence). By contrast, it is also possible that the reason most ‘good’ species have strong intrinsic reproductive isolation is because strong intrinsic postzygotic isolation is essential to the speciation process. For example, species pairs with strong intrinsic postzygotic isolation may be less likely to collapse owing to hybridization upon secondary contact than weakly isolated species (e.g. Templeton effect [136]; reviewed in [16,37,137]). As species accumulate divergence, genetic incompatibilities are expected to increase in complexity and redundancy (box 1; [21,22]). This increased redundancy can greatly reduce the probability of introgression, and therefore the probability of species collapse (figure 1). Alternatively, intrinsic postzygotic barriers may play a generative role via reinforcement or positive feedbacks between pre- and postzygotic barriers [e.g. 138]. Determining whether the presence of strong intrinsic postzygotic barriers in divergent species is a deterministic outcome of time, or whether intrinsic postzygotic barriers play an essential role in species persistence are challenging, but essential goals in speciation biology.
We posit that intrinsic postzygotic barriers may play a dynamic role throughout the speciation process. Through time the underlying genetic architecture of intrinsic postzygotic barriers is expected to change, and this in itself can influence how these barriers contribute to speciation (figure 1; box 1). Early on, the evolutionary forces that give rise to intrinsic postzygotic barriers will generate polymorphic incompatibilities, some of which will fix between species (figure 1; box 1). Through time, these simple incompatibilities are expected to increase in genetic complexity and redundancy (figure 1; box 1). As these barriers increase in complexity, so too does the ability of intrinsic postzygotic barriers to prevent introgression at genome-wide scales. The presence of strong intrinsic barriers—whether simple or complex—can also generate reinforcing selection, which can ultimately complete speciation.
4. Intrinsic postzygotic barriers can contribute to speciation in multiple ways
The contribution of intrinsic postzygotic isolation to speciation is a contentious issue. While there is substantial theoretical work on this topic, empirical tests are challenging, and may require a knowledge of barrier alleles, the ability to detect genome-wide rates of introgression, large comparative datasets and/or the ability to perform many controlled crosses. Here, we outline three important ways that intrinsic postzygotic barriers can contribute to speciation.
(a). Limiting introgression
The first and most obvious way in which intrinsic postzygotic barriers contribute to speciation is by dampening gene flow between diverging populations. By definition, all reproductive barriers should restrict introgression between species, but barriers may vary in the rate at which they prevent introgression and the extent to which particular barriers prevent introgression can be influenced by many factors. Secondary contact zones—whether natural, synthetic or simulated—present a unique opportunity to test how effective different types of barriers are, as well as the factors that affect the ability of particular barriers to restrict introgression [16,139]. Here, we discuss the efficacy of intrinsic postzygotic reproductive isolation as a barrier to gene flow by comparing intrinsic postzygotic barriers to other barriers, and discussing the factors that can influence the ability of intrinsic postzygotic reproductive barriers to prevent introgression.
(i). How well do intrinsic postzygotic barriers prevent introgression relative to prezygotic barriers?
Researchers have argued that prezygotic barriers are sufficient to maintain species boundaries [140], intrinsic postzygotic barriers are more effective than prezygotic barriers [40,141,142], both are equally efficient at preventing introgression [65,143], or that both are necessary to prevent species collapse [37,144]. Different conclusions mainly stem from differences in underlying assumptions (e.g. the genetic architecture of reproductive isolation) or what is considered evidence for the maintenance of species boundaries (e.g. whether hybrid zones remain ‘bimodal’ [140], or the diffusion of alleles relative to dispersal [40,141,145]). Empirical studies describe hybrid zones maintained predominantly by prezygotic barriers [146–151], postzygotic barriers [152–156] or a combination [157–163]. Thus, either prezygotic or postzygotic reproductive barriers may be sufficient to maintain a stable hybrid zone if sufficiently strong. Conversely, both prezygotic and postzygotic barriers are susceptible to gene flow, and either may dissolve if sufficiently weak [66,67,145,164].
One approach to estimate the efficacy of different barriers in preventing introgression is to assess how easily specific barrier alleles cross species boundaries relative to the rest of the genome. Alleles that play no role in reproductive isolation should diffuse across a secondary contact zone in a manner that is proportional to the migration rate, and as such should exhibit generally low differentiation [145,165]. By contrast, alleles that maintain species boundaries should show sharper clines [145,166], and should represent differentiated regions of the genome (although we note that this is not a valid assumption for single allele mate preference [167]). By comparing the rate of diffusion of alleles that contribute to pre- versus intrinsic postzygotic isolation, we may begin to understand how effective each of these types of barriers are. While seemingly straightforward, only a handful of studies have assessed the outcome of known barrier loci in hybrid zones (e.g. [62,64,168,169]), and studies comparing clines of known pre- versus intrinsic postzygotic barriers are lacking. Genomic scans for highly differentiated loci have been used to identify regions of the genome that appear resistant to gene flow and thus may contribute to reproductive isolation (e.g. [170–173]), but these analyses are also likely to contain regions of high differentiation that are unrelated to speciation [165,174,175]. In addition, genomic scans can only assess what alleles resist introgression. Barrier alleles that introgress between species cannot be identified using this approach. In order to holistically assess what alleles maintain species boundaries and which ones succumb to introgression, we suggest that researchers use both sympatric and allopatric populations to understand the distribution of both reproductive barriers (e.g. [68,176]), as well as the underlying alleles. While certainly a challenging endeavour, integrating quantitative genetic mapping methods to identify barrier loci in allopatric populations with population genomic tools to assess their fate in sympatry is a powerful approach to determine how different types of barrier loci fair in secondary contact.
Ultimately, speciation biologists are interested in understanding how reproductive barriers can allow for divergence to accumulate, and for gene flow to be prevented not just at specific barrier loci, but genome-wide. Theory predicts that even moderate intrinsic postzygotic isolation can substantially decrease the rate of introgression of both barrier and linked neutral loci [40,65,166]. One valuable test of the relative efficacy of pre- versus intrinsic postzygotic barriers in nature would be to characterize the extent of introgression and the distribution of introgressed alleles in secondary contact zones when the involved species are reproductively isolated via prezygotic, postzygotic or both types of barriers. With the advent of inexpensive sequencing technologies in combination with advanced computational methods for ancestry assignment (e.g. [158,177–179]), detecting and quantifying introgression across a wide variety of secondary contact zones is an achievable task, and in fact has been done to assess the genomic outcomes of hybridization, as well as map incompatibility loci in hybrid zones (e.g. [180,181]).
(ii). What factors affect the ability of intrinsic postzygotic barriers to impede introgression?
A rich theoretical literature suggests that the ability of intrinsic postzygotic barriers to prevent introgression depends on three aspects of barrier loci: (i) the genetic architecture, (ii) the genetic context, and (iii) the mechanism of evolution. We discuss each of these in turn.
Genetic architecture- or simply, the number and location of incompatibility loci, and their mode of action (i.e. dominance, additivity) can effect rates of introgression. Polygenic intrinsic postzygotic barriers should be more efficient than simple incompatibilities at hampering introgression across the genome (although this will also depend on the dominance, additivity and effect size of the loci involved; [67,145,166,182,183]). The rate of introgression for a neutral locus will depend on both the degree of linkage with and the amount of selection against linked incompatibility alleles. Therefore, if incompatibilities are randomly distributed throughout the genome, then as the number of incompatibility loci increases, so does the proportion of the genome that is linked to an incompatibility allele, and is thus protected against introgression (although, selection against any particular incompatibility locus will be lower for polygenic incompatibilities than simple ones if selection against hybrids is equal). Curiously, the proportion of the genome that is protected against introgression may increase nonlinearly with time, as the number of loci involved in incompatibilities is expected to increase nonlinearly (assuming that an increase in the number of loci involved in an incompatibility also increases reproductive isolation or the genetic redundancy of strong intrinsic postzygotic barriers; [22,24,25,30]).
In addition, the dominance of incompatibility alleles, as well as whether they are sex-linked or autosomal will influence the generation (i.e. F1, F2 or later) that an incompatibility is revealed and the proportion of hybrids that are afflicted by the incompatibility. In theory, incompatibilities that manifest in the first generation of hybrids, or involve more dominant loci should be more effective at preventing introgression [30,67]. While there are few empirical tests of this prediction, recent work has shown that hybrid lethality involving two recessive alleles is relatively ineffective at preventing gene flow in sympatric populations of Mimulus [62]. Dominance and linkage can interact to influence the manifestation and maintenance of barriers. For dominant incompatibility loci, linkage between interacting alleles can maintain reproductive isolation in the face of substantial gene flow [164,184]. However, for incompatibilities involving two recessive loci, tight linkage among them will ensure a low probability that hybrids are homozygous for both incompatibility loci, and therefore the incompatibility will rarely manifest.
The genomic context of barrier loci can also impact patterns of introgression. Gene density and recombination can interact to influence rates of introgression such that regions of low recombination are more protected against introgression than regions of high recombination. This is because regions of high recombination have an increased probability that neutral alleles will recombine away from incompatibility alleles, particularly in gene-rich regions which are more likely to house incompatibility loci [180]. This has been shown theoretically [145] and empirically across scales: including variation in local recombination rate along the genome [180,181], within versus outside of structural changes that reduce recombination (e.g. inversions [185–187]), and between chromosomes that vary in the extent of recombination they experience (e.g. sex chromosomes versus autosomes: [168,188], although reduced introgression on sex chromosomes may also be a function of the higher density of incompatibility loci on sex chromosomes than autosomes; reviewed in [189]). The recombination landscape can vary within and between populations [190,191], and this may have implications for population or individual differences in the rates and landscape of introgression.
