Abstract
Objective:
Identifying effective treatment for papillomatosis is limited by a lack of animal models, and there is currently no preclinical model for testing potential therapeutic agents. We hypothesized that xenografting of papilloma may facilitate in vivo drug testing to identify novel treatment options.
Methods:
A biopsy of fresh tracheal papilloma was xenografted into a NOD-scid-IL2Rgammanull (NSG) mouse.
Results:
The xenograft began growing after 5 weeks and was serially passaged over multiple generations. Each generation showed a consistent log-growth pattern, and in all xenografts, the presence of the human papillomavirus (HPV) genome was confirmed by polymerase chain reaction (PCR). Histopathologic analysis demonstrated that the squamous architecture of the original papilloma was maintained in each generation. In vivo drug testing with bevacizumab (5 mg/kg i.p. twice weekly for 3 weeks) showed a dramatic therapeutic response compared to saline control.
Conclusion:
We report here the first successful case of serial xenografting of a tracheal papilloma in vivo with a therapeutic response observed with drug testing. In severely immunocompromised mice, the HPV genome and squamous differentiation of the papilloma can be maintained for multiple generations. This is a feasible approach to identify therapeutic agents in the treatment of recurrent respiratory papillomatosis.
Keywords: bevacizumab, drug testing, laryngeal papilloma, larynx, papilloma, pulmonary papilloma, recurrent respiratory papillomatosis, xenograft
Introduction
Recurrent respiratory papillomatosis (RRP) is a disease characterized by repeated growth of benign, exophytic lesions of the upper respiratory tract caused by human papillomavirus (HPV) types 6 and 11.1 Currently, surgical debulking of the papilloma is the primary method of treatment, but these tumors tend to return unpredictably and require repeated surgical excisions.2 In a small subset of patients, the papilloma spreads further down the respiratory tree to involve the trachea and lung parenchyma. For these patients, the therapeutic and surgical options are limited, and the development of effective systemic treatments has been impeded by the lack of animal models for RRP.
Cell culture techniques have been used for decades to investigate the biologic behavior of laryngeal papilloma and the molecular mechanisms by which the HPV virus drives cell growth.3 However, recent studies have shown that tumors grown in vitro are not representative of the patient-derived tumor,4 and animal models with xenografted human tumors provide a more stable and applicable method for drug screening and development.5 In RRP, various adjuvant therapies have been used to attempt to reduce the severity of this disease,6–8 including localized injections of bevacizumab, an anti-angiogenesis drug.9,10 An accessible means of testing these therapeutic agents against patient-derived tissue could have significant clinical implications for patients with RRP.
The aim of this study was to develop a xenograft animal model of RRP for therapeutic drug testing. In order to be clinically relevant and robust as a model for RRP, this xenograft must be stably passaged across multiple generations, maintain squamous differentiation in vivo, and continue to contain the type-specific HPV genome as detected through molecular methods. Specifically, we hypothesized that we would be able to graft tissue specimens from a patient with RRP into immunocompromised mice and to observe a therapeutic response with drug testing to identify potential clinical therapeutic targets.
Materials and Methods
Human Subject and Animal Research
All research protocols and tissue collection were approved by the institutional review board of Johns Hopkins Hospital. All animal procedures were performed according to approved animal protocols and in accordance with the recommendations for the proper ethical use and care of laboratory animals.
Tissue Collection
During surgery, a sample of tracheal papilloma was collected from the subject and then transferred to the research laboratory in cold RPMI-1640 medium. Pieces (3 mm) of the tissue were saved in cold RPMI medium before grafting. The remaining tissue was frozen in an optimum cutting temperature (OCT) compound within 8 hours of resection. Frozen samples were stored at −80°C until required.
Animal Studies
Female NOD-scid-IL2Rgammanull (NSG) mice were purchased for animal studies (Jackson Laboratory, Bar Harbor, Maine, USA). Papilloma tissue samples obtained from the surgeon were kept in cold RPMI medium and then implanted into NSG mice within 8 hours of resection. Mice were anesthetized with isoflurane and exposed to it intermittently during the grafting procedure. The right flank was disinfected with iodine solution, and a small skin incision was made. A fresh 3-mm papilloma sample was transplanted subcutaneously, and wounds were closed using 9-mm clips. The tumor volume (length × width × height) was measured 2 times weekly using a caliper. Ten weeks after grafting, the tumors were excised and cut into small sections (3 mm) for serial transplantation. Before each passage, part of the tumors were frozen in OCT compound and saved in −80°C. Mice from the final tumor passage were used for in vivo drug testing with bevacizumab. After initial signs of tumor growth, bevacizumab (5 mg/kg) was administered through intraperitoneal (i.p.) injection. The control group received an equal volume of saline (0.9% sodium chloride) solution through i.p. injection. Bevacizumab and saline were given twice weekly for a total of 6 doses. Tumor volume was measured 2 times weekly with a caliper.
