Abstract
Purpose
To determine if there is a biologic rationale for using checkpoint inhibitor drugs targeting programmed cell death ligand 1 (PD-L1) and PD-L2 in the treatment of adenoid cystic carcinoma of the orbit.
Methods
Twenty-three cases of adenoid cystic carcinoma involving the orbit (13 primary lacrimal gland, 5 secondarily extending into the orbit, and 5 unspecified) were examined histopathologically. Immunohistochemistry for PD-L1, PD-L2, and CD8 was performed. Charts were reviewed for clinical correlations.
Results
Expression of PD-L1 and of PD-L2 was overall low in adenoid cystic carcinoma (mean expression 1.4?±0.9 of 5 for PD-L1, mean 0.83±1.1 of 5 for PD-L2), and tumor-infiltrating CD8-positive T-lymphocytes were sparse (mean 1.1?±0.51 of 3). Only 13 of the 23 (57%) cases expressed PD-L1 as a combined positive score ≥1 of cells. No associations were found between expression levels of these markers and patient sex, tumor site of origin, Tumor, Node, Metastasis stage, or patient outcome. A significant association was observed between stromal PD-L1 expression and tumor histopathologic subtype (p = 0.05), and between tumor PD-L1 expression and prior exposure to radiation (p = 0.03).
Conclusions
Checkpoint inhibitor drugs may have limited impact in the treatment and clinical course of orbital adenoid cystic carcinoma based on the low frequency of CD8 infiltrate and low expression of PD-L1 and PD-L2. Pretreatment with radiation, however, may improve tumor response to checkpoint inhibitor drugs.
Checkpoint inhibitor drugs have been employed successfully in the treatment of many neoplasms, including cutaneous melanoma, renal cell carcinoma, non–small-cell lung carcinoma, squamous cell carcinomas of the head and neck, urothelial carcinoma, hepatocellular carcinoma, and Hodgkin lymphoma.1 Expression of programmed cell death ligand 1 (PD-L1) and PD-L2, degree of CD8-positive T-lymphocyte infiltration and tumor mutational burden have been found to predict tumor response to therapy with a class of drugs called immune checkpoint inhibitors (ICIs).2,3 Expression of these markers has also been found to correlate with prognosis for certain tumors.4 With regard to ocular adnexal malignancies, these markers are expressed in conjunctival invasive squamous cell carcinoma5,6 and sebaceous carcinoma.7–10
Adenoid cystic carcinoma (ACC) is an ocular adnexal malignancy with a different cell of origin and distinct behavior from squamous and sebaceous carcinomas. Despite extensive investigations, treatment of localized primary lacrimal gland ACC remains mostly limited to radiotherapy complementing surgery,11 but recurrent and metastatic disease remains a no man’s land devoid of any preferred and well-tested efficacious therapy. Studies investigating the expression of PD-L1 and PD-L2 in nonlacrimal gland ACC of the head and neck have found mostly low expression levels of these markers, suggesting a minimal role for checkpoint inhibitor drugs.12–17 This would suggest that checkpoint inhibitor drugs should correspondingly have a minimal role in the treatment of orbital ACCs. However, the results of studies from nonperiocular head and neck malignancies do not always translate directly to ocular adnexal malignancies.18 For example, lacrimal gland ACCs displayed a higher mutational burden than reported in ACCs of the head and neck,19,20 suggesting that there may be subtle differences between ACCs in these different areas.
The purpose of the current investigation was to assess the expression of PD-L1, PD-L2, and CD8-positive T-lymphocytes in ACC involving the orbit and ocular adnexa to determine whether there is a rationale for the use of ICIs in the treatment of primary ACC of the lacrimal gland or secondary tumors invading the orbit from the sinus. A secondary goal of this study was to determine whether any patient or tumor characteristics were associated with tissue PD-L1 or PD-L2 expression.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Massachusetts General Hospital and Partners Healthcare (Institutional Review Board #2014P000478) and is compliant with the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. A search of the Massachusetts General Hospital (MGH) and Massachusetts Eye and Ear (MEE) pathology files using the terms “adenoid cystic carcinoma” and “orbit” was performed for cases submitted between 1990 and 2017. Forty cases were identified. Pathology reports, existing histopathologic slides, and tissue blocks were reviewed. Eighteen cases were found to have sufficient material in tissue blocks for immunohistochemical studies. An additional 5 cases of orbital ACC were supplied by the Wills Eye Hospital, bringing the total number of cases to 23. Thirteen cases from the MGH and MEE represented primary lacrimal gland ACC; 5 cases from MGH and MEE were ACC of the sinus or lacrimal sac with secondary extension or recurrence in the orbit. Clinical history, including prior radiation history, was available and reviewed for the 18 cases from MGH and MEE. The origin of the 5 cases from the Wills Eye Hospital was unspecified and no clinical history was available. Three normal lacrimal glands were selected as controls.
