Abstract
This study evaluated health outcomes among people who inject drugs who are infected with hepatitis C virus using an artificial intelligence platform. Mean (SD) cumulative adherence (visual confirmation of administration) was 91.3% (10.5%). Most subjects (88.2%) achieved ≥80% adherence to treatment, and 88.2% (15 of 17) achieved a sustained virologic response.
Keywords: adherence, HCV, PWID
Globally, it is estimated that 71 million people have chronic hepatitis C virus (HCV) infection, including an estimated 7.5 million people who have recently injected drugs [1]. Morbidity and mortality due to HCV-related liver disease continue to increase, despite the advent of well-tolerated, interferon-free direct-acting antiviral (DAA) HCV regimens with cure rates >95% [2]. Though DAAs promise a cure for this population, treatment uptake remains particularly low in the drug-using population [3, 4]. This untreated group of PWID acts as a virus reservoir, continuing to spread the disease, and PWID are responsible for the main burden of incident HCV disease in developed countries. Therefore, improved models of HCV care for PWID must be a priority.
Few PWID are offered DAA therapy, due in part to concerns about suboptimal adherence to antiviral therapy [5]. Directly observed treatment (DOT; where every dose of medication is taken under direct observation) compared with self-administered treatment has been shown to be associated with improved adherence to both interferon-based and DAA regimens in HCV-infected PWID enrolled in opioid agonist treatment programs [6–8]. However, DOT is less feasible for PWID who are not enrolled in opioid agonist treatment programs. Video conference solutions for remote observation (VDOT) and asynchronous solutions have also been piloted to monitor adherence to tuberculosis. While these modalities are effective for ensuring treatment adherence, they are resource-intensive and require a high number of human review hours per patient [9].
The artificial intelligence (AI) platform AiCure (New York, NY, USA) uses computer vision and machine learning to capture and analyze audio and video data related to medication administration to detect critical steps in medication ingestion without the need for human input. The platform can be downloaded as an application on any smartphone. To date, the AI platform is being deployed in Phase I–IV drug development studies to monitor and increase adherence [10]. It is also being used as a trial enrichment strategy to improve data quality postrandomization [11]. In real-world settings, the platform is used as a cost-effective alternative to DOT to measure and maximize adherence to TB treatment in several county public health departments and adherence to HCV therapy in patients who are commercially insured [12]. The platform has not been studied in HCV-infected PWID receiving antiviral therapy.
To our knowledge, this is the first study to evaluate the use of an artificial intelligence (AI) platform to optimize adherence to HCV therapy in PWID. The pilot was implemented in a methadone treatment program that offered on-site HCV treatment by primary care providers. The primary objective of the pilot was to determine whether the use of an AI platform was feasible in PWID, including those actively using drugs, and whether smartphone applications delivering AI DOT would be associated with high adherence (≥80%) to HCV therapy.
METHODS
Participants and Setting
This pilot study was conducted in the Division of Substance Abuse at 1 of 3 methadone maintenance treatment programs administered by the Montefiore Medical Center in the Bronx, New York. These programs were the site for study recruitment and all research visits. The medical team at each program provided comprehensive primary medical care, including HCV and HIV care.
Eligible study subjects were infected with HCV, received HCV medical care at the methadone program, planned to initiate HCV treatment within the next 3 months, and had genotype 1 HCV. HCV viral loads and genotype were obtained by chart review. Substance use was assessed by self-report at baseline and by urine toxicology monthly during HCV treatment.
Study Design
This was a prospective single-arm trial in which HCV-infected subjects used the AI smartphone DOT platform in order to self-administer HCV therapy—ledipasvir and sofosbuvir.
HCV Treatment Protocol
The HCV evaluation consisted of HCV viral load assessments at the following time points: baseline (before HCV treatment initiation), after HCV treatment initiation (4, 8, and 12 weeks if applicable), and 12 weeks after HCV treatment completion. Quantitative HCV viral load was detected using the COBAS Ampliprep/Taqman assay, version 2.0 (Roche Molecular Systems, Pleasanton, CA, USA), which quantifies HCV VL 15 IU/mL to 100 million IU/mL. HCV genotype was assessed before enrollment using the Abbott RealTime HCV Genotype II assay (Abbott Molecular Inc., Abbott Park, IL, USA). All study subjects self-administered fixed-dose ledipasvir and sofosbuvir (LDV/SOF) for 8 to 12 weeks as per American Association for the Study of Liver Diseases/Infectious Diseases Society of America guidelines and were given mobile devices with the AI application downloaded to be used during self-administration of medication. Medication adherence was monitored via the AI application.
Outcomes
The primary outcome was adherence. Participants were credited with taking medication if there was computer visual confirmation of ingestion between 12:00 am and 11:59 pm on any specific day. Secondary outcomes were treatment completion, end of treatment response (ETR), and sustained virologic response 12 weeks after completion of treatment (SVR12). The study was approved by an institutional review board at Albert Einstein College of Medicine. All participants signed an informed consent document.
