Abstract
Background
Incarcerated persons are a special population with higher hepatitis C virus (HCV) prevalence and should be prioritized for microelimination. In this study, we investigate the seroprevalence and evaluate the effectiveness and safety of direct-acting antiviral (DAA) therapy in custodial settings.
Methods
Incarcerated persons in Yunlin Prison were recruited to receive anti-HCV antibody screening. Patients with positive HCV ribonucleic acid (RNA) were treated with glecaprevir/pibrentasvir (GLE/PIB) in our special chronic hepatitis C (CHC) clinic in prison. The primary endpoint was sustained virologic response at week 12 off therapy (SVR12).
Results
A total of 1402 incarcerated persons were invited to anti-HCV screening and 824 (58.7%) accepted. The prevalence of anti-HCV positivity was 33.5% (276 of 824), and the viremic rate (detectable HCV RNA) was 69.2% (191 of 276). According to fibrosis index based on 4 factors, patients with F3 stage were 6 (3.1%), but none met the criteria of F4 stage. However, 6 (3.1%) had liver cirrhosis with splenomegaly, confirmed by findings of ultrasonography. The median log10 HCV RNA level at baseline was 6.235 (2.394–7.403). Genotype (GT) 6 was predominant (39.3%), followed by GT 1a (22.0%) and 1b (14.1%). Mixed GT HCV infection accounted for 3.6% of total infections. In total, 165 patients received GLE/PIB therapy. The overall SVR12 rates were 100%.
Conclusions
Direct-acting antiviral therapy is highly effective and safe for incarcerated patients in Taiwan. Our special prison-based CHC clinic, linking universal screening to medical care, can serve as a model for microelimination of HCV in custodial settings.
Keywords: chronic hepatitis C, direct-acting antiviral agent, hepatitis C virus, prison, Taiwan
We established a special hepatitis C clinic in prison and offered universal hepatitis C screening for incarcerated persons. The prevalence of anti-HCV positivity was 33.5%, and the viremic rate (detectable HCV RNA) was 69.2%. All treated patients achieved SVR.
Chronic hepatitis C virus (HCV) infection is a significant cause of liver-related morbidity and mortality worldwide. In 2015, an estimated 71.1 million people had chronic HCV infection globally, corresponding to a prevalence of 0.1% [1]. Taiwan has one of the highest HCV prevalence rates in Northeast Asia [2]. From 1996 to 2005, the prevalence of anti-HCV antibody (anti-HCV) in the general population of Taiwan was 4.4% [3]. Hepatitis C virus infection remains one of the most serious public health issues in Taiwan’s healthcare system.
In most countries, the prevalence of HCV infection is higher in the incarcerated population than in the general population, with estimated prevalence ranging from 3% to 38% [4]. This phenomenon is probably related to the chaotic life of this special population, specifically, the frequent injection substance use among incarcerated persons [5]. Tattooing and risky sexual behavior also expose this vulnerable group to risk of HCV infection. A large proportion of the prison population in Taiwan is composed of criminalized persons with injection substance use. In 2019, 27 893 incarcerated persons were convicted of substance use-related crimes, accounting for 49.5% of the total prison population. Injection substance use is prohibited in Taiwan’s prison system. Those incarcerated patients got HCV infection before they entered the prison. In Taiwan, human immunodeficiency virus (HIV) screen testing is routinely performed for all incarcerated persons, but HCV is not included. Therefore, without routine anti-HCV screening, the prevalence of HCV infection in the custodial setting of Taiwan is unknown.
Given the high prevalence of HCV infection in custodial settings, the World Health Organization Hepatitis C 2018 guidelines classified people in prison as a priority group for HCV treatment [6]. In fact, incarceration can be viewed as an opportunity for providing HCV screening and therapeutic interventions. The adequate duration of prison sentences permits the completion of a full-course antiviral treatment. However, a previous study indicated that loss to follow-up upon release from prison is a significant barrier to HCV treatment for incarcerated patients [7]. Therefore, early and effective HCV elimination in prison facilitates improving linkage to care and increasing the treatment rates of this vulnerable group, which is a key goal that is unmet and necessary for HCV elimination.
In the past, few studies have evaluated the efficacy of pegylated interferon (Peg-IFN) plus ribavirin (RBV) in HCV-infected incarcerated patients worldwide. The overall sustained virologic response (SVR) rate was approximately 36%–69% [8, 9]. In Taiwan, the overall SVR rate of Peg-IFN/RBV therapy in prison population varied from 65.3% to 84.5% [10, 11]. Incarcerated patients have been shown to be as likely to be treated for HCV and as likely to achieve SVR as nonincarcerated patients [12]. Unfortunately, incarcerated patients in most countries have fewer opportunities to receive medical assistance than other citizens [5, 13]. In fact, the healthcare system in prison cannot be effectively used to develop general screening programs or diagnostic and therapeutic approaches for HCV-infected incarcerated populations. The HCV-infected incarcerated patients used to receive out-of-prison medical treatment on bail for their chronic hepatitis C (CHC). This process required a lot of guard manpower and had some guarding risks.