Finally, the degree to which intrinsic postzygotic barriers can prevent gene flow is also a product of the evolutionary drivers responsible for incompatibilities. Most theory suggests that incompatibility alleles that evolved neutrally are unlikely to be maintained in secondary contact [67,145]. This is because derived incompatibility alleles will experience a fitness cost when in the wrong genomic background, while ancestral alleles should be equally fit on all genomic backgrounds. By contrast, if incompatibilities have evolved via selection, the selective benefit of each incompatibility allele may outweigh the fitness cost when those alleles are found in the wrong genetic background. Yet, little work has been done to explore how different evolutionary drivers of incompatibilities can influence the stability of incompatibility alleles under scenarios of gene flow. It has been argued that incompatibilities which have evolved as a byproduct of adaptation to the same or similar selective pressures (e.g. mutation-order speciation) are unlikely to be maintained in the face of gene flow, as allelic combinations that have the highest global fitness will eventually spread to both species [15,192,193]. While this is intuitive for situations in which incompatibilities arise when populations are adapting to the same optima (e.g. [194]), it may not accurately describe incompatibilities that result from other selective processes (such as genomic conflicts and other co-evolutionary processes). Yet, to our knowledge, this has not been explicitly modelled. Therefore, new theory and simulations assessing how different evolutionary drivers affect the stability of different barriers in secondary contact are needed.
(b). Early onset of species boundaries via rapid evolution
The second major contribution of intrinsic postzygotic barriers to speciation is that these barriers can play a prominent role when they evolve early in the speciation process. Above, we point out that hybrid sterility and inviability often evolve rapidly and many incompatibility alleles exhibit signatures of rapid evolution (box 1). The rapid evolution of intrinsic barriers is perhaps unsurprising in light of the potential evolutionary drivers of incompatibilities. While only a handful of studies have determined the evolutionary drivers of incompatibility alleles, of the few examples amassed, co-evolutionary, conflict-driven processes appear to dominate (reviewed in [19,87,88]). Theory predicts that conflict-driven evolution should promote arms-race dynamics [195,196], which in turn would result in rapid evolution that is detectable at the molecular level. Several intrinsic reproductive barriers that are thought to evolve via conflict-driven processes also exhibit rapid evolution—either by appearing in recently diverged species [118,126] or by showing signals of positive molecular evolution [50,53,83,85].
While this review has focused on more severe hybrid defects, there is a host of less obvious intrinsic hybrid deficiencies that may appear even earlier in the speciation process. For example, in animals, hybrids have been shown to display transgressive metabolic phenotypes [46,47,197–199], and neurological defects [43–45,200]. In plants, many closely related crop varieties exhibit reduced vegetative growth, malformed roots and/or reduced fertility (sometimes referred to as ‘hybrid weakness’; e.g. [201–204]; synthesized in [20]). The fitness consequences of these defects have rarely been studied (though see [197,200]), but may impose substantial selection against hybrids between recently diverged taxa (e.g. in crop plants, hybrid weakness has evolved within thousands of years). Determining the timing of when these types of barriers evolve, their commonality in natural populations, the degree of selection against them, and the evolutionary drivers responsible for them are all essential aims for speciation biologists.
(c). Reinforcement
Lastly, the third major role intrinsic postzygotic reproductive isolation can play in the speciation process is the generation of subsequent reproductive isolation. Specifically, the presence of strong intrinsic postzygotic barriers between sympatric taxa can lead to selection favouring increased prezygotic reproductive isolation owing to low fitness hybrids (reinforcement; [138,205]). While we focus on examples and theory in which reinforcing selection results from intrinsic barriers, reinforcement may also be a consequence of extrinsic postzygotic reproductive isolation (reviewed in [31]). Reinforcement has primarily been studied in the context of increased mate preference in sympatry ([206–209]; or similarly, pollinator shifts in plants; [210–213]), but can manifest as any reproductive barrier that prevents parental investment in unfit hybrids, including increased gametic incompatibilities [214,215], ecological divergence that decreases the probability of heterospecific matings [216], or early embryo abortion in systems with substantial parental care [217]. Reinforcement can also have more direct consequences of the generation on biodiversity through processes such as cascading reinforcement (the increase in reproductive isolation between parapatric conspecific populations as a result of reinforcing selection between sympatric heterospecific interactions; e.g. [209,218–220]).
Reinforcement may be a common phenomenon across taxonomic groups, and is considered by some to be an essential step in speciation [205,221]. Patterns consistent with reinforcement have been described in a number of natural systems (e.g. [9,206–208,210–213,218,219,222–227]), and reinforcement has been experimentally evolved many times (e.g. [228–232]; reviewed in [233]). Reinforcement is often considered a final step in the speciation process; reinforcing selection can cause an increase in prezygotic reproductive isolation that finalizes speciation and allows coexistence between close relatives. Because of its association with the completion of reproductive isolation, there is a connotation that reinforcement may take a long time to evolve (e.g. [144,234]). However, three classes of empirical results show that reinforcement need not be a process that occurs only between highly divergent species. Firstly, in many experimental evolution studies of Drosophila, reinforcement can evolve in fewer than 10 generations [228–231]. Secondly, in large surveys of the accumulation of reproductive isolation through time (such as [9,222,235]), evidence for reinforcement rests on the observation that prezygotic barriers evolves more rapidly in sympatry than allopatry. In Drosophila, allopatric species pairs often exhibit strong prezygotic isolation at low to moderate levels of divergence [9,222]. Therefore, evidence of reinforcement in Drosophila stems from the observation that strong prezygotic reproductive isolation evolves between very recently diverged sympatric species pairs [9,222]. Of course, studies which have scored both pre- and postzygotic reproductive isolation for a large number of sympatric and allopatric species pairs are scarce, and so broad conclusions about the rate of evolution of reinforcement from large-scale comparative work are limited. Thirdly, reinforcement has been documented between recently diverged species pairs, such as in the Drosophila subquinaria species complex [208,236]. Thus, while reinforcement may signify later stages of speciation, the presence of reinforcing selection is not restricted to highly divergent species pairs.
Reinforcement may also manifest as ecological divergence that decreases the probability of heterospecific matings. This phenomenon is well described in the context of polyploids and their diploid progenitors (‘minority cytotype principle’; [216]), but can also extend to diploid species. In essence, when two species co-occur and one is substantially less common than the other, the less common species can experience a potential cost to reproduction as it is more likely to engage in heterospecific matings than the more common species. Selection to reduce interspecific mating can result in ecological divergence [216]. In the context of polyploids and their diploid progenitors, ecological divergence is common [237–241] and perhaps immediate [242]. Patterns of ecological divergence as a consequence of strong intrinsic postzygotic barriers among diploids are less established, but have been described in Mimulus [243].
5. Moving forward as a field
We outline three contributions of intrinsic postzygotic barriers to speciation and highlight how their role may change through time. Still, there are many remaining questions which can further our understanding of the role of intrinsic postzygotic barriers in speciation (box 2). Gaps in our knowledge can be categorized as questions pertaining to the origin, commonality, and strength of different types of intrinsic postzygotic barriers, the relationship between intrinsic postyzogtic barriers and introgression, and the role of intrinsic postzygotic barriers in species persistence and diversification. Answering these questions will require the integration of new theory and simulations, as well as the collection of more datasets that address both broad scale patterns of reproductive isolation and detailed dissections of specific barriers. Below we highlight how integrative studies can further our understanding of intrinsic postzygotic barriers.
Box 2. Outstanding questions in speciation biology.
| question | potential approach(es) | |
|---|---|---|
| origin, commonality, and importance of intrinsic postzygotic barriers | 1. what are the major evolutionary drivers of intrinsic barriers and how frequent are they in nature? | a. genetically dissect reproductive barriers to determine if genes involved provide information of the evolutionary drivers (such as [50,85,91]) |
| b. compare the extent of reproductive isolation or diversification rates between groups that are known to differ in proxies for particular evolutionary drivers (e.g. mating system as a proxy for parental conflict and the evolution of hybrid seed inviability, circa [135] | ||
| 2. how common are less obvious intrinsic barriers (e.g. deficits in hybrid metabolism, neurology, or general ‘hybrid weakness’)? At what stage are these barriers important? | a. case studies quantifying hybrid defects, and when possible, the amount of selection against hybrids who carry them [e.g. 200] | |
| b. comparative studies that analyse the relative age of species pairs that produce hybrids with ‘hybrid weakness’ or other transgressive phenotypes | ||
| 3. is there interplay between intrinsic barriers and ecology? How important is this for speciation? | a. generate hybrids under multiple biologically realistic environmental conditions and measure viability or sterility [e.g. 41] | |
| b. assess hybrid sterility of viability in natural populations across an environmental gradient | ||
| intrinsic postzygotic barriers and introgression | 4. how effective are intrinsic barriers at preventing introgression in nature? | a. map the genetic basis of incompatibility loci in allopatric populations, assess the fate of incompatibility alleles in contact zones (e.g. whether or not they are still present, or assess the steepness of clines across a hybrid zone for incompatibility loci versus neutral loci). Genetic mapping will of course be easiest in model systems in which laboratory crosses are possible. Although not as robust, if researchers are using non-model systems in which laboratory crosses are unattainable, using field collected hybrids for admixture mapping or RNAseq of barrier tissues (e.g. gametes) may be informative. We caution, however, that these natural hybrids may represent a non-random collection of incompatibility alleles |
| b. experimentally evolve hybrid swarms between parental species with different types of reproductive barriers. Assess the prevalence of reproductive isolation through time and determine how quickly different types of barriers are lost from hybrid populations (or whether some persist) | ||
| c. simulate genome-wide patterns of introgression for species pairs with differing types of reproductive barriers | ||
| 5. what is the relationship between the amount of introgression (or the composition of introgressed alleles) and divergence time? | a. assess rates and timing of introgression in natural or synthetic contact zones from species pairs of differing ages | |
| b. the same as above, but can use genomes of broadly sympatric taxa rather than explicit contact zones | ||
| c. simulate genome-wide patterns of introgression between pairs of populations with differing divergence | ||
| 6. how do different evolutionary drivers that are responsible for the evolution of intrinsic barriers influence the stability of these barriers in the face of gene flow? | a. simulate secondary contact when incompatibilities are driven by different evolutionary mechanisms (e.g. neutrality, local adaptation, systems drift, conflict or other co-evolutionary dynamics), and assess the stability of simulated incompatibility alleles | |
| intrinsic postzygotic barriers and species persistence and diversification | 7. how important are intrinsic postzygotic barriers for species coexistence and diversification? | a. compare rates of accumulation of postzygotic intrinsic barriers in allopatry versus sympatry to determine if sympatric taxa are more likely to be strongly isolated (consistent with Templeton effect [136]) |
| b. simulate secondary contact between populations that are weakly or strongly reproductively isolated with pre- and/or postzygotic reproductive barriers and determine the probability of extinction | ||
| c. determine whether levels of reproductive isolation are correlated with diversification rates [such as 244] |
Several questions remain on the origin, commonality and strength of different types of intrinsic barriers (box 2, questions 1–3). A potential major contributor to intrinsic postzygotic isolation are subtle intrinsic postzygotic barriers that affect hybrid physiological or cognitive ability, or general ‘hybrid weakness’ (box 2, question 2). Case studies that detail the diversity of hybrid defects with measurements of selection against these transgressive phenotypes, as well as comparative studies that assess the rate of accumulation of these types of barriers (such as [9,235]) can inform us of the commonality, strength, and timing of these types of barriers in nature.