Study Intervention
All participants received devices with the AI application predownloaded. Participants received dosing reminders. If they did not dose at the patient-specified daily time through the app, a further 4 reminders in the form of a text message within the app were sent every 15 minutes for an hour. A late-dose reminder was sent approximately two-thirds of the way between the original reminder time and the end of the dosing window (11:59 pm). Real-time data were captured, encrypted, and transmitted to cloud-based dashboards for analysis and intervention. In addition, notifications were pushed automatically to clinic staff via email or SMS text message if doses were missed or data were pending. Specifically, clinic staff was notified if (1) the participant missed the prior day’s dose; (2) no data were available from the participant’s device the prior day; (3) the participant had not used the system for 2 doses in the prior 7 days; and (4) adherence dropped below 80% over the prior 7 days. After notifications, clinic staff had the option to conduct brief adherence discussions (either by phone or in person) with participants who demonstrated suboptimal adherence. Dosing data fell into 5 mutually exclusive categories: (1) visual confirmation of ingestion (basis for the adherence calculation); (2) self-reported dose on the AI application; (3) self-reported dose by phone to the clinic staff; (4) missed dose; and (5) dose taken in clinic.
RESULTS
Seventeen PWIDs infected with HCV genotype-1 were enrolled between November 2016 and March 2017. Subjects were 70.6% male, 76.5% Latino, and 23.5% black, with a mean (SD) age of 51.2 (11.9) years. Twelve (70.6%) subjects reported substance use in the 6 months before HCV treatment (heroin, cocaine, crack cocaine, benzodiazepines, and/or prescription opioids), and 8 (47.1%) self-reported substance use in the 30 days before the baseline interview. Nine (52.9%) reported a current psychiatric comorbidity (depression, anxiety disorder, psychotic disorder, or bipolar disorder). Fifteen subjects received 12 weeks of HCV treatment, and 2 received 8 weeks. The overall retention in the study was 100%.
Treatment Adherence and Virologic Outcomes
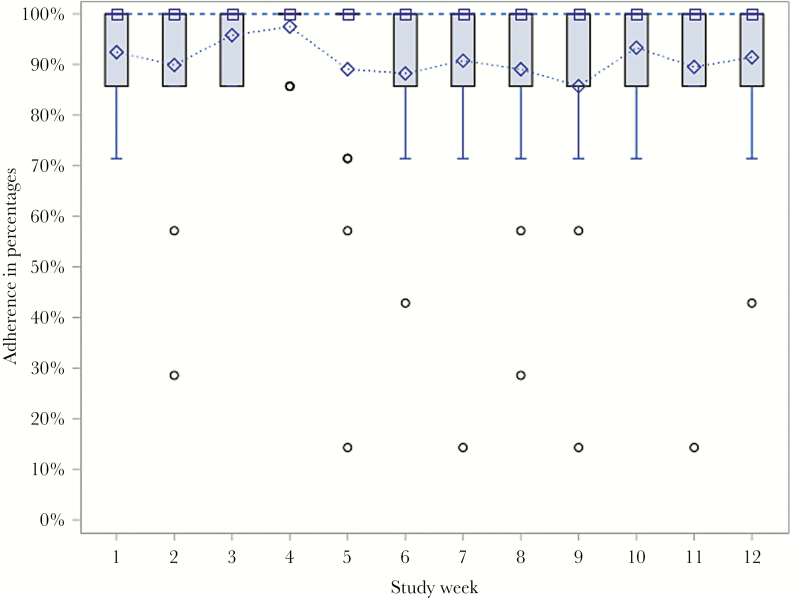
Mean (SD) cumulative adherence (visual confirmation of drug administration using the AI application) was 91.3% (10.5%). Overall, 15 subjects (88.2%) achieved ≥80% adherence (Figure 1), and 100% completed treatment. Sixteen (94.1%) achieved ETR, and 15 (88.2%) achieved SVR12. Subjects who failed to achieve SVR12 had 98% and 100% adherence, as measured by the AiCure application. One subject who failed had advanced fibrosis (METAVIR Stage 3 disease) and received 8 weeks of antiviral treatment. The other subject who failed had cirrhosis, received 12 weeks of antiviral treatment, and used cocaine in the 30 days before the baseline interview. Drug use was detected by urine toxicology in 9 subjects (52.9%) during HCV treatment including opiates (n = 8), cocaine (n = 6), and benzodiazepines (n = 2).
Figure 1.
Weekly adherence levels: Boxplot. The median adherence levels are denoted by the square markers in the boxes and connected by the dashed line. The mean adherence levels are denoted by the diamond markers and connected by the dotted line. The outliers are denoted by the round markers outside the whiskers. The lower and upper ends of the boxes represent the first (Q1) and third (Q3) quartiles, respectively. The widths of the boxes are the interquartile ranges (IQRs). The lengths of the whiskers are the smaller of 1.5*IQR and the distance from Q1 to the outermost point within 1.5*IQR. In this study, all of the medians, third quartiles, and maximums were equal to 100% for all weeks.