In recent studies, the introduction of direct-acting antivirals (DAAs) has revolutionized the management of CHC with high SVR rates and favorable tolerability in the general population [14–19]. Direct-acting antiviral therapy is preferred in custodial settings because DAAs are more effective and safer and allow for shorter treatment courses, compared with Peg-IFN/RBV therapy. However, only a few reports have evaluated the outcomes of HCV DAA treatment in the prison environment [20–24].
To the best of our knowledge, no studies have been conducted to examine DAA use in the custodial settings of Taiwan. Direct-acting antiviral treatment response in incarcerated patients with HCV infection remains unknown. The current study investigates the seroprevalence and genotype (GT) distribution of HCV and evaluates the effectiveness and safety of DAA therapy in Taiwan’s custodial settings.
METHODS
Study Population
In this prospective cohort study, incarcerated persons in Yunlin Prison were invited to participate in hepatitis B surface antigen and anti-HCV screening between February and June 2019. Written informed consent was obtained before screening. All anti-HCV-positive incarcerated persons were referred to the special CHC clinic in Yunlin Prison for further evaluation. Patients with positive HCV ribonucleic acid (RNA) were treated with glecaprevir/pibrentasvir (GLE/PIB) and followed up according to the Taiwan National Health Insurance (NHI) clinical practice guidelines. Glecaprevir/pibrentasvir was selected for this study because it was the only available pangenotypic DAA in our hospital during the study period. In addition, pangenotypic DAA is the choice of treatment for possible mixed GT HCV infections in prison. Patients with less than 6 months remaining on their sentence were excluded from this study because they would be released from prison before completing the study protocol.
Special Clinic for Chronic Hepatitis C in Prison
Since January 2017, DAAs have been reimbursed by the Taiwan NHI program for CHC patients with advanced hepatic fibrosis or compensated cirrhosis. On January 1, 2019, the Taiwan NHI authorized the prescription of DAAs to all Taiwanese citizens with CHC. Currently in Taiwan, DAAs can only be prescribed by hepatologists and infection specialists. However, healthcare services in prison clinics in Taiwan are generally provided by family physicians instead of hepatologists, rendering DAA therapy inaccessible to incarcerated patients with HCV infection in Taiwan. To address this issue, we established a special CHC clinic in Yunlin Prison in February 2019, after universal screening for HCV infection. Two hepatologists, 1 registered nurse, 1 case manager, and 2 assistants were stationed at the clinic. We also equipped the prison clinic with a portable ultrasound machine for abdominal ultrasonography.
This study was approved by the Ethics Committee of the National Taiwan University Hospital (201810078RINC). Confidentiality of the enrolled patients was protected in accordance with the principles of Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
Assessments
We collected demographic and clinical characteristics at baseline, including HCV viral load and GT, stage of hepatic fibrosis, prior HCV treatment experience, past injection substance use, HIV, and hepatitis B virus (HBV) infection, for risk factor analysis. Human immunodeficiency virus-positive patients were referred to infection subspecialist for further medical care.
Serum HCV RNA level was determined by Cobas TaqMan HCV Test v2.0 (Roche Molecular Diagnostics, Pleasanton, CA) with a lower limit of quantification (LLOQ) of 15 IU/mL. Hepatitis C virus GT was determined by Cobas HCV GT (Roche Molecular Diagnostics). Advanced hepatic fibrosis (fibrosis stage F3) was assessed using fibrosis index based on 4 factors (FIB-4) test ≥3.25. Abdominal ultrasonography was performed to detect the presence of liver cirrhosis and for hepatocellular carcinoma surveillance. Baseline laboratory tests were performed within 3 months before the initiation of GLE/PIB treatment. Patients were followed every 4 weeks until the end of treatment (EOT) and at week 12 after treatment completion. Treatment-emergent adverse events (AEs) were recorded at every follow-up appointment. Safety data and laboratory abnormalities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE).
Statistical Analysis
Statistical analyses were performed using SPSS Statistics version 22.0 (IBM Corp., Armonk, NY). Baseline characteristics were reported in median (range) and frequencies (percentages), as appropriate. The on-treatment and off-treatment viral response rates and safety data were expressed in number and percentage. Univariate analysis was performed using the χ 2 test, the Fisher’s exact test, or the Student’s t test, as appropriate. A 2-sided P < .05 was considered statistically significant.