Ten tumors from MGH and MEE had no prior exposure to radiation. Nine of these were primary lacrimal gland tumors and one was of sinonasal origin with extension into the orbit.
Four tumors had exposure to radiation within a month prior to resection (ranging from 1 to 4 weeks). The first was a primary lacrimal land tumor that had undergone an incisional biopsy and been treated with 8 Gray of radiation at an outside institution. The patient then transferred care to MEE and underwent surgical debulking of the residual tumor 4 weeks after radiation (specimen used in the current study), followed by an additional 64 Gray of postoperative radiation. The second case was a primary lacrimal gland tumor that underwent an incisional biopsy at an outside institution. The patient was then treated with 20 Gray of preoperative radiation followed by surgical excision of residual tumor 3 weeks after radiation (specimen used in the current study). This patient was treated with 54 Gray of postoperative radiation. The third case was a lacrimal gland tumor that underwent incisional biopsy and was treated with 72 Gray of radiation at an outside institution without definitive tumor excision. One year after the completion of radiation, the tumor increased in size. The tumor was treated at MEE with 20 Gray of radiation followed by surgical excision 1 week after radiation (specimen used in the current study); the tumor bed was exposed with postoperative brachytherapy. The fourth patient had a lacrimal gland tumor that was treated at an outside institution with subtotal resection followed by 71 Gray of radiation. The tumor recurred and was treated with 54 Gray of radiation followed by surgical excision 1.5 weeks after radiation (specimen used in the current study).
Four tumors had a remote history of radiation prior to resection. In all 4 cases, these were recurrent tumors. Three tumors were initially of sinonasal origin with recurrences in the orbit, while one was of lacrimal gland origin with a recurrence in the orbit. Two tumors were resected 6 years after prior radiation, 1 was resected 9 years after prior radiation, and 1 was resected 18 years after prior radiation.
Five-micron paraffin sections were immunostained at the Massachusetts General Hospital’s Immunopathology Laboratory. The following antibodies were used: PD-L1 (rabbit monoclonal antibody clone E1L3N, 1:30 dilution, Cell Signaling Technology, Danvers, MA), PD-L2 (rabbit monoclonal antibody clone D7U8C, 1:100 dilution, Cell Signaling Technology, Danvers, MA), and CD8 (rabbit polyclonal antibody, #ab4055, 1:200 dilution, Abcam, Cambridge, MA).
Immunostained slides were scored by 2 pathologists using 2 scoring systems.5,21 The first scoring system finds more usefulness in research, as it reveals fine gradations in tissue expression of markers, but it is more time-consuming.5 The second scoring system, the combined positive score (CPS), is less time-consuming and is now commonly used for scoring PD-L1 expression in clinical trials and clinical practice; it is the scoring method used as a companion diagnostic for ICIs in various solid tumor types, including head and neck squamous cell carcinoma.21
As previously described, with the first scoring system, overall tissue expression of PD-L1 and of PD-L2 was graded on a 0 to 5 scale. Zero indicated no positive cells; 1 rare individual positive cells; 2 infrequent small clusters of positive cells within or directly adjacent to tumor tissue; 3 a single large cluster, multiple smaller clusters, or a moderately dense diffuse infiltration; 4 indicated a single very large dense cluster, multiple large clusters or dense diffuse infiltration; and 5 designated staining of coalescing clusters or dense infiltration throughout the tumor tissue. For each sample, an assessment was also made on a 0 to 5 scale of whether the PD-L1 or PD-L2 immunostaining was present or absent in the tumor cells, stromal cells, or endothelial cells. Overall CD8 immunostaining was graded on a 0 to 3 scale, with 0 indicating no infiltration: 1 indicating low, 2 moderate, and 3 high levels of infiltration. A separate 0 to 3 score was given for CD8 staining in the tumor and in the stroma.
The CPS was determined as follows: the proportion of all PD-L1-expressing cells (tumor, macrophage, and lymphocytes) over the total number of viable tumor cells × 100 was assessed. A sample was considered positive if the score was ≥1 and negative if the score was <1.21
Immunohistochemical results were analyzed about clinical characteristics, including patient sex, tumor origin, tumor size, patient outcome, history of radiation, and tumor histopathology. Statistical analyses were performed with TIBCO Statistica 13.5 (Palo Alto, CA). Cross-tabulation with the Fisher exact test was used for comparing categorical variable. Ordinal scores were compared between groups using 1-way analysis of variance. Relationships between ordinal variables were tested using Pearson and Spearman correlation. A p value of <0.05 was considered significant.