While there is substantial theory highlighting the efficacy of intrinsic postzygotic barriers against introgression, explicit empirical work is needed (box 2, question 4). One approach is to combine quantitative genetics to map the genetic basis of reproductive isolation and population genomics to infer patterns of introgression of these alleles. Another approach is to pair large-scale quantifications the accumulation of intrinsic postzygotic isolation through time (as in [9,111–117,245–247]), with comparative population genomic studies to assess how the extent of introgression tracks with divergence times (as in [130]) and the accumulation of reproductive isolation. The later approach can also be used to test whether the extent of introgression is also nonlinear through time (box 2, question 5; [21,22,30]).
Lastly, intrinsic postzygotic barriers may be essential for species persistence [37,234], but empirical tests are needed (box 2, question 7). For example, the Templeton effect posits that only strongly isolated species pairs can persist in sympatry, as weakly isolated species pairs are more likely to go extinct or collapse via introgression [37,136,137,234]. One approach to test this would be to use large-scale comparative datasets to assess the rate of accumulation of intrinsic postzygotic isolation in sympatric and allopatric species pairs, with the prediction that sympatric species pairs should show higher intrinsic postzygotic isolation than similarly divergent allopatric species pairs. The role of reproductive barriers in speciation at macro-evolutionary time scales is also not well understood. For example, reproductive isolation is often abundant, but may not predict patterns of diversification [244,248]. Understanding if intrinsic postzygotic barriers contribute to macroevolutionary patterns of diversity, and what factors affect this process, is an important, but rarely studied aspect of speciation.
Acknowledgements
We thank the Matute laboratory for useful discussions of the state of speciation genetics. We are also grateful to Leonie Moyle and Kyle Christie for providing access to data from previously published work. This review was greatly improved by insightful comments from Jonna Kulmuni and Darren Irwin, as well as one anonymous reviewer.
Data accessibility
All data presented in this paper were accessed from previously published datasets. These datasets have been referenced throughout the manuscript.
Authors' contributions
J.M.C. and D.R.M. both conceived of the ideas and contributed substantially to the writing of this manuscript.
Competing interests
We declare we have no competing interests.
Funding
J.M.C. and D.R.M. are supported by NSF DEB grant no. 1737752.
References
- 1.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 2.Dobzhansky T. 1936. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21, 113–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stebbins GL. 1958. The inviability, weakness, and sterility of interspecific hybrids. Adv. Genet. 9, 147–215. ( 10.1016/S0065-2660(08)60162-5) [DOI] [PubMed] [Google Scholar]
- 4.Prager EM, Wilson AC. 1975. Slow evolutionary loss of the potential for interspecific hybridization in birds: a manifestation of slow regulatory evolution. Proc. Natl Acad. Sci. USA 72, 200–204. ( 10.1073/pnas.72.1.200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey J, Bradshaw HD, Schemske DW. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57, 1520–1534. ( 10.1111/j.0014-3820.2003.tb00360.x) [DOI] [PubMed] [Google Scholar]
- 6.Nosil P, Vines TH, Funk DJ. 2013. Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59, 705–719. ( 10.1111/j.0014-3820.2005.tb01747.x) [DOI] [PubMed] [Google Scholar]
- 7.Sobel JM, Chen GF. 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68, 1511–1522. ( 10.1111/evo.12362) [DOI] [PubMed] [Google Scholar]
- 8.Arnegard ME, et al. 2014. Genetics of ecological divergence during speciation. Nature 511, 307–311. ( 10.1038/nature13301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne JA, Orr HA. 1989. Patterns of speciation in Drosophila. Evolution 43, 362–381. ( 10.1111/j.1558-5646.1989.tb04233.x) [DOI] [PubMed] [Google Scholar]
- 10.Coyne JA, Orr HA. 1997. Patterns of speciation in Drosophila ‘ revisited. Evolution 51, 295–303. ( 10.1111/j.1558-5646.1997.tb03650.x) [DOI] [PubMed] [Google Scholar]
- 11.Mayr E. 1963. Animal species and evolution. Cambridge, MA: Harvard University Press. [Google Scholar]
- 12.Grant PR, Grant BR. 1997. Genetics and the origin of bird species. Proc. Natl Acad. Sci. USA 94, 7768–7775. ( 10.1073/pnas.94.15.7768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin DE. 2000. Song variation in an avian ring species. Evolution 54, 998–1010. ( 10.1111/j.0014-3820.2000.tb00099.x) [DOI] [PubMed] [Google Scholar]
- 14.Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH. 2008. The strength and genetic basis of reproductive isolating barriers in flowering plants. Phil. Trans. R. Soc. B 363, 3009–3021. ( 10.1098/rstb.2008.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieseberg LH, Willis JH. 2008. Plant speciation. Science 317, 910–914. ( 10.1126/science.1137729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer. [Google Scholar]
- 17.Maheshwari S, Barbash DA. 2011. The genetics of hybrid incompatibilities. Annu. Rev. Genet. 45, 331–355. ( 10.1146/annurev-genet-110410-132514) [DOI] [PubMed] [Google Scholar]
- 18.Cutter AD. 2012. The polymorphic prelude to Bateson–Dobzhansky–Muller incompatibilities. Trends Ecol. Evol. 27, 210–219. ( 10.1016/j.tree.2011.11.004) [DOI] [PubMed] [Google Scholar]
- 19.Presgraves DC. 2010. Molecular evolutionary basis of species formation. Nat. Rev. Genet. 11, 175–180. ( 10.1038/nrg2718) [DOI] [PubMed] [Google Scholar]
- 20.Bomblies K, Weigel D. 2007. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nat. Rev. Genet. 8, 382–393. ( 10.1038/nrg2082) [DOI] [PubMed] [Google Scholar]
- 21.Orr HA. 1995. Population genetics of speciation. Genetics 139, 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orr HA, Turelli M. 2001. The evolution of postzygotic isolation: accumulating Dobzhansky-Muller incompatibilities. Evolution 55, 1085–1094. ( 10.1111/j.0014-3820.2001.tb00628.x) [DOI] [PubMed] [Google Scholar]
- 23.Gourbiere S, Mallet J. 2009. Are species real? The shape of the species boundary with expondential failure, reinforcement, and the ‘missing snowball’. Evolution 64, 1–24. ( 10.1111/j.1558-5646.2009.00844.x) [DOI] [PubMed] [Google Scholar]
- 24.Matute DR, Butler IA, Turissini DA, Coyne JA. 2010. A test of the snowball theory or the rate of evolution of hybrid incompatibilities. Science 329, 1518–1521. ( 10.1126/science.1193440) [DOI] [PubMed] [Google Scholar]
- 25.Moyle LC, Nakazato T. 2010. Hybrid incompatibility ‘snowballs’ between Solanum species. Science 329, 1521–1523. ( 10.1126/science.1193063) [DOI] [PubMed] [Google Scholar]
- 26.Matute DR, Gavin-Smyth J. 2014. Fine mapping of dominant X-linked incompatibility alleles in Drosophila hybrids. PLoS Genet. 10, 1–15. ( 10.1371/journal.pgen.1004270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RJ, White MA, Payseur BA. 2015. The pace of hybrid incompatibility in house mouse. Evolution 201, 229–242. ( 10.1534/genetics.115.179499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero RF, Muir CD, Josway S, Moyle LC. 2017. Pervasive antagonistic interactions among hybrid incompatibility loci. PLoS Genet. 13, e1006817 ( 10.1371/journal.pgen.1006817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Presgraves DC. 2010. Speciation genetics: search for the missing snowball. Curr. Biol. 20, R1073–R1074. ( 10.1016/j.cub.2010.10.056) [DOI] [PubMed] [Google Scholar]
- 30.Muirhead CA, Presgraves DC. 2016. Hybrid incompatibilities, local adaptation, and the genomic distribution of natural introgression between species. Am. Nat. 187, 249–261. ( 10.1086/684583) [DOI] [PubMed] [Google Scholar]
- 31.Servedio MR, Noor MAF. 2003. The role of reinforcement in speciation: theory and data. Ann. Rev. Ecol. Evol. Syst. 34, 339–364. ( 10.1146/annurev.ecolsys.34.011802.132412) [DOI] [Google Scholar]
- 32.Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. 2009. The frequency of polyploid speciation in vascular plants. Proc. Natl Acad. Sci. USA 106, 13 875–13 879. ( 10.1073/pnas.0811575106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueshima R, Asami T. 2003. Single-gene speciation by left–right reversal. Nature 425, 2003 ( 10.1038/425679a) [DOI] [PubMed] [Google Scholar]
- 34.Slotte T, Huang H, Lascoux M, Ceplitis A. 2008. Polyploid speciation did not confer instant reproductive isolation in Capsella (Brassicaceae). Mol. Biol. Evol. 25, 1472–1481. ( 10.1093/molbev/msn092) [DOI] [PubMed] [Google Scholar]
- 35.Sutherland BL, Galloway LF. 2017. Postzygotic isolation varies by ploidy level within a polyploid complex. New Phytol. 213, 404–412. ( 10.1111/nph.14116) [DOI] [PubMed] [Google Scholar]