RESULTS
Demographic Characteristics
During February to June 2019, we conducted 3 thorough briefings at Yunlin Prison and approached the potential participants of this study with an offer of enrollment. A total of 1402 incarcerated persons, including those imprisoned during the study period, were invited. It equaled the full number of incarcerated individuals during study period. Among them, 824 incarcerated persons (58.8%) agreed to enrollment for HCV screening, and 276 incarcerated persons (33.5%, 276 of 824) were anti-HCV positive, 191 (69.2%, 191 of 276) of which were viremic. The baseline demographic and risk factor characteristics of the incarcerated persons with positive anti-HCV are shown in Table 1. Less IFN and DAA experience was found in the positive HCV RNA group. Hepatitis B virus coinfection was more prominent in the negative HCV RNA group. The baseline demographics and clinical characteristics of those CHC patients with positive HCV RNA are shown in Table 2. The median age of the CHC population was 45.6 years. Five (2.6%) patients were IFN-experienced. Six (3.1%) patients had a fibrosis stage of F3, according to the FIB-4 index. None of the patients met the F4 stage criteria of the FIB-4 index. However, 6 (3.1%) patients had liver cirrhosis with splenomegaly, as confirmed by ultrasonography findings. One patient showed prolonged international normalized ratio (2.12) due to concomitant warfarin use.
Table 1.
Descriptive Characteristics of the Incarcerated Persons With Positive Anti-HCVa
| Characteristics | All Patients (n = 276) |
Positive HCV RNA (n = 191) |
Negative HCV RNA (n = 85) |
P Valueb |
|---|---|---|---|---|
| Age, years | 45.5 (30–73) | 45.6 (30–73) | 45.3 (30–64) | .5843 |
| IFN-experienced | 14 (5.1%) | 5 (2.6%) | 9 (10.6%) | .0133 |
| DAA-experienced | 1 (0.4%) | 0 (0%) | 1 (1.2%) | .3080 |
| Hepatic Fibrosis on Fibrosis-4 Score | ||||
| F3 | 8 (2.9%) | 6 (3.1%) | 2 (2.4%) | 1 |
| F4 | 0 | 0 | 0 | |
| HBV | 35 (12.7%) | 9 (4.7%) | 26 (30.6%) | <.0001 |
| HIV | 23 (8.3%) | 17 (8.9%) | 6 (7.1%) | .6093 |
| PWID | 233 (84.4%) | 166 (86.9%) | 67 (78.8%) | .0872 |
Abbreviations: DAA, direct acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; PWID, people who inject drugs; RNA, ribonucleic acid.
aData are expressed as n (%) or median (range). Categorical variables were compared by the χ 2 test or the Fisher’s exact test; continuous variables were compared by the Student’s t test.
bComparison was made between positive HCV RNA and negative HCV RNA groups; significant P values are shown in bold text.
Table 2.
Descriptive Characteristics of the Incarcerated Persons With Positive HCV RNAa
| Characteristics | All Patients (N = 191) |
Treated Patients (N = 165) |
Untreated Patients (N = 26) |
P Valueb |
|---|---|---|---|---|
| Age (years) | 45.6 (30–73) | 45.8 (30–73) | 44.3 (33–63) | .38219776 |
| IFN-experienced | 5 (2.6%) | 5 (3.0%) | 0 (0%) | 1 |
| Hepatic Fibrosis on Fibrosis-4 score | ||||
| F3 | 6 (3.1%) | 6 (3.6%) | 0 (0%) | 1 |