RESULTS
PD-L1, PD-L2, and CD8 Expression in Tumor and Stroma
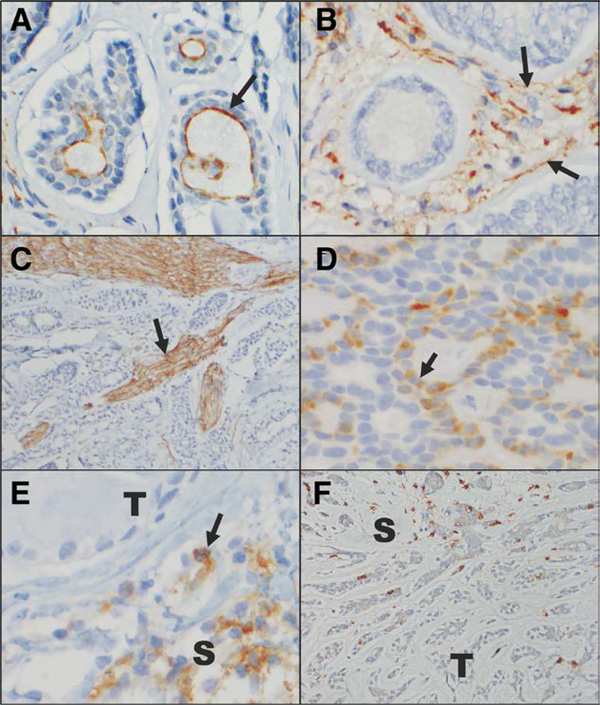
Twenty-three cases of ACC involving the orbit were identified; 13 were of lacrimal gland origin, 5 were of sinonasal origin with extension or local recurrence in the orbit, and 5 were orbital ACCs of unspecified primary location. Seventeen of 23 (74%) cases displayed tubular and/or cribriform morphology, while 6 were solid type (basaloid) or contained a component of solid type. Immunostaining for PD-L1 expression revealed low overall tissue expression levels of PD-L1 (mean 1.4±0.9) (Table 1). Similarly, PD-L1 expression was low in the different tissue subcompartments when graded on a 0 to 5 scale (mean 0.73±1.1 in tumor cells, mean 1.1±1.1 in the associated stroma, and mean 0.96±1.1 in endothelial cells) (Fig. 1A, B, see Fig. 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A218). With the CPS, which is a less stringent scoring system, PD-L1 expression was positive in 13 (57%) cases and negative in 10 (43%). The PD-L1 expression on tumor cells was membranous, with a predominance of expression on the apices of cells. Curiously, the current anti-PD-L1 antibody also prominently highlighted nerves, a likely artifact of this clone (Fig. 1C).
TABLE 1.
Expression of PD-L1 and PD-L2, and CD8 T-lymphocyte infiltration
| Marker | Degree of expression (mean ± standard deviation); n = 23 samples |
|---|---|
| PD-L1 overall* | 1.4 ± 0.9 (n = 23); expression was 0 in 2 samples |
| PD-L1 tumor | 0.73 ± 1.1 |
| PD-L1 stroma | 1.1 ± 1.1 |
| PD-L1 endothelium | 0.96 ± 1.1 |
| PD-L2 overall† | 0.83 ± 1.1 (n = 23); expression was 0 in 4 samples |
| PD-L2 tumor | 0.22 ± 0.85 |
| PD-L2 stroma | 0.87 ± 1.2 |
| PD-L2 endothelium | 0.04 ± 0.21 |
| CD8 overall‡ | 1.1 ± 0.51 (n = 23); expression was 0 in 2 samples |
| CD8 tumor | 0.39 ± 0.5 |
| CD8 stroma | 1.3 ± 0.62 |
| Combined positive score | Degree of expression (mean ± standard deviation); n = 23 samples |
| PD-L1 ≥ 1 | 57% (n = 13 samples) |
| PD-L1 < 1 | 43% (n = 10 samples) |
PD-L1 scored on a 0–5 scale.
PD-L2 scored on a 0–5 scale.
CD8 scored on a 0–3 scale.
PD-L, programmed celi death ligand.
FIG. 1.
Immunohistochemical staining for PD-L1, PD-L2, and CD8 in adenoid cystic carcinoma. A, PD-L1 positively immunnonstains the apical membranes of some tumor cells (arrow). B, PD-L1 positively immunostains the stromal cells (arrow) surrounding some tumors. In this case, the tumor cells do not express PD-L1. C, This anti-PD-L1 antibody also prominently highlights nerve fascicles in a number of the tumor samples examined (arrow). D, PD-L2 positively immunostains the cytoplasm and membranes of some tumor cells (arrow). E, PD-L2 positively immunostains clusters of S. In this case, the T do not express PD-L2. F, Rare CD8-positive T-lymphocytes are present infiltrating the tumor, but greater numbers are appreciated in the surrounding stroma (A–F, immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, original magnification ×40, ×40, ×10, ×40, ×40, ×20, with additional optical magnification of ×3 for A–E and ×2 for F). PD-L, programmed cell death ligand; S, stromal cells; T, tumor cells.