- 36.Richards PM, Morii Y, Kimura K, Hirano T, Chiba S, Davison A. 2017. Single-gene speciation: mating and gene flow between mirror-image snails. Evol. Lett. 1, 282–291. ( 10.1002/evl3.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todesco M, et al. 2016. Hybridization and extinction. Evol. Appl. 9, 892–908. ( 10.1111/eva.12367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kearns AM, et al. 2018. Genomic evidence of speciation reversal in ravens. Nat. Commun. 9, 906 ( 10.1038/s41467-018-03294-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matute DR, et al. 2020. Rapid and predictable evolution of admixed populations between two Drosophila species pairs. Genetics 214, 211–230. ( 10.1534/genetics.119.302685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irwin DE. 2020. Assortative mating in hybrid zones is remarkably ineffective in promoting speciation. Am. Nat. 195 ( 10.1086/708529) [DOI] [PubMed] [Google Scholar]
- 41.Bundus JD, Alaei R, Cutter AD. 2015. Gametic selection, developmental trajectories, and extrinsic heterogeneity in Haldane's rule. Evolution 69, 2005–2017. ( 10.1111/evo.12708) [DOI] [PubMed] [Google Scholar]
- 42.Miller CJJ, Matute DR. 2017. The effect of temperature on Drosophila hybrid fitness. G3: Genes, Genomes, Genetics 7, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice AM, McQuillan MA. 2018. Maladaptive learning and memory in hybrids as a reproductive isolating barrier. Proc. R. Soc. B 285, 20180542 ( 10.1098/rspb.2018.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turissini DA, Comeault AA, Liu G, Lee YCG, Matute DR. 2017. The ability of Drosophila hybrids to locate food declines with parental divergence. Evolution 71, 960–973. ( 10.1111/evo.13180) [DOI] [PubMed] [Google Scholar]
- 45.McQuillan MA, Ii TCR, Huynh AV, Rice AM. 2018. Hybrid chickadees are deficient in learning and memory. Evolution 72, 1155–1164. ( 10.1111/evo.13470) [DOI] [PubMed] [Google Scholar]
- 46.Ellison CK, Niehuis O, Gadau J. 2008. Hybrid breakdown and mitochondrial dysfunction in hybrids of Nasonia parasitoid wasps. J. Evol. Biol. 21, 1844–1851. ( 10.1111/j.1420-9101.2008.01608.x) [DOI] [PubMed] [Google Scholar]
- 47.Ellison CK, Burton RS. 2008. Interpopulation hybrid breakdown maps to the mitochondrial genome. Evolution 62, 631–638. ( 10.1111/j.1558-5646.2007.00305.x) [DOI] [PubMed] [Google Scholar]
- 48.Dey A, Jin Q, Chen YC, Cutter AD. 2014. Gonad morphogenesis defects drive hybrid male sterility in asymmetric hybrid breakdown of Caenorhabditis nematodes. Evol. Dev. 372, 362–372. ( 10.1111/ede.12097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hämälä T, Mattila TM, Leinonen PH, Kuittinen H, Savolainen O. 2017. Role of seed germination in adaptation and reproductive isolation in Arabidopsis lyrata. Mol. Ecol. 26, 3484–3496. ( 10.1111/mec.14135) [DOI] [PubMed] [Google Scholar]
- 50.Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D. 2007. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 5, 1962–1972. ( 10.1371/journal.pbio.0050236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chae E, et al. 2014. Species-wide genetic incompatibility analysis identifies immune genes as hot spots of deleterious epistasis. Cell 159, 1341–1351. ( 10.1016/j.cell.2014.10.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corbett-Detig RB, Zhou J, Clark AG, Hartl DL, Ayroles JF. 2013. Genetic incompatibilities are widespread within species. Nature 504, 135–137. ( 10.1038/nature12678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Case AL, Finseth FR, Barr CM, Fishman L. 2016. Selfish evolution of cytonuclear hybrid incompatibility in Mimulus. Proc. R. Soc. B 283, 20161493 ( 10.1098/rspb.2016.1493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sweigart AL, Mason AR, Willis JH. 2007. Natural variation for a hybrid incompatibility between two species of Mimulus. Evolution 61, 141–151. ( 10.1111/j.1558-5646.2007.00011.x) [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Fernandez H, Bolnick DI. 2007. What causes partial F1 hybrid viability? Incomplete penetrance versus genetic variation. PLoS ONE 12, e1294 ( 10.1371/journal.pone.0001294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Good JM, Handel MA, Nachman MW. 2007. Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62, 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozlowska JL, Ahmad AR, Jahesh E, Cutter AD. 2012. Genetic variation for postzygotic reproductive isolation between Caenorhabditis briggsae and Caenorhabditis sp. 9. Evolution 66, 1180–1195. ( 10.1111/j.1558-5646.2011.01514.x) [DOI] [PubMed] [Google Scholar]
- 58.Turner LM, Schwahn DJ, Harr B. 2011. Reduced male fertility is common byt highly variable in form and severity in natural house mouse hybrid zone. Evolution 66, 443–458. ( 10.1111/j.1558-5646.2011.01445.x) [DOI] [PubMed] [Google Scholar]
- 59.Matute DR, Gavin-Smyth J, Liu G. 2014. Variable post-zygotic isolation in Drosophila melanogaster/D. simulans hybrids. J. Evol. Biol. 27, 1691–1705. ( 10.1111/jeb.12422) [DOI] [PubMed] [Google Scholar]
- 60.Sicard A, Kappel C, Josephs EB, Lee YW, Marona C, Stinchcombe JR, Wright SI, Lenhard M. 2015. Divergent sorting of a balanced ancestral polymorphism underlies the establishment of gene-flow barriers in Capsella. Nat. Commun. 6, 7960 ( 10.1038/ncomms8960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnard-Kubow KB, Galloway LF. 2017. Variation in reproductive isolation across a species range. Ecol. Evol. 7, 9347–9357. ( 10.1002/ece3.3400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuellig MP, Sweigart AL. 2018. A two-locus hybrid incompatibility is widespread, polymorphic, and active in natural populations of Mimulus. Evolution 72, 1–12. ( 10.1111/evo.13596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bracewell RR, Bentz BJ, Sullivan BT, Good JM. 2017. Rapid neo-sex chromosome evolution and incipient speciation in a major forest pest. Nat. Commun. 8, 1593 ( 10.1038/s41467-017-01761-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson EL, et al. 2018. The evolution of polymorphic hybrid incompatibilities in house mice. Genetics 209, 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gavrilets S. 1997. Single locus clines. Evolution 51, 979–983. ( 10.1111/j.1558-5646.1997.tb03678.x) [DOI] [PubMed] [Google Scholar]
- 66.Kondrashov AS. 2003. Accumulation of Dobzhansky-muller incompatibilities within a spatially structured population. Evolution 57, 151–153. ( 10.1111/j.0014-3820.2003.tb00223.x) [DOI] [PubMed] [Google Scholar]
- 67.Bank C, Bürger R, Hermisson J. 2012. The limits to parapatric speciation: Dobzhansky-Muller incompatibilities in a continent-island model. Genetics 191, 845–863. ( 10.1534/genetics.111.137513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostevik KL, Rifkin JL, Xia H, Rausher MD. 2019. Asymmetric gene flow causes cascading reproductive isolation. BioRxiv ( 10.1101/767970) [DOI]
- 69.Darwin C. 1859. On the origin of species. London, UK: John Murray. [Google Scholar]
- 70.Bateson W. 1909. Heredity and variation in modern lights. In Danvin and modmz science (ed. Seward AC.), pp. 85–101. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 71.Dobzhansky TG. 1937. Genetics and the origin of species. New York, NY: Columbia University Press. [Google Scholar]
- 72.Muller HJ. 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71–125. [Google Scholar]
- 73.Greig D. 2009. Reproductive isolation in Saccharomyces. Heredity 102, 39–44. ( 10.1038/hdy.2008.73) [DOI] [PubMed] [Google Scholar]
- 74.Blackman BK. 2016. Speciation genes. Encycl. Evol. Biol. 4, 166–175. ( 10.1016/B978-0-12-800049-6.00066-4) [DOI] [Google Scholar]
- 75.Landry CR, Hartl DL, Ranz JM. 2007. Genome clashes in hybrids: insights from gene expression. Heredity 99, 483–493. ( 10.1038/sj.hdy.6801045) [DOI] [PubMed] [Google Scholar]
- 76.Barbash DA, Awadalla P, Tarone AM. 2004. Functional divergence caused by ancient positive selection of a Drosophila hybrid incompatibility locus. PLoS Biol. 2, 839–848. ( 10.1371/journal.pbio.0020142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cattani MV, Presgraves DC. 2009. Genetics and lineage-specific evolution of a lethal hybrid incompatibility between Drosophila mauritiana and its sibling species. Genetics 181, 1545–1555. ( 10.1534/genetics.108.098392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sweigart AL, Fishman L, Willis JH. 2006. A simple genetic incompatibility causes hybrid male sterility in Mimulus. Genetics 172, 2465–2479. ( 10.1534/genetics.105.053686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Presgraves DC. 2003. A fine-scale genetic analysis of hybrid incompatibilities in Drosophila. Genetics 163, 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ting C, Tsaur S-C, Wu M-L, Wu C-I. 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282, 1501–1504. ( 10.1126/science.282.5393.1501) [DOI] [PubMed] [Google Scholar]