DNA Extraction
Frozen tissue samples were brought to −20°C and cut into 5-μm sections. The sections were stained with hematoxylin and eosin (H&E) stain and reviewed under light microscopy by a head and neck pathologist. Ten sections were cut and digested with Proteinase K at 48°C and purified with phenol chloroform. Ethanol precipitation was performed with 100% ethanol, ammonium acetate (NH4OAc), and glycogen. Tris-HCl/EDTA solution was added to resuspend the samples overnight. Frozen tissue was not available for 1 xenograft tissue, so DNA extraction was performed using formalin-fixed and paraffin-embedded (FFPE) tissues. Formalin-fixed and paraffin-embedded tissues were deparaffinized using 100% xylene and then washed with 100%, 70%, and then 50% ethanol. Tissues were then digested with Proteinase K at 48°C. The precipitation, extraction, and purification steps were performed as described above for the frozen samples.
Polymerase Chain Reaction
The presence of the HPV genome in the extracted DNA samples was determined by both consensus and type-specific polymerase chain reaction (PCR). To detect HPV DNA, consensus primers, Gp 5+ (5’-TTTGTTACTGTGGTAGATACTAC-3’) and Gp 6+ (5’-GAAAAATAAACTGTAAATCATATTC-3’), were used. To determine the HPV genotype, L1-type-specific PCRs were performed for HPV 6 and HPV 11. The primers used were the following: HPV 6-specific primers (forward 5’-ATC CGT AAC TAC ATC TTC CA-3’, reverse 5’-GGC CTG TGA CTG CAC ATA CC-3’) and HPV 11-specific primers (forward 5’-TCT GTG TCT AAA TCT GCT AC-3’, reverse 5’-GGG TTT CTG ACA GGT AAT GGC-3’). Platinum Taq DNA polymerase was used for all reactions, and PCR products were visualized with ethidium bromide staining on 2% agarose gels.