Expression levels of PD-L2 were similarly overall low (mean 0.83±1.13), with low expression levels in tumor cells (mean 0.22?±?0.85), low levels in the associated stroma (mean 0.87±1.2) and in endothelial cells (mean 0.04±0.21) (Table 1, Fig. 1D, F; see Fig. 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A218). Immunostaining for CD8 revealed an overall low level of CD8-positive T-lymphocyte infiltration (mean 1.1±0.51). Lymphocytes were scarce in the tumor (mean 0.39±0.5) and more numerous in the associated stroma (mean 1.3±0.62) (Table 1, Fig. 1F, see Fig. 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A218). Three tissue samples did not express any PD-L1, while 12 did not express any PD-L2. In summary, PD-L1 and PD-L2 are expressed in ACC, but the degree of their expression is low.
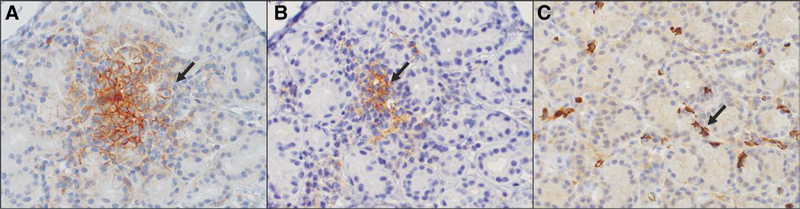
Three normal human lacrimal glands were immunostained for the same markers as the tumors (Fig. 2A–C). Expression of PD-L1, PD-L2, and CD8-positive T-lymphocytes was similarly low in the control lacrimal gland tissue, but the number of samples was too small to draw any meaningful conclusions. Programmed cell death ligand 1 expression, when present, was membranous. The number of immunostained lacrimal glands was similarly too small to draw statistical conclusions about differences in PD-L1, PD-L2, and CD8 expression of ACC compared with normal tissue.
FIG. 2.
Immunohistochemical staining for PD-L1, PD-L2, and CD8 in normal lacrimal gland. A, PD-L1 positively immunostains occasional lacrimal gland acini in a membranous pattern (arrow). B, PD-L2 positively immunostains the cytoplasm and membranes of small clusters of stromal cells (arrow) distributed among the lacrimal gland acini. C, Low numbers of CD8-positive T-lymphocytes are scattered throughout the lacrimal gland (A–C, immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, original magnification ×20, ×20, ×20, with additional ×3 optical magnification for A–C). PD-L, programmed cell death ligand.
Clinical Correlations With PD-L1, PD-L2, and CD8 Expression
The immunohistochemical data were examined within the context of the clinical characteristics including tumor origin, patient sex, tumor histopathology, exposure to preceding radiation, patient survival, and Tumor, Node, Metastasis stage to determine whether any relationships could be derived. Comparative analyses were performed for both the CPS and for the more finely graded score. Clinical data were available for 18 of the 23 patients. It should be noted that due to small sample size, these analyses may be underpowered to detect certain relationships.
The mean age of the 18 patients at the time of initial tumor diagnosis was 40.1 years (± 19.7 years) (Table 2). Nine patients were male and 9 were female. Thirteen tumors were of primary lacrimal gland origin, while 5 arose in the lacrimal sac or were of sinonasal origin with secondary extension into the orbit. Ten biopsies had been obtained prior to any radiation therapy; 4 biopsies were performed within a month of radiation (“immediately”), while 4 were performed several years (ranging from 6 to 18 years) after preceding radiation. The radiation histories are detailed in the Materials and Methods section. Twelve tumors (67%) were at T2 stage or less, while 6 (33%) were at greater than T2 stage. Six patients died of their disease at a median of 12.9 years (standard deviation = 9.2 years) after completion of initial therapy. Eleven patients are alive at a median of 9.1 years (standard deviation = 7.1 years) after completion of initial therapy (Table 2). Of the 11 who are alive, 9 have not experienced a recurrence or metastasis.
TABLE 2.