- 81.Barbash DA, Siino DF, Tarone AM, Roote J. 2003. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc. Natl Acad. Sci. USA 100, 5302–5307. ( 10.1073/pnas.0836927100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Presgraves DC, Balagopalan L, Abmayr SM, Orr HA. 2003. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423, 715–719. ( 10.1038/nature01679) [DOI] [PubMed] [Google Scholar]
- 83.Tang S, Presgraves DC. 2009. Evolution of the Drosophila nuclear pore complex results in multiple hybrid incompatibilities. Science 6, 779–783. ( 10.1126/science.1169123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliver PL, et al. 2009. Accelerated evolution of the Prdm9 speciation gene across diverse metazoan taxa. PLoS Genet. 5, 1–14. ( 10.1371/journal.pgen.1000753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phadnis N, Baker EP, Cooper JC, Frizzell KA, Hsieh E, de la Cruz AFA, Schendure J, Kitzman JO, Malik HS. 2015. An essential cell cycle regulation gene causes hybrid inviability in Drosophila. Science 350, 1552–1555. ( 10.1126/science.aac7504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sweigart AL, Flagel LE. 2015. Evidence of natural selection acting on a polymorphic hybrid incompatibility locus in Mimulus. Genetics 199, 543–554. ( 10.1534/genetics.114.171819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson NA. 2010. Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet. 26, 317–325. ( 10.1016/j.tig.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 88.Nosil P, Crespi BJ. 2012. Conflictual speciation: species formation via intragenomic conflict. Trends Ecol. Evol. 28, 48–57. [DOI] [PubMed] [Google Scholar]
- 89.Masly JP, Jones CD, Noor MAF, Locke J, Orr HA. 2006. Gene transposition as a novel cause of hybrid male sterility. Science 313, 1448–1450. ( 10.1126/science.1128721) [DOI] [PubMed] [Google Scholar]
- 90.Bikard D, Patel D, Le Mette C, Giorgi V, Camilleri C, Bennett MJ, Loudet O. 2009. Divergent evolution of duplicate genes leads to genetic incompatibilities within A. thaliana. Science 323, 623–626. ( 10.1126/science.1165917) [DOI] [PubMed] [Google Scholar]
- 91.Phadnis N, Orr HA. 2009. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323, 376–379. ( 10.1126/science.1163934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuellig MP, Sweigart AL. 2018. Gene duplicates cause hybrid lethality between sympatric species of Mimulus. PLoS 14, e1007130 ( 10.1371/journal.pgen.1007130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bolnick DI, Near TJ, Wainwright PC. 2006. Body size divergence promotes post-zygotic reproductive isolation in centrarchids. Evol. Ecol. Res. 8, 903–913. [Google Scholar]
- 94.Funk DJ, Nosil P, Etges WJ. 2006. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. Natl Acad. Sci. USA 103, 3209–3213. ( 10.1073/pnas.0508653103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dettman JR, Sirjusingh C, Kohn LM, Anderson JB. 2007. Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature 447, 585–588. ( 10.1038/nature05856) [DOI] [PubMed] [Google Scholar]
- 96.Agrawal AF, Feder JL, Nosil P. 2011. Ecological divergence and the origins of intrinsic postmating isolation with gene flow. Int. J. Ecol. 2011, 1–15. ( 10.1155/2011/435357) [DOI] [Google Scholar]
- 97.Wright KM, Lloyd D, Lowry DB, Macnair MR, Willis JH. 2013. Indirect evolution of hybrid lethality due to linkage with selected locus in Mimulus guttatus. PLoS Biol. 11, e1001497 ( 10.1371/journal.pbio.1001497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kulmuni J, Westram AM. 2017. Intrinsic incompatibilities evolving as a by-product of divergent ecological selection: considering them in empirical studies on divergence with gene flow. Mol. Ecol. 26, 3093–3103. ( 10.1111/mec.14147) [DOI] [PubMed] [Google Scholar]
- 99.True JR, Haag ES. 2001. Developmental system drift and flexibility in evolutionary trajectories. Evol. Dev. 3, 109–119. ( 10.1046/j.1525-142x.2001.003002109.x) [DOI] [PubMed] [Google Scholar]
- 100.Mack KL, Nachman MW. 2016. Gene regulation and speciation. Trends Genet. 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiffman JS, Ralph PL.. 2018. System drift and speciation. BioRxiv ( 10.1101/231209) [DOI]
- 102.Werth CR, Windham MD. 1991. A model for divergent, allopatric speciation of polyploid pteridophytes resulting from silencing of duplicate-gene expression. Am. Nat. 137, 515–526. ( 10.1086/285180) [DOI] [Google Scholar]
- 103.Lynch M, Force AG. 2000. The origin of interspecific genomic incompatibility via gene duplication. Am. Nat. 156, 590–605. ( 10.1086/316992) [DOI] [PubMed] [Google Scholar]
- 104.Moyle LC, Muir CD, Han MV, Hahn MW. 2010. The contribution of gene movement to the ‘two rules of speciation’. Evolution 64, 1541–1557. ( 10.1111/j.1558-5646.2010.00990.x) [DOI] [PubMed] [Google Scholar]
- 105.Bomblies K. 2009. Too much of a good thing? Hybrid necrosis as a by-product of plant immune system diversification. Botany 87, 1013–1022. ( 10.1139/B09-072) [DOI] [Google Scholar]
- 106.Frank SA. 1991. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution 45, 262–267. [DOI] [PubMed] [Google Scholar]
- 107.Hurst LD, Pomiankowski A. 1991. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics 128, 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Charlesworth B, Coyne JA, Orr HA. 1993. Meiotic drive and unisexual hybrid sterility: A comment [1]. Genetics 133, 421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coyne JA, Orr HA. 1993. Further evidence against meiotic-drive models of hybrid sterility. Evolution 47, 685–687. ( 10.1111/j.1558-5646.1993.tb02123.x) [DOI] [PubMed] [Google Scholar]
- 110.Fuller ZL, Leonard CJ, Young RE, Schaeffer SW, Phadnis N. 2018. Ancestral polymorphisms explain the role of chromosomal inversions in speciation. PLoS Genet. 14, 1–26. ( 10.1371/journal.pgen.1007526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Presgraves DAC. 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56, 1168–1183. ( 10.1111/j.0014-3820.2002.tb01430.x) [DOI] [PubMed] [Google Scholar]
- 112.Price TD, Bouvier MM. 2002. The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089. ( 10.1111/j.0014-3820.2002.tb00133.x) [DOI] [PubMed] [Google Scholar]
- 113.Malone JH, Fontenot BE. 2008. Patterns of reproductive isolation in toads. PLoS ONE 3, e3900 ( 10.1371/journal.pone.0003900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jewell C, Papineau AD, Freyre R, Moyle LC. 2012. Patterns of reproductive isolation in Nolana (Chilean bellflower). Evolution 66, 2628–2636. ( 10.1111/j.1558-5646.2012.01607.x) [DOI] [PubMed] [Google Scholar]
- 115.Owens GL, Rieseberg LH. 2013. Hybrid incompatibility is acquired faster in annual than in perennial species of sunflower and tarweed. Evolution 68, 893–900. ( 10.1111/evo.12297) [DOI] [PubMed] [Google Scholar]
- 116.Christie K, Strauss SY. 2018. Along the speciation continuum: quantifying intrinsic and extrinsic isolating barriers across five million years of evolutionary divergence in California jewelflowers. Evolution 72, 1063–1079. ( 10.1111/evo.13477) [DOI] [PubMed] [Google Scholar]
- 117.Bolnick DI, Near TJ. 2005. Tempo of hybrid inviability in Centrarchid fishes (Teleostei: Centrarchidae). Evolution 59, 1754–1767. ( 10.1111/j.0014-3820.2005.tb01824.x) [DOI] [PubMed] [Google Scholar]
- 118.Christianson SJ, Swallow JG, Wilkinson GS. 2005. Rapid evolution of postzygotic reproductive isolation in stalk-eyed flies. Evolution 59, 849–857. ( 10.1554/04-291) [DOI] [PubMed] [Google Scholar]
- 119.Bracewell RR, Pfrender ME, Mock KE, Bentz BJ. 2010. Cryptic postzygotic isolation in an eruptive species of bark beetle. Evolution 65, 961–975. ( 10.1111/j.1558-5646.2010.01201.x) [DOI] [PubMed] [Google Scholar]
- 120.Moyle LC, Levine M, Stanton ML, Wright JW. 2012. Hybrid sterility over tens of meters between ecotypes adapted to serpentine and non-serpentine soils. Evol. Biol. 39, 207–218. ( 10.1007/s11692-012-9180-9) [DOI] [Google Scholar]
- 121.Skrede I, Brochmann C, Borgen L, Rieseberg LH. 2008. Genetics of intrinsic postzygotic isolation in a circumpolar plant species, Draba nivalis (Brassicaceae). Evolution 62, 1840–1851. ( 10.1111/j.1558-5646.2008.00418.x) [DOI] [PubMed] [Google Scholar]
- 122.Gustafsson ALS, Skrede I, Rowe HC, Gussarova G, Borgen L, Rieseberg LH, Brochmann C, Parisod C. 2014. Genetics of cryptic speciation within an Arctic mustard, Draba nivalis. PLoS ONE 9, e93834 ( 10.1371/journal.pone.0093834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scopece G, Widmer A, Cozzolino S, Biologiche S. 2008. Evolution of postzygotic reproductive isolation in a guild of deceptive orchids. Am. Nat. 171, 315–326. ( 10.1086/527501) [DOI] [PubMed] [Google Scholar]
- 124.Briscoe Runquist B, Chu E, Iverson JL, Kopp JC, Moeller DA. 2014. Rapid evolution of reproductive isolation between incipient outcrossing and selfing Clarkia species. Evolution 68, 2885–2900. ( 10.1111/evo.12488) [DOI] [PubMed] [Google Scholar]