Results
Patient-derived papilloma tissue was successfully grafted into immunodeficient mice and serially transplanted multiple times. In each generation, no visible tumor was seen for approximately 6 weeks, and then it began to expand in a log-growth pattern. The tumor was removed, divided, and repassaged at the peak of this log-growth curve, when the approximate tumor volume was 1000 mm3. Resection of the tissues taken from each passage revealed papillomatous characteristics on the surface of the tumors (Figure 1). Hematoxylin and eosin staining revealed typical papillary squamous epithelium in the original papilloma sample and maintained human squamous epithelium within a murine fibrous background in all generations of the xenografted tissue (Figure 2).
Figure 1.
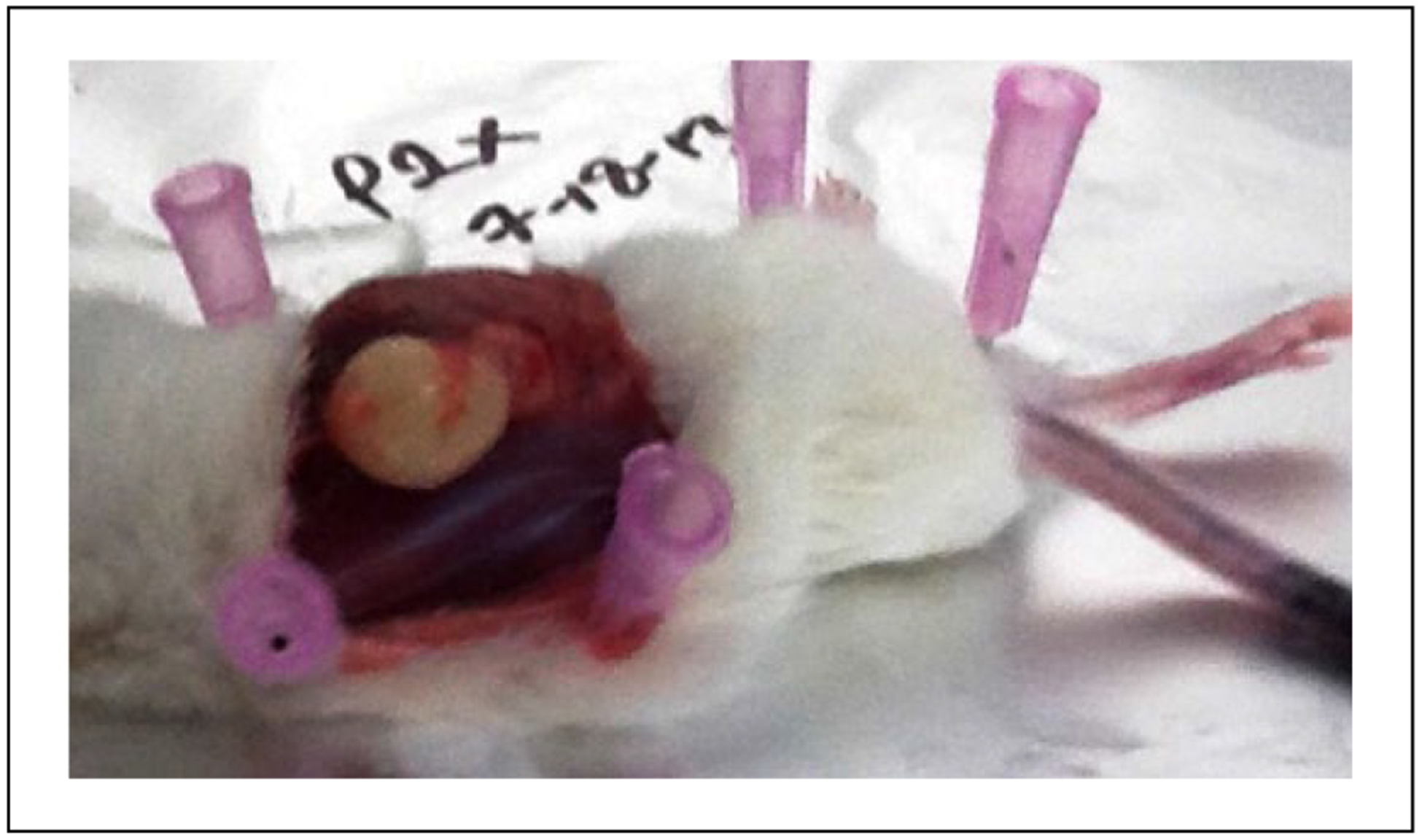
Xenografted papilloma tissue. Tissue is harvested from the subcutaneous tissue at 12 weeks postimplantation to passage to the next generation and for tissue analysis.
Figure 2.
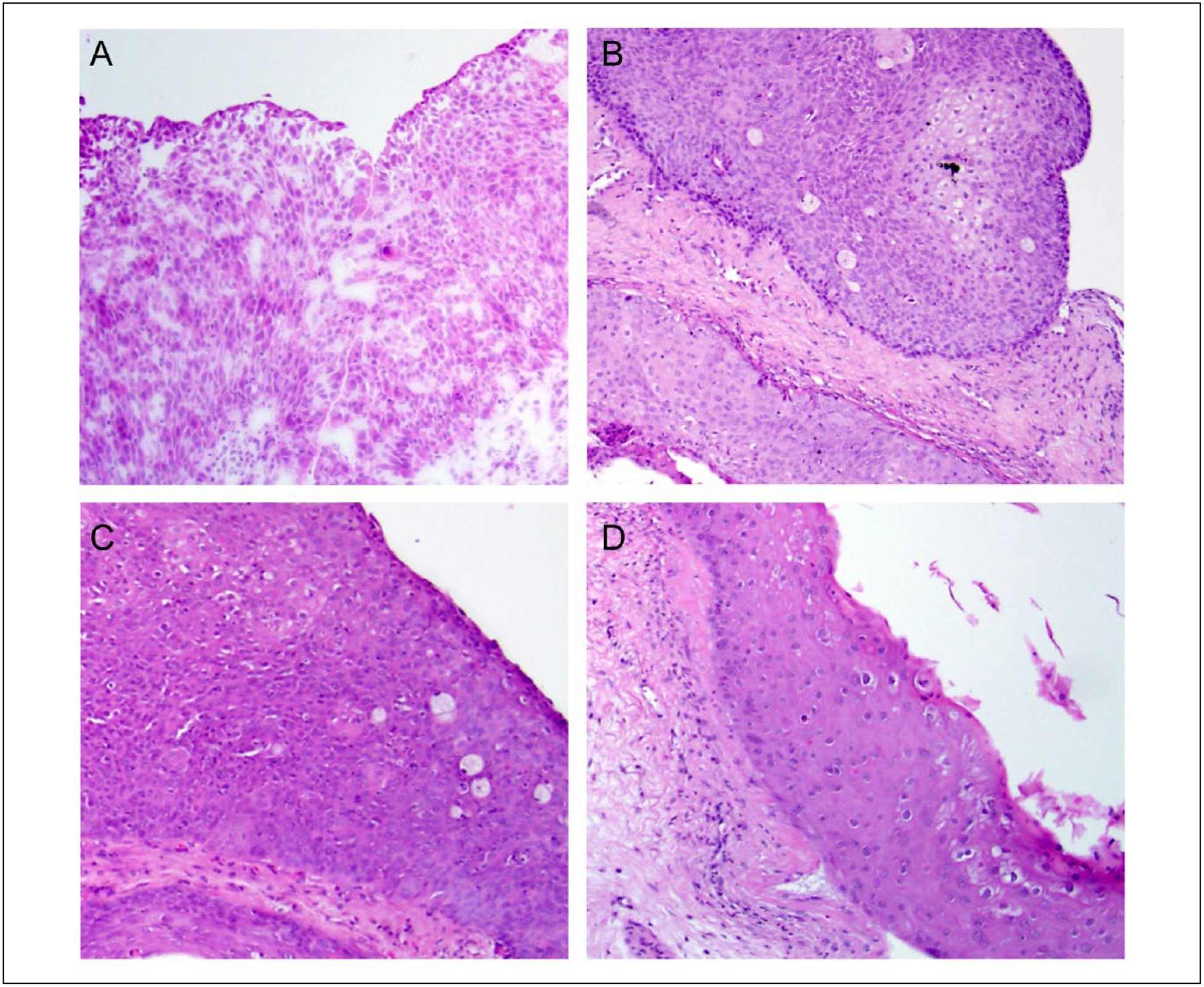
Papilloma xenograft photomicrographs. Representative hematoxylin and eosin (H&E)-stained sections of human pulmonary papilloma harvested after (A) resection from the patient, (B) first xenograft passage (P0), (C) second xenograft passage (P1), and (D) fourth xenograft passage (P3). All magnification 400×.