Patient clinical characteristics (n = 18)
| Clinical characteristic | |
|---|---|
| Age at diagnosis | 40.1 ± 19.7 |
| Sex | |
| Male | 9 (50%) |
| Female | 9 (50%) |
| Tumor origin | |
| Lacrimal gl and | 13 (72%) |
| Sinonasal or lacrimal sac | 5 (28%) |
| Timing of biopsy | |
| Before radiation | 10 (56%) |
| Immediately after radiation (1–4 weelcs) | 4 (22%) |
| Years after radiation (6–18 years) | 4 (22%) |
| Tumor stage | |
| T2 or less | 12 (67%) |
| Greater than T2 | 6 (33%) |
| Patient survival (years) | |
| Died | 12.9 years median (standard deviation 9.2 years; n = 6) |
| Alive (with or without recurrence) | 9.1 years median (standard deviation 7.1 years; n = 11) |
There were no associations found between PD-L1, PD-L2, or CD8 expression and tumor origin (lacrimal gland vs. nonlacrimal gland from surrounding sinuses), patient gender (male vs. female), Tumor, Node, Metastasis stage (≤T2 vs. >T2), or patient outcome (alive without recurrence vs. died or tumor recurred) for both the CPS and the more finely graded score (see Table 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A219).
A relationship was identified between the expression of PD-L1 in the stroma and tumor histopathology. Tumors with tubular-cribriform histopathology tended to have a greater expression of stromal PD-L1 (mean 1.35, standard deviation 1.17) as compared with tumors that contained a solid component (mean 0.33, standard deviation 0.52; p = 0.05 (see Table 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A219). No other relationships were identified between immunohistochemical markers and tumor histopathology.
A relationship was also identified between PD-L1 expression and tumor exposure to radiation with both the CPS and the more refined score. Tumors were separated into 3 groups, the first without radiation prior to the biopsy, the second with radiation immediately preceding the biopsy (1–4 weeks), and the third with radiation years before the biopsy (6–18 years). A significant relationship was identified with the PD-L1 CPS (p = 0.02) (see Table 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A219). Eight of 10 (80%) tumors without prior radiation had PD-L1 scores of <1, while 4 of 4 (100%) tumors with radiation years prior had PD-L1 expression of ≥1. Of the tumors that had radiation immediately prior to biopsy, 2 had PD-L1 expression of <1, while 2 had PD-L1 expression of ≥1. A similar association was found with the more refined scale. Tumor PD-L1 expression was significantly greater in tumors that had radiation many years before biopsy (mean 2.25, standard deviation 1.71) as compared with tumors that had no prior radiation (mean 0.89, standard deviation 1.23), p = 0.03. No other significant relationships were identified between the expression of histopathologic markers and radiation history.
No relationships were observed between overall PD-L1 and PD-L2 expression or overall PD-L1 and CD8 expression; however, a correlation was observed between overall PD-L2 and overall CD8 expression (see Table 2, Supplemental Digital Content, available at http://links.lww.com/IOP/A220).
DISCUSSION
Despite surgical excision and radiotherapy, a significant number of patients with ACC develop local recurrences and metastases.11,22–24 Given the successes in treating other malignancies with checkpoint inhibitor drugs, there has been hope that these drugs may find usefulness in the treatment of lacrimal gland and orbital ACC. Expression of checkpoint inhibitor markers such as PD-L1, tumor infiltration with CD8 T-lymphocytes, and tumor mutational burden have been discovered to correlate with tumor response to immunotherapy; PD-L1 expression has been used in deciding whether patients are eligible for participating in clinical trials with checkpoint inhibitor drugs.1,3,4,25–27
Our current study found that PD-L1 and PD-L2 are both expressed in very low levels in ACC involving the orbit (overall mean 1.4±0.9 of 5 for PD-L1, and overall mean 0.8±1.1 of 5 for PD-L2). In addition, the number of CD8-positive infiltrating T-lymphocytes was low (overall mean 1.1±0.51 of 3). Occasional tumors focally expressed higher levels of PD-L1 or PD-L2. These results, in conjunction with the overall relatively low mutational burden of ACC,20,22,28,29 suggest that although a small subset of tumors may have the potential to respond to checkpoint inhibitors, most will not. The CPS for PD-L1, on the other hand, revealed that 57% of tumors had scores of =1, which in clinical practice would indicate that up to 57% could be eligible for treatment with checkpoint inhibitors.
Experience with checkpoint inhibitor drugs for nonophthalmic ACC has been greater with head and neck tumors; several studies have already examined the expression of PD-L1 and PD-L2 in such cases (Table 3). Three studies have examined the expression of both PD-L1 and PD-L2,12–14 while 3 have explored the expression of PD-L1 alone.15–17 Four studies discovered no expression of PD-L1 in ACC tumor cells, while 2 identified very low levels of expression of PD-L1. These studies suggested a limited role for PD-L1 inhibitors with respect to ACC. The 3 studies that examined PD-L2 found positive expression in 45% to 100% of samples. The latter suggested a possible role for PD-L2 or PD-1 inhibitors in the treatment of ACC. The results of the foregoing studies contrast with the results of our study in which higher expression of PD-L1 was seen over PD-L2. Possible explanations for the varying results include differences in the anti-PD-L1 and anti-PD-L2 antibodies used and the various scoring methods.
TABLE 3.