| F4 | 0 | 0 | 0 | |
| Liver cirrhosis with splenomegaly on ultrasonography | 6 (3.1%) | 6 (3.6%) | 0 | 1 |
| HCV Genotype | ||||
| 1a | 42 (22.0%) | 36 (21.8%) | 6 (23%) | .8855 |
| 1b | 27 (14.1%) | 24 (14.5%) | 3 (11.5%) | 1 |
| 2 | 20 (10.5%) | 17 (10.3%) | 3 (11.5%) | .7397 |
| 3 | 20 (10.5%) | 19 (11.5%) | 1 (3.8%) | .3203 |
| 6 | 75 (39.3%) | 64 (38.7%) | 11 (42.3%) | .7327 |
| 1a + 1b | 2 (1.0%) | 1 (0.6%) | 1 (3.8%) | .2543 |
| 1b + 2 | 2 (1.0%) | 1 (0.6%) | 1 (3.8%) | .2543 |
| 2 + 6 | 3 (1.6%) | 3 (1.8%) | 0 (0%) | 1 |
| HCV RNA (log10 IU/mL) | 6.235 (2.394–7.403) |
6.246 (2.394–7.403) |
6.165 (4.139–7.306) |
.67385888 |
| Platelet count (k/μL) | 218.1 (50–408) | 218.2 (50–408) | 216.9 (93–349) | .90530737 |
| ALT (U/L) | 49.2 (7–350) | 47.9 (7–350) | 58.0 (10–315) | .44189620 |
| AST (U/L) | 34.2 (9–175) | 34.0 (9–175) | 35.5 (14–115) | .78307864 |
| Total bilirubin (mg/dL) | 0.77 (0.37–1.97) |
0.77 (0.37–1.97) |
0.73 (0.44–1.41) |
.44094274 |
| Albumin (g/dL) | 4.47 (3.6–5.3) | 4.47 (3.6–5.1) | 4.45 (4.0–5.3) | .77880776 |
| INR | 0.97 (0.88–2.12) | 0.98 (0.88–2.12) | 0.96 (0.91–1.07) | .08209333 |
| Creatinine (mg/dL) | 0.92 (0.6–2.2) | 0.92 (0.6–2.2) | 0.89 (0.7–1.1) | .17604307 |
| CKD stage 4–5 | 0 | 0 | 0 | |
| HBV | 9 (4.7%) | 7 (4.2%) | 2 (7.6%) | .3528 |
| HIV | 17 (8.9%) | 16 (9.6%) | 1 (3.8%) | .4766 |
| PWID | 166 (86.9%) | 143 (86.6%) | 23 (88.4%) | 1 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD, chronic kidney disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; INR, international normalized ratio; PWID, people who inject drugs; RNA, ribonucleic acid.
aData are presented as no. (%) or median (range). Categorical variables were compared using the χ 2 test or the Fisher’s exact test. Continuous variables were compared using the Student’s t test.
bComparison was made between treated and untreated patient groups. Significant P values are shown in bold text.
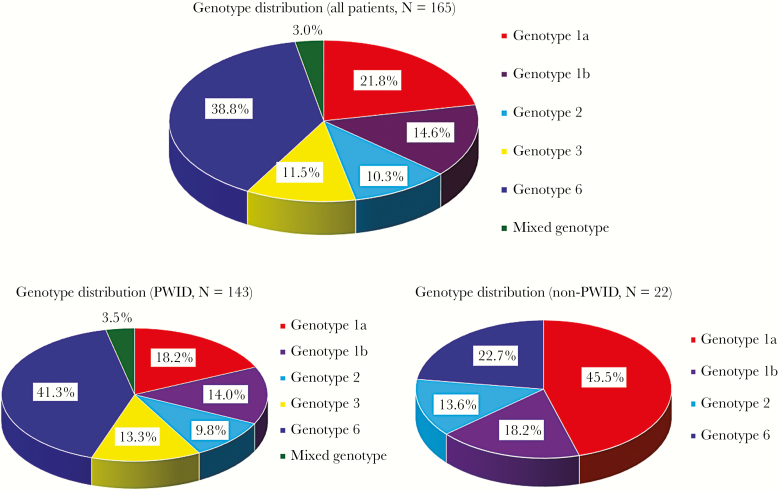
The median log10 HCV RNA level at baseline was 6.235 (2.394–7.403). The GT distribution was 39.3% (75 of 191) GT 6, 22.0% (42 of 191) GT 1a, 14.1% (27 of 191) GT 1b, 10.5% (20 of 191) GT 2, and 10.5% (20 of 191) GT 3. Seven patients had mixed GT HCV infections, including 3 with GT 2 + 6 (1.6%), 2 with GT 1a + 1b (1.0%), and 2 with GT 1b + 2 (1.0%). Seventy-four (38.7%) patients had elevation of alanine aminotransferase before treatment. The GT distribution of treated patients is shown in Figure 1. Genotype 6 was predominant in treated patients of persons who inject drugs (PWID), followed by GTs 1a, 1b, and 3.
Figure 1.
Chronic hepatitis C genotype distribution of treated patient group in Yunlin prison. PWID, people who inject drugs.
Nine (4.7%) and 17 (8.9%) patients were coinfected with HBV and HIV, respectively; during the interview, 166 patients (86.9%) admitted to injection substance use in the past. One patient had a confirmed diagnosis of advanced colon cancer with multiple liver metastases.