The tumors from the final passage started growing at about 5 weeks postgrafting, and bevacizumab was administered at 6 weeks. The tumor in the bevacizumab-treated mouse grew until week 7 and then showed a dramatic decrease in volume after the third dose (Figure 3). The control mouse displayed an overall increase in tumor volume in a log-growth pattern seen in previous generations of the xenograft. At the end of 10 weeks, the tumor volume for the bevacizumab-treated mouse was 23.7 mm3 compared to 1039.5 mm3 for the control mouse.
Figure 3.
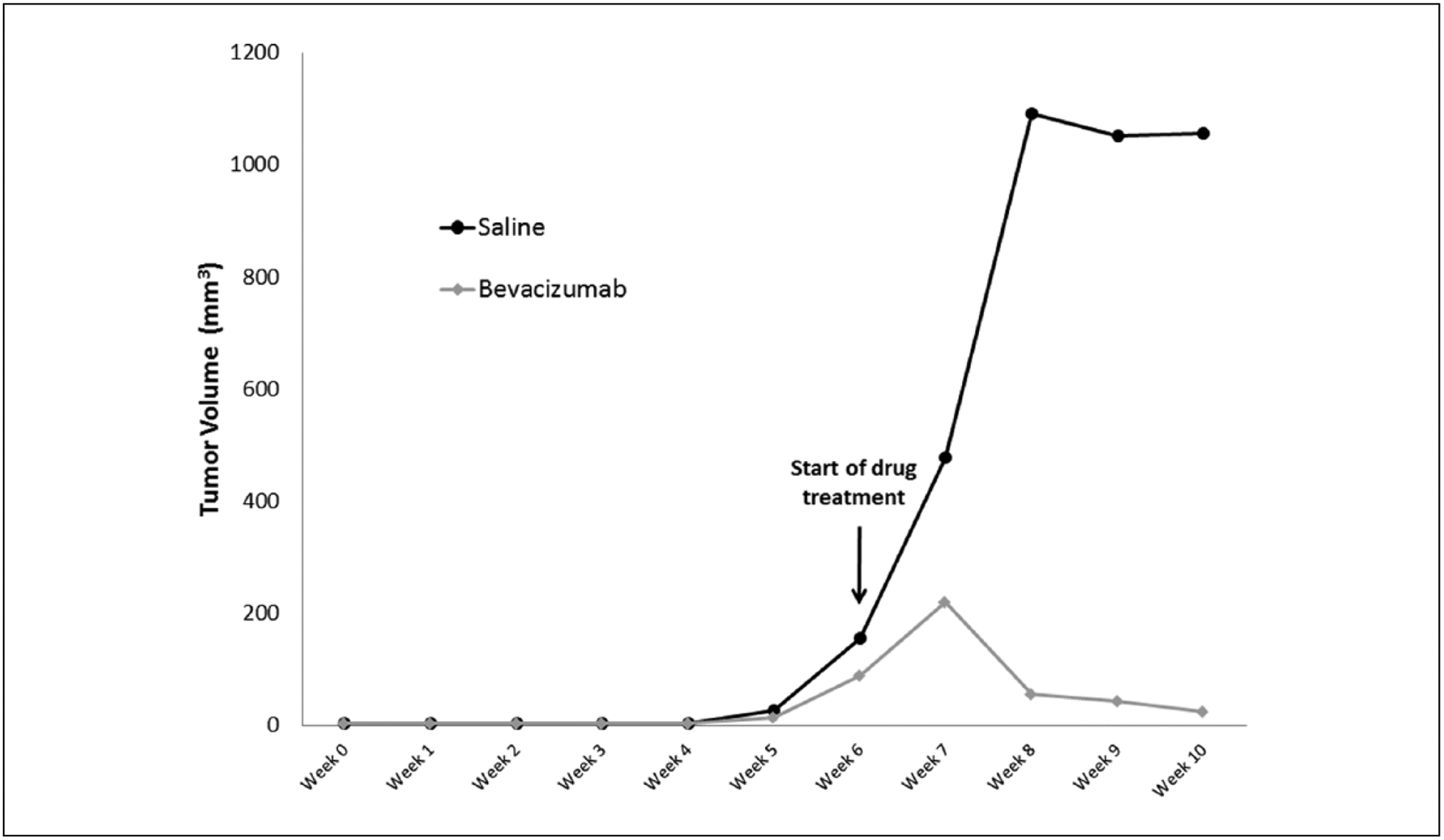
Xenograft growth kinetics. Two xenografts derived from the previous generation (P2) were grafted into 2 mice simultaneously (P3). Once the tumor was palpable (week 6), treatment began with intraperitoneal bevacizumab (5 mg/kg) or saline control. Tumor growth was measured with calipers weekly. The bevacizumab-treated xenograft showed dramatic tumor regression compared to saline control.