Review of previous studies of PD-L1 or PD-L2 expression in adenoid cystic carcinoma
| Author, year citation | Tumors studied | Positive PD-L1 expression (no. positive/total) | Positive PD-L2 expression (no. positive/total) | CD8 expression |
|---|---|---|---|---|
| Mosconi et al. (2019)12 | 36 ACCs | Greater than 1% expression by IHC: | Greater than 10% expression by IHC: | Intratumoral: |
| −10 parotid | Low: 19/36 | |||
| −9 sublingual | ACC tumor PD-L1: 0/36 | ACC tumor PD-L2: 36/36 | High: 17/36 | |
| −5 submandibular | ACC stroma PD-L1; 0/36 | ACC stroma PD-L1; 29/36 | Intrastromal: | |
| −4 palate | Low: 22/36 | |||
| −4 tongue | High: 14/36 | |||
| −4 jugal mucosa (primary and recurrences) | ||||
| Nakano et al. (2019)13 | 11 ACCs (out of 30 head and neck salivary gland tumors) | Greater than 1% expression by IHC: | Positive by in situ hybridization | ACC: |
| Low: 11/11 | ||||
| ACC tumor PD-L1: 0/11 | ACC tumor PD-L2: 5/11 | High: 0/11 | ||
| Non-ACC tumor PD-L1: 11/19 | Non-ACC tumor PD-L2: 6/17 | Non-ACC: Low: 9/19 High: 10/19 | ||
| Sridliaran et al. (2016)14 | 28 ACCs (from 21 patients) (mixture of primary and metastatic tumors to the lung—primary site unspecified; 7 patients had both primary and metastatic tumor sample) | Greater than 5% expression by IHC: | Greater than 10% expression by IHC: | High: 42% |
| ACC tumor PD-L1: 0/28 | ACC tumor PD-L2: | |||
| −9/15 of primary | ||||
| −8/11 of metastatic | ||||
| Mukaigawa et al. (2016)15 | 53 ACCs (out of 219 salivary gland tumors of oral cavity ) | Greater than 5% expression by IHC: | Not studied | Unspecified |
| ACC tumor PD-L1: 1/53 | ||||
| ACC TIMC PD-L1 : 0/53 | ||||
| Tapias et al. (2019)16 | 14 ACCs (out of 23 primary tracheal tumors) | Greater than 1% expression by IHC: ACC tumor: 0/14 | Not studied | Low: 14/14 |
| High: 0/14 | ||||
| Harada et al. (2018)17 | 25 ACCs (out of 47 malignant salivary gland tumors) | Greater than 5% expression by IHC: PD-L1 tumor: 11/25 ACC | Not studied | Unspecified |
ACC, adenoid cystic carcinomas; IHC, immunohistochemistry; PD-L, programmed cell death ligand; TIMC, tumor-infiltrating mononuclear cell.
Many different scales have been used in the literature and in clinical trials to assess tumor expression levels of PD-L1 and PD-L2, some more complex than others. For PD-L1, the most widely used scale in clinical practice now is either the tumor proportion score or CPS, the latter of which grades the overall number of positive cells in both the tumor and surrounding stroma as ≥1 or <1.21 Although easily applied in clinical practice, this scale does not provide the detailed information of other scales5 that may be more advantageous for advancing basic research. For PD-L2, there is still significant variability in terms of which scales are used. For the purposes of the current study, a finely graded scale was applied to both PD-L1 and PD-L2 scoring, while also applying the commonly used CPS for PD-L1.
With regard to the current study, PD-L1, PD-L2, and CD8 expression and potential relationships to clinical characteristics such as patient gender, tumor origin (lacrimal gland vs. nonlacrimal gland sinonasal origin), Tumor, Node, Metastasis stage, patient outcome (no recurrence, local recurrence or metastasis), tumor histopathology (tubular-cribriform vs. any component of solid type), and radiation prior to biopsy (no radiation vs. radiation immediately prior vs. history of radiation several years prior) were explored. The current study lacks the power to draw strong conclusions based on the small sample size (only 18 patients had clinical data for analysis); however, several interesting trends were identified that may warrant closer examination in future studies.
The first association that warrants closer investigation is the relationship between tumor histopathologic subtype and PD-L1 expression. The current study found that PD-L1 expression in the stroma of tumors with tubular-cribriform histopathology was greater than in the stroma of tumors with a solid-type component (p = 0.05) (see Table 1, Supplemental Digital Content, available at http://links.lww.com/IOP/A219). Tumors with tubular-cribriform histopathology in general portend a better clinical course than those with solid-type histopathology.23 This result may further indicate that tubular-cribriform tumors may respond better to checkpoint inhibitor drugs than solid-type tumors.