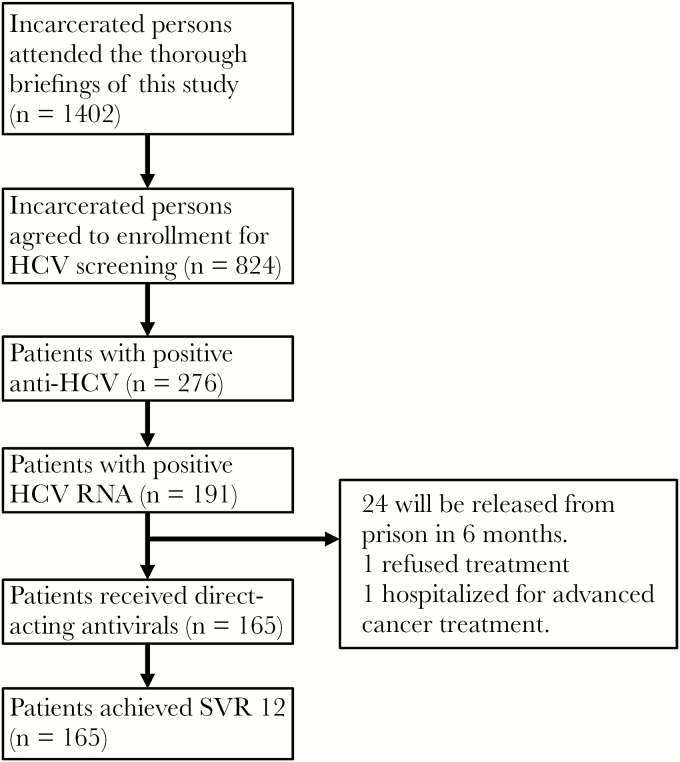
A total of 165 CHC patients received GLE/PIB therapy between February and June 2019. Twenty-six patients were excluded in this study, including 24 patients who will be released from prison in 6 months, 1 who refused antiviral therapy, and 1 who was transferred to a hospital for advanced cancer therapy. We excluded incarcerated patients who had less than 6 months sentence remaining because their SVR12 data cannot be obtained. The NHI would not reimburse the cost of treatments if these data are not available. However, further treatment after release was recommended. In univariate analyses, no statistically significant differences in baseline demographic characteristics were found among those who were treated and those who were not treated. All enrolled patients were treated according to the NHI clinical practice guidelines; 159 (96.4%) and 6 (3.6%) patients were treated for 8 and 12 weeks, respectively.
Treatment Effectiveness
All 165 patients completed the treatment course. All (100%) patients had HCV RNA level below LLOQ at EOT. The overall SVR12 rates were 100%, regardless of baseline characteristics or treatment duration. The patient selection protocol and treatment outcome of patients treated for HCV infection in Yunlin Prison are summarized in Figure 2.
Figure 2.
Selection protocol and outcome of patients treated for hepatitis C virus (HCV) infection in prison. RNA, ribonucleic acid; SVR12, sustained virologic response at week 12 off therapy.
Safety Profile
In our study, 16 (9.7%) patients experienced pruritis as the only AE. No anorexia and fatigue were reported during the course of treatment and no severe AEs occurred. Regarding laboratory abnormalities, only 1 Grade ≥3 (>5 × upper limit of normal, ULN) elevation in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) level was observed. The ALT and AST level peaked at 304 and 179 U/L, respectively, and dropped to normal limits at EOT. Elevation in total bilirubin level (>1.5 × ULN) during the treatment period was detected in 19 patients (11.5%). Among them, 2 patients exhibited Grade 3 (>3 × ULN) elevation in total bilirubin level (1.2%), which peaked at 3.59 mg/dL and 3.33 mg/dL, respectively, and both patients completed the treatment without interruption. All enrolled patients completed treatment without premature termination. None of the patients experienced death or hepatic encephalopathy in our cohort. The AEs and laboratory abnormalities are summarized in Table 3.
Table 3.
Safety Summary of the 165 Patients Treated With GLE/PIBa
| Adverse Events | All Treated Patients (n = 165) |
|---|---|
| Pruritus | 16 (9.7) |
| Anorexia | 0 (0) |
| Fatigue | 0 (0) |
| Deaths | 0 (0) |
| Laboratory Abnormalitiesb | |
| ALTc >5 × ULN | 1 (0.6) |
| ASTc >5 × ULN | 1 (0.6) |
| Total Bilirubin | |
| >1.5–3 × ULN | 17 (10.3) |
| >3 × ULN | 2 (1.2) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GLE/PIB, glecaprevir/pibrentasvir; ULN, upper limit of normal.
aData are presented as no. (%).
bPost-baseline laboratory abnormalities.
cPostnadir increase to >5 × ULN.
DISCUSSION
This study showed that microelimination of CHC in prison is possible with universal screening of anti-HCV for incarcerated persons. In addition, we linked universal screening to the standard of care for incarcerated patients with positive HCV RNA.