The original tissue and all xenograft passages were successfully amplified using Gp5+/6+ primers, confirming HPV DNA presence (Figure 4). To verify the HPV geno-type of the original papilloma tissue, L1-type-specific primers for HPV 6 and 11 were used. The HPV 6 primer was able to amplify the original sample, and negative results were seen with the HPV 11 primer (Figure 5). Therefore, HPV 6 type-specific PCR was then carried out with the xenograft samples. The results showed 235 bp fragments for all 4 xenograft tissues, confirming the presence of HPV 6 DNA.
Figure 4.
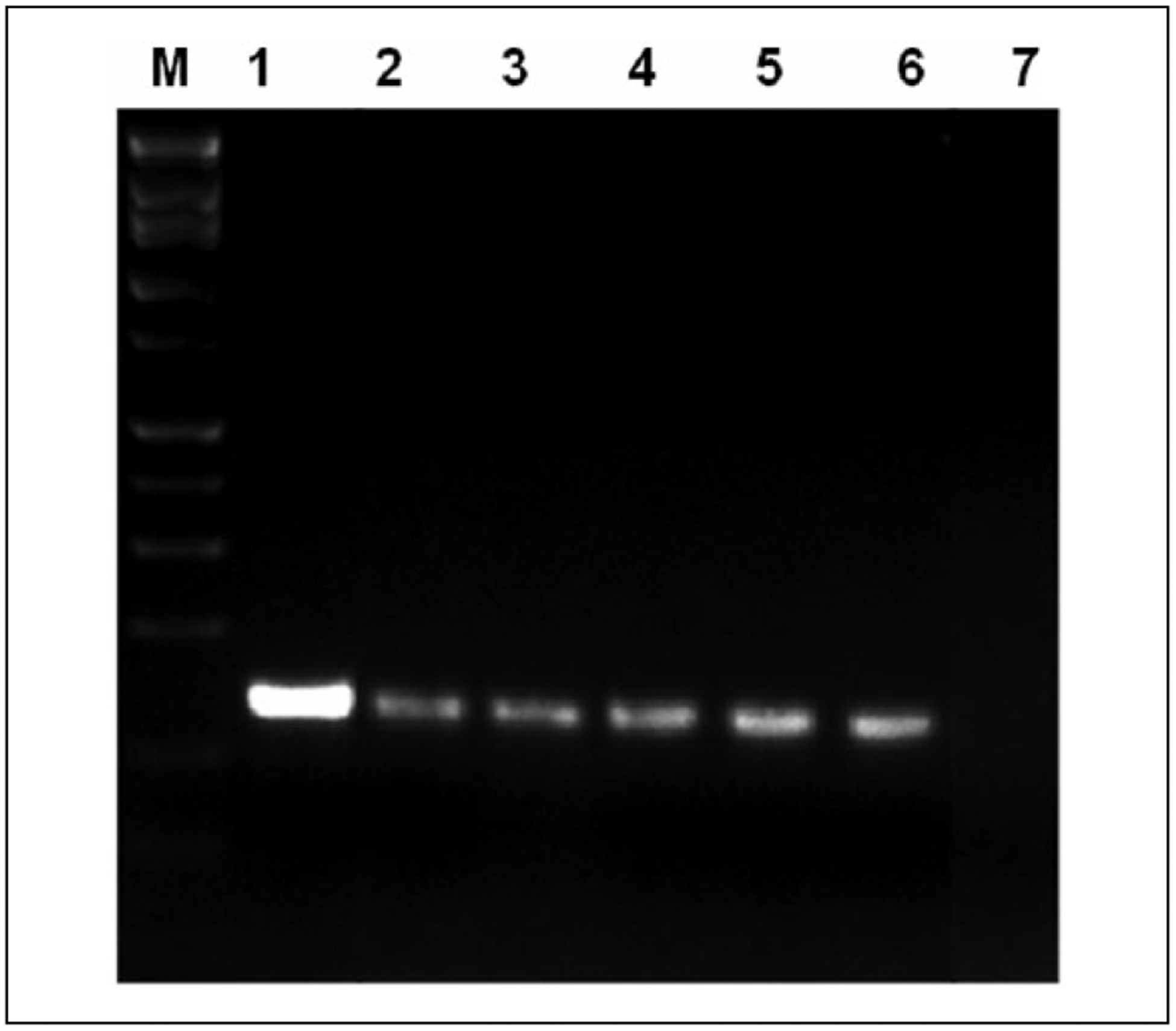
Gp5+/6+ human papillomavirus (HPV) consensus primer polymerase chain reaction demonstrates HPV DNA in all xenograft generations. Human pulmonary papilloma tissue and serial xenograft passages show positive DNA bands using HPV consensus primers. Lane 1—positive control; lane 2—original papilloma tissue; lanes 3–6—first (P0), second (P1), third (P2), and fourth (P3) passages, respectively; and lane 7—negative control.