- 125.Macnair MR, Christie P. 1983. Reproductive isolation as a pleiotropic effect of copper tolerance in Mimulus guttatus? Heredity 50, 295–302. ( 10.1038/hdy.1983.31) [DOI] [Google Scholar]
- 126.Coughlan JM, Wilson Brown M, Willis J. 2019. Patterns of hybrid seed inviability in the Mimulus guttatus sp. complex reveal a potential role of parental conflict in reproductive isolation. Curr. Biol. 30, 83–93. ( 10.1016/j.cub.2019.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barnard-Kubow KB, So N, Galloway LF. 2016. Cytonuclear incompatibility contributes to the early stages of speciation. Evolution 70, 2752–2766. ( 10.1111/evo.13075) [DOI] [PubMed] [Google Scholar]
- 128.Brekke TD, Good JM. 2014. Parent-of-origin growth effects and the evolution of hybrid inviability in dwarf hamsters. Evolution 68, 3134–3148. ( 10.1111/evo.12500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mallet J, Beltrán M, Neukirchen W, Linares M. 2007. Natural hybridization in heliconiine butterflies: the species boundary as a continuum. BMC Evol. Biol. 16, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roux C, Fra C, Romiguier J, Anciaux Y, Galtier N, Bierne N. 2016. Shedding light on the grey zone of speciation along a continuum of genomic divergence. PLoS Biol. 14, 1–22. ( 10.1371/journal.pbio.2000234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Riesch R, et al. 2017. Transitions between phases of genomic differentiation during stick-insect speciation. Nat. Ecol. Evol. 1, 1–13. ( 10.1038/s41559-017-0082) [DOI] [PubMed] [Google Scholar]
- 132.Wilson AC, Maxson LR, Sarich VM. 1974. Two types of molecular evolution: evidence from studies of interspecific hybridization. Proc. Natl Acad. Sci. USA 71, 2843–2847. ( 10.1073/pnas.71.7.2843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fitzpatrick BM. 2004. Rates of evolution of hybrid inviability in birds and mammals. Evolution 58, 1865–1870. ( 10.1111/j.0014-3820.2004.tb00471.x) [DOI] [PubMed] [Google Scholar]
- 134.Yukilevich R. 2013. Tropics accelerate the evolution of hybrid male sterility in Drosophila. Evolution 67, 1805–1814. ( 10.1111/evo.12056) [DOI] [PubMed] [Google Scholar]
- 135.Brandvain Y, Haig D. 2005. Divergent mating systems and parental conflict as a barrier to hybridization in flowering plants. Am. Nat. 166, 330–338. ( 10.1086/432036) [DOI] [PubMed] [Google Scholar]
- 136.Templeton AR. 1981. Mechanisms of speciation: a population genetic approach. Annu. Rev. Ecol. Syst. 12, 23–48. ( 10.1146/annurev.es.12.110181.000323) [DOI] [Google Scholar]
- 137.Rhymer JM, Simberloff D. 1996. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 27, 83–109. ( 10.1146/annurev.ecolsys.27.1.83) [DOI] [Google Scholar]
- 138.Servedio MR, Sætre G. 2003. Speciation as a positive feedback loop between postzygotic and prezygotic barriers to gene flow. Proc. R. Soc. Lond. B 270, 1473–1479. ( 10.1098/rspb.2003.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harrison R. 1990. Hybrid zones: windows on evolutionary process. In Oxford surveys in evolutionary biology (eds Futuyma D, Antonovics J), pp. 69–128. New York, NY: Oxford University Press. [Google Scholar]
- 140.Jiggins CD, Mallet J. 2000. Bimodal hybrid zones and speciation. Trends Evol. Ecol. 15, 250–255. ( 10.1016/S0169-5347(00)01873-5) [DOI] [PubMed] [Google Scholar]
- 141.Kruuk LEB, Baird SJE, Gale KS, Barton NH. 1999. A comparison of multilocus clines maintained by environmental adaptation or by selection against hybrids. Genetics 153, 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sadedin S, Littlejohn MJ. 2003. A spatially explicit individual-based model of reinforcement in hybrid zones. Evolution 57, 962–970. ( 10.1111/j.0014-3820.2003.tb00308.x) [DOI] [PubMed] [Google Scholar]
- 143.Barton NH, Gale KS. 1993. Genetic analysis of hybrid zones. In Hybrid zones and the evolutionary process (ed. Harrison R.), pp. 13035 New York, NY: Oxford University Press. [Google Scholar]
- 144.Liou LW, Price TD. 1994. Speciation by reinforcement of premating isolation. Evolution 48, 1451–1459. ( 10.1111/j.1558-5646.1994.tb02187.x) [DOI] [PubMed] [Google Scholar]
- 145.Barton NH, Bengtsson BO. 1986. The barrier to genetic exchange between hybridising populations. Heredity 57, 357–376. ( 10.1038/hdy.1986.135) [DOI] [PubMed] [Google Scholar]
- 146.Stankowski S, Sobel JM, Streisfeld MA. 2015. The geography of divergence-with-gene-flow facilitates multi-trait adaptation and the evolution of pollinator isolation in Mimulus aurantiacus. Evolution 69, 3054–3068. ( 10.1111/evo.12807) [DOI] [PubMed] [Google Scholar]
- 147.Sobel JM, Stankowski S, Streisfeld MA. 2019. Variation in ecophysiological traits might contribute to ecogeographic isolation and divergence between parapatric ecotypes of Mimulus aurantiacus. J. Evol. Biol. 32, 604–618. ( 10.1111/jeb.13442) [DOI] [PubMed] [Google Scholar]
- 148.Toews DPL, Taylor SA, Vallender R, Brelsford A, Butcher BG, Messer PW, Lovette IJ. 2016. Plumage genes and little else distinguish the genomes of hybridizing warblers. Curr. Biol. 26, 2313–2318. ( 10.1016/j.cub.2016.06.034) [DOI] [PubMed] [Google Scholar]
- 149.Poelstra JW, et al. 2014. The genomic landscape underlying phenotypic integrity in the face of gene flow in crows. Science 344, 1410–1414. ( 10.1126/science.1253226) [DOI] [PubMed] [Google Scholar]
- 150.Larson EL, Andres JA, Bogdanowicz SM, Harrison RG. 2013. Differential introgression in a mosaic hybrid zone reveals candidate barrier genes. Evolution 67, 3653–3661. ( 10.1111/evo.12205) [DOI] [PubMed] [Google Scholar]
- 151.Larson EL, Hume GL, Andre JA, Harrison RG. 2012. Post-mating prezygotic barriers to gene exchange between hybridizing field crickets. J. Evol. Biol. 25, 174–186. ( 10.1111/j.1420-9101.2011.02415.x) [DOI] [PubMed] [Google Scholar]
- 152.Szymura J, Barton NH. 1991. The genetic structure of the hybrid zone between the fire-bellied toads Bombina bombina and B. variegata: comparisons between transects and between loci. Evolution 45, 237–261. ( 10.1111/j.1558-5646.1991.tb04400.x) [DOI] [PubMed] [Google Scholar]
- 153.Kruuk LEB, Gilchrist JS, Barton NH. 1998. Hybrid dysfunction in fire-bellied toads. Evolution 53, 1611–1616. [DOI] [PubMed] [Google Scholar]
- 154.Singhal S, Moritz C. 2012. Strong selection against hybrids maintains a narrow contact zone between morphologically cryptic lineages in a rainforest lizard. Evolution 66, 1474–1489. ( 10.1111/j.1558-5646.2011.01539.x) [DOI] [PubMed] [Google Scholar]
- 155.Pulido-Santacruz P, Aleixo A, Weir JT. 2018. Morphologically cryptic Amazonian bird species pairs exhibit strong postzygotic reproductive isolation. Proc. R. Soc. B 285, 2017–2081. ( 10.1098/rspb.2017.2081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brelsford A, Irwin DE. 2009. Incipient speciation despite little assortative mating: the yellow-rumped warbler hybrid zone. Evolution 63, 3050–3060. ( 10.1111/j.1558-5646.2009.00777.x) [DOI] [PubMed] [Google Scholar]
- 157.Cooper BS, Sedghifar A, Nash WT, Comeault AA, Matute DR. 2017. A maladaptive combination of traits contributes to the maintenance of a stable hybrid zone between two divergent species of Drosophila. Curr. Biol. 28, 2940–2947. ( 10.1016/j.cub.2018.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schumer M, Cui R, Powell DL, Dresner R, Rosenthal GG, Andolfatto P. 2014. High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species. ELife 3, 1–21. ( 10.7554/eLife.02535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schumer M, Powell DL, Delclós PJ, Squire M, Cui R, Andolfatto P. 2017. Assortative mating and persistent reproductive isolation in hybrids. Proc. Natl Acad. Sci. USA 114, 10 936–10 941. ( 10.1073/pnas.1711238114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cruzan MB, Arnold ML. 1994. Assortative mating and natural selection in an iris hybrid zone. Evolution 48, 1946–1958. ( 10.1111/j.1558-5646.1994.tb02225.x) [DOI] [PubMed] [Google Scholar]
- 161.Rieseberg LH, Baird SJE, Desrochers AM. 1998. Patterns of mating in wild sunflower hybrid zones. Evolution 52, 713–726. ( 10.1111/j.1558-5646.1998.tb03696.x) [DOI] [PubMed] [Google Scholar]
- 162.Teeter KC, et al. 2008. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Resour. 18, 67–76. ( 10.1101/gr.6757907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smadja C, Ganem G. 2002. Subspecies recognition in the house mouse: a study of two populations from the border of a hybrid zone. Behav. Ecol. 13, 312–320. ( 10.1093/beheco/13.3.312) [DOI] [Google Scholar]
- 164.Gavrilets S. 1997. Hybrid zones with Dobzhansky-type epistatic selection. Evolution 51, 1027–1035. ( 10.1111/j.1558-5646.1997.tb03949.x) [DOI] [PubMed] [Google Scholar]
- 165.Ravinet M, Faria R, Butlin RK, Galindo J, Bierne N, Rafajlović M, Noor MAF, Mehlig B, Westram AM. 2017. Interpreting the genomic landscape of speciation: finding barriers to gene flow. J. Evol. Biol. 30, 1450–1477. ( 10.1111/jeb.13047) [DOI] [PubMed] [Google Scholar]