Several studies reported that in most countries, the prevalence of HCV infection is higher in prisons than in community settings. The estimated prevalence varies from 3% to 38% [4]. However, the true seroprevalence and GT distribution of HCV in Taiwan’s prison system are unknown due to the lack of research data. In Taiwan, the anti-HCV prevalence among incarcerated persons without substance use disorder was estimated to be 8.4% [25]. Among incarcerated persons with intravenous heroin dependence, the prevalence of HCV infection was 78.1% [26]. These studies focused on the HCV seroprevalence of subgroups rather than of all incarcerated persons in prisons. Our study is the first in Taiwan to use opt-in screening inside a prison to evaluate the seroprevalence and GT distribution of HCV. The results showed that the prevalence of HCV infection in the prison population was 33.5%, which was considerably higher than that of the general population.
The distribution of HCV GTs might vary between prison and general populations. Genotypes 1b and 2 are predominant in the general population of Taiwan [27]. Previous studies conducted outside of Taiwan showed that HCV GTs 1 and 3 are more predominant in prison [28–34]. By contrast, 1 study in Taiwan reported that GT 2a was the most predominant (58.9%), followed by 1a (17.3%), among incarcerated persons with intravenous heroin use [26]. Another study in Taiwan indicated that GT 1 was the most predominant (41.4%), followed by 3 (25.9%), among incarcerated patients who received Peg-IFN/RBV treatment [11]. Most of the incarcerated patients, who were enrolled in these studies, were transferred out of prison to receive medical therapy at medical facilities. Selection bias might exist in these studies.
Our universal screening showed that GT 6 (39.3%) was predominant, followed by GT 1a (22.0%). This result differs from the findings of other countries and previous studies in Taiwan. The mode of viral transmission may influence the predominance of certain GTs in incarcerated persons. In our study, incarcerated persons with past substance dependency accounted for 86.9% of the chronic HCV-infected prison population. According to previous research, a higher prevalence of HCV GT 6 (41.0%), followed by 1a (18.5%) and 1b (13.8%), was reported in people with injection substance use in Taiwan [35]. This result highlights the association between the route of HCV transmission and GT distribution. In addition, the coinfection rates of HBV and HIV were 4.7% and 8.9%, respectively, probably because of similarities in the viral transmission routes. In Taiwan, the prevalence of HCV and HIV coinfection among people with injection substance use is relatively high and is gradually increasing [36]. As a result of high-risk behaviors, PWID might commonly harbor mixed GT HCV infection and at risk of reinfection after treatment. Our study also found a relationship between mixed GT infection and PWID. In the treated patient group, mixed GT infection was 3.5% among PWID but none among non-PWID. These findings indicate that injection substance use is a crucial risk factor. Therefore, effective strategies, such as syringe services program and opioid agonist therapy, are required to prevent HCV transmission among PWID. Moreover, aggressive diagnostic and therapeutic approaches for HCV-infected incarcerated populations are also required, particularly in the new era of DAA therapy, which provides shorter treatment regimens in correctional settings.
Previously, CHC incarcerated patients had limited access to medical treatments because of obstacles such as medical accessibility, lack of disease awareness, and low financial support. However, therapeutic effects would not change in prison. Some studies showed comparable or more favorable treatment responses to Peg-IFN/RBV therapy in incarcerated persons than in community-based patients [11, 12]. In the new era of DAA therapy, SVR, virologic failure, and discontinuation rates were reported to be similar in patients in the prison and community settings [23]. Because of its fewer side effects and improved efficacy, DAA therapy is preferred over IFN-based therapy for incarcerated patients. The NHI authorized the prescription of DAAs to all Taiwanese citizens, including incarcerated patients, with confirmed CHC, and drug availability no longer poses a problem for treating CHC in prisons. However, DAAs can only be prescribed by hepatologists or infection specialists, and this limitation is possibly a major barrier to CHC treatments for incarcerated patients. We addressed this issue by establishing a special CHC clinic in prison. This special CHC clinic, linking HCV screening to care, can be used as a model for treating incarcerated patients with CHC.
In our study, the overall SVR rates were 100% and no discontinuation of therapy was reported. This superior treatment response was probably attributed to favorable medical compliance in prison, which is partly due to the effective administration works in prison. No severe AEs were observed and pruritis (9.7%) was the only AE in our study. Although anorexia and fatigue (approximately 5%) during GLE/PIB therapy were reported in real-world studies in Taiwan [37, 38], no such complaint was recorded in our study group, possibly because younger age, less comorbidities, and the simple prison life of our treated group might have alleviated such side effects. Regarding laboratory abnormalities, overall Grade ≥2 elevation in aminotransferase and total bilirubin level occurred in 12.7% of our patients. All patients recovered to normal limits after completing therapy. These findings indicate that GLE/PIB is effective, safe, and well tolerated in incarcerated patients with chronic HCV infection in Taiwan. Our study demonstrates that prison is an ideal place for microelimination of CHC; otherwise, incarcerated patients with CHC may have limited access to therapy once they are released from prison.