Figure 5.
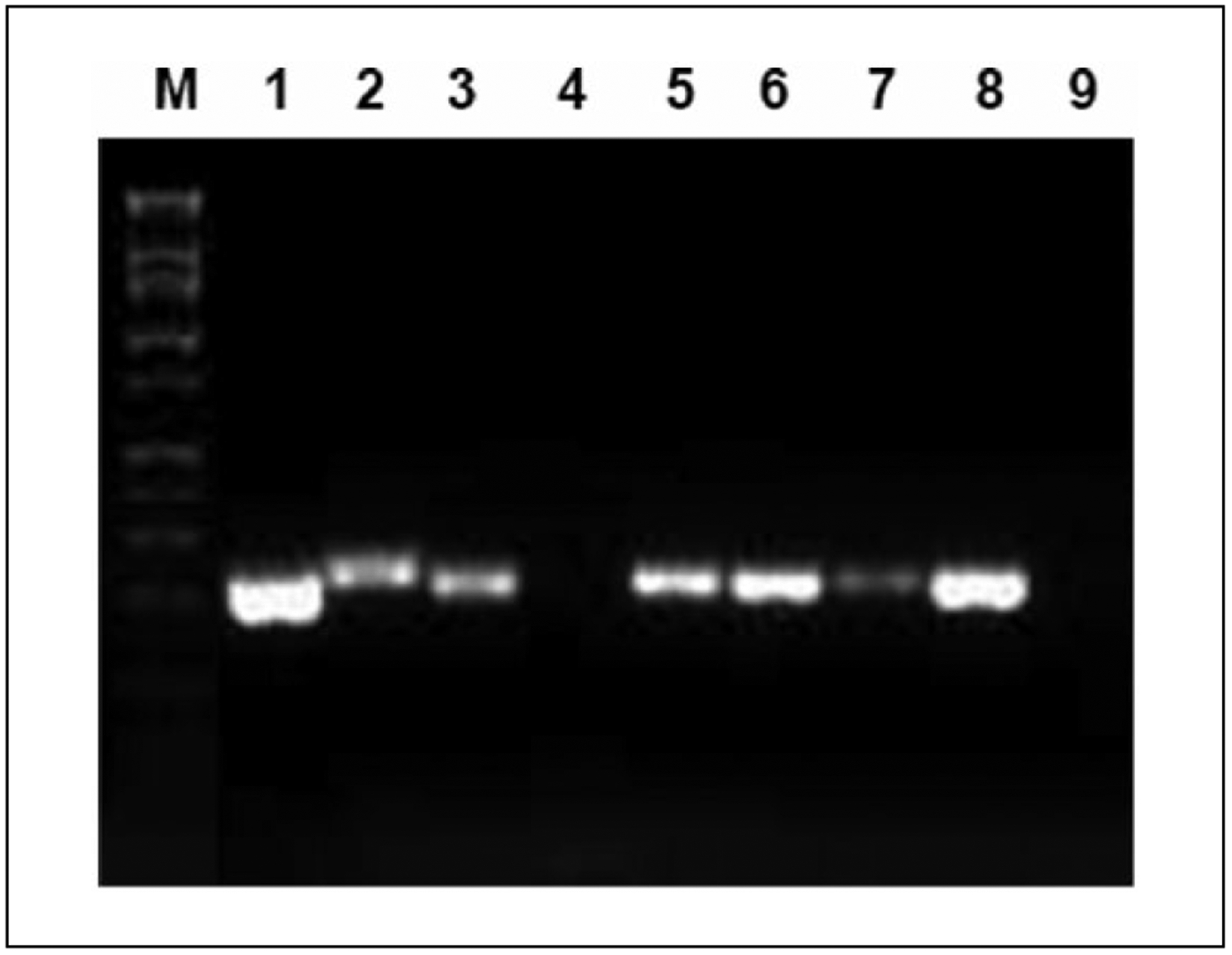
L1-type-specific polymerase chain reaction demonstrates human papillomavirus (HPV) 6 DNA in all xenograft generations. Human pulmonary papilloma tissue and serial xenograft passages show HPV DNA using type-specific primers. Lanes 1 and 2—HPV 6 and HPV 11 positive controls; lanes 3 and 4—original papilloma tissue showing HPV 6 positivity (lane 3) but not HPV 11 (lane 4); lanes 5–8—first (P0), second (P1), third (P2), and fourth (P3) passages all positive for HPV 6 DNA; and lane 9—negative control.