Interventions
AiCure staff logged a total of 63 interventions with study staff. Study staff in turn logged a total of 30 interventions directly with 9 subjects (52.9%)—8 in person and 22 phone calls. These 9 subjects received between 1 and 8 interventions throughout the study period. The 2 participants who did not achieve SVR did not receive any interventions as they achieved high adherence. All interventions by AiCure and study staff were captured on the AiCure dashboards.
DISCUSSION
This study utilized a novel AI platform to assess and optimize medication adherence in PWID receiving HCV therapy. Unlike other HCV studies in PWID, which have used strict inclusion/exclusion criteria or implemented in-person DOT to improve adherence and SVR, this pilot study used an AI platform in a real-world setting. All subjects completed treatment, and overall adherence was >90%. Adherence as measured by the AI platform was similar to adherence (measured by electronic monitors) observed in the DOT arm of the PREVAIL study (83%), in which patients enrolled in opioid agonist treatment programs received in-person DOT, and higher than adherence observed in the self-administered arm of the study (75%) [8]. Few studies have deployed smartphone applications to optimize adherence in vulnerable patient populations including PWID. Two subjects failed to achieve SVR despite high rates of adherence. One participant with advanced fibrosis may have achieved suboptimal duration of treatment, and the second participant may have had decreased intracellular concentrations of the active metabolite of sofosbuvir due to ongoing cocaine use [13]. The limitations of the study included the modest sample size and lack of control arm. Nevertheless, consistent use and high adherence over the course of 8 to 12 weeks observed in this study underscore the possibility of harnessing new technologies to optimize adherence in patients and reduce the burden of advanced liver disease in settings where in-person DOT is less feasible, including federally qualified health centers and syringe service programs. In addition, smartphone DOT applications leveraging artificial intelligence may require less staff-based monitoring than video-observed therapy. Increasing the use of such technology may help to achieve the World Health Organization goal of HCV elimination by 2030 [14].
Acknowledgments
The authors acknowledge Aubri Charboneau of Sage Scientific Writing, LLC, for editing support for the manuscript.
Financial support. This work was supported by the National Institute on Drug Abuse (R01DA034086).
Potential conflicts of interest. Laura Shafner is an employee of AiCure, New York, NY, USA. Alain Litwin has served on advisory boards for and received research grants from Gilead Sciences and Merck Pharmaceuticals. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis 2011; 43:66–72. [DOI] [PubMed] [Google Scholar]
- 3. Midgard H, Bramness JG, Skurtveit S, et al. Hepatitis C treatment uptake among patients who have received opioid substitution treatment: a population-based study. PLoS One 2016; 11:e0166451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alavi M, Raffa JD, Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver Int 2014; 34:1198–206. [DOI] [PubMed] [Google Scholar]
- 5. Asher AK, Portillo CJ, Cooper BA, et al. Clinicians’ view of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inject drugs. Subst Use Misuse 2016; 51:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frieden TR, Sbarbaro JA. Promoting adherence to treatment for tuberculosis: the importance of direct observation. Bull World Health Organ 2007; 85:407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDermott CL, Lockhart CM, Devine B. Outpatient directly observed therapy for hepatitis C among people who use drugs: a systematic review and meta-analysis. J Virus Erad 2018; 4:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akiyama MJ, Norton BL, Arnsten JH, et al. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chuck C, Robinson E, Macaraig M, et al. Enhancing management of tuberculosis treatment with video directly observed therapy in New York City. Int J Tuberc Lung Dis 2016; 20:588–93. [DOI] [PubMed] [Google Scholar]
- 10. Bain EE, Shafner L, Walling DP, et al. Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth Uhealth 2017; 5:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goelder C, Shafner L, Umbricht D. Assessing the value of an AI platform during screening to predict adherence to study drug during treatment in an ongoing proof-of-concept study in schizophrenia. Paper presented at: International Society for CNS Clinical Trials and Methodology, 14th Annual Scientific Meeting; 20–22 February 2018, Washington DC. [Google Scholar]
- 12. Salcedo J, Rosales M, Guevara R, et al. Cost-effectiveness of novel artificial intelligence platform AiCure for active tuberculosis treatment in Los Angeles county. Paper presented at: Society for Medical Decision Making 40th Annual North American Meeting; 13–17 October 2018; Montreal, QC, Canada. [Google Scholar]
- 13. Brooks KM, Castillo-Mancilla JR, Morriw M, et al. Intracellular sofosbuvir (SOF) concentrations in persons with HCV and cocaine. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 4–7 March 2019, Seattle, Washington. [Google Scholar]
- 14.World Health Organization. Prevention and control of viral hepatitis infection: framework for global action Available at: http://www.who.int/hiv/pub/hepatitis/Framework/en/. Accessed 2 July 2018.