- 166.Barton NH, Hewitt GM. 1985. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 16, 113–148. ( 10.1146/annurev.es.16.110185.000553) [DOI] [Google Scholar]
- 167.Moore. 1979. A single locus mass-action model of assortative mating, with comments on the process of speciation. Heredity 42, 173–186. ( 10.1038/hdy.1979.21) [DOI] [Google Scholar]
- 168.Payseur BA, Renz JAGK, Nachman MW. 2004. Differential patterns of introgression across the X chromosome in a hybrid zone between species of house mice. Evolution 58, 2064–2078. ( 10.1111/j.0014-3820.2004.tb00490.x) [DOI] [PubMed] [Google Scholar]
- 169.Stankowski S, Sobel JM, Streisfeld MA. 2017. Geographic cline analysis as a tool for studying genome-wide variation: a case study of pollinator-mediated divergence in a monkeyflower. Mol. Ecol. 26, 107–122. ( 10.1111/mec.13645) [DOI] [PubMed] [Google Scholar]
- 170.Turner TL, Hahn MW, Nuzhdin SV. 2005. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 3, 1572–1578. ( 10.1371/journal.pbio.0030285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nosil P, Funk DJ, Ortíz-Barrientos D. 2009. Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402. ( 10.1111/j.1365-294X.2008.03946.x) [DOI] [PubMed] [Google Scholar]
- 172.Nadeau NJ, et al. 2012. Genome-wide patterns of divergence and gene flow across a butterfly radiation. Mol. Ecol. 22, 814–826. ( 10.1111/j.1365-294X.2012.05730.x) [DOI] [PubMed] [Google Scholar]
- 173.Ellegren H, et al. 2012. The genomic landscape of species divergence in Ficedula flycatchers. Nature 491, 756–760. ( 10.1038/nature11584) [DOI] [PubMed] [Google Scholar]
- 174.Noor MAF, Bennett SM. 2009. Islands of speciation or mirages in the desert? Examining the role of restricted recombination in maintaining species. Heredity 103, 439–444. ( 10.1038/hdy.2009.151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cruickshank TE, Hahn MW. 2014. Reanalysis suggests that genomic islands of speciation are due to reduced diversity, not reduced gene flow. Mol. Ecol. 23, 3133–3157. ( 10.1111/mec.12796) [DOI] [PubMed] [Google Scholar]
- 176.Rifkin JL, Castillo AS, Liao IT, Rausher MD. 2019. Gene flow, divergent selection and resistance to introgression in two species of morning glories (Ipomoea). Mol. Ecol. 28, 1709–1729. ( 10.1111/mec.14945) [DOI] [PubMed] [Google Scholar]
- 177.Turissini DA, Matute DR. 2017. Fine scale mapping of genomic introgressions within the Drosophila yakuba clade. PLoS Genet. 13, e1006971 ( 10.1371/journal.pgen.1006971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schrider DR, Ayroles J, Matute DR, Kern AD. 2018. Supervised machine learning reveals introgressed loci in the genomes of Drosophila simulans and D. sechellia. PLoS Genet. 14, e1007341 ( 10.1371/journal.pgen.1007341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Corbett-Detig RB, Nielsen R. 2017. A hidden Markov model approach for simultaneously estimating local ancestry and admixture time using next generation sequence data in samples of arbitrary ploidy. PLoS Genet. 13, e1006529 ( 10.1371/journal.pgen.1006529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schumer M, et al. 2018. Natural selection interacts with recombination to shape the evolution of hybrid genomes. Science 360, 656–660. ( 10.1126/science.aar3684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Martin SH, Davey JW, Salazar C, Jiggins CD. 2019. Recombination rate variation shapes barriers to introgression across butterfly genomes. PLoS Biol. 17, e2006288 ( 10.1371/journal.pbio.2006288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barton NH, Charlesworth B. 1984. Genetic revolutions, founder effects, and speciation. Annu. Rev. Syst. 15, 133–164. ( 10.1146/annurev.es.15.110184.001025) [DOI] [Google Scholar]
- 183.Barton NH. 1979. The dynamics of hybrid zones. Heredity 43, 341–359. ( 10.1038/hdy.1979.87) [DOI] [Google Scholar]
- 184.Noor MAF, Grams KL, Bertucci LA, Reiland J. 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl Acad. Sci. USA 98, 12 084–12 088. ( 10.1073/pnas.221274498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Noor MAF, Garfield DA, Schaeffer SW, Machado CA. 2007. Divergence between the Drosophila pseudoobscura and D. persimilis genome sequences in relation to chromosomal inversions. Genetics 177, 1417–1428. ( 10.1534/genetics.107.070672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McGaugh SE, Noor MAF. 2012. Genomic impacts of chromosomal inversions in parapatric Drosophila species. Phil. Trans. R. Soc. B 367, 422–429. ( 10.1098/rstb.2011.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Twyford AD, Friedman J. 2015. Adaptive divergence in the monkey flower Mimulus guttatus is maintained by a chromosomal inversion. Evolution 69, 1476–1486. ( 10.1111/evo.12663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sæther SA, et al. 2007. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97. ( 10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- 189.Presgraves DC. 2008. Sex chromosomes and speciation in Drosophila. Trends Genet. 24, 336–343. ( 10.1016/j.tig.2008.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Comeron JM, Ratnappan R, Bailin S. 2012. The many landscapes of recombination in Drosophila melanogaster. PLoS Genet. 8, 33–35. ( 10.1371/journal.pgen.1002905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Samuk K, Manzano-Winkler B, Ritz KR, Noor MAF. 2020. Natural selection shapes variation in genome-wide recombination rate in Drosophila pseudoobscura. Curr. Biol. 30, 1517–1528. ( 10.1016/j.cub.2020.03.053) [DOI] [PubMed] [Google Scholar]
- 192.Schluter D. 2009. Evidence for ecological speciation and its alternate. Science 323, 737–741. ( 10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- 193.Nosil P, Flaxman SM. 2011. Conditions for mutation-order speciation. Proc. R. Soc. B 278, 399–407. ( 10.1098/rspb.2010.1215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ono J, Gerstein AC, Otto SP. 2017. Widespread genetic incompatibilities between first-step mutations during parallel adaptation of Saccharomyces cerevisiae to a common environment. PLoS Biol. 15, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haig D, Westoby M. 1989. Parent-specific gene expression and the triploid endosperm. Am. Nat. 134, 147–155. ( 10.1086/284971) [DOI] [Google Scholar]
- 196.Gavrilets S. 2000. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889. ( 10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- 197.Barreto FS, Burton RS, Barreto FS. 2013. Elevated oxidative damage is correlated with reduced fitness in interpopulation hybrids of a marine copepod. Proc. R. Soc. B 280, 20131521 ( 10.1098/rspb.2013.1521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mcfarlane SE, Sirkiä PM, Murielle Å, Qvarnström A. 2016. Hybrid dysfunction expressed as elevated metabolic rate in male Ficedula flycatchers. PLoS ONE 11, 1–10. ( 10.1371/journal.pone.0161547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prokic MD, Despotovic SG, Vuc TZ, Petrovic TG, Gavric JP. 2018. Oxidative cost of interspecific hybridization: a case study of two Triturus species and their hybrids. J. Exp. Biol. 221, 1–7. ( 10.1242/jeb.182055) [DOI] [PubMed] [Google Scholar]
- 200.Delmore KE, Irwin DE. 2014. Hybrid songbirds employ intermediate routes in a migratory divide. Ecol. Lett. 17, 1211–1218. ( 10.1111/ele.12326) [DOI] [PubMed] [Google Scholar]
- 201.Gepts P, Bliss FA. 1985. F1 hybrid weakness in the common bean. J. Hered. 76, 447–450. ( 10.1093/oxfordjournals.jhered.a110142) [DOI] [Google Scholar]
- 202.Chen C, Chen H, Shan J, Zhu M, Shi M, Gao J, Lin H. 2013. Genetic and physiological analysis of a novel type of interspecific hybrid weakness in rice. Mol. Plant 6, 716–728. ( 10.1093/mp/sss146) [DOI] [PubMed] [Google Scholar]
- 203.Chen C, et al. 2014. A two-locus interaction causes interspecific hybrid weakness in rice. Nat. Commun. 5, 1–11. ( 10.1038/ncomms4357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xue W, et al. 2019. Hybrid decay: A transgenerational epigenetic decline in vigor and viability triggered in backcross populations of teosinte with maize. Genetics 213, 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dobzhansky TG. 1940. Genetics and the origin of species. New York, NY: Columbia University Press. [Google Scholar]
- 206.Noor MAF. 1995. Speciation driven by natural selection in Drosophila. Nature 375, 674–675. ( 10.1038/375674a0) [DOI] [PubMed] [Google Scholar]
- 207.Sætre GP, Moum T, Bureš S, Král M, Adamjan M, Moreno J. 1997. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–592. ( 10.1038/42451) [DOI] [Google Scholar]
- 208.Dyer KA, White BE, Sztepanacz JL, Bewick ER, Rundle HD. 2014. Reproductive character displacement of epicuticular compounds and their contribution to mate choice in Drosophila subquinaria and Drosophila recens. Evolution 68, 1163–1175. ( 10.1111/evo.12335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Comeault AA, Venkat A, Matute DR. 2016. Correlated evolution of male and female reproductive traits drive a cascading effect of reinforcement in Drosophila yakuba. Proc. R. Soc. B 283, 23–48. ( 10.1098/rspb.2016.0730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hopkins R, Rausher MD. 2011. Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii. Nature 469, 411–414. ( 10.1038/nature09641) [DOI] [PubMed] [Google Scholar]
- 211.Hopkins R, Rausher MD. 2012. Pollinator-mediated selection on flower color allele drives reinforcement. Science 335, 1090–1092. ( 10.1126/science.1215198) [DOI] [PubMed] [Google Scholar]
- 212.Grossenbacher DL, Whittall JB. 2011. Increased floral divergence in sympatric monkeyflowers. Evolution 65, 2712–2718. ( 10.1111/j.1558-5646.2011.01306.x) [DOI] [PubMed] [Google Scholar]
- 213.Grossenbacher DL, Stanton ML. 2014. Pollinator-mediated competition influences selection for flower-color displacement in sympatric monkeyflowers. Am. J. Bot. 101, 1915–1924. ( 10.3732/ajb.1400204) [DOI] [PubMed] [Google Scholar]
- 214.Matute DR. 2010. Reinforcement of gametic isolation in Drosophila. PLoS Biol. 8, e1000341 ( 10.1371/journal.pbio.1000341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Castillo DM, Moyle LC. 2019. Conspecific sperm precedence is reinforced but sexual selection weakened in sympatric populations of Drosophila. Proc. R. Soc. B 286, 20182535 ( 10.1098/rspb.2018.2535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fowler NL, Levin DA. 1984. Ecological constraints on the establishment of a novel polyploid in competition with its diploid progenitor. Am. Nat. 124, 703–711. ( 10.1086/284307) [DOI] [Google Scholar]
- 217.Coyne JA. 1974. The evolutionary origin of hybrid inviability. Evolution 28, 505–506. ( 10.2307/2407181) [DOI] [PubMed] [Google Scholar]
- 218.Hoskin CJ, Higgie M, McDonald KR, Moritz C. 2005. Reinforcement drives rapid allopatric speciation. Nature 437, 1353–1356. ( 10.1038/nature04004) [DOI] [PubMed] [Google Scholar]
- 219.Kozak GM, Roland G, Rankhorn C, Falater A, Berdan EL, Fuller RC. 2015. Behavioral isolation due to cascade reinforcement in Lucania killifish. Am. Nat. 185, 491–506. ( 10.1086/680023) [DOI] [PubMed] [Google Scholar]
- 220.Humphreys DP, Rundle HD, Dyer KA. 2016. Patterns of reproductive isolation in the Drosophila subquinaria complex: can reinforced premating isolation cascade to other species? Cur. Zool. 62, 183–191. ( 10.1093/cz/zow005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hudson EJ, Price TD. 2014. Pervasive reinforcement and the role of sexual selection in biological speciation. J. Hered. 105, 821–833. ( 10.1093/jhered/esu041) [DOI] [PubMed] [Google Scholar]
- 222.Yukilevich R. 2012. Asymmetrical patterns of speciation uniquely support reinforcement in Drosophila. Evolution 66, 1430–1446. ( 10.1111/j.1558-5646.2011.01534.x) [DOI] [PubMed] [Google Scholar]
- 223.Rundle HD, Schluter D. 1998. Reinforcement of stickleback mate preference: sympatry breed contempt. Evolution 52, 200–208. ( 10.1111/j.1558-5646.1998.tb05153.x) [DOI] [PubMed] [Google Scholar]
- 224.Nosil P, Crespi BJ, Sandoval CP. 2003. Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement. Proc. R. Soc. Lond. B 270, 1911–1918. ( 10.1098/rspb.2003.2457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Albert AYK, Schluter D. 2004. Reproductive character displacement of male stickleback mate preference: reinforcement of direct selection? Evolution 58, 1099–1107. ( 10.1111/j.0014-3820.2004.tb00443.x) [DOI] [PubMed] [Google Scholar]
- 226.Ortiz-Barrientos D, Counterman BA, Noor MAF. 2004. The genetics of speciation by reinforcement. PLoS Biol. 2, e416 ( 10.1371/journal.pbio.0020416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weber MG, Cacho NI, Phan MJQ, Disbrow C, Ramírez SR, Strauss SY. 2018. The evolution of floral signals in relation to range overlap in a clade of California jewelflowers (Streptanthus s.l.) Evolution 72, 798–807. ( 10.1111/evo.13456) [DOI] [PubMed] [Google Scholar]
- 228.Koopman KF. 1950. Natural selection for reproductive isolation between Drosophila pseudoobscura and Drosophila persimilis. Evolution 4, 135–148. ( 10.1111/j.1558-5646.1950.tb00048.x) [DOI] [Google Scholar]
- 229.Higgie M, Chenoweth S, Blows MW, Higgie M, Chenoweth S, Blows MW. 2000. Natural selection and the reinforcement of mate recognition. Science 290, 519–521. ( 10.1126/science.290.5491.519) [DOI] [PubMed] [Google Scholar]
- 230.Matute DR. 2010. Reinforcement can overcome gene flow during speciation in Drosophila. Curr. Biol. 20, 2229–2233. ( 10.1016/j.cub.2010.11.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Matute DR. 2015. Noisy neighbors can hamper the evolution of reproductive isolation by reinforcing selection. Am. Nat. 185, 253–269. ( 10.1086/679504) [DOI] [PubMed] [Google Scholar]
- 232.Myers EM, Frankino WA. 2012. Time in a bottle: The evolutionary fate of species discrimination in sibling Drosophila species. PLoS ONE 7, e31759 ( 10.1371/journal.pone.0031759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.White NJ, Snook RR, Eyres I. 2019. The past and future of experimental speciation. Trends Ecol. Evol. 35, 10–21. ( 10.1016/j.tree.2019.08.009) [DOI] [PubMed] [Google Scholar]
- 234.Price TD. 2010. The roles of time and ecology in the continental radiation of the Old World leaf warblers (Phylloscopus and Seicercus). Phil. Trans. R. Soc. B 365, 1749–1762. ( 10.1098/rstb.2009.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Moyle LC, Olson MS, Tiffin P. 2004. Patterns of reproductive Iiolation in three angiosperm genera. Evolution 58, 1195–1208. ( 10.1111/j.0014-3820.2004.tb01700.x) [DOI] [PubMed] [Google Scholar]
- 236.Ginsberg PS, Humphreys DP, Dyer KA. 2019. Ongoing hybridization obscures phylogenetic relationships in the Drosophila subquinaria species complex. J. Evol. Biol. 32, 1093–1105. ( 10.1111/jeb.13512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Johnson MTJ, Husband BC, Burton TL. 2003. Habitat differentiation between diploid and tetraploid Galax urceolata (Diapensiaceae). Int. J. Plant Sci. 164, 703–710. ( 10.1086/376813) [DOI] [Google Scholar]
- 238.Meirmans PG, Vlot EC, Den Nijs JCM, Menken SBJ. 2003. Spatial ecological and genetic structure of a mixed population of sexual diploid and apomictic triploid dandelions. J. Evol. Biol. 16, 343–352. ( 10.1046/j.1420-9101.2003.00515.x) [DOI] [PubMed] [Google Scholar]
- 239.Lumaret R, Guillerm J, Delay J, Loutfi AAL, Izco J, Jay M. 1987. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain). Oecologia 73, 436–446. ( 10.1007/BF00385262) [DOI] [PubMed] [Google Scholar]
- 240.Richardson ML, Hanks LM. 2011. Differences in spatial distribution, morphology, and communities of herbivorous insects among three cytotypes of Solidago altissima (Asteraceae). Am. J. Bot. 98, 1–7. ( 10.3732/ajb.1100018) [DOI] [PubMed] [Google Scholar]
- 241.Coughlan JM, Han S, Stefanović S, Dickinson TA. 2017. Widespread generalist clones are associated with range and niche expansion in allopolyploids of Pacific northwest hawthorns (Crataegus L). Mol. Ecol. 20, 5484–5499. ( 10.1111/mec.14331) [DOI] [PubMed] [Google Scholar]
- 242.Ramsey J. 2011. Polyploidy and ecological adaptation in wild yarrow. Proc. Natl Acad. Sci. USA 108, 7096–7101. ( 10.1073/pnas.1016631108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Toll K, Willis JH. 2019. Hybrid inviability and differential submergence tolerance drive habitat segregation between two congeneric monkeyflowers. Ecology 99, 2776–2786. ( 10.1002/ecy.2529) [DOI] [PubMed] [Google Scholar]
- 244.Rabosky DL, Matute DR. 2013. Macroevolutionary speciation rates are decoupled from the evolution of intrinsic reproductive isolation in Drosophila and birds. Proc. Natl Acad. Sci. USA 110, 15 354–15 359. ( 10.1073/pnas.1305529110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sasa MM, Chippindale PT, Johnson NA, Picado C, Rica UDC, Jose S, Rica C. 1998. Patterns of postztgotic isolation in frogs. Evolution 52, 1811–1820. ( 10.1111/j.1558-5646.1998.tb02258.x) [DOI] [PubMed] [Google Scholar]
- 246.Stelkens RB, Young KA, Seehausen O. 2009. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution 64, 617–633. ( 10.1111/j.1558-5646.2009.00849.x) [DOI] [PubMed] [Google Scholar]
- 247.Giraud T, Gourbiere S. 2012. The tempo and modes of evolution of reproductive isolation in fungi. Heredity 109, 204–214. ( 10.1038/hdy.2012.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rosenblum EB, Sarver BAJ, Brown JW, Roches SD, Hardwick KM, Hether TD, Eastman JM, Pennell MW, Harmon LJ. 2012. Goldilocks meets Santa Rosalia: an ephemeral speciation model explains patterns of diversification across time scales. Evol. Biol. 39, 255–261. ( 10.1007/s11692-012-9171-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this paper were accessed from previously published datasets. These datasets have been referenced throughout the manuscript.