Incarcerated patients, particularly those with injection substance use, were reported to have high overall incidence of reinfection after successful treatment [39, 40]. This issue warrants further investigation. In our study, we could not estimate the reinfection rate after SVR, because a few of our treated patients were lost to regular follow-up after being released from prison or transferred to other prisons. Nevertheless, released incarcerated patients are presumed to have a high risk of reinfection due to their chaotic postprison lifestyle in the community. Therefore, community-based health interventions for the elimination of HCV must be provided to this vulnerable group.
This study has several limitations. First, more than 40% of incarcerated persons in Yunlin Prison did not agree to participate in this study because the opt-in screening approach was adopted. Routine screening for all incarcerated persons is recommended as the aggressive diagnostic approach for HCV-infected incarcerated populations. Second, to comply with the Taiwan NHI program, several patients were excluded from treatment because of the short length of their remaining sentence. Third, we did not assess the total direct medical and nonmedical costs. Therefore, the cost-effectiveness of IFN-free DAA therapy for the prison population could not be analyzed.
CONCLUSIONS
We determined that the prevalence of HCV infection in the prison population was 33.5%, which was considerably higher than that of the general population in Taiwan. Genotype 6 (39.3%) was predominant. Direct-acting antiviral therapy was highly effective and safe for incarcerated patients with CHC in Taiwan. Our special CHC clinic in prison, linking HCV screening to care, can serve as a model for HCV microelimination.
Acknowledgments
We thank the Agency of Corrections, Ministry of Justice, Taiwan and the Yunlin Prison. We also thank Hsiu-Yen Yang and Hsueh-Hsia Liao for assistance in clinical data management.
Financial support. This work was funded by the National Taiwan University Hospital Yunlin Branch (NTUHYL108. X005).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 2. Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31 (Suppl 2):61–80. [DOI] [PubMed] [Google Scholar]
- 3. Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc 2007; 106:148–55. [DOI] [PubMed] [Google Scholar]
- 4. Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology 2013; 58:1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zampino R, Coppola N, Sagnelli C, Di Caprio G, Sagnelli E. Hepatitis C virus infection and prisoners: epidemiology, outcome and treatment. World J Hepatol 2015; 7:2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva: World Health Organization, 2018. [PubMed] [Google Scholar]
- 7. Post JJ, Arain A, Lloyd AR. Enhancing assessment and treatment of hepatitis C in the custodial setting. Clin Infect Dis 2013; 57(Suppl 2):S70–4. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd AR, Clegg J, Lange J, et al. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis 2013; 56:1078–84. [DOI] [PubMed] [Google Scholar]
- 9. Sterling RK, Hofmann CM, Luketic VA, et al. Treatment of chronic hepatitis C virus in the Virginia Department of Corrections: can compliance overcome racial differences to response? Am J Gastroenterol 2004; 99:866–72. [DOI] [PubMed] [Google Scholar]
- 10. Chen CP, Cheng CY, Zou H, et al. Evaluation of cost-effectiveness of peginterferon plus ribavirin for chronic hepatitis C treatment and direct-acting antiviral agents among HIV-infected patients in the prison and community settings. J Microbiol Immunol Infect 2019; 52:556–62. [DOI] [PubMed] [Google Scholar]
- 11. Cheng CH, Lin CC, Chen HL, et al. Genotype distribution and treatment response among incarcerated drug-dependent patients with chronic hepatitis C infection. PLoS One 2018; 13:e0191799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rice JP, Burnett D, Tsotsis H, et al. Comparison of hepatitis C virus treatment between incarcerated and community patients. Hepatology 2012; 56:1252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bretschneider W, Elger BS. Expert perspectives on Western European prison health services: do ageing prisoners receive equivalent care? J Bioeth Inq 2014; 11:319–32. [DOI] [PubMed] [Google Scholar]
- 14. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–98. [DOI] [PubMed] [Google Scholar]
- 15. Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014; 370: 1983–92. [DOI] [PubMed] [Google Scholar]
- 16. Liu CJ, Chuang WL, Sheen IS, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology 2018; 154:989–97. [DOI] [PubMed] [Google Scholar]
- 17. Liu CH, Liu CJ, Su TH, et al. Real-world effectiveness and safety of sofosbuvir and ledipasvir with or without ribavirin for patients with hepatitis C virus genotype 1 infection in Taiwan. PLoS One 2018; 13:e0209299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu CH, Liu CJ, Su TH, et al. Real-world effectiveness and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with or without ribavirin for patients with chronic hepatitis C virus genotype 1b infection in Taiwan. J Gastroenterol Hepatol 2018; 33:710–7. [DOI] [PubMed] [Google Scholar]
- 19. Hong CM, Liu CH, Su TH, et al. Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C in Taiwan: real-world data. J Microbiol Immunol Infect 2018; S1684-1182(18)30403-1. doi: 10.1016/j.jmii.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 20. Aspinall EJ, Mitchell W, Schofield J, et al. A matched comparison study of hepatitis C treatment outcomes in the prison and community setting, and an analysis of the impact of prison release or transfer during therapy. J Viral Hepat 2016; 23:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bartlett SR, Fox P, Cabatingan H, et al. Demonstration of near-elimination of hepatitis c virus among a prison population: the Lotus Glen Correctional Centre Hepatitis C Treatment Project. Clin Infect Dis 2018; 67:460–3. [DOI] [PubMed] [Google Scholar]
- 22. Hochstatter KR, Stockman LJ, Holzmacher R, et al. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Justice 2017; 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marco A, Roget M, Cervantes M, et al. In and Out of Prison Study Group Comparison of effectiveness and discontinuation of interferon-free therapy for hepatitis C in prison inmates and noninmates. J Viral Hepat 2018; 25:1280–6. [DOI] [PubMed] [Google Scholar]
- 24. Pontali E, Fiore V, Ialungo AM, et al. ; Gruppo Infettivologi Penitenziari Treatment with direct-acting antivirals in a multicenter cohort of HCV-infected inmates in Italy. Int J Drug Policy 2018; 59:50–3. [DOI] [PubMed] [Google Scholar]
- 25. Liao KF, Lai SW, Chang WL, Hsu NY. Screening for viral hepatitis among male non-drug-abuse prisoners. Scand J Gastroenterol 2006; 41:969–73. [DOI] [PubMed] [Google Scholar]
- 26. Liao KF, Lai SW, Lin CY, et al. Diversity of hepatitis C virus genotypes among intravenous heroin users in Taiwan. Am J Med Sci 2011; 341:110–2. [DOI] [PubMed] [Google Scholar]
- 27. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10:553–62. [DOI] [PubMed] [Google Scholar]
- 28. Keten D, Emin Ova M, Sirri Keten H, et al. The prevalence of hepatitis B and C among prisoners in Kahramanmaras, Turkey. Jundishapur J Microbiol 2016; 9:e31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santos BF, de Santana NO, Franca AV. Prevalence, genotypes and factors associated with HCV infection among prisoners in Northeastern Brazil. World J Gastroenterol 2011; 17:3027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuadrado A, Llerena S, Cobo C, et al. Microenvironment eradication of hepatitis C: a novel treatment paradigm. Am J Gastroenterol 2018; 113:1639–48. [DOI] [PubMed] [Google Scholar]
- 31. Meyer MF, Wedemeyer H, Monazahian M, et al. Prevalence of hepatitis C in a German prison for young men in relation to country of birth. Epidemiol Infect 2007; 135:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walker MR, Li H, Teutsch S, et al. Incident hepatitis C virus genotype distribution and multiple infection in Australian prisons. J Clin Microbiol 2016; 54:1855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adland E, Jesuthasan G, Downs L, et al. Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort. BMC Infect Dis 2018; 18:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sterling RK, Cherian R, Lewis S, et al. Treatment of HCV in the Department of Corrections in the era of oral medications. J Correct Health Care 2018; 24:127–36. [DOI] [PubMed] [Google Scholar]
- 35. Hsieh MH, Tsai JJ, Hsieh MY, et al. Hepatitis C virus infection among injection drug users with and without human immunodeficiency virus co-infection. PLoS One 2014; 9:e94791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu JY, Lin HH, Liu YC, et al. Extremely high prevalence and genetic diversity of hepatitis C virus infection among HIV-infected injection drug users in Taiwan. Clin Infect Dis 2008; 46:1761–8. [DOI] [PubMed] [Google Scholar]
- 37. Hsu SJ, Chiu MC, Fang YJ, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in Asian patients with chronic hepatitis C. J Formos Med Assoc 2019; 118:1187–92. [DOI] [PubMed] [Google Scholar]
- 38. Liu CH, Liu CJ, Hung CC, et al. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection: real-world effectiveness and safety in Taiwan. Liver Int 2020; 40:758–68. [DOI] [PubMed] [Google Scholar]
- 39. Marco A, Guerrero RA, Vergara M, et al. Reinfection in a large cohort of prison inmates with sustained virological response after treatment of chronic hepatitis C in Catalonia (Spain), 2002–2016. Int J Drug Policy 2019; 72:189–94. [DOI] [PubMed] [Google Scholar]
- 40. Bhandari R, Morey S, Hamoodi A, et al. High rate of hepatitis C reinfection following antiviral treatment in the North East England Prisons. J Viral Hepat 2020; 27:449–52. [DOI] [PubMed] [Google Scholar]