The second association gleaned from the current study warranting further investigation is the relationship between radiation and PD-L1 expression. Both the CPS and the more refined scale that the authors employed established that tumors that had previous radiation treatment displayed a significantly greater PD-L1 expression than tumors that had not had exposure to radiation (p = 0.01 and p = 0.02). Of interest was the fact that tumors that had been treated many years earlier with radiation maintained an increased level of PD-L1 expression. The relationship between PD-L1 expression and radiation is of clinical importance in patient treatment and is an area of active exploration in the Immuno-oncology field. Studies in non-ACC human tissues and in animal models have demonstrated that PD-L1 and other immune checkpoints are transiently elevated after radiation and that this effect can be dose-dependent.30,31 Moreover, combining radiation with checkpoint inhibitor drugs can result in a synergistic effect, allowing tumors that are usually minimally responsive to radiation or checkpoint inhibitors alone to be retarded and prolonging survival.32 Such findings suggest that tumors like ACC, which have inherently low mutational burden, low PD-L1 expression, and low levels of CD8 T-lymphocyte infiltration, might become responsive to ICIs if the drug is administered soon after pretreatment with radiation.
Clinical experience with ICIs in the treatment of ACC has been even more limited than immunohistochemical studies. The CTLA-4 inhibitor, ipilimumab, has been used to treat metastatic ACC in only 1 case33; the patient exhibited a slight clinical response to treatment but then developed a granulomatous dacryoadenitis. Programmed cell death ligand 1 immunostaining was performed and revealed 0% membranous staining. In another case,34 PD-1 inhibitor pembrolizumab was used in combination with radiotherapy to treat ACC of the parotid gland that had metastasized to the brain; the long-term outcome in this case is unknown.
Two patients with metastatic ACC have been treated with the PD-1 inhibitor pembrolizumab in a phase 1b clinical trial.35 KEYNOTE-028 is a large clinical study in which patients with many different metastatic tumors not responsive to other therapies have been enrolled. To qualify, patients had to have a PD-L1 positivity level of membranous staining in >1% of scorable cells in the tumor samples. Of 102 patients with head and neck tumors that were screened, 33 patients manifested PD-L1 positivity; 26 were eventually enrolled in the study. Of those enrolled, only 2 patients had ACC. Three enrolled patients demonstrated a partial response to treatment, but they were not patients with ACC.35
A phase II clinical trial was performed at the Dana Farber Cancer Center and the MGH to assess the effects of pembrolizumab with or without radiation in ACC (NCT03087019; study number 16–609; Pembrolizumab With or Without Radiation in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma). The interim analysis revealed that pembrolizumab monotherapy or with hypofractionated radiation therapy was well tolerated.36 No objective responses were observed; however, prolonged stable disease was achieved in a subset of patients.
The currently available clinical results reinforce the concept that checkpoint inhibitors may have a limited beneficial role in a small subset of patients with ACC. For most patients, however, other therapeutic avenues will need to be evaluated.37
The poor response of certain tumors such as ACC to immunotherapy may in part be due to the low number of associated inflammatory cells37 and the absence of immunogenic tumor antigens due to a low mutational tumor burden.19,20 Our study, along with others,12–16 has demonstrated low numbers of tumor-associated inflammatory cells in ACC. Some investigators have speculated that treatment with radiation may potentiate the effects of immunotherapies by increasing the inflammatory cell infiltrate,30–32 but this remains an unresolved issue and may depend on the dose of radiation administered.
Supplementary Material
Footnotes
The authors have no financial or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.op-rs.com.).