Discussion
We report here the first successful case of serial xenografting of a tracheal papilloma in vivo with a therapeutic response observed with drug testing. Bevacizumab treatment resulted in dramatic tumor regression compared to the untreated xenografted tissue, and pathologically and molecularly, the transplanted tissue maintained human squamous differentiation with a maintained HPV genome.
The development of therapeutic agents for RRP and HPV-related diseases in general has been hampered by the requirement of the HPV virus for differentiating human tissue to complete its life cycle.11 A variety of techniques to identify targets for HPV-related diseases have been developed, some of which have led directly to therapeutic trials in humans. Cell culture experiments have led to clinical trials for celecoxib,12 and a recent report used reprogrammed cells to screen therapeutic targets for pulmonary papillomatosis.13 The drawback to these approaches is that they are able to screen only for compounds that are directly or indirectly cytotoxic in cell culture. There is no tumor architecture or immunologic microenvironment in these methods that can recapitulate the particular biology of a growing papilloma. Furthermore, in vitro culturing itself causes changes in the biologic behavior and genetic makeup of the tumor itself due to the demands of growth in dispersed cell media culture.4 Xenograft models for drug screening therefore offer an alternate method to evaluate therapeutics in a more biologically relevant manner.
NOD-scid-IL2Rgammanull mice are the most severely immunocompromised strain of mice commercially available. They lack B cells, T cells, natural killer (NK) cells, and complement, with defective dendritic cells and macrophages. Because of this severely compromised immune system, they are suitable for tumor xenografting in a wide variety of tumor models, including ovarian cancer,14 breast cancer,15 melanoma,16 and Ewing sarcoma.17 In all of these systems, the tumor retains a remarkable degree of biologic behavior relevant to the tumor model, including maintenance of phenotype, metastasis patterns similar to the original tumor,16 tumor acistes, and the persistence of human fibroblasts and lymphocytes that are part of the tumor’s immunologic microenvironment.14 In RRP, which is primarily a localized immunologic deficiency,18 the maintenance of immunologic characteristics could play an important role in determining the response to treatment.
Based on this literature and experience in other tumor models, NSG mice were selected for our initial trial of subcutaneous implantation of a patient-derived papilloma. In this xenograft model, we were able to achieve consistent log-growth from the implanted papilloma in 4 serial generations of tumor, with expansion of the original sample into multiple mice in each generation. Human papillomavirus DNA was identified by consensus primer PCR, and just as the patient’s laryngeal lesion was shown by type-specific PCR analysis to contain HPV 6 DNA, all representative sections from each passage also showed HPV 6 positivity. Histological analysis was confirmed by H&E staining, which demonstrated that the squamous architecture of the tumors was retained through all generations within a murine fibrous background. These results are consistent with previous studies that demonstrated high molecular and pathologic similarity between human-derived adenocarcinoma xenograft tissues and the original tumors5 and highlight the feasibility of propagating the HPV genome through patient-derived xenografts.
Once the xenograft was successfully passaged and phenotype confirmed, we were further able to investigate the effect of bevacizumab on tumor growth. Bevacizumab was selected for our initial therapeutic trial because of its demonstrated efficacy in RRP9 and its mechanism of action through inhibition of neovascularization, which makes it unsuitable to test in dispersed cell culture. Systemic administration was chosen as the method of drug administration because the pharmacokinetics of intraperitoneal bevacizumab have been well studied, with a known half-life of 5.5 days and systemic bioavailability of 93%.19 The pharmacokinetics of sublesional bevacizumab are not known, neither the systemic bioavailability nor the residence time in local tissue, even though this is the primary method of reported administration in humans for RRP.
Bevacizumab was able to decrease the size of the tumor in the xenograft model compared to saline control. Therefore, even after several passages of the patient-derived papilloma, the xenograft system is an effective method for in vivo drug testing. Further proof of principle of this approach would be correlation of this tumor response seen in the xenograft to a patient response to systemic bevacizumab, but the patient from whom the papilloma was derived has not yet undergone this systemic treatment.
The results demonstrated in this study are consistent with previous findings in other groups using xenograft models testing bevacizumab.20 Combination therapy of bevacizumab with other treatments demonstrated additional indications of tumor inhibition, including decreases in the density and diameter of the blood vessels. Histologically, we could not examine vascular endothelial growth factor expression or changes in papilloma vasculature, given that the treated papilloma xenograft essentially completely disappeared under bevacizumab treatment, but further experiments could examine these histologic changes.
Conclusion
This study revealed that bevacizumab treatment is able to elicit a significant therapeutic response in immunodeficient NSG mice with patient-derived papilloma. This finding and the stability of the human-derived papilloma through multiple xenograft passages direct attention to the importance of studying the pharmacodynamics of drugs in patient- originated in vivo tumors. We describe an animal model that replicates the molecular and histological characteristics of a human tracheal lesion and thus allows for personalized testing of therapeutic agents, which can then be clinically translatable to human trials.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Larson DA, Derkay CS. Epidemiology of recurrent respiratory papillomatosis. APMIS. 2010;118(6–7):450–454. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesan NN, Pine HS, Underbrink MP. Recurrent respiratory papillomatosis. Otolaryngol Clin North Am. 2012;45(3):671–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg BM, Abramson AL, Meade RP. Culture of human laryngeal papilloma cells in vitro. Otolaryngol Head Neck Surg. 1982;90(6):728–735. [DOI] [PubMed] [Google Scholar]
- 4.Daniel VC, Marchionni L, Hierman JS, et al. A primary xenograft model of small-cell lung cancer reveals irrevers ible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69(8):3364–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattie M, Christensen A, Chang MS, et al. Molecular characterization of patient-derived human pancreatic tumor xenograft models for preclinical and translational development of cancer therapeutics. Neoplasia. 2013;15(10):1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadha NK, James A. Adjuvant antiviral therapy for recurrent respiratory papillomatosis. Cochrane Database Syst Rev. 2012;12:CD005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen CA, Bryson PC. Indole-3-carbinol for recurrent respiratory papillomatosis: long-term results. J Voice. 2004;18(2):248–253. [DOI] [PubMed] [Google Scholar]
- 8.Leventhal BG, Kashima HK, Mounts P, et al. Long-term response of recurrent respiratory papillomatosis to treatment with lymphoblastoid interferon alfa-N1. Papilloma Study Group. N Engl J Med. 1991;325(9):613–617. [DOI] [PubMed] [Google Scholar]
- 9.Zeitels SM, Barbu AM, Landau-Zemer T, et al. Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol. 2011;120(10):627–634. [DOI] [PubMed] [Google Scholar]
- 10.Best SR, Friedman AD, Landau-Zemer T, et al. Safety and dosing of bevacizumab (Avastin) for the treatment of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2012;121(9):587–593. [DOI] [PubMed] [Google Scholar]
- 11.Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118(6–7):422–449. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Coniglio SJ, Chan A, Symons MH, Steinberg BM. Up-regulation of Rac1 by epidermal growth factor mediates COX-2 expression in recurrent respiratory papillomas. Mol Med. 2007;13(3–4):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan H, Myers S, Wang J, et al. Use of reprogrammed cells to identify therapy for respiratory papillomatosis. N Engl J Med. 2012;367(13):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bankert RB, Balu-Iyer SV, Odunsi K, et al. Humanized mouse model of ovarian cancer recapitulates patient solid tumor progression, ascites formation, and metastasis. PLoS One. 2011;6(9):e24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez KE, Fan F, Smith W, Allred DC, Medina D, Behbod F. Human primary ductal carcinoma in situ (DCIS) sub-type-specific pathology is preserved in a mouse intraductal (MIND) xenograft model. J Pathol. 2011;225(4):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintana E, Piskounova E, Shackleton M, et al. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med. 2012;4(159):159ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vormoor B, Knizia HK, Batey MA, et al. Development of a preclinical orthotopic xenograft model of ewing sarcoma and other human malignant bone disease using advanced in vivo imaging. PLoS One. 2014;9(1):e85128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and −11. APMIS. 2010;118(6–7):455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah DK, Veith J, Bernacki RJ, Balthasar JP. Evaluation of combined bevacizumab and intraperitoneal carboplatin or paclitaxel therapy in a mouse model of ovarian cancer. Cancer Chemother Pharmacol. 2011;68(4):951–958. [DOI] [PubMed] [Google Scholar]
- 20.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–680. [PubMed] [Google Scholar]