REFERENCES
- 1.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- 2.Pai SI, Zandberg DP, Strome SE. The role of antagonists of the PD-1:PD-L1/PD-L2 axis in head and neck cancer treatment. Oral Oncol 2016;61:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Xu J, Du C, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol 2019; 9:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolkow N, Jakobiec FA, Afrogheh AH, et al. Programmed cell death 1 ligand 1 and programmed cell death 1 ligand 2 are expressed in conjunctival invasive squamous cell carcinoma: therapeutic implications. Am J Ophthalmol 2019;200: 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagarajan P, El-Hadad C, Gruschkus SK, et al. PD-L1/PD1 expression, composition of tumor-associated immune infiltrate, and HPV status in conjunctival squamous cell carcinoma. Invest Ophthalmol Vis Sci 2019;60:2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S, Yu H, Fu G, et al. Programmed death receptor ligand 1 expression in eyelid sebaceous carcinoma: a consecutive case series of 41 patients. Acta Ophthalmol 2019;97:e390–e396. [DOI] [PubMed] [Google Scholar]
- 8.Kandl TJ, Sagiv O, Curry JL, et al. High expression of PD-1 and PD-L1 in ocular adnexal sebaceous carcinoma. Oncoimmunology 2018;7:e1475874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaraj P, Sen S. Evaluation of PD-L1 and PD-1 expression in aggressive eyelid sebaceous gland carcinoma and its clinical significance. Indian J Ophthalmol 2019;67:1983–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen RC, Lawson BM, Jody NM, et al. The programmed death pathway in ocular adnexal sebaceous carcinoma. Ophthalmic Plast Reconstr Surg 2019. [DOI] [PubMed]
- 11.Wolkow N, Jakobiec FA, Lee H Long-term outcomes of globe-preserving surgery with proton beam radiation for adenoid cystic carcinoma of lacrimal gland. Am J Ophthalmol 2019;201:84–85. [DOI] [PubMed] [Google Scholar]
- 12.Mosconi C, de Arruda JAA, de Farias ACR, et al. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol 2019;88:95–101. [DOI] [PubMed] [Google Scholar]
- 13.Nakano T, Takizawa K, Uezato A, et al. Prognostic value of programmed death ligand-1 and ligand-2 co-expression in salivary gland carcinomas. Oral Oncol 2019;90:30–37. [DOI] [PubMed] [Google Scholar]
- 14.Sridharan V, Gjini E, Liao X, et al. Immune profiling of adenoid cystic carcinoma: PD-L2 expression and associations with tumor-infiltrating lymphocytes. Cancer Immunol Res 2016;4:679–687. [DOI] [PubMed] [Google Scholar]
- 15.Mukaigawa T, Hayashi R, Hashimoto K, et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol 2016;114:36–43. [DOI] [PubMed] [Google Scholar]
- 16.Tapias LF, Shih A, Mino-Kenudson M, et al. Programmed death ligand 1 and CD8+ immune cell infiltrates in resected primary tracheal malignant neoplasms. Eur J Cardiothorac Surg 2019;55:691–698. [DOI] [PubMed] [Google Scholar]
- 17.Harada K, Ferdous T, Ueyama Y. PD-L1 expression in malignant salivary gland tumors. BMC Cancer 2018;18:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stagner AM, Afrogheh AH, Jakobiec FA, et al. p16 Expression is not a surrogate marker for high-risk human papillomavirus infection in periocular sebaceous carcinoma. Am J Ophthalmol 2016;170:168–175. [DOI] [PubMed] [Google Scholar]
- 19.Sant DW, Tao W, Field MG, et al. Whole exome sequencing of lacrimal gland adenoid cystic carcinoma. Invest Ophthalmol Vis Sci 2017;58:BIO240–BIO246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho AS, Kannan K, Roy DM, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet 2013;45:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019;143:330–337. [DOI] [PubMed] [Google Scholar]
- 22.Tse DT, Kossler AL, Feuer WJ, et al. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology 2013;120:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolkow N, Jakobiec FA, Lee H, et al. Long-term outcomes of globe-preserving surgery with proton beam radiation for adnoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol 2018;195:43–62. [DOI] [PubMed] [Google Scholar]
- 24.Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck–an update. Oral Oncol 2015;51: 652–661. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y, Liang H, Hu J, et al. PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer 2018;9:2938–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 2015;3:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahal M, Pleasance E, Grewal J, et al. Personalized oncogenomic analysis of metastatic adenoid cystic carcinoma: using whole-genome sequencing to inform clinical decision-making. Cold Spring Harb Mol Case Stud 2018;4:a002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Zhao BR, Liu SQ, et al. Mutational landscape and clonal diversity of pulmonary adenoid cystic carcinoma. Cancer Biol Ther 2018;19:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J, Le TQ, Massarelli E, et al. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer 2018;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly RJ, Zaidi AH, Smith MA, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg 2018;268:992–999. [DOI] [PubMed] [Google Scholar]
- 32.Oweida A, Lennon S, Calame D, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 2017;6:e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ileana Dumbrava E, Smith V, Alfattal R, et al. Autoimmune granulomatous inflammation of lacrimal glands and axonal neuritis following treatment with ipilimumab and radiation therapy. J Immunother 2018;41:336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garber ST, Khoury L, Bell D, et al. Metastatic adenoid cystic carcinoma mimicking butterfly glioblastoma: a rare presentation in the splenium of the corpus callosum. World Neurosurg 2016;95:621.e13–9. [DOI] [PubMed] [Google Scholar]
- 35.Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol 2018. February 21. doi: 10.1097/COC.0000000000000429. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenfeld J, Mahmood U, Chen YH, et al. A randomized phase II study of pembrolizumab with or without radiation in patients with recurrent or metastatic adenoid cystic carcinoma. ASCO Annual Meeting; June 1, 2019, 2019; Chicago, IL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrarotto R, Heymach JV, Glisson BS. MYB-fusions and other potential actionable targets in adenoid cystic carcinoma. Curr Opin Oncol 2016;28:195